Abstract
The prognosis of patients suffering from pancreatic cancer is still poor and novel therapeutic options are urgently needed. Recently, the transcription factor signal transducer and activator of transcription 5b (STAT5b) was associated with tumor progression in human solid cancer. Hence, we assessed whether STAT5b might serve as an anticancer target in ductal pancreatic adenocarcinoma (DPAC). We found that nuclear expression of STAT5b can be detected in approximately 50% of DPAC. Blockade of STAT5b by stable shRNA-mediated knockdown showed no effects on tumor cell growth in vitro. However, inhibition of tumor cell motility was found even in response to stimulation with epidermal growth factor or interleukin-6. These findings were paralleled by a reduction of prometastatic and proangiogenic factors in vitro. Subsequent in vivo experiments revealed a strong growth inhibition on STAT5b blockade in subcutaneous and orthotopic models. These findings were paralleled by impaired tumor angiogenesis in vivo. In contrast to the subcutaneous model, the orthotopic model revealed a strong reduction of tumor cell proliferation that emphasizes the meaning of assessing targets in an appropriate microenvironment. Taken together, our results suggest that STAT5b might be a potential novel target for human DPAC.
Introduction
Ductal pancreatic adenocarcinoma (DPAC) is one of the major leading causes of cancer-related death in the Western world [1]. As conventional cancer treatments have little impact on disease course, almost all patients diagnosed with pancreatic cancer eventually develop metastases and die [2,3]. To date, only surgical resection (possible in about 10–20% of patients) can increase the 5-year survival rate from almost 0% to 20%; systemic chemotherapy and/or radiation may only allow a marginal increase in survival [4]. Therefore, new therapies based on the molecular biology of pancreatic cancer are urgently needed to improve overall survival of patients with this aggressive disease.
Activation of several signaling pathways and transcription factors is commonly seen in human malignant tumors, including pancreatic cancer [4,5]. Among these factors, signal transducers and activators of transcription (STATs) comprise a family of seven transcription factors (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6) and at least STAT3, STAT5a, and STAT5b are implicated in solid tumor growth [6,7]. In particular, STAT3 has been involved in angiogenesis, metastases, and apoptosis in various cancer entities, including pancreatic cancer [8–10]. Regarding STAT5a, this transcription factor has been reported to be involved in apoptosis of breast cancer cells and a dominant-negative STAT5a mutant showed induction of cell death and reduced invasive properties of prostate cancer cells [11–14]. In contrast, STAT5b has been associated with advanced tumor stage in colorectal cancer, glioblastoma, and hepatocellular carcinoma [15–18]. Moreover, experimental data suggest a role for STAT5b in apoptosis and epithelial-mesenchymal transition [15,19]. In ovarian cancer, Chen et al. also describe a role for STAT5 activation and angiogenesis [20]. However, little is known about the effects of STAT5a and STAT5b in pancreatic cancer. So far, some reports indicate a role for STAT5a in the development of endocrine pancreatic diseases, e.g., diabetes [21], whereas STAT5b has been shown to be expressed in intraductal papillary mucinous neoplasms [22]. The role of STAT5b in human DPAC remains to be determined.
In the present study, we show that nuclear expression of STAT5b occurs in about half of DPACs investigated. Furthermore, experimental knockdown of STAT5b expression in pancreatic cancer cells led to strong inhibition of tumor growth in subcutaneous and orthotopic tumor models, which was linked to impaired angiogenesis. In addition, down-regulation of STAT5b diminished tumor cell motility in vitro and impaired metastasis formation in vivo. STAT5b, therefore, represents an interesting novel target for anticancer therapy in pancreatic cancer.
Materials and Methods
Immunohistochemical Assessment of STAT5b Expression in Human DPAC
To study the expression of STAT5b in human DPAC, we obtained tumor specimens from patients who underwent surgery for pancreatic adenocarcinoma between 2000 and 2008 in the Department of Surgery, University Hospital Regensburg, Germany. Representative formalin-fixed, paraffin-embedded tissue sections of 80 DPACs of various tumor stages were immunostained using a STAT5b-specific antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Slides were deparaffinized in xylene, followed by treatment with a graded series of alcohol washes [100%, 96%, 70% ethanol/ddH2O (vol/vol)], rehydration in phosphate-buffered saline (pH 7.5), and blocked against endogenous peroxidase with H2O2. Slides were incubated with primary antibody (1:25 dilution) at 4°C overnight. After washing with phosphate-buffered saline, biotinylated antibody (1:50 dilution; Vectastain Universal Elite ABC Kit, Vector Laboratories, Burlingame, CA) was added to tissue sections. STAT5b was visualized by Vectastain Elite ABC reagent (Vector Laboratories), followed by incubation with DAB. Nuclei were counterstained with hematoxylin. Negative controls were performed by omitting the primary antibody. Nuclear immunoreactivity for STAT5b was scored as absent (-; <10% of cancer cells) or positive (+; >10% of cancer cells) in accordance with a published scoring system for STAT5b expression [22].
Cell Culture and Cytokines
The human pancreatic cancer cell line HPAF-II was obtained from the American Type Culture Collection and L3.6pl cells were kindly provided by Dr I. J. Fidler (The University of Texas M.D. Anderson Cancer Center). Tumor cells were cultured in Dulbecco's modified Eagle's medium (DMEM; PAA Laboratories, Pasching, Austria) supplemented with 15% fetal calf serum (FCS) and maintained in 5% CO2 at 37°C, as described [23]. Recombinant human interleukin-6 (IL-6) and epidermal growth factor (EGF) were purchased from R&D Systems (Wiesbaden, Germany).
Stable Transfection
HPAF-II and L3.6pl cells were stably transfected with either STAT5b-shRNA (TIB MOLBIOL, Berlin, Germany) or a luciferase-shRNA (Luc-shRNA) expression plasmid by using Lipofectamin transfection reagent (Invitrogen, Karlsruhe, Germany), as described before [24]. Cells were maintained and expanded in selective medium containing neomycin (G418; Sigma Aldrich, Deisenhofen, Germany). Knockdown of STAT5b was verified by Western blot analysis and by polymerase chain reaction (PCR) for successful silencing. Two different sequences for STAT5b knockdown were used for further experiments to reduce potential off-target effects by certain sequences.
Measurement of Tumor Cell Growth In Vitro
To evaluate the effects of STAT5b, we seeded knockdown tumor cells in 96-well plates (1 x 103 per well) for 24, 48, and 72 hours under full medium and serum-reduced conditions (1% FCS in DMEM). We used 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay to assess cell growth [25]. Effects of STAT5b inhibition on growth of tumor cells in vitro were determined in a cell-counting assay. Briefly, 106 cells were seeded into six-well dishes; after 24, 48, and 72 hours, cells were trypsinized and counted. Furthermore, [3H]thymidine assays were used to assess proliferation in HPAF-II clones with STAT5b knockdown, as previously described [26].
Migration Assays
To determine the effect of STAT5b inhibition on cell motility, we performed migration assays using modified Boyden chambers [25]. Briefly, 5 x 104 tumor cells were resuspended in 1% FCS-DMEM and seeded into inserts with 8-µm pores (Becton Dickinson Biosciences, Heidelberg, Germany). EGF (50 ng/ml) and IL-6 (50 ng/ml) were used as chemoattractants. Cells were fixed after 24 and 48 hours, and migrated cells were stained (Diff-Quick reagent; Dade Behring, Newark, NJ). Cells that migrated through the filters were counted in four random fields and average numbers were calculated.
Western Blot Analyses
Experiments were performed at a cellular density of 60% to 70%. Whole-cell lysates were prepared as described elsewhere [23,25]. Protein was prepared from tumor tissue for Western blot analyses [25]. Protein samples (40 µg) were subjected to Western blot analysis on a denaturating 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Membranes were sequentially probed to determine signaling intermediates with antibodies against phospho-AKTSer473, Akt, c-Myc, phospho-STAT3Tyr705, STAT3, Bim, Caspase-3, Puma, phospho-BadSer136, Bad (Cell Signaling Technology, Beverly, MA), and STAT5b and β-actin (Santa Cruz Biotechnology). Antibodies were detected by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).
Real-time PCR Analysis
PCR was used to determine the effects of STAT5b knockdown on target genes. Total RNA was isolated using TRIzol reagent (Invitrogen) and subsequently purified by ethanol precipitation. For each RNA sample, 1 µg of aliquot was reverse transcribed into cDNA using the Superscript II Kit (Qiagen, Hilden, Germany). Selected primer pairs for PCR were given as follows: STAT5b (5′-TGAAGGCCACCATCATCAG and 3′-TGTTCAAGATCTCGCCACTG), caveolin-1 (CAV-1; 5′-GAAAGAAGATGGGGGAGGAG and 3′-AAAGTCCCCAAAGGCAGAAT), urokinase-type plasminogen activator receptor (uPAR; 5′-GCCTTACCGAGGTTGTGTGT and 3′-GCTTCGGGAATAGGTGACAG), IL-6 (5′-CCCAGTACCCCCAGGAGAAGA and 3′-GTTGGGTCAGGGGTGGTTATTG), hypoxia-inducible factor-1β (HIF-1β; 5′-TACCATGCCCCAGATTCAGGAT and 3′-TCAGTGGTGGCAGTGGTAGTGG), vascular endothelial growth factor-A (VEGF-A; 5′-GCAGCTTGAGTTAAACGAACG and 3′-GGTTCCCGAAACCCTGAG), vascular endothelial growth factor-D (VEGF-D; 5′-GGTGCAGGCTCCAGTAATGA and 3′-AGGGCTTGAAGAATGTGTTG), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 5′-GCGGGGCTCCCAGAACATCAT and 3′-CCAGCCCCAGCGTCAAAGGTG). Primers were optimized for MgCl2 and annealing, and PCR products were confirmed by gel electrophoresis. Reverse transcription-PCR was performed by using the LightCycler system and Roche FastStart LightCycler-Master Hybridization Probes master mix (Roche Diagnostics, Mannheim, Germany).
Animal Models
Eight-week-old male athymic nude mice (BALB/cnu/nu; Charles River, Sulzfeld, Germany) were used for experiments, as approved by the Institutional Animal Care and Use Committee of the University of Regensburg and the regional authorities. In addition, experiments were conducted according to the “Guidelines for the Welfare of Animals in Experimental Neoplasia” published by the United Kingdom Coordinating Committee on Cancer Research. Effects of selective STAT5b inhibition were first evaluated in a subcutaneous pancreatic cancer model using HPAF-II and L3.6pl pancreatic cancer cells stably transfected with Luc-shRNA and two STAT5b-shRNA vectors (different STAT5b inhibitory sequences). Cells (1 x 106) were injected into the subcutis (right flank) of nude mice (n = 6–8/group). Tumor diameters were measured every other day and tumor volumes were determined by using the formula width2 x length x 0.5. Subcutaneous tumors were excised and weighed when the experiment was terminated.
Subsequently, the effects of STAT5b inhibition were evaluated in an orthotopic tumor model. Briefly, 1 x 106 HPAF-II human pancreatic cancer cells (Luc-shRNA-transfected cells and STAT5b-shRNA-transfected cells) were injected into the pancreatic tail of mice (n = 5–6/group). Mice were sacrificed after 32 days; tumors were excised and weighed, and the incidence of liver and lymph node metastases was determined. Tumors were either paraffin-embedded or ornithine carbamoyltransferase-embedded for immunohistochemical analyses or stored for protein extraction. Knockdown of STAT5b in tumor cells was determined by Western blot analysis and PCR before injection of tumor cells into mice. In addition, STAT5b expression was determined from tumor tissue after termination of the experiments by Western blot analysis.
Immunohistochemical Analysis of Tumor Vascularization and Tumor Cell Proliferation
Multiple cryosections were obtained from tumors for all immunohistochemical analyses. CD31+ vessel area was assessed using rat antimouse CD31/PECAM-1 antibody (Pharmingen, San Diego, CA) and peroxidase-conjugated goat anti-rat immunoglobulin G (IgG) (Jackson Research Laboratories, West Grove, PA), as previously described [27]. Antibody binding was visualized using stable DAB. Images were obtained in four different quadrants of each tumor section (2 mm inside the tumor-normal tissue interface) at 40x magnification. The area of CD31-stained vessels was determined by converting images to grayscale and setting a consistent threshold for all slides using ImageJ software (version 1.33; National Institutes of Health). Vessel areas were expressed as pixels per high-power field [25,27]. To define the amount of proliferating tumor cells, mice received intraperitoneal injections of bromodeoxyuridine (BrdU; Sigma Aldrich; 1 mg/mouse) 2 hours before termination of animal studies. A commercially available BrdU detection kit (Becton Dickinson) was used to visualize BrdU uptake of cells in tumor sections. Briefly, sections were incubated with anti-BrdU antibody solution, followed by streptavidin-conjugated HRP-linked goat anti-mouse IgG2. Antibody binding was visualized by incubating slides with DAB followed by hematoxylin counterstaining. BrdU-positive tumor cells were counted in four fields per tumor section at 20x magnification and averages were calculated [25].
For analyses of tumor cell apoptosis, a commercially available terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) detection kit was used (Promega, Mannheim, Germany). After the staining procedure, four fields at 20x magnification were selected in each tumor at the proliferation front, and identifiable TUNEL-positive cells were counted.
Statistics
Statistical analyses were carried out by using SigmaStat (Version 3.0). Results of in vivo experiments were analyzed for significant outliers using the Grubb's test (www.graphpad.com). Tumor-associated variables of in vivo experiments were tested for statistical significance using the Mann-Whitney U test for nonparametric data or analysis of variance followed by Tukey's multiple comparison tests for more than two groups. The two-sided Student's t test was applied for analysis of in vitro data. Patient survival was determined using the Cox regression analyses. All results are expressed as the mean ± SEM.
Results
STAT5b Is Expressed in DPAC
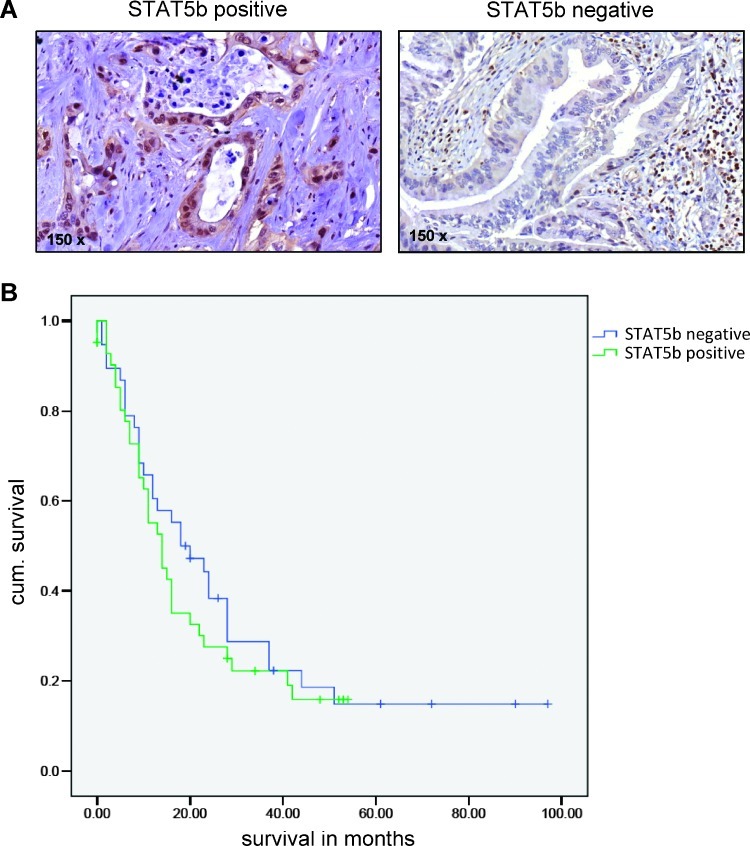
Because increased STAT5b expression was observed in intraductal papillary mucinous neoplasm progressing to intraductal papillary mucinous carcinoma, we asked whether this transcription factor might also play a role in DPAC. To evaluate this issue, we determined the expression of STAT5b protein by immunohistochemistry in DPAC (Figure 1A). Using a collection of 80 tumor samples, 42 tumors showed nuclear expression of STAT5b (>10% of cancer cells). No association was found regarding tumor localization, histologic grading, tumor stage, or lymph node stage (Table 1). Tumors with nuclear positive STAT5b expression showed a trend (not statistically significant) toward reduced patient survival (Figure 1B). Furthermore, weak positive STAT5b staining was found in normal pancreatic ducts adjacent to the tumor tissue in some areas. In addition, some peritumoral stromal cells showed positive nuclear staining. Nevertheless, results show that STAT5b is expressed in cancer cells in about half of human DPACs, representing a potential target for anticancer therapy.
Figure 1.
STAT5b expression in human DPAC. (A) Left: Strong nuclear expression of STAT5b in the majority of cancer cells. Right: No nuclear STAT5b expression in cancer cells. (B) Patients where nuclear STAT5b expression was present in >10% of cancer cells showed a trend toward reduced survival as determined by Kaplan-Meier analysis. This did not reach statistical significance (P = 0.204).
Table 1.
A Total of 80 Human DPACs Were Assessed for Nuclear STAT5b Expression.
| Nuclear Immunoreactivity (Number of Patients) | ||||
| Variable | Number | STAT5b Positive | STAT5b Negative | P value |
| Total | 80 | 42 | 38 | |
| Sex | ||||
| Male | 52 | 27 | 25 | 1.0 |
| Female | 28 | 15 | 13 | |
| Localization | ||||
| Pancreatic head | 58 | 29 | 29 | .6 |
| Pancreatic tail | 22 | 13 | 9 | |
| Histologic grading | ||||
| G1 | 5 | 2 | 3 | .8 |
| G2 | 39 | 20 | 19 | |
| G3 | 36 | 20 | 16 | |
| pT stage | ||||
| T1, T2 | 8 | 3 | 5 | .5 |
| T3, T4 | 72 | 39 | 33 | |
| N stage | ||||
| N0 | 22 | 12 | 10 | 1.0 |
| N1 | 58 | 30 | 28 | |
No statistical differences were found regarding gender, localization, grading, pT stage, and lymph node status.
Inhibition of STAT5b by Stable shRNA Vector in Pancreatic Cancer Cell Lines Has No Effects on Growth of Tumor Cells In Vitro
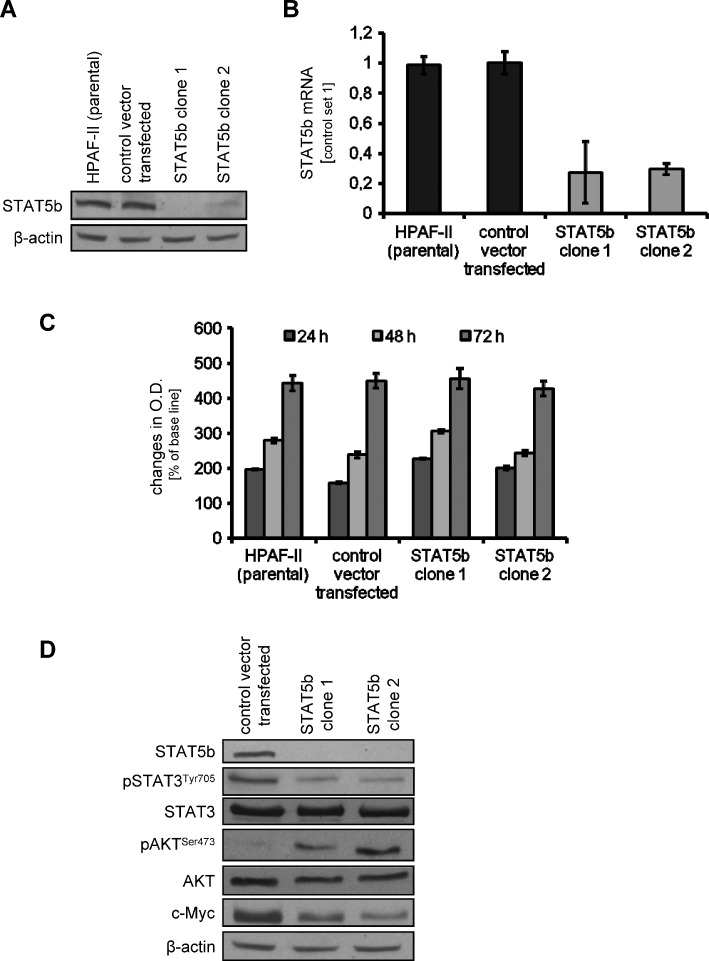
The effect of STAT5b inhibition on pancreatic cancer cell lines was assessed. In the absence of a commercially available STAT5b inhibitor, STAT5b-shRNA plasmids were used. After successful transfection of HPAF-II and L3.6pl pancreatic cancer cell lines, inhibition of STAT5b expression was determined by Western blot analysis and PCR (Figure 2, A and B, for HPAF-II and Figures W1A and W3A for L3.6pl). Stable transfection led to inhibition of STAT5b expression in approximately 70% to 80% of HPAF-II and L3.6pl pancreatic cancer cells. We next sought to evaluate the impact of STAT5b inhibition on the growth of tumor cells in vitro. Interestingly, we found no difference between parental tumor cells, Luc-shRNA-transfected tumor cells, and STAT5b-shRNA-transfected tumor cells by MTT assays (HPAF-II and L3.6pl; Figures 2C and W2A). These effects were confirmed by using a cell counting method for both cancer cell lines (Figure W2, B and C). In addition, [3H]thymidine incorporation assays revealed no significant effects of STAT5b blockade on HPAF-II cell proliferation with STAT5b knockdown (Figure W2D). However, when comparing activation of signaling intermediates on STAT5b blockade, we detected reduced phosphorylation of STAT3 and slightly reduced expression of c-Myc (Figures 2D and W1B). Surprisingly, phosphorylation of AKTSer473 was increased on STAT5b inhibition, indicating possible activation of survival signaling in tumor cells. Nevertheless, results from these experiments show that targeting STAT5b has no direct effect on the growth of pancreatic cancer cells in vitro; we also conclude that STAT5b inhibition influences various signaling pathways.
Figure 2.
Effects of targeting STAT5b on human pancreatic cancer cell line HPAF-II in vitro. (A and B) Knockdown of STAT5b in HPAF-II pancreatic cancer cell line using an shRNA approach was verified by Western blot analysis (A) and PCR (B). STAT5b expression was reduced by 70% to 80% as determined by both methods. (C) Knockdown of STAT5b in HPAF-II has no effects on the growth of tumor cells in vitro, as determined by MTT assay. (D) Inhibition of STAT5b leads to a reduction of constitutive STAT3 phosphorylation and c-Myc expression in HPAF-II cancer cells. However, phosphorylation of AKTSer473 was observed on STAT5b blockade in vitro. Results are shown for HPAF-II pancreatic cancer cells; similar results were obtained from L3.6pl cells. Bars, SEM.
Impairment of Tumor Cell Motility by Down-regulation of STAT5b
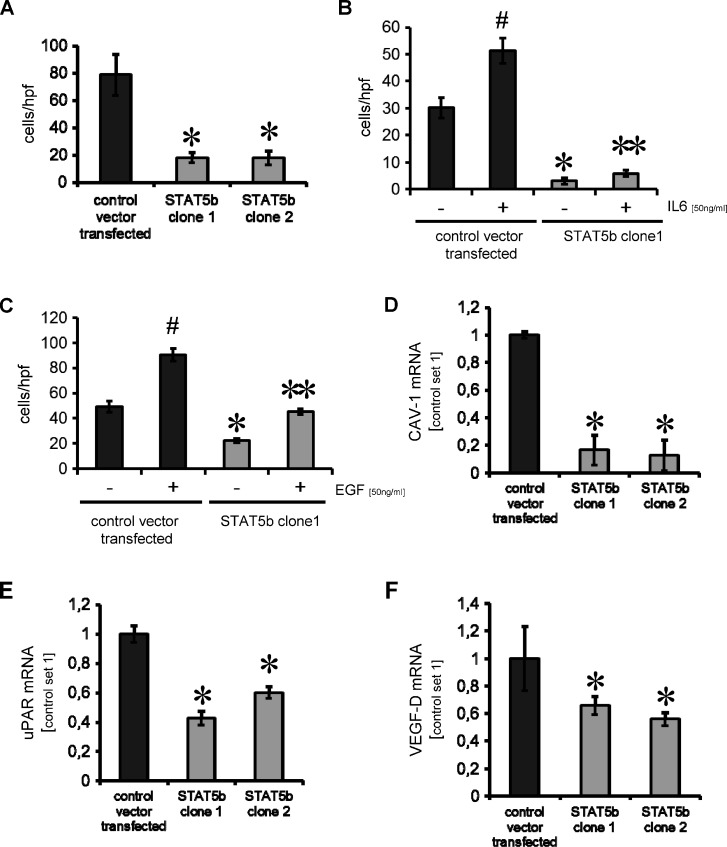
Because STAT5b is known to modulate migration and invasion of tumor cells, we evaluated the effects of STAT5b inhibition on motility of stably transfected pancreatic cancer cells [15,28]. Boyden chamber assays show that inhibition of STAT5b leads to a significant reduction in constitutive HPAF-II tumor cell migration (Figure 3A). In addition, because IL-6 and EGF are both known to induce motility of pancreatic cancer cell lines at least in part through activation of janus kinase (JAK)/STAT signaling, the effect of STAT5b inhibition on IL-6- and EGF-induced motility was determined. As previously shown, IL-6 and EGF led to an increase in tumor cell motility that was impaired by knockdown of STAT5b in the pancreatic cancer cell line HPAF-II (Figure 3, B and C). Similar results were obtained from L3.6pl pancreatic cancer cells with stable STAT5b knockdown (data not shown). In summary, down-regulation of STAT5b impairs constitutive and IL-6-/EGF-induced motility of pancreatic cancer cell lines in vitro.
Figure 3.
Targeting STAT5b in pancreatic cancer cell line HPAF-II impairs tumor cell motility and modulates mediators of metastases. (A) Inhibition of STAT5b by shRNA results in a significant decrease in constitutive tumor cell motility in vitro (*P < .05). Results are shown for two HPAF-II clones with stable STAT5b knockdown. (B) Stimulation with IL-6 leads to an increase in HPAF-II motility (#P < .05). Knockdown of STAT5b impairs both constitutive and IL-6-induced migration (*, **P < .05). (C) Similar to IL-6, EGF induces HPAF-II cell motility (#P < .05). This effect is blocked by knockdown of STAT5b (*, **P < .05). (D–F) Stable shRNA-mediated knockdown of STAT5b significantly reduces mRNA expression of metastatic factors CAV-1, uPAR, and VEGF-D in the human pancreatic cancer cell line HPAF-II (*P < .05). All results are shown for HPAF-II human pancreatic cancer cells; similar results were obtained from L3.6pl cells. Bars, SEM.
Regulation of Metastasis Mediators by STAT5b Inhibition
In view of the fact that STAT5b inhibition impairs tumor cell motility, we assessed whether it also affects the expression of prometastatic factors CAV-1, uPAR, and VEGF-D [29–32]. Indeed, knockdown of STAT5b led to a significant reduction of CAV-1 and uPAR mRNA in HPAF-II cells (Figure 3, D and E). Furthermore, expression of VEGF-D was significantly reduced in the pancreatic cancer cell line HPAF-II on STAT5b inhibition (Figure 3F). These results were confirmed in L3.6pl human cancer cells stably transfected with STAT5b-shRNA plasmid (Figure W3, B–D). We conclude that targeting STAT5b may reduce tumor cell motility and impair expression of prometastatic factors in tumor cells.
Effects of Targeting STAT5b on Mediators of Tumor Angiogenesis
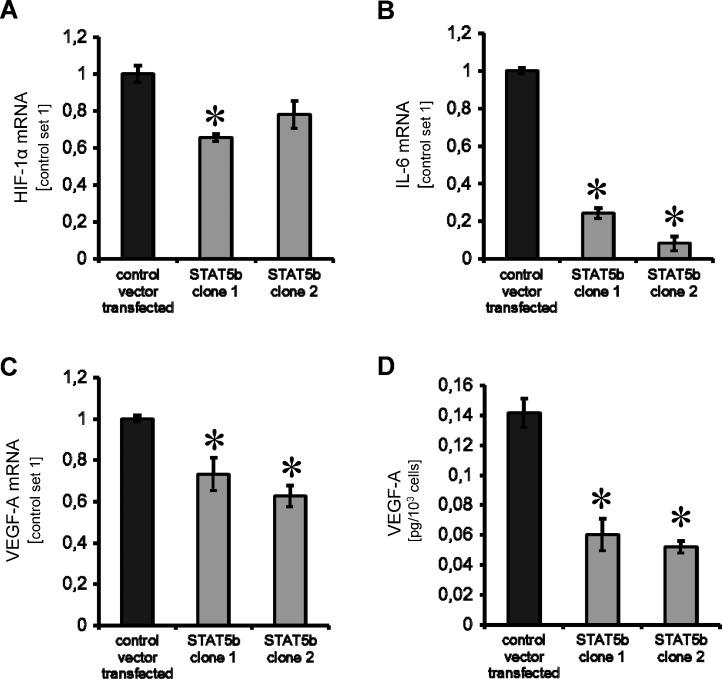
Because the role of STAT5b in pancreatic cancer angiogenesis has not been investigated, we determined the impact of STAT5b on angiogenic factors. We found a slight, but significant, reduction of HIF-1β expression in one of the two HPAF-II STAT5b-shRNA knockdown clones (Figure 4A). More consistently, a significant reduction of IL-6 and VEGF-A mRNA was detected in both HPAF-II STAT5b-shRNA clones (Figure 4, B and C). This observation was further supported by the significant reduction of VEGF-A secretion from tumor cells as determined by ELISA (Figure 4D). Nevertheless, effects on HIF-1β expression were not found in L3.6pl STAT5b-shRNA-transfected cells, whereas reduction of IL-6 and VEGF-A mRNA, as well as VEGF-A secretion, was confirmed (Figure W3, E–H). Taken together, specific inhibition of STAT5b may also affect tumor angiogenesis through effects on VEGF-A secretion rather than effects on HIF-1α expression in pancreatic cancer cells.
Figure 4.
Modulation of angiogenic factors on STAT5b blockade. (A) A significant reduction of HIF-1α mRNA was only observed in one of the HPAF-II STAT5b knockdown clones tested (*P < .05). (B) Inhibition of STAT5b leads to a significant reduction of IL-6 mRNA expression in HPAF-II pancreatic cancer cells (*P < .05). (C) Importantly, VEGF-A mRNA was markedly reduced in both HPAF-II STAT5b clones investigated (*P < .05). (D) These results were confirmed by significant reduction of VEGF-A secretion from HPAF-II cancer cells in vitro (*P < .05). All results are shown for HPAF-II human pancreatic cancer cells; results for L3.6pl cells are shown in Figure W3. Bars, SEM.
Impact of STAT5b Down-regulation on Tumor Growth In Vivo
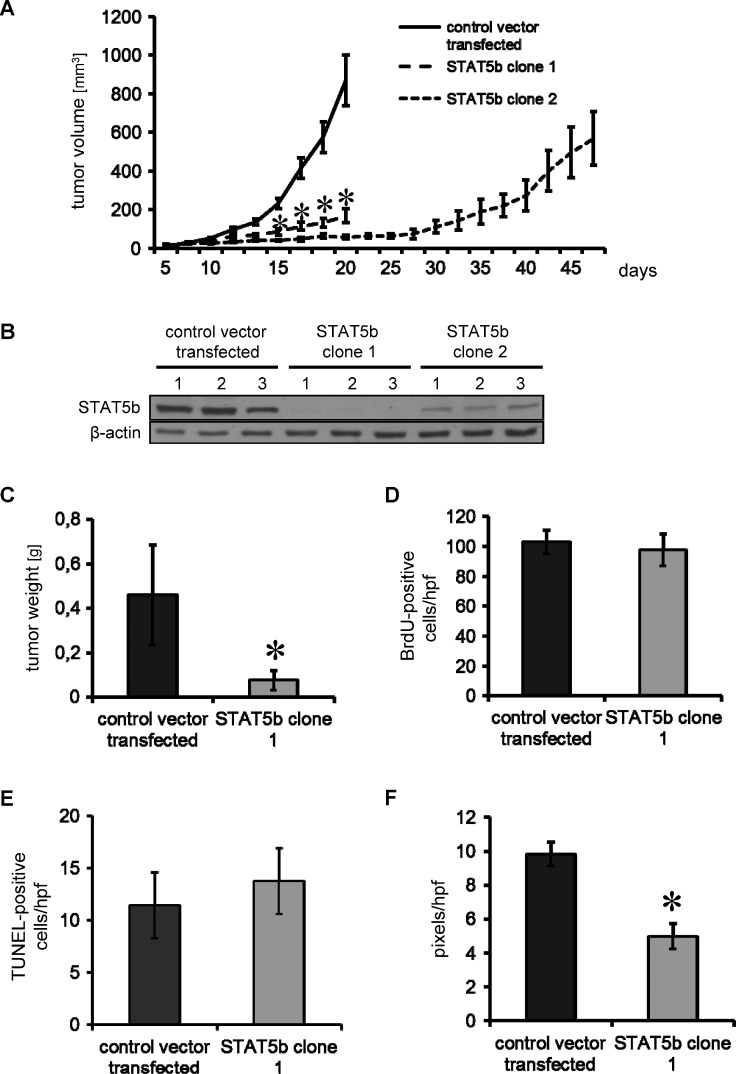
The effects of STAT5b inhibition on tumor growth in vivo were first determined in subcutaneous tumor models. While HPAF-II cells with Luc-shRNA (control vector transfected) showed exponential growth (Figure 5A), inhibition of STAT5b significantly impaired tumor growth in both HPAF-II STAT5b-shRNA clones tested (STAT5b clone 1 and STAT5b clone 2; Figure 5A). After 21 days, control mice and STAT5b clone 1 mice were sacrificed and the weight of STAT5b clone 1 tumors was found to be significantly reduced (Figure 5C). Similar results were obtained with L3.6pl cells with stable STAT5b-shRNA-induced knockdown (Figure W4, A and B). To further evaluate the duration of growth inhibition in HPAF-II cells by STAT5b knockdown, the second clone was continued in mice. Results show that these tumors started growing after around 5 weeks (Figure 5A). Western blot analyses of STAT5b expression in tumor tissue showed that STAT5b was not detectable in STAT5b clone 1, whereas control vector-transfected cells had strong STAT5b expression. In contrast, the STAT5b clone 2 continued until regrowth had detectable STAT5b expression; however, the overall STAT5b protein level was lower than in the control vector-transfected tumors (Figure 5B). We conclude from these results that the STAT5b inhibition was sufficient to impair tumor growth in vivo and that regrowth of tumors is at least in part due to loss of the STAT5b knockdown plasmid from the tumor cells. It is also possible that escape mechanisms associated with STAT5b inhibition might play a role.
Figure 5.
Inhibition of tumor growth on STAT5b knockdown in the subcutaneous tumor model (HPAF-II). (A) Stable shRNA-mediated knockdown of STAT5b leads to a significant inhibition of tumor growth as determined in a subcutaneous tumor model. Results are shown for two different shRNA sequences to avoid possible off-target effects. Starting from day 15 after tumor cell inoculation, tumors in both groups with shRNA-mediated STAT5b knockdown were significantly smaller than control vector-transfected tumors (*P < .05). Animals injected with STAT5b-shRNA clone 2 cells were left until tumor progression was observed. (B) Western blot analyses from tumor tissue showed no STAT5b expression in STAT5b clone 1 compared to control vector-transfected tumors. In contrast, in STAT5b clone 2 continued until regrowth, STAT5b expression was detectable at lower levels compared to controls. (C) Animals with control tumors and STAT5b-shRNA clone1 tumors were sacrificed after 21 days. Inhibition of tumor growth was reflected by final tumor weight (*P < .05). (D) Cell proliferation as measured by BrdU showed no difference between the control and STAT5b clone 1 tumors. (E) Furthermore, the number of apoptotic (TUNEL-positive) cells was not different between control and STAT5b clone 1 tumors. (F) Inhibition of STAT5b leads to a significant reduction of tumor vascularization as determined by CD31 vessel area (*P < .05). Bars, SEM.
The effects of STAT5b on tumor cell proliferation, angiogenesis, and apoptosis in vivo were determined by BrdU, CD31, and TUNEL staining in tissue obtained from control vector-transfected and STAT5b clone 1-derived tumors. Consistent with our in vitro results, no difference regarding tumor cell proliferation (BrdU) and apoptosis (TUNEL) was observed (Figure 5, D and E). In contrast, tumor angiogenesis as determined by CD31 vessel area was significantly reduced on STAT5b inhibition (Figure 5F).
Effects of STAT5b Inhibition in an Orthotopic Pancreatic Cancer Model
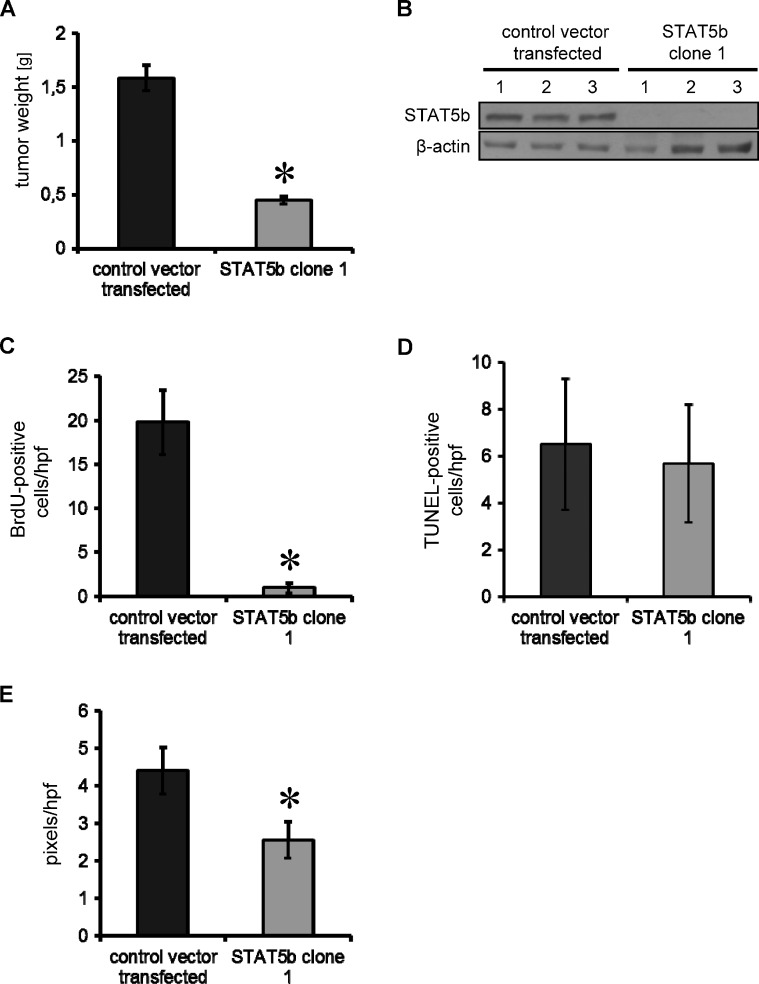
Because the microenvironment has a strong impact on tumor growth, we next assessed the effects of STAT5b inhibition in an orthotopic tumor model using HPAF-II pancreatic cancer cells stably transfected with control vector and STAT5b-shRNA plasmid. After 31 days, mice were sacrificed and tumors were excised. Similar to the subcutaneous model, we found a significant inhibition of tumor growth in vivo as determined by final tumor weight (Figure 6A). Western blot analyses from tumor tissue confirmed persistent STAT5b knockdown when the experiment was terminated (Figure 6B). Interestingly, growth inhibition was paralleled by a trend toward reduced metastases in STAT5b knockdown tumors, although this did not reach statistical significance (lymph node: two of six positive in controls, zero of five positive in STAT5b-shRNA clone 1; liver: three of six positive in controls, zero of five positive in STAT5b-shRNA clone 1). Results from our experiments indicate that inhibition of STAT5b in pancreatic cancer cells has strong inhibitory effects on orthotopic tumor growth in vivo.
Figure 6.
Effects of STAT5b blockade in an orthotopic tumor model. (A) Orthotopic implantation of HPAF-II pancreatic cancer cells into the pancreatic tail of nude mice results in inhibition of tumor growth after 31 days, as determined by final tumor weight (*P < .05). (B) STAT5b knockdown was persistent after 31 days (Western blot analysis). (C) Blockade of transcription factor STAT5b leads to a significant reduction in tumor cell proliferation (BrdU incorporation; *P < .05). (D) No effect was observed on tumor cell apoptosis (TUNEL staining). (E) However, tumor vascularization, measured by CD31 vessel area, was reduced on STAT5b inhibition (*P < .05). Bars, SEM.
To further assess whether inhibition of STAT5b has effects on tumor cell proliferation, apoptosis and angiogenesis in the orthotopic model, BrdU, TUNEL, and CD31 stainings were performed in relevant tissue sections. Unexpectedly, tumor cell proliferation was significantly impaired in the orthotopic model, which contrasts with our data from in vitro experiments and from the subcutaneous tumor model that showed no difference between luciferase-transfected control cells and the STAT5b clone 1 (Figure 6C). In line with the subcutaneous model results, no effect on tumor cell apoptosis was found (Figure 6D) and no difference was detected (Western blot analysis) concerning the expression of apoptotic markers Puma, Caspase 3, Bim, phospho- BadSer136, and Bad (data not shown). Also consistent with the subcutaneous model, vessel area (CD31 staining) was significantly reduced on STAT5b inhibition (Figure 6E). These results indicate that inhibition of STAT5b impairs pancreatic cancer growth through antiangiogenic mechanisms in vivo. Conflicting results regarding in vitro versus in vivo tumor cell proliferation suggest that the in vivo pancreatic microenvironment may have a yet unknown role that enhances STAT5b involvement.
Discussion
The prognosis of patients with DPAC remains poor with an overall 5-year survival rate below 2%. In general, pancreatic cancer is refractory to chemotherapy and radiotherapy. To date, only surgical resection provides increased tumor control, but overall survival rates are still very poor [4]. Therefore, novel therapeutic approaches based on the molecular biology of pancreatic cancer are urgently needed to improve the prognosis for these patients. Within the present study, we investigated STAT5b as a potential target for antineoplastic therapy in pancreatic cancer models. Our results show that STAT5b is expressed in half of the patient DPAC specimens. In preclinical models, specific knockdown of STAT5b in pancreatic cancer cell lines led to strong inhibition of tumor growth and a decrease of metastatic potential in vitro and in vivo. Together, our results suggest that STAT5b might be a novel interesting target to improve outcome of patients with pancreatic cancer.
Although several studies have investigated the expression of STAT5b in solid cancers [16,17,22], the function of STAT5b in human DPAC has not been addressed. With regard to pancreatic malignancies, Kataoka et al. have shown increased nuclear expression of STAT5b in intraductal papillary mucinous carcinoma compared to intraductal papillary mucinous adenoma and normal pancreatic ducts [22]. In the present study, we found that about half of DPACs stain positive for nuclear STAT5b. Expression of STAT5b has been associated with poor prognosis or advanced tumor stage in several solid tumor entities including colon cancer, hepatocellular carcinoma, and glioblastoma multiforme [15–18], but our exploratory clinical study could not confirm this statistically regarding DPAC. However, even with our limited patient numbers, a trend toward reduced survival was evident in patients with nuclear STAT5b expression. We emphasize caution when interpreting these results because of the small number of cases included into the study. Furthermore, tumor samples were obtained from surgical resections with curative intents and one might speculate that STAT5b expression is stronger in advanced stages where resections are not performed anymore. Nevertheless, our results clearly show that STAT5b is expressed in a subset of DPAC and therefore might serve as a target for anticancer therapy.
Tumor growth cannot be sustained unless the supply of oxygen and nutrients is assured by the formation of new blood vessels [33,34]. Although pancreatic cancer is characterized by low vascular density, induction of tumor angiogenesis is still essential for this tumor entity. In particular, secretion of VEGF-A from tumor cells is an important factor for development of new vessels [35,36], and VEGF-A has been associated with a poor prognosis in DPAC [37–39]. Interestingly, we found a significant reduction of VEGF-A production in tumor cells on STAT5b inhibition in vitro, which was paralleled by a clear reduction of vessel area in subcutaneous and orthotopic mouse models. These results are in line with previous reports from Chen et al. that described a connection between STAT5 activation and VEGF expression in ovarian cancer [20]. However, no discrimination between STAT5a and STAT5b was performed in their study. Moreover, Liang et al. found a reduction of VEGF-A secretion from glioblastoma multiforme cells on STAT5b inhibition, but their findings were not evaluated in vivo [16]. In summary, our results show that inhibition of STAT5b in pancreatic cancer cells has the potential to diminish tumor growth at least in part through effects on VEGF-A and tumor angiogenesis.
Metastasis formation in DPAC is a major issue because patients either present with metastatic disease or very quickly develop metastases even after curative resection [3,40]. Although several reports have connected STAT5b expression to motility and metastasis in solid tumor entities, this had not yet been investigated in DPAC [15,16,18,41]. In glioblastoma multiforme, reduction of cancer cell invasiveness by STAT5b inhibition was linked to reduction of FAK expression [16]; in hepatocellular carcinoma, Lee et al. found that STAT5b is essential for epithelial-mesenchymal transition associated with metastasis formation [15,19]. Results from our study further support the function of STAT5b as a modulator of tumor cell motility, even in response to various stimuli. Moreover, we found a reduction of prometastatic factors CAV-1, uPAR, and VEGF-D on STAT5b inhibition [29,30,32]. These in vitro data are consistent with the fact that we found no liver or lymph node metastases in the orthotopic pancreatic cancer model on STAT5b blockade. Our results provide strong indications that STAT5b plays a role in metastasis formation in DPAC.
An important addition to present knowledge was our STAT5b blockade in an orthotopic pancreatic cancer model, where we observed significant growth inhibition similar to the subcutaneous mouse model (Figures 5 and 6). Interestingly, analyses of the tumor tissue from the orthotopic model revealed a strong antiproliferative effect of STAT5b blockade, which was not observed in vitro or in the subcutaneous model. One can speculate that inhibition of STAT5b in tumor cells modulates the local pancreatic microenvironment, which in turn leads to reduced tumor cell proliferation and therefore emphasizes the need to verify results in appropriate orthotopic mouse models as proposed recently [42]. However, we observed constitutive phosphorylation of Akt, a known promoter of survival and proliferation in tumor cells, on STAT5b blockade in vitro [43]. This might indicate a potential escape mechanism from STAT5b-mediated growth inhibition at least in our cell culture experiments. Nevertheless, these findings clearly warrant further investigation.
In conclusion, we provide evidence that STAT5b is frequently expressed in DPAC. Furthermore, we show that STAT5b is as an interesting target to impair tumor growth and metastasis formation in this devastating disease, at least in part through effects on tumor vascularization. Therefore, inhibitors to STAT5b in pancreatic cancer should be tested as soon as they are available in preclinical and clinical settings.
Supplementary Material
Acknowledgments
The authors thank Christine Wagner, Eva Scheiffert, Katrin Enderle, and Kathrin Stengel for excellent technical assistance.
Abbreviations
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- DMEM
Dulbecco's modified Eagle's medium
- FCS
fetal calf serum
- VEGF-A
vascular endothelial growth factor-A
- VEGF-D
vascular endothelial growth factor-D
- STAT5b
signal transducer and activator of transcription 5b
- uPAR
urokinase-type plasminogen activator receptor
- IL-6
interleukin-6
- CAV-1
caveolin-1
- HIF-1α
hypoxia-inducible factor-1α
- DPAC
ductal pancreatic adenocarcinoma
Footnotes
These studies were supported in part by the German Research Council [Deutsche Forschungsgemeinschaft (DFG/KFO262) to S.A.L. and M.P.K.] and grants from the University of Regensburg, Medical Faculty (ReForM-A and ReForM-C to S.A.L. and C.M.).
This article refers to supplementary materials, which are designated by Figures W1 to W4 and are available online at www.neoplasia.com.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 4.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cree IA. Cancer biology. Methods Mol Biol. 2011;731:1–11. doi: 10.1007/978-1-61779-080-5_1. [DOI] [PubMed] [Google Scholar]
- 6.Santos CI, Costa-Pereira AP. Signal transducers and activators of transcription—from cytokine signalling to cancer biology. Biochim Biophys Acta. 2011;1816:38–49. doi: 10.1016/j.bbcan.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Yu H, Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 8.Glienke W, Hausmann E, Bergmann L. Targeting STAT3 signaling in pancreatic cancer promotes antiapoptotic gene expression. Pancreas. 2011;40:323–324. doi: 10.1097/MPA.0b013e318204ea7b. [DOI] [PubMed] [Google Scholar]
- 9.Huang C, Jiang T, Zhu L, Liu J, Cao J, Huang KJ, Qiu ZJ. STAT3-targeting RNA interference inhibits pancreatic cancer angiogenesis in vitro and in vivo. Int J Oncol. 2011;38:1637–1644. doi: 10.3892/ijo.2011.1000. [DOI] [PubMed] [Google Scholar]
- 10.Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–329. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 11.Ahonen TJ, Xie J, LeBaron MJ, Zhu J, Nurmi M, Alanen K, Rui H, Nevalainen MT. Inhibition of transcription factor Stat5 induces cell death of human prostate cancer cells. J Biol Chem. 2003;278:27287–27292. doi: 10.1074/jbc.M304307200. [DOI] [PubMed] [Google Scholar]
- 12.Kazansky AV, Spencer DM, Greenberg NM. Activation of signal transducer and activator of transcription 5 is required for progression of autochthonous prostate cancer: evidence from the transgenic adenocarcinoma of the mouse prostate system. Cancer Res. 2003;63:8757–8762. [PubMed] [Google Scholar]
- 13.Peck AR, Witkiewicz AK, Liu C, Stringer GA, Klimowicz AC, Pequignot E, Freydin B, Tran TH, Yang N, Rosenberg AL, et al. Loss of nuclear localized and tyrosine phosphorylated Stat5 in breast cancer predicts poor clinical outcome and increased risk of antiestrogen therapy failure. J Clin Oncol. 2011;29:2448–2458. doi: 10.1200/JCO.2010.30.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran TH, Utama FE, Lin J, Yang N, Sjolund AB, Ryder A, Johnson KJ, Neilson LM, Liu C, Brill KL, et al. Prolactin inhibits BCL6 expression in breast cancer through a Stat5a-dependent mechanism. Cancer Res. 2010;70:1711–1721. doi: 10.1158/0008-5472.CAN-09-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee TK, Man K, Poon RT, Lo CM, Yuen AP, Ng IO, Ng KT, Leonard W, Fan ST. Signal transducers and activators of transcription 5b activation enhances hepatocellular carcinoma aggressiveness through induction of epithelial-mesenchymal transition. Cancer Res. 2006;66:9948–9956. doi: 10.1158/0008-5472.CAN-06-1092. [DOI] [PubMed] [Google Scholar]
- 16.Liang QC, Xiong H, Zhao ZW, Jia D, Li WX, Qin HZ, Deng JP, Gao L, Zhang H, Gao GD. Inhibition of transcription factor STAT5b suppresses proliferation, induces G1 cell cycle arrest and reduces tumor cell invasion in human glioblastoma multiforme cells. Cancer Lett. 2009;273:164–171. doi: 10.1016/j.canlet.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Mao Y, Li Z, Lou C, Zhang Y. Expression of phosphorylated Stat5 predicts expression of cyclin D1 and correlates with poor prognosis of colonic adenocarcinoma. Int J Colorectal Dis. 2011;26:29–35. doi: 10.1007/s00384-010-1090-7. [DOI] [PubMed] [Google Scholar]
- 18.Xiong H, Su WY, Liang QC, Zhang ZG, Chen HM, Du W, Chen YX, Fang JY. Inhibition of STAT5 induces G1 cell cycle arrest and reduces tumor cell invasion in human colorectal cancer cells. Lab Invest. 2009;89:717–725. doi: 10.1038/labinvest.2009.11. [DOI] [PubMed] [Google Scholar]
- 19.Koppikar P, Lui VW, Man D, Xi S, Chai RL, Nelson E, Tobey AB, Grandis JR. Constitutive activation of signal transducer and activator of transcription 5 contributes to tumor growth, epithelial-mesenchymal transition, and resistance to epidermal growth factor receptor targeting. Clin Cancer Res. 2008;14:7682–7690. doi: 10.1158/1078-0432.CCR-08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Ye D, Xie X, Chen B, Lu W. VEGF, VEGFRs expressions and activated STATs in ovarian epithelial carcinoma. Gynecol Oncol. 2004;94:630–635. doi: 10.1016/j.ygyno.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 21.Jackerott M, Moldrup A, Thams P, Galsgaard ED, Knudsen J, Lee YC, Nielsen JH. STAT5 activity in pancreatic β-cells influences the severity of diabetes in animal models of type 1 and 2 diabetes. Diabetes. 2006;55:2705–2712. doi: 10.2337/db06-0244. [DOI] [PubMed] [Google Scholar]
- 22.Kataoka TR, Ioka T, Tsukamoto Y, Matsumura M, Ishiguro S, Nishizawa Y. Nuclear expression of STAT5 in intraductal papillary mucinous neoplasms of the pancreas. Int J Surg Pathol. 2007;15:277–281. doi: 10.1177/1066896907302233. [DOI] [PubMed] [Google Scholar]
- 23.Lang SA, Moser C, Gaumann A, Klein D, Glockzin G, Popp FC, Dahlke MH, Piso P, Schlitt HJ, Geissler EK, et al. Targeting heat shock protein 90 in pancreatic cancer impairs insulin-like growth factor-I receptor signaling, disrupts an interleukin-6/signal-transducer and activator of transcription 3/hypoxia-inducible factor-1β autocrine loop, and reduces orthotopic tumor growth. Clin Cancer Res. 2007;13:6459–6468. doi: 10.1158/1078-0432.CCR-07-1104. [DOI] [PubMed] [Google Scholar]
- 24.Hackl C, Lang SA, Moser C, Mori A, Fichtner-Feigl S, Hellerbrand C, Dietmeier W, Schlitt HJ, Geissler EK, Stoeltzing O. Activating transcription factor-3 (ATF3) functions as a tumor suppressor in colon cancer and is up-regulated upon heat-shock protein 90 (Hsp90) inhibition. BMC Cancer. 2010;10:668. doi: 10.1186/1471-2407-10-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taeger J, Moser C, Hellerbrand C, Mycielska ME, Glockzin G, Schlitt HJ, Geissler EK, Stoeltzing O, Lang SA. Targeting FGFR/PDGFR/VEGFR impairs tumor growth, angiogenesis, and metastasis by effects on tumor cells, endothelial cells, and pericytes in pancreatic cancer. Mol Cancer Ther. 2011;10:2157–2167. doi: 10.1158/1535-7163.MCT-11-0312. [DOI] [PubMed] [Google Scholar]
- 26.Gottfried E, Rogenhofer S, Waibel H, Kunz-Schughart LA, Reichle A, Wehrstein M, Peuker A, Peter K, Hartmannsgruber G, Andreesen R, et al. Pioglitazone modulates tumor cell metabolism and proliferation in multicellular tumor spheroids. Cancer Chemother Pharmacol. 2011;67:117–126. doi: 10.1007/s00280-010-1294-0. [DOI] [PubMed] [Google Scholar]
- 27.Lang SA, Moser C, Fichnter-Feigl S, Schachtschneider P, Hellerbrand C, Schmitz V, Schlitt HJ, Geissler EK, Stoeltzing O. Targeting heat-shock protein 90 improves efficacy of rapamycin in a model of hepatocellular carcinoma in mice. Hepatology. 2009;49:523–532. doi: 10.1002/hep.22685. [DOI] [PubMed] [Google Scholar]
- 28.Bernaciak TM, Zareno J, Parsons JT, Silva CM. A novel role for signal transducer and activator of transcription 5b (STAT5b) in β1-integrin-mediated human breast cancer cell migration. Breast Cancer Res. 2009;11:R52. doi: 10.1186/bcr2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchler P, Reber HA, Tomlinson JS, Hankinson O, Kallifatidis G, Friess H, Herr I, Hines OJ. Transcriptional regulation of urokinase-type plasminogen activator receptor by hypoxia-inducible factor 1 is crucial for invasion of pancreatic and liver cancer. Neoplasia. 2009;11:196–206. doi: 10.1593/neo.08734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C, Qiu Z, Wang L, Peng Z, Jia Z, Logsdon CD, Le X, Wei D, Huang S, Xie K. A novel FoxM1-caveolin signaling pathway promotes pancreatic cancer invasion and metastasis. Cancer Res. 2012;72:655–665. doi: 10.1158/0008-5472.CAN-11-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koch M, Dettori D, Van Nuffelen A, Souffreau J, Marconcini L, Wallays G, Moons L, Bruyere F, Oliviero S, Noel A, et al. VEGF-D deficiency in mice does not affect embryonic or postnatal lymphangiogenesis but reduces lymphatic metastasis. J Pathol. 2009;219:356–364. doi: 10.1002/path.2605. [DOI] [PubMed] [Google Scholar]
- 32.Kurahara H, Takao S, Maemura K, Shinchi H, Natsugoe S, Aikou T. Impact of vascular endothelial growth factor-C and -D expression in human pancreatic cancer: its relationship to lymph node metastasis. Clin Cancer Res. 2004;10:8413–8420. doi: 10.1158/1078-0432.CCR-04-0379. [DOI] [PubMed] [Google Scholar]
- 33.Garcea G, Lloyd TD, Gescher A, Dennison AR, Steward WP, Berry DP. Angiogenesis of gastrointestinal tumours and their metastases—a target for intervention? Eur J Cancer. 2004;40:1302–1313. doi: 10.1016/j.ejca.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 35.Kim HJ, Tsoyi K, Heo JM, Kang YJ, Park MK, Lee YS, Lee JH, Seo HG, Yun-Choi HS, Chang KC. Regulation of lipopolysaccharide-induced inducible nitric-oxide synthase expression through the nuclear factor-κB pathway and interferon-β/tyrosine kinase 2/Janus tyrosine kinase 2-signal transducer and activator of transcription-1 signaling cascades by 2-naphthylethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline (THI 53), a new synthetic isoquinoline alkaloid. J Pharmacol Exp Ther. 2007;320:782–789. doi: 10.1124/jpet.106.112052. [DOI] [PubMed] [Google Scholar]
- 36.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuwahara K, Sasaki T, Kuwada Y, Murakami M, Yamasaki S, Chayama K. Expressions of angiogenic factors in pancreatic ductal carcinoma: a correlative study with clinicopathologic parameters and patient survival. Pancreas. 2003;26:344–349. doi: 10.1097/00006676-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Niedergethmann M, Hildenbrand R, Wostbrock B, Hartel M, Sturm JW, Richter A, Post S. High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas. 2002;25:122–129. doi: 10.1097/00006676-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Rahbari NN, Schmidt T, Falk CS, Hinz U, Herber M, Bork U, Buchler MW, Weitz J, Koch M. Expression and prognostic value of circulating angiogenic cytokines in pancreatic cancer. BMC Cancer. 2011;11:286. doi: 10.1186/1471-2407-11-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yachida S, Iacobuzio-Donahue CA. The pathology and genetics of metastatic pancreatic cancer. Arch Pathol Lab Med. 2009;133:413–422. doi: 10.5858/133.3.413. [DOI] [PubMed] [Google Scholar]
- 41.Gu L, Vogiatzi P, Puhr M, Dagvadorj A, Lutz J, Ryder A, Addya S, Fortina P, Cooper C, Leiby B, et al. Stat5 promotes metastatic behavior of human prostate cancer cells in vitro and in vivo. Endocr Relat Cancer. 2010;17:481–493. doi: 10.1677/ERC-09-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Man S, Munoz R, Kerbel RS. On the development of models in mice of advanced visceral metastatic disease for anti-cancer drug testing. Cancer Metastasis Rev. 2007;26:737–747. doi: 10.1007/s10555-007-9087-6. [DOI] [PubMed] [Google Scholar]
- 43.Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.