Abstract
Background
There are no established reports about the expression of the Piwil gene, a subfamily of the Piwi gene involved in RNA silencing and self-renewal, in colorectal carcinomas. It is known that the degree of PIWIL2 expression is higher in colorectal carcinomas. But its clinicopathologic significance remains undetermined. This study reassessed the relationship between PIWIL2 expression and the clinicopathologic parameters in colorectal carcinomas.
Methods
An immunohistochemistry of PIWIL2 expression was done in 60 cases of colorectal carcinoma. This was followed by an analysis of the correlation between PIWIL2 expression and clinicopathologic features and a survival analysis.
Results
There were 44 cases (73.3%) where the degree of PIWIL2 expression was relatively higher. The high degree of PIWIL2 expression was significantly correlated with the lower degree of differentiation (p=0.039), deep invasion (p=0.019) and perineural invasion (p=0.027). The overall survival was longer in patients with the lower degree of PIWIL2 expression than in those with the higher degree of PIWIL2 expression.
Conclusions
Our results showed that the degree of PIWIL2 expression was relatively higher in colorectal carcinomas and it was significantly correlated with variable clinicopathologic indicators for a poor prognosis. This indicates that PIWIL2-positive cells contribute to the progression of colorectal cancer.
Keywords: PIWIL2, Colorectal cancer, Piwi gene
Recently, much interest has centered on stem cells and their ability to predict outcome and response to treatment, the hallmarks of biomarkers in various diseases. Similarly, interest has also focused on colorectal carcinoma, one of the leading causes of cancer mortality, despite remarkable advances in cancer diagnostics and treatment.1
Various proteins and enzymes for stem cells have been detected and then tested to examine whether they are useful biomarkers, one of which is the Argonaute gene family.2 Humans have eight ARGONAUTE-like proteins, four of which fall into the eIF2C/AGO subfamily (EIF2C1/hAGO1, EIF2C2/hAGO2, EIF2C3/hAGO3, and EIF2C4/hAGO4), while the remainder are in the PIWI subfamily (PIWIL1/HIWI, PIWIL2/HILI, PIWIL3, and PIWIL4/HIWI2).3,4 To date, several human ARGONAUTE proteins have been identified. But relatively little is known about their function in human disease. Several studies have detected Piwi genes in various types of human and animal tumor cell lines, thus making them potential biomarkers for various types of cancer. These biomarkers include prostate, breast, gastrointestinal, ovarian and endometrial cancers in humans and breast tumors, rhabdomyosarcoma and medulloblastoma in mice.5-7 To date, however, only a limited number of reports have been made on the PIWIL2 expression in colorectal cancer.8 In addition, no comprehensive studies have been conducted to determine the clinical significance of PIWIL2 expression in colorectal cancer. Li et al.8 first conducted a systematic immunohistochemical study of human ARGONAUTE proteins in a cohort of 75 colon cancer specimens. According to these authors, there was a significant correlation between an increased expression of PIWIL2 and the occurrence of colon cancer tissue. Furthermore, these authors also noted that PIWIL2 might be a novel marker with early diagnostic significance in patients with colorectal cancer.
Given the above background, we conducted this comprehensive study to determine the correlation between PIWIL2 expression and a set of clinicopathologic parameters including the rate and duration of patient survival based on the immunohistochemical findings in patients with colorectal cancer.
MATERIALS AND METHODS
Patient selection and sample collection
Primary tumor samples were collected from 60 patients (n=60) who had been diagnosed with colorectal adenocarcinoma at Kosin University Gospel Hospital in Busan, Korea. The patients had undergone a colectomy between January 2006 and December 2010, where cases were identified retrospectively from clinicopathologic data. Inclusion criteria were the histopathologic diagnosis of colorectal adenocarcinoma, the availability of clinical follow-up data and the availability of paraffin-embedded tissue specimens. The following clinicopathologic characteristics were evaluated for their relevance to protein expression or long-term survival: age (>65 years vs ≤65 years), sex, tumor location (proximal, distal, and rectal), invasion depth (T1, T2 vs T3, T4), pathologic stage (p-stage; I, II vs III, IV), lymph node status, distant metastasis, lymphatic invasion, venous invasion, and perineural invasion. The tumor differentiation grade was classified into two groups: the low-grade (well-to-moderately differentiated tumors) group and the high-grade (poorly-differentiated tumors) one. No patients received chemoradiation treatment preoperatively. Invasion depth and staging were determined using tumor, node and metastasis system of the American Joint Committee on Cancer.9 In our series, the median follow-up period was 28 months (range, 2 to 72 months). The overall survival (OS) was calculated from the date of surgery to the date of last follow-up or death (whichever occurred first).
Immunohistochemistry
For the immunohistochemical analysis, 4-µm thick sections were cut consecutively from the blocks. This was followed by deparaffinization in xylene and rehydration in a graded alcohol series. The following steps were performed by BOND-MAX autostaining system (Leica Microsystems, Wetzlar, Germany). Antigen retrieval was performed in pH 6.0 citratephosphate buffer using a microwave. The primary antibody against PIWIL2 was a rabbit polyclonal antibody against PIWIL2 (1:100, Abcam, Cambridge, UK). Slides were counter-stained with Meyer's hematoxylin. Negative controls were treated similarly without primary antibodies.
Evaluation of immunohistochemical staining
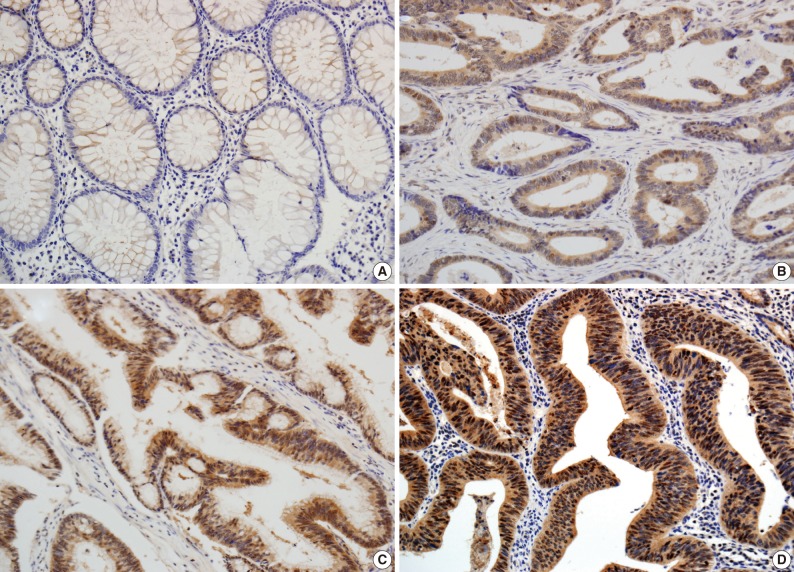
The scoring method of He et al.6 was used for the immunohistochemical staining evaluation of both the intensity and the proportion of reactive tumor cells. Slides were examined under low power (×40 to ×100) microscopy to identify the regions containing the highest percentage of PIWIL2-positive cells (hot spot) in the cancer nest. Ten hot spots inside the tumor tissue were selected, which was followed by a semi-quantitative evaluation of the expression of PIWIL2 with high power (×400) microscopy. The cell-positive score was graded: <5%, 0 (-); 5-25%, 1 (+); 25-50%, 2 (++); and >50%, 3 (+++). In order to evaluate the intensity of PIWIL2, tumor cells were compared with normal epithelium. Cytoplasmic and nuclear staining in tumor cells, whose staining property was stronger than that in normal epithelium, was considered positive. The staining intensity score was graded: 0, negative (-); 1, weak (+); 2, moderate (++); and 3, strong staining (+++) (Fig. 1). Immunoreactive scores were calculated by adding the cell-positive score and staining intensity score and then dividing by 2. Those with low (immunoreactive scores <2) and high (immunoreactive scores ≥2) were grouped separately for statistical analysis. The evaluation was performed twice, with the evaluator being blind to the clinical details.
Fig. 1.
Immunohistochemical analysis of PIWIL2 expression in colon cancer and adjacent non-cancer tissue (×200). PIWIL2 protein shows a negative expression in normal glands (A). In colon cancer tissue, PIWIL2 expression shows the cytoplasmic and cytonuclear pattern. The intensity of PIWIL2 expression is graded as weakly positive (B), moderately positive (C), and strongly positive (D).
Statistical analysis
Statistical analyses were performed using SPSS ver. 20.0 (SPSS Inc., Chicago, IL, USA). The correlation between protein expression and categorical variables was compared, if appropriate, using the Pearson χ2 test or Fisher exact probability test. Survival curves were estimated using the Kaplan-Meier method. The distribution of survival was comparatively studied using the log-rank test. Independent prognostic factors were determined using Cox's proportional hazard model. Statistical significance was set at p<0.05.
RESULTS
Clinicopathologic findings
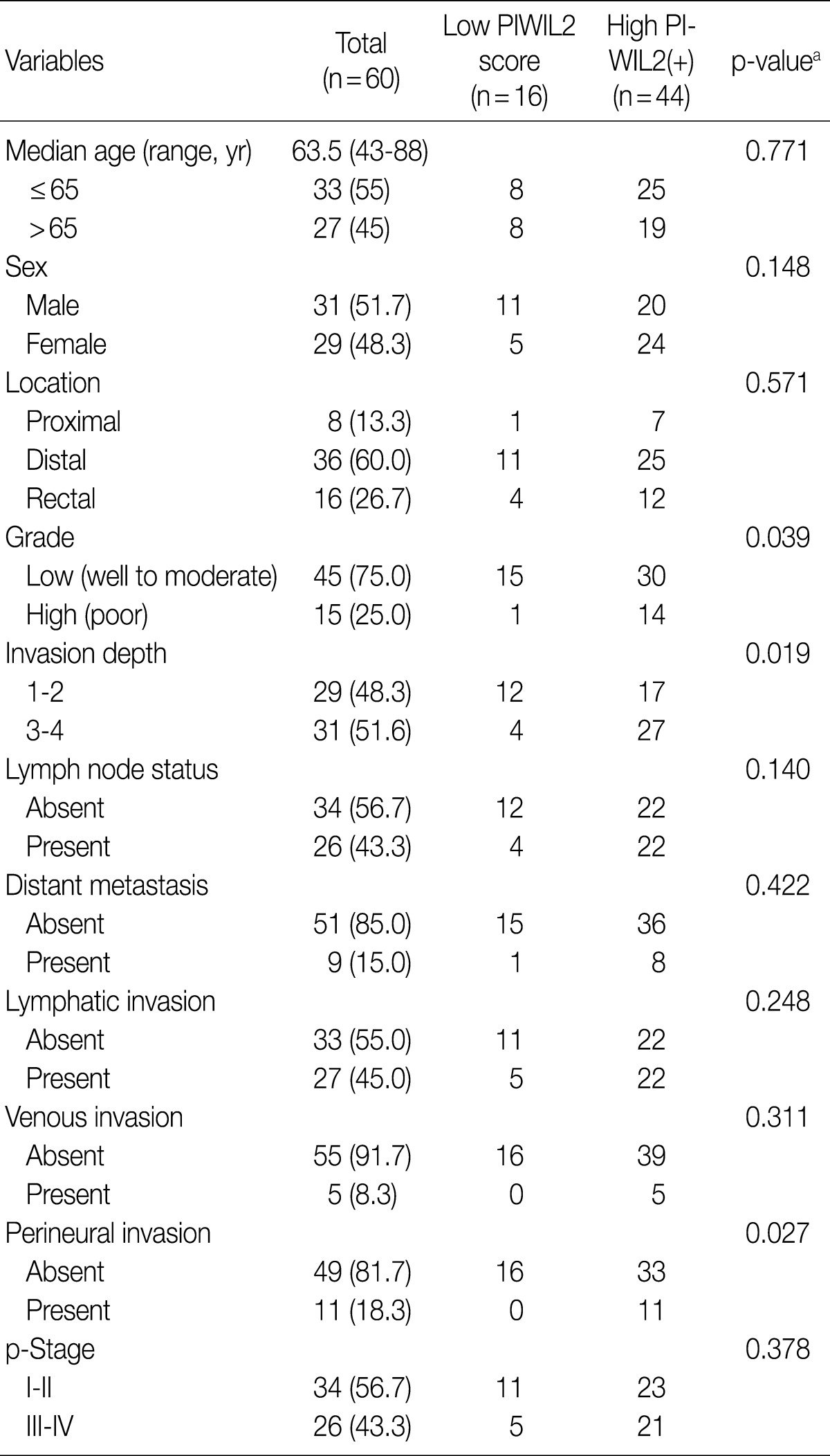
In this retrospective study, we reviewed clinicopathologic findings from the 60 patients (31 men and 29 women). The mean age was 63.5 years (range, 43 to 88 years) and the mean follow-up period was 28 months (range, 2 to 72 months). Of the 60 resected cases of colorectal cancer, the locations of the cancers were the proximal colon (from the cecum to the ascending colon) in 8 patients, the distal colon (from the transverse colon to the rectosigmoid junction) in 36 patients and the rectum in 16 patients. In addition, there were 15 cases of colorectal cancer with a high-grade differentiation. The invasion depth was limited (T1 or T2) in 29 patients and advanced (T3 or T4) in 31 patients. There were 26 cases of colorectal cancer with a regional lymph metastasis and 9 cases with a distant metastasis. In addition, there were 27 cases of lymphatic invasion, 5 cases of vascular invasion and 11 cases of perineural invasion. Furthermore, there were 26 cases of colorectal cancer with a high p-stage (III and IV) (Table 1).
Table 1.
Correlation between PIWIL2 expression and clinicopathologic parameters in patients with colorectal carcinoma
p-Stage, pathologic stage.
aFisher exact test, p<0.05.
Expression of PIWIL2
In the non-cancerous surface and glandular epithelium of the colon, immunohistochemistry was positive for PIWIL2; it was stained in the nuclear and cytonuclear pattern, whose intensity ranged from entirely negative to weakly positive. In the neoplastic glands, PIWIL2 showed the same immunohistochemistry expression as the adjacent non-cancerous glands but it had a much stronger intensity. In contrast, PIWIL2 expression was mainly seen in the cytoplasmic regions on the surface of tumor and in the cytonuclear pattern of the invasive front of the colorectal cancer. There were 44 cases (73.3%) of colorectal cancer with high degree of PIWIL2 expression of an immunoreactive score ≥2.
An independent comparison between PIWIL2 immunoreactivity and clinicopathologic characteristics
Table 1 shows the correlation between PIWIL2 expression and clinicopathologic features in our clinical series of patients with colorectal cancer. The degree of PIWIL2 expression was higher in patients with a higher T stage (p=0.019). The higher degree of PIWIL2 expression was significantly more correlated with the lower degree of differentiation as compared with the lower degree of PIWIL2 expression (p=0.039). In addition, there was a significant correlation between the high degree of PIWIL2 expression and perineural invasion (p=0.027). There were no significant differences in such clinicopathologic features as age, sex, location, tumor grade, lymph node status, distant metastasis, lymphatic or venous invasion, and p-stage between the group of patients with lower PIWIL2 scores and that of their counterparts with higher PIWIL2 scores. There was a tendency, however, that the higher expression of PIWIL2 protein was correlated with older age, distal location, lymph node metastasis, distant metastasis, lymphatic invasion, venous invasion, and adverse pathological stages.
Survival analysis
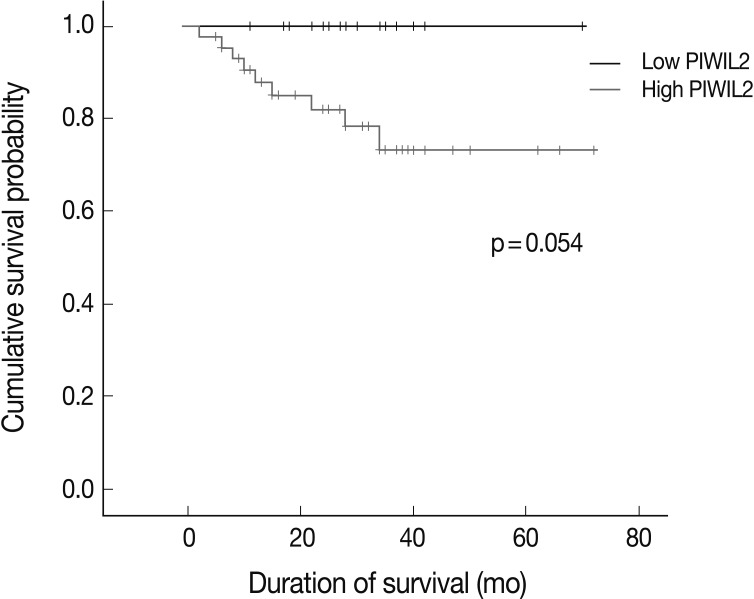
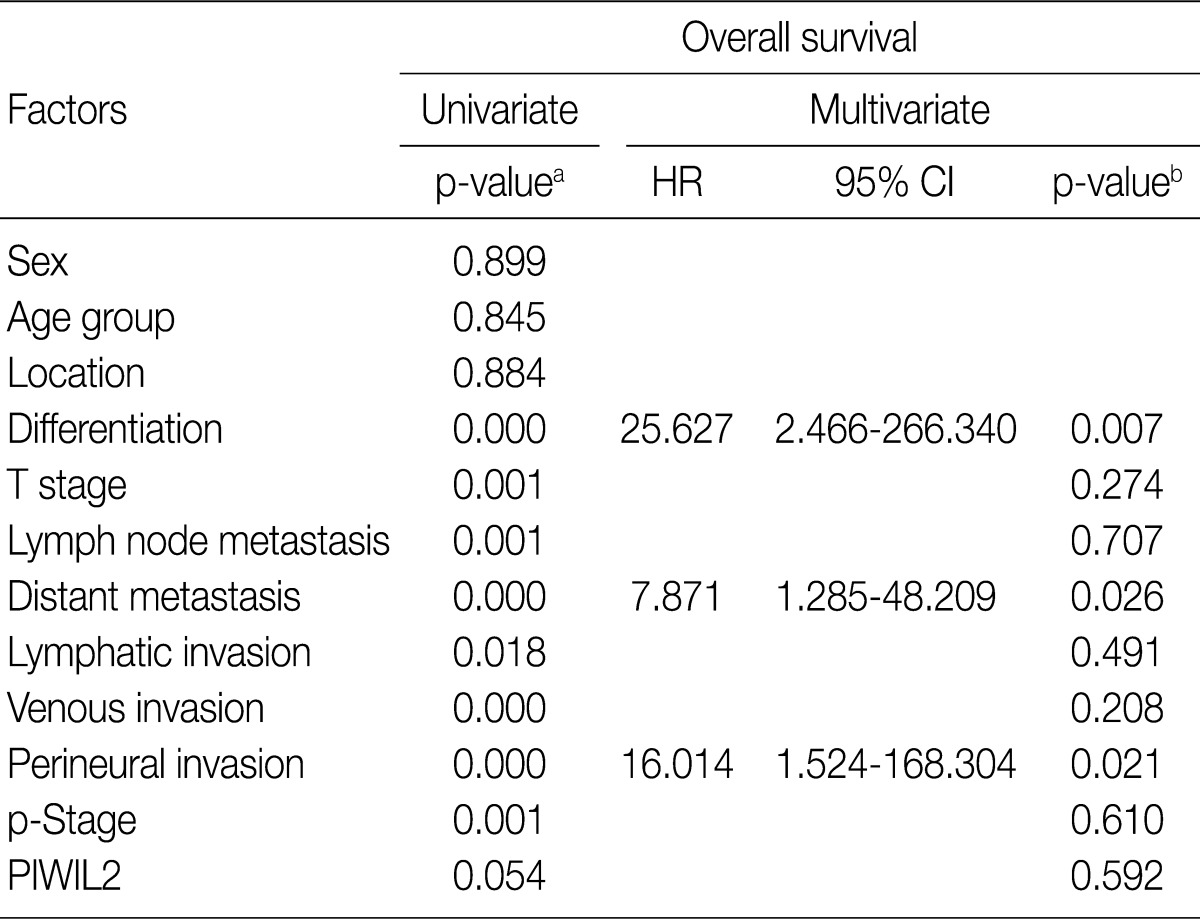
For the survival analysis, Kaplan-Meier curves were plotted for univariate analysis. At the time of analysis, there were 9 cases (15%) of cancer-specific deaths. Cancer-specific survival was highly correlated with tumor differentiation, depth of invasion, lymph node metastasis, distant metastasis, lymphatic invasion, venous invasion, perineural invasion, and p-stage. Patients with low PIWIL2 scores had a longer OS compared to their counterparts high PIWIL2 scores (median survival period, 28 months vs 12 months, respectively), although survival benefits showed a statistically borderline significance (p=0.054) (Fig. 2). Multivariate analysis using the Cox proportional hazard model was performed to evaluate an independent predictor in patients with colorectal cancer. As shown in Table 2, there was a correlation between the OS and various clinicopathologic parameters. The OS was poorly correlated with sex, age and tumor location. But it was highly correlated with tumor differentiation, T stage, lymph node metastasis, distant metastasis, lymphatic invasion, venous invasion, perineural invasion and p-stage. On multivariate analysis, tumor differentiation (p=0.007), distant metastasis (p=0.026), and perineural invasion (p=0.021) were significantly associated with low OS. On multivariate analysis, however, PIWIL2 was not an independent prognostic factor (p=0.592).
Fig. 2.
Univariate survival analysis in 60 cases of colorectal adenocarcinoma. Patients with low PIWIL2 scores have a longer overall survival than their counterparts with high PIWIL2 scores although survival benefits show a statistically borderline significance (p=0.054).
Table 2.
Univariate and multivariate analysis of overall survival
HR, harzard ratio; CI, confidence interval; p-Stage, pathologic stage.
aKaplan-Meier method using log-rank test, p<0.05; bCox proportional hazard model, p<0.05.
DISCUSSION
PIWIL2 belongs to the Piwi gene family, whose members are characterized by conserved PAZ and PIWI domains and play crucial roles in the self-renewal mechanism of stem and germ cells, RNA silencing and translational regulation in different organisms during evolution.4,10,11 These genes are highly conserved during evolution and play essential roles in stem-cell self-renewal, gametogenesis and RNA interference (RNAi) in diverse organisms ranging from Arabidopsis to humans.10,12
Recent studies have shown that PIWIL2 is widely expressed in various types of human cancer and these include prostate, breast, gastrointestinal, ovarian, and endometrial cancers. It has also been reported that it is also prevalently expressed in breast tumor, rhabdomyosarcoma, and medulloblastoma in an animal experimental model using mice.5-7 Several previous studies have reported that Piwi genes play an important role in the tumorigenesis of various cancer cells. Liu et al.13 showed that the proportion of PIWIL1-positive cells was increased from 10% in normal gastric tissues to 76% in gastric cancer. Some investigators have also studied Piwi gene expression in breast and cervical cancers, thus describing it as a highly promising factor for its application to the cancer diagnosis and treatment.6,7,14
Although identification of biomarkers for the detection of colon cancer is a critical issue for the treatment and cure of colon cancer, there are only a limited number of reports on PIWIL2 expression in colorectal cancers. Li et al.8 first conducted a systematic immunohistochemical study of human ARGONAUTE proteins in a cohort of 75 colon cancer specimens. According to these authors, ARGONAUTE proteins were over-expressed in colon cancer relative to its adjacent non-cancer tissue. These authors indicate that the expression of EIF2C2-4 and PIWIL4 might be increased in advanced tumors with a distant metastasis. This suggests that both genes might be involved in the promotion of tumor invasion. Furthermore, the above authors also suggested that the higher degree of PIWIL2 expression was significantly correlated with the occurrence of colon cancer and PIWIL2 might be a novel marker with early diagnostic significance in patients with colorectal cancer. But they failed to discover a significant correlation between Piwi expression and clinicopathologic parameters (age, sex, histological grade, lymph node status or p-stage). In our series, however, a positive immunohistochemistry of PIWIL2 was significantly correlated with a poorer differentiation of the tumor, direct tumor invasion and perineural invasion. The significance of PIWIL2 expression as a prognostic factor remains undetermined in patients with colorectal cancer despite its clear correlation with several clinicopathologic parameters of a prognostic value.
He et al.6 suggested that PIWIL2 be used as a biomarker for early detection of cervical cancers because it appeared in various stages of cervical neoplasia. In this study, the degree of PIWIL2 expression was relatively higher in adenocarcinoma tissues as compared with their adjacent non-neoplastic ones (Fig. 1). These findings indicate that PIWIL2 might play an important role in tumor development. But we failed to determine whether PIWIL2 expression could solely be used as a biomarker in making a diagnosis of early colon cancer. This is because we did not examine it in the precancerous lesions. Further extensive studies are therefore warranted to establish the diagnostic value of PIWIL2 including precancerous lesions such as adenoma in patients with early colon cancer.
How do ARGONAUTE proteins play an important role in colonic carcinogenesis? One possible mechanism is the regulation of PIWIL2 over the expression of genes related to proliferation and apoptosis. PIWIL2 mediates gene regulation through small RNA and methylation.15 A subset of small RNA species, termed Piwi-interacting RNAs, has been characterized in mammalian testis; these small RNAs interact with PIWI proteins (PIWIL1 and PIWIL2) to silence transpositional function in the germ cell genome.5 This implies that Piwi-interacting RNAs determines the specificity for DNA methylation, which is known to be a key component of gene regulation in cancer cells.
For the specific mechanism of PIWIL2-mediating gene regulation in colorectal tumorigenesis, at the molecular level, several studies on the Piwi family5,11,16 demonstrated that PIWIL2 acts as an oncogene by inhibition of apoptosis and promotion of proliferation via STAT3/Bcl-XL signaling. PIWIL2 activates the expression of Bcl-XL, a member of the bcl-2 gene family, which serves as regulators of cell death. PIWIL2 was also able to induce STAT3 expression, which functions as downstream effectors of cytokine and growth factor receptor signaling. Persistent signaling of specific STATs has been demonstrated to directly contribute to the oncogenesis by stimulating cell proliferation and preventing apoptosis. Lee et al.14 demonstrated that PIWIL2 is expressed predominantly in breast cancer stem cells modulating their proliferation and anti-apoptotic state through the STAT3/Bcl-XL signaling pathway. Our results support that PIWIL2 expression is associated with the progression of colorectal cancer. But further studies are warranted to identify correlation between PIWIL2 and other factors related to cell proliferation and apoptosis.
In this study, an immunohistochemistry was the only measure that we took to disclose the role of PIWIL2 in colorectal cancer. But the immunohistochemical findings are not necessarily consistent with the results of chromosomal analysis. Further complementary molecular studies are therefore warranted to advocate our results about PIWIL2 expression. This should particularly apply to the function of PIWIL2 as a cancer stem cell and the mechanisms by which PIWIL2 regulates the pathways of proliferation and anti-apoptosis, specifically through the STAT3/Bcl-XL signaling pathway.
In conclusion, our results showed that the degree of PIWIL2 expression was relatively higher in colorectal cancer tissues and it was significantly correlated with a variety of clinicopathologic indicators for a poor prognosis. This indicates that PIWIL2-positive cells contribute to the progression of colorectal cancer. Our study demonstrated that the PIWIL2 protein may be used as a prognostic factor in the context of colorectal cancer. But further studies are warranted to examine the clinical significance of PIWIL2 and its usefulness as a biomarker.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Gao JX. Cancer stem cells: the lessons from pre-cancerous stem cells. J Cell Mol Med. 2008;12:67–96. doi: 10.1111/j.1582-4934.2007.00170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmell MA, Xuan Z, Zhang MQ, Hannon GJ. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 2002;16:2733–2742. doi: 10.1101/gad.1026102. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki T, Shiohama A, Minoshima S, Shimizu N. Identification of eight members of the Argonaute family in the human genome small star, filled. Genomics. 2003;82:323–330. doi: 10.1016/s0888-7543(03)00129-0. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Schütte D, Wulf G, et al. Stem-cell protein Piwil2 is widely expressed in tumors and inhibits apoptosis through activation of Stat3/Bcl-XL pathway. Hum Mol Genet. 2006;15:201–211. doi: 10.1093/hmg/ddi430. [DOI] [PubMed] [Google Scholar]
- 6.He G, Chen L, Ye Y, et al. Piwil2 expressed in various stages of cervical neoplasia is a potential complementary marker for p16. Am J Transl Res. 2010;2:156–169. [PMC free article] [PubMed] [Google Scholar]
- 7.Liu JJ, Shen R, Chen L, et al. Piwil2 is expressed in various stages of breast cancers and has the potential to be used as a novel biomarker. Int J Clin Exp Pathol. 2010;3:328–337. [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Yu C, Gao H, Li Y. Argonaute proteins: potential biomarkers for human colon cancer. BMC Cancer. 2010;10:38. doi: 10.1186/1471-2407-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. pp. 143–164. [Google Scholar]
- 10.Unhavaithaya Y, Hao Y, Beyret E, et al. MILI, a PIWI-interacting RNA-binding protein, is required for germ line stem cell self-renewal and appears to positively regulate translation. J Biol Chem. 2009;284:6507–6519. doi: 10.1074/jbc.M809104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JH, Engel W, Nayernia K. Stem cell protein Piwil2 modulates expression of murine spermatogonial stem cell expressed genes. Mol Reprod Dev. 2006;73:173–179. doi: 10.1002/mrd.20391. [DOI] [PubMed] [Google Scholar]
- 12.Kuramochi-Miyagawa S, Kimura T, Ijiri TW, et al. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development. 2004;131:839–849. doi: 10.1242/dev.00973. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Sun Y, Guo J, et al. Expression of hiwi gene in human gastric cancer was associated with proliferation of cancer cells. Int J Cancer. 2006;118:1922–1929. doi: 10.1002/ijc.21575. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Jung C, Javadian-Elyaderani P, et al. Pathways of proliferation and antiapoptosis driven in breast cancer stem cells by stem cell protein piwil2. Cancer Res. 2010;70:4569–4579. doi: 10.1158/0008-5472.CAN-09-2670. [DOI] [PubMed] [Google Scholar]
- 15.Kuramochi-Miyagawa S, Watanabe T, Gotoh K, et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 2008;22:908–917. doi: 10.1101/gad.1640708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye Y, Yin DT, Chen L, et al. Identification of Piwil2-like (PL2L) proteins that promote tumorigenesis. PLoS One. 2010;5:e13406. doi: 10.1371/journal.pone.0013406. [DOI] [PMC free article] [PubMed] [Google Scholar]