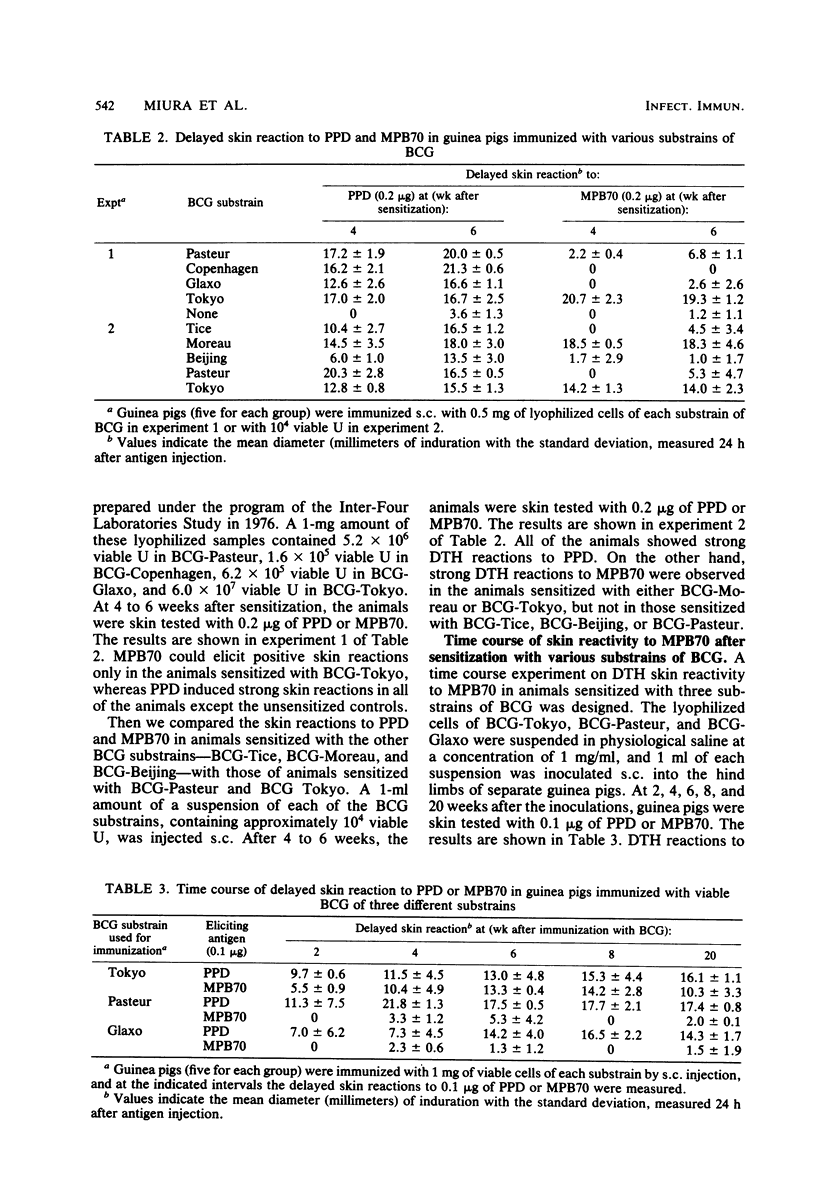
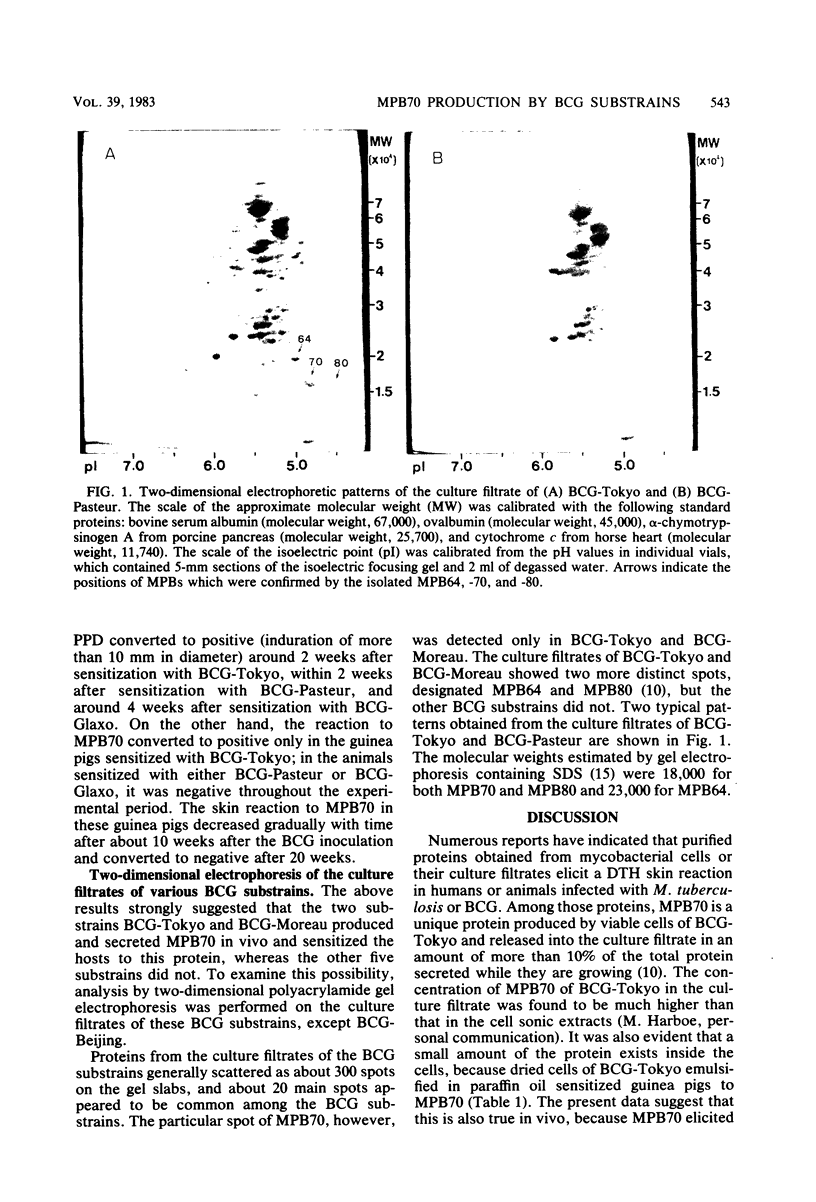
Abstract
A protein, isolated and purified from the unheated culture filtrate of Mycobacterium bovis BCG (substrain Tokyo 172) and designated MPB70, elicited a delayed skin reaction in guinea pigs sensitized with viable cells of BCG but not in those sensitized with heat-killed cells. The skin reaction reached the maximum 4 to 8 weeks after the inoculation of the BCG and then decreased gradually, resulting in conversion to negative after 20 weeks, whereas the skin reaction to purified protein derivative (PPD) continued to be positive. Guinea pigs immunized with viable cells of various substrains of BCG were skin tested with MPB70 and PPD. Guinea pigs immunized with the BCG substrain Tokyo 172 and the substrain Moreau (Brazil) showed strong delayed skin reactions to both MPB70 and PPD. On the other hand, guinea pigs immunized with the Pasteur substrain 1173P2, the Glaxo substrain 1077, the Copenhagen substrain 1331, the Tice substrain, or the Beijing substrain 64-42 showed negative skin reactions to MPB70, whereas they were strongly positive to PPD. In a two-dimensional acrylamide gel electrophoretic analysis of proteins from the culture filtrates of the BCG substrains, the culture filtrates of the Tokyo and Moreau substrains showed the spot of MPB70 on the gel slabs, whereas those of the other BCG substrains did not.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augier J., Augier-Gibory S. Analyse électrophorétique bidimensionnelle en gel de polyacrylamide des tuberculoprotéines présentes dans L'IP 48 et dans diverses autres préparations de PPD. Ann Inst Pasteur (Paris) 1969 May;116(5):713–726. [PubMed] [Google Scholar]
- Chaparas S. D., Hedrick S. R. Comparison of strains of BCG. I. Antigenic analysis and tuberculin reactivity. Infect Immun. 1973 May;7(5):777–780. doi: 10.1128/iai.7.5.777-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparas S. D., Janicki B. W., Good R., Johnson A. H., Wright G., Goldstein R., Daniel T. M., Alling D. W. In vitro and in vivo reactivity and specificity of fractions from sonicates of Mycobacterium tuberculosis separated by gradient acrylamide gel electrophoresis. Am Rev Respir Dis. 1980 Oct;122(4):533–542. doi: 10.1164/arrd.1980.122.4.533. [DOI] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Cohn M. L., Davis C. L., Middlebrook G. Comparison of freeze-dried daughter strains of BCG by aerogenic immunization and virulent challenge. Tubercle. 1966 Sep;47(3):263–268. doi: 10.1016/s0041-3879(66)80004-1. [DOI] [PubMed] [Google Scholar]
- DUBOS R. J., PIERCE C. H., SCHAEFER W. B. Differential characteristics in vitro and in vivo of several substrains of BCG. III. Multiplication and survival in vivo. Am Rev Tuberc. 1956 Nov;74(5):683–698. doi: 10.1164/artpd.1956.74.5.683. [DOI] [PubMed] [Google Scholar]
- LIND A. Serological studies of mycobacteria by means of diffusion-in-gel techniques. 3. A difference in precipitinogenic content found in substrains of BCG. Int Arch Allergy Appl Immunol. 1960;17:1–9. doi: 10.1159/000229107. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Auclair D. J., Lagrange P. H. Immunopotentiation with BCG. I. Immune response to different strains and preparations. J Natl Cancer Inst. 1973 Nov;51(5):1655–1667. doi: 10.1093/jnci/51.5.1655. [DOI] [PubMed] [Google Scholar]
- Nagai S., Matsumoto J., Nagasuga T. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect Immun. 1981 Mar;31(3):1152–1160. doi: 10.1128/iai.31.3.1152-1160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Sher N. A., Chaparas S. D., Pearson J., Chirigos M. Virulence of six strains of Mycobacterium bovis (BCG) in mice. Infect Immun. 1973 Nov;8(5):736–742. doi: 10.1128/iai.8.5.736-742.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wright G. L., Jr, Affronti L. F., Reich M. Characterization and comparison of mycobacterial antigens by two-dimensional polyacrylamide gel electrophoresis. Infect Immun. 1972 Apr;5(4):482–490. doi: 10.1128/iai.5.4.482-490.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G. L., Jr, Roberts D. B. Two-dimensional immunoelectrophoresis of mycobacterial antigens. Comparison with a reference system. Am Rev Respir Dis. 1974 Feb;109(2):306–310. doi: 10.1164/arrd.1974.109.2.306. [DOI] [PubMed] [Google Scholar]




