Abstract
Objective
The aim of this study was to investigate significant clinical, tumour-related and dosimetric factors among patients with grade 0–1, grade 2 and grade 3 radiation pneumonitis (RP) after stereotactic body radiotherapy (SBRT) for lung tumours.
Methods
Patients (n=128) with a total of 133 lung tumours treated with SBRT of 50 Gy in 5 fractions were analysed. RP was graded according to the Common Terminology Criteria for Adverse Events v.3.0. Significant factors were identified by univariate and multivariate analyses. Threshold dose–volume histograms (DVHs) were constructed to identify the incidence of RP.
Results
The median follow-up period was 12 months (range, 6–45 months). In univariate analyses, gender, operability, forced expiratory volume in 1 s (FEV1), internal target volume, lung volumes treated with doses >5–30 Gy (V5–30) and mean lung dose were significant factors differentiating between grade 0–1 and grade 2 RP, and V15–30 were significant factors differentiating between grade 2 and grade 3. However, no factors were significant between grade 0–1 and grade 3 RP. Multivariate analysis showed that female gender, high FEV1 and high V15 were significant factors differentiating between grade 0–1 and grade 2 RP. Threshold DVH curves were created based on ≤5% and ≤15% risk of grade 2 RP among patients with grade 0–2 RP.
Conclusions
Grade 0–2 RP was dose–volume dependent, and female gender and high FEV1 were significant predictive clinical factors for grade 2 RP among patients with grade 0–2 RP. However, incidences of V15–30 in grade 3 RP were significantly lower than those in grade 2 RP, and no significant clinical or tumour-related factors were found. Further studies are needed to identify the mechanism underlying the development of grade 3 RP after SBRT for lung tumours.
Previously, we investigated the clinical and dosimetric factors that correlate with severe radiation pneumonitis (RP) in patients with lung tumours treated with stereotactic body radiotherapy (SBRT) [1]. We found that, among a variety of factors, only a short latent period was a significant correlate of severe RP.
Other reports [2-6] have also analysed the clinical and dosimetric factors correlated with RP after SBRT. Various dosimetric factors were reported to significantly correlate with RP after SBRT, which included the mean dose in the ipsilateral lung, V7 and V10 by Kyas et al [2], normal tissue complication probability (NTCP) by Ricardi et al [3], lung volumes treated with doses higher than 2.5–50 Gy (V2.5–50) by Guckenberger et al [4], mean lung dose by Barriger et al [5] and contralateral V5 by Ong et al [6].
We found a discrepancy in the significant clinical and dosimetric factors between the results of these five studies on low-grade RP [2-6] and our study on severe RP [1]. We speculated that the mechanism underlying the development of grade ≥3 RP might be different from that of grade 2 RP. Additionally, the treatment of grade ≥3 RP was much more critical than that of grade 2 RP. Most patients with grade ≥3 RP needed to be admitted to hospital and steroids or oxygen therapy were administered. By contrast, patients with grade 2 RP were simply followed up carefully without administration of medication as outpatients.
In the present study, to ascertain this discrepancy, we analysed the clinical and dosimetric factors that correlated with RP after SBRT among patients with grade 0–1, grade 2 and grade 3 RP in the same sample of patients as that included in our previous study [1].
Methods and materials
Patients
Between February 2005 and November 2008, SBRT was performed at our institutions for a total of 163 lung tumours in 157 patients with Stage I and II primary lung cancers and solitary metastatic lung tumours. All patients provided written informed consent. From our database, we retrospectively collected the data for patients who had a minimum follow-up period of 6 months. As of February 2009, a total of 128 patients with 133 lung tumours were included in this study. Five patients who had two metachronous lesions were treated with SBRT twice at different times. Thus, we investigated 133 cases of SBRT.
The patients' backgrounds were described in the previous report [1] (Table 1). There were 111 primary lung cancers and 22 metastatic lung tumours. Regarding the primary lung cancers, pathologically proven cases included 41 adenocarcinomas, 25 squamous cell carcinomas, 6 non-small cell lung cancers (NSCLCs) and 4 small cell lung cancers. The remaining 35 patients were considered to have lung cancer without pathologically proven evidence, based on successive increases in tumour sizes obtained by CT, as well as by uptake on positron emission tomography and/or elevated levels of tumour markers (carcinoembryonic antigen, squamous cell carcinoma, cytokeratin 19 fragment, sialyl Lewis-x antigen, neuron specific enolase or pro-gastrin-releasing peptide). Among the metastatic lung tumours, the primary sites were the colon (10) and the lung (6), with 6 tumours occurring in other sites. There were 34 operable and 99 inoperable cases.
Table 1. Clinical or tumour-related factors evaluated as possible contributors to grade 0–1, 2 and 3 radiation pneumonitis.
| Median (range) |
Tukey's HSD test |
|||||
| Grade 0–1 | Grade 2 | Grade 3 | 0–1 vs 2 | 0–1 vs 3 | 2 vs 3 | |
| Factors | (n=105) | (n=21) | (n=7) | p-value | p-value | p-value |
| Clinical | ||||||
| Gender | 79/26 | 8/13 | 5/2 | 0.002a | 0.974 | 0.205 |
| Age (years) | 77 (52–92) | 77 (43–86) | 79 (70–85) | 0.264 | 0.93 | 0.466 |
| Operability | 82/23 | 11/10 | 6/1 | 0.036a | 0.893 | 0.182 |
| Emphysema | 73/32 | 19/2 | 5/2 | 0.121 | 0.993 | 0.587 |
| LDH (IU l–1) | 198 (136–429) | 198 (147–297) | 200 (177–277) | 0.838 | 0.813 | 0.667 |
| CRP (mg dl–1) | 0.13 (0–5.7) | 0.06 (0. –3.3) | 0.1 (0–3.8) | 0.773 | 0.97 | 0.985 |
| KL-6 (U ml–1) | 324 (124–816) | 274 (178–515) | 410 (177–620) | 0.386 | 0.326 | 0.107 |
| SP-D (ng ml–1) | 50 (17–158) | 38 (17–116) | 54 (36–64) | 0.555 | 0.898 | 0.980 |
| Pulmonary function test | ||||||
| FEV1 (l) | 1.48 (0.46–3.56) | 1.97 (1.06–3.12) | 1.5 (0.61–2.64) | 0.041a | 0.587 | 0.850 |
| VC (l) | 2.32 (1.28–4.4) | 2.57 (1.55–4.5) | 1.85 (1.76–3.32) | 0.568 | 1.0 | 0.836 |
| Tumour-related | ||||||
| Upper vs lower | 53/52 | 7/14 | 3/4 | 0.328 | 0.920 | 0.901 |
| Central vs peripheral | 29/76 | 15/6 | 5/2 | 0.996 | 0.998 | 1.0 |
| ITV (ml) | 8.7 (0.2–62.8) | 15.0 (1.8–66.8) | 10.6 (2.2–35.0) | 0.018a | 0.922 | 0.471 |
| Dosimetric | ||||||
| V5 | 17.7 (4.5–40.9) | 23.3 (9.5–40.1) | 23.3 (11.5–33.1) | <0.0001a | 0.117 | 0.760 |
| V10 | 9.3 (2.5–28.9) | 14.3 (3.3–25.1) | 11.3 (6.1–13.8) | <0.0001a | 0.709 | 0.121 |
| V15 | 5.6 (1.2–19.9) | 9.4 (2.1–18.7) | 6.0 (4.0–6.8) | <0.0001a | 0.958 | 0.009a |
| V20 | 3.7 (0.7–11.6) | 6.2 (1.5–13.0) | 3.8 (2.9–4.9) | <0.0001a | 0.983 | 0.004a |
| V25 | 2.6 (0.4–8.1) | 4.5 (1.1–8.7) | 2.7 (2.2–4.0) | <0.0001a | 1.0 | 0.004a |
| V30 | 1.8 (0.2–6.0) | 3.2 (0.9–6.6) | 1.9 (1.6–3.3) | <0.0001a | 0.974 | 0.008a |
| Mean lung dose (cGy) | 354 (137–829) | 500 (192–753) | 422 (228–538) | <0.0001a | 0.714 | 0.102 |
CRP, C-reactive protein; FEV1, forced expiratory volume in 1 s; HSD, honestly significant difference; ITV, internal target volume; KL-6, sialylated carbohydrate antigen; LDH, lactate dehydrogenase; SP-D, surfactant protein D; VC, vital capacity.
aStatistically significant (p<0.05).
Before treatment, a pulmonary function test was performed for all patients. Lactate dehydrogenase (LDH) and C-reactive protein (CRP) levels were obtained. Sialylated carbohydrate antigen (KL-6) and surfactant protein D (SP-D) were also monitored on the wards from February 2006 and March 2007, respectively.
Treatment
We have described our methods for SBRT in previous reports [7,8]. To summarise briefly, long-scan-time CT was used to directly visualise the internal target volume (ITV) after immobilising the patient with a vacuum pillow. A planning target volume (PTV) was determined by adding a margin of 6–8 mm to the ITV. Dynamic conformal multiple arc irradiation was used for SBRT. The leaf margins were modified to ensure that the PTV was included in the 80% isodose surface. The dose calculation was determined using a superposition algorithm. The prescribed doses were defined as 80% isodoses of the maximum doses; in a previous study, they were found to be nearly equivalent to the dose that covered 95% of the PTV (D95) [8].
As for the prescribed dose, we principally used 50 Gy per 5 fractions for patients with a peripheral NSCLC lesion. For patients with a central NSCLC lesion [9], we used 50 Gy per 10 fractions until the end of March 2007, and then used 40 Gy per 5 fractions from April 2007 onwards. For patients with radioresistant solitary metastatic lung tumours such as from colon cancer [10], we employed 60 Gy per 5 fractions.
This policy resulted in 2 patients treated with a prescribed dose of 60 Gy per 5 fractions, 98 with 50 Gy per 5 fractions, 29 with 40 Gy per 5 fractions and 4 with 50 Gy per 10 fractions.
Follow-up
For all patients, graphical appearances of RP were monitored monthly on an outpatient basis with chest X-radiograph examinations until either clinical and radiograph findings had stabilised or 6 months had passed after SBRT. CT scans were performed at 1 and 3 months after SBRT and thereafter at 3 month intervals during the first 2 years, even in the absence of clinical symptoms. Because most patients with toxic events (87%) developed the end point within 6 months after SBRT [2], those without toxic events were also required to have a minimum follow-up at 6 months. RP was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v.3.0 by a single radiation oncologist (AT) within a follow-up period of 1 year, and was retrospectively reviewed.
Statistical analysis
Clinical, tumour-related and dosimetric factors were assessed for correlations among three groups: grade 0–1 RP, grade 2 RP and grade 3 RP. Clinical factors were patient gender, age, operability status, presence of emphysema, LDH, CRP, KL-6, SP-D and pulmonary function test results, including forced expiratory volume in 1 s (FEV1) and vital capacity (VC). Tumour-related factors included tumour location (upper lung vs lower lung or central lesion vs peripheral lesion) and ITV. Dosimetric factors included V5 (in increments of 5 Gy) and mean lung dose. Differences among the three groups were analysed with Tukey's honestly significant difference test. The variables that were significantly different (p<0.05) between grade 0–1 RP and grade 2 RP by univariate analysis were then included in a multivariate analysis using a logistic regression test. Data were analysed with SPSS 17.0 [IBM Corporation (formerly SPSS Inc.), Armonk, NY]. Differences by univariate and multivariate analysis were regarded as statistically significant at p<0.05.
Results
Patient characteristics
The median follow-up period was 12 months (range, 6–45 months). Among the patients in this study, RP developed as follows: grade 0 in 36 patients (27%), grade 1 in 69 patients (52%), grade 2 in 21 patients (16%) and grade 3 in 7 patients (5%). No patient had RP of grade ≥4.
Comparisons among grade 0–1, grade 2 and grade 3 RP
The results of the analyses of clinical, tumour-related and dosimetric factors are given in Table 1. Gender, operability, FEV1, ITV and all of the dosimetric factors were significant predictive risk factors between grade 0–1 and grade 2 RP. V15–30 were significant predictive risk factors between grade 2 and grade 3 RP. However, no clinical or dosimetric factors were significant between grade 0–1 and grade 3 RP.
Subsequent multivariate analysis between grade 0–1 and grade 2 RP was performed with the variables that showed univariate significance (p<0.05). V15 was used as a surrogate for the dosimetric factors because V20 in conventional fractionated radiotherapy (CFRT) with 50 Gy, delivered in 2 Gy per fraction, approximates V15 for SBRT of 50 Gy delivered in 10 Gy per fraction using the linear quadratic model, with an α/β ratio of 3.3±1.5 Gy [11]. As shown in Table 2, the significant factors were gender, FEV1 and V15. The risk of grade 2 RP was higher in females than in males (33.3% vs 9.2%, respectively). Figure 1 shows the relationship between the grade of RP and FEV1. The FEV1 was analysed in two clusters: one for which FEV1 was ≤1.8 l and another for which FEV1 was >1.8 l. The risk of grade 2 RP was significantly higher in the FEV1 >1.8 l group than in the FEV1 ≤1.8 l group (26.3% vs 10.1%, respectively). The V15 was significantly higher in patients with grade 2 RP than in patients with grade 0–1 RP; meanwhile, there were no differences between grade 0–1 and grade 3 RP (Figure 2).
Table 2. Multivariate analysis of factors affecting grade 2 radiation pneumonitis.
| 95% confidence interval for HR |
||||
| Factor | p-value | HR | Lower | Upper |
| Gender | 0.032 | 5.895 | 1.160 | 29.961 |
| Operability | 0.169 | 2.600 | 0.666 | 10.150 |
| FEV1 | 0.018 | 5.760 | 1.343 | 24.701 |
| ITV | 0.264 | 1.030 | 0.978 | 1.084 |
| V15 | 0.035 | 1.275 | 1.017 | 1.599 |
FEV1, forced expiratory volume in 1 s; HR, hazard ratio; ITV, internal target volume; V15, lung volumes treated with doses >15 Gy.
Figure 1.
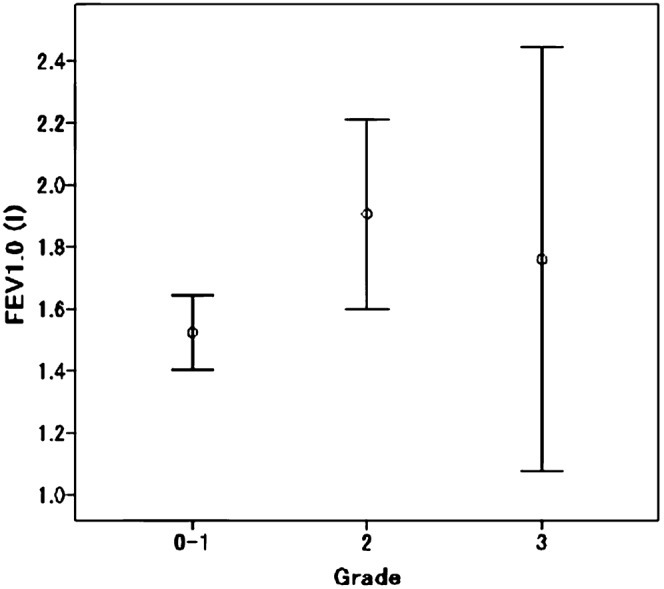
Relationship between the grade of radiation pneumonitis and FEV1. The centre circle indicates the mean FEV1 and the error bars indicate the 95% confidence interval. FEV1, forced expiratory volume in 1 s.
Figure 2.
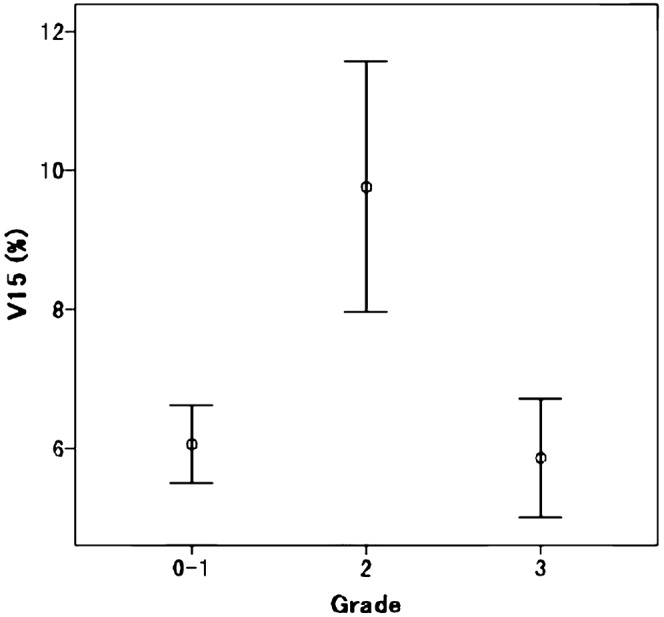
Relationship between the grade of radiation pneumonitis and V15. The centre circle indicates the mean V15 and the error bars indicate the 95% confidence interval.
Dose–volume parameters
For each dose level in the range of 5–25 Gy (in increments of 5 Gy), the risk of grade 2 RP was obtained for patients in whom the given dose covered above or below a given lung volume. By examining different lung volume cut points for a given dose, patients were separated into two groups: those who were less than or equal to and those who were above the volume threshold. This volume threshold was determined based on findings of ≤5% and ≤15% risk of grade 2 RP in the low-volume group.
The risks of developing grade 2 RP with the volume cut points for the above dose levels are listed in Table 3. For example, 15 Gy delivered to ≤6% of the lung resulted in a 5.4% rate of grade 2 RP vs 32.2% for volumes >6% (p=0.002). Figure 3 graphically shows the volume cut point which indicates that at those doses the risks of grade 2 RP were ≤5% or ≤15%. The development of grade 2 RP was found to be significantly dependent upon the volume of a given dose.
Table 3. Relationship between percentage volume of lung receiving a given dose and risk of developing grade 2 radiation pneumonitis.
| Grade 2 radiation pneumonitis rate (%) |
||||
| Dose (Gy) | Lung volume cut point (%) | ≤Volume cut point | >Volume cut point | p-value |
| 5 | 18 | 5.5 | 32.3 | 0.007 |
| 24 | 13 | 45.4 | 0.003 | |
| 10 | 10.5 | 5.8 | 38.5 | 0.003 |
| 15.8 | 13.6 | 61.9 | 0.003 | |
| 15 | 6 | 5.4 | 32.2 | 0.002 |
| 9.8 | 15 | 50.0 | 0.007 | |
| 20 | 3.6 | 5 | 37.2 | 0.006 |
| 6.5 | 14 | 46.8 | 0.003 | |
| 25 | 2.5 | 4.2 | 29.9 | 0.001 |
| 5.2 | 4.8 | 65.0 | 0.001 | |
Figure 3.
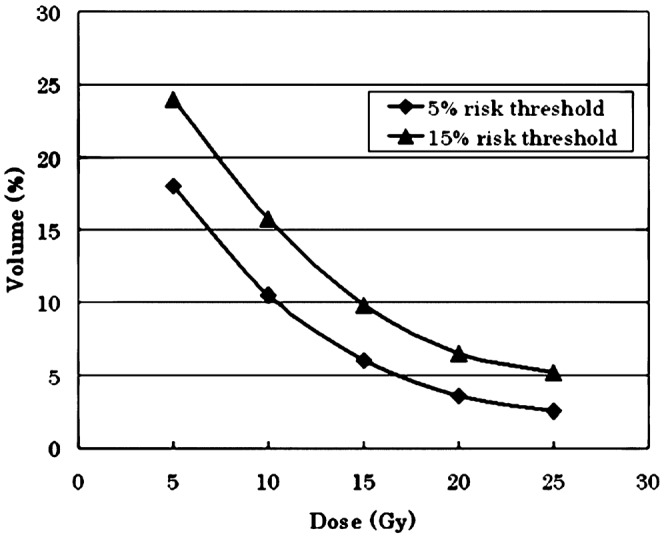
Threshold dose curve estimating the risk of grade 2 radiation pneumonitis.
Discussion
SBRT for treating lung cancer results in high local control rates and allows for a painless ambulatory treatment with minimal toxicity by focusing high radiation doses onto the target and by sparing the surrounding normal tissue [12-15]. SBRT is indicated for small lung tumours, i.e. Stage I primary lung cancer and oligometastatic lung tumours. By contrast, CFRT is usually indicated for Stage II or III lung cancer. Therefore, with SBRT, the PTV is much smaller, and a much higher dose can be irradiated to the PTV than with CFRT.
Severe RP after CFRT is often a dose-limiting factor in treating Stage II or III lung cancer; therefore, it has been well studied [16]. On the other hand, we revealed that grade 3 RP after SBRT was only correlated with a short latent period and that other dosimetric factors were not statistically significant [8]. From this context, we investigated differences among grades 0–1, 2 and 3 RP in terms of correlations with clinical, tumour-related and dosimetric factors. In addition, we will discuss to what extent the mechanism of RP after SBRT is similar to that of RP after CFRT and on which points they differ.
Dosimetric factors for radiation pneumonitis after conventional fractionated radiotherapy
For CFRT, many dosimetric factors were reported to correlate significantly with RP [16]. Table 4 shows the toxicity criteria for pneumonitis. Some differences exist between the same grades for each criterion.
Table 4. Toxicity scales for radiation pneumonitis.
| Criteria | 1 | 2 | 3 | 4 |
| CTCAE v.2.0 | Radiographic changes but asymptomatic or symptoms not requiring steroids | Radiographic changes and requiring steroids | Requiring oxygen | Requiring assisted ventilation |
| CTCAE v.3.0 | Asymptomatic; radiographic findings only | Symptomatic; not interfering with ADL | Symptomatic; interfering with ADL; O2 indicated | Life-threatening; ventilatory support indicated |
| RTOG/EORTC (LENT-SOMA) | Asymptomatic or mild symptoms (dry cough), with radiographic findings | Moderately symptomatic (severe cough, fever) | Severely symptomatic | Severe respiratory insufficiency; continuous oxygen/assisted ventilation |
| SWOG | Asymptomatic or symptoms not requiring steroids, with radiographic findings | Initiation or increase in steroids required | O2 required | Assisted ventilation necessary |
ADL, activities of daily living; CTCAE, Common Terminology Criteria for Adverse Events; EORTC, European Organisation for Research and Treatment of Cancer; LENT-SOMA, Late effects of normal tissue: subjective, objective, management and analysis; RTOG, Radiation Therapy Oncology Group; SWOG, Southwest Oncology Group.
Among dosimetric factors, V20 has been a well-known and significant factor for RP in various evaluations with repetition, i.e. by means of Radiation Therapy Oncology Group (RTOG) grade 1 [17], RTOG grade 2 [17,18], CTCAE v. 2.0 [19,20], CTCAE v. 3.0 [21] and Southwest Oncology Group (SWOG) grade 2 [22]. Mean lung dose (MLD) was also reported to be a significant factor by means of RTOG grade 1 [17], RTOG grade 2 [17], RTOG grade 3 [23], CTCAE v.1.0 [24], CTCAE v.2.0 [20] and SWOG grade 2 [25]. Recently, lung volumes that were treated with doses >5 Gy were also found to be significant factors in CFRT with chemotherapy [26,27]. In addition, Jin et al [21] studied grade ≥3 RPs by CTCAE v.3.0 and showed threshold DVH curves defined by V20 ≤25%, V25 ≤20%, V35 ≤15% and V50 ≤10%. Patients with lung DVHs satisfying these constraints had only a 2% incidence of grade ≥3 RP.
Dosimetric factors in SBRT
Only a few studies regarding factors correlating significantly with RP after SBRT have been reported. Kyas et al [2] studied a total of 64 patients with NSCLC treated with single doses of 20–30 Gy to estimate the risk of RP. They reported that V7 and V10 could be used to predict the risk of lung toxicity after SBRT [2]. Their end points were the occurrence or non-occurrence of perifocal changes in the lung detected by CT, which corresponded to grade ≥1 RPs by CTCAE v.3.0. Ricardi et al [3] studied RP after SBRT with either a dose of 45 Gy in 3 fractions over 5 days or a dose of 26 Gy in a single fraction. They divided patients into grade 0–1 and grade 2–3 RP by RTOG lung toxicity scores. They found a statistically significant difference between the two groups for MLD calculated by an equivalent dose in 2 Gy fractions. The results in our study showed that all of the dosimetric factors (V5–30 and MLD) we analysed correlated with grade 2 RP among grade 0–2 RP. We also identified threshold DVH curves from these dosimetric factors. In this way, V7 and V10 by Kyas, MLD by Ricardi and V5–30 and MLD in this study, which were evaluated by low-grade RP, were revealed to be significant. By contrast, no dosimetric factors were significant between grade 0–1 and grade 3 RP.
Discrepancy between low-grade and high-grade RP
There was a discrepancy between the results of the three studies concerning low-grade RP and those concerning high-grade RP. Clinically, grade ≥3 RP is much more critical than grade 2 RP, according to CTCAE v.3.0. For patients with grade 3 RP, steroids or oxygen therapy should be administered in the hospital. On the other hand, patients with grade 2 RP were followed carefully with no medication or with only antitussive medicine as outpatients. The results that no dosimetric factors were significant between grade 0–1 and grade 3 RP may be because the number of patients with grade ≥3 RP was too small to extract any significant factors. However, Figure 2 demonstrates clearly that the values of V15 in grade 0–1 RP were as low as those in grade 3 RP, although those in grade 2 were significantly higher than those of grade 0–1 and grade 3. A possible explanation is that the mechanism for the development of grade ≥3 RP after SBRT may be different from that of low-grade RP. That is, low-grade RP after SBRT was dose–volume dependent and, thus, corresponded to the classic RP after CFRT. In contrast, grade ≥3 RP was dose–volume independent, and often associated with out-of-field RP, which is considered to be a disease process that is pathophysiologically different from classic RP [28-30]. The PTVs of SBRT in this study were so small that V15 and V20 ranged from 1.2 to 19.9% and from 0.7 to 13.0%, respectively, with our treatment methods [8]. All of the values in this study were far smaller than the values for the dose–volume threshold curve with CFRT presented by Jin et al [21], who reported that the incidence of RP among patients whose DVHs were under the threshold curve was 2% for grade ≥3 according to CTCAE v.3.0.
We used CTCAE v.3.0 to evaluate RP after SBRT, which may impose potential limitations. Given that the main criteria distinguishing grade 3 pneumonitis from grade 2 pneumonitis are “interference with activities of daily living” and “oxygen indication”, there is a large subjective component in making this distinction. However, in our patients, it was very easy to differentiate those grades, because those patients who had grade 3 RP had symptoms that were much more severe than those of patients with grade 2 RP. When we thought oxygen was indicated for a patient owing to dyspnoea, we checked the partial pressure of oxygen in the arterial blood (PaO2) and ascertained that the value was <60 torr. The distinction between grades 1 and 2 is also unclear, because patients often have comorbid pulmonary disease and are elderly. Sometimes they have pulmonary symptoms, i.e. cough and effort dyspnoea, before treatment. We carefully interviewed patients with regard to whether the symptoms occurred after treatment or if they were stable before treatment.
Further investigation is needed to identify the significant clinical, tumour-related and dosimetric factors that correlate with grade ≥3 RP. When clarifying risk factors of severe RP and excluding patients with such risk factors, we may be able to treat larger lesions and/or treat with dose escalation with feasibility.
Clinical factors with CFRT and SBRT
Many clinical factors were also reported to be significant for RP evaluated by various criteria and grades [16]. These included no history of smoking, female gender, low performance status, low pulmonary function, low PaO2, co-morbid pulmonary disease, tumours in the lower lung field and concurrent chemotherapy. However, clinical factors for RP after conventional radiotherapy have not been demonstrated consistently across different studies [16].
To our knowledge, there has been only one report that studied clinical risk factors correlating with RP after SBRT. Kimura et al [31] reported that most of their patients with no evidence of increased density patterns and scar-like patterns had significant pulmonary emphysema. Other reports have also suggested that poor pulmonary function or comorbidity of pulmonary emphysema did not lead to poor outcome in toxicity. Baumann et al [32] reported that grade 1–2 RP and grade ≥3 RP according to CTCAE v.2.0 occurred in 7 patients and no patients, respectively, and in 40 patients with chronic obstructive pulmonary disease treated with SBRT. Henderson et al [33] reported that poor baseline pulmonary function did not predict decreased survival or pulmonary function after SBRT. Paludan et al [34] found no association between DVH parameters and changes in dyspnoea nor any consistent temporal variations of dyspnoea after SBRT. In our study, multivariate analysis showed that female gender and high FEV1 correlated significantly with grade 2 RP. Patients with low FEV1, who typically had a comorbidity of pulmonary emphysema, were at low risk for grade 2 RP. This corresponded with the results that no poor pulmonary function or comorbidity of pulmonary emphysema led to poor outcome in toxicity and may often be advantageous for treating patients who have comorbid moderate pulmonary emphysema.
Female gender was also a significant factor for RP, with inconsistency across some studies. Robnett et al [35] reported that female gender was significant; by contrast, Claude et al [17] reported that gender was not significant for RP. Robnett et al proposed two reasons for its significance. One was that most females have smaller lung volumes (and smaller FEV values), and the other was that RP may represent a hypersensitivity reaction, similar in some ways to an autoimmune disease, and many autoimmune diseases are more common in females. We believe that there is an underlying mechanism for development of grade 3 RP after SBRT for lung tumours, and that further study should be undertaken to identify it.
Conclusions
Grade 0–2 RP was dose–volume dependent, and female gender and high FEV1 values were significant predictive clinical factors for grade 2 RP among patients with grade 0–2 RP. However, grade 3 RP was dose–volume independent, and no significant clinical and tumour-related factors were found. Further studies are required to identify the underlying mechanism for the development of grade 3 RP after SBRT for lung tumours.
References
- 1.Takeda A, Ohashi T, Kunieda E, Enomoto T, Sanuki N, Takeda T, et al. Early graphical appearance of radiation pneumonitis correlates with the severity of radiation pneumonitis after stereotactic body radiotherapy (SBRT) in patients with lung tumors. Int J Radiat Oncol Biol Phys 2010;77:685–90 [DOI] [PubMed] [Google Scholar]
- 2.Kyas I, Hof H, Debus J, Schlegel W, Karger CP. Prediction of radiation-induced changes in the lung after stereotactic body radiation therapy of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2007;67:768–74 [DOI] [PubMed] [Google Scholar]
- 3.Ricardi U, Filippi AR, Guarneri A, Giglioli FR, Mantovani C, Fiandra C, et al. Dosimetric predictors of radiation-induced lung injury in stereotactic body radiation therapy. Acta Oncol 2009;48:571–7 [DOI] [PubMed] [Google Scholar]
- 4.Guckenberger M, Baier K, Polat B, Richter A, Krieger T, Wilbert J, et al. Dose-response relationship for radiation-induced pneumonitis after pulmonary stereotactic body radiotherapy. Radiother Oncol 2010;97:65–70 [DOI] [PubMed] [Google Scholar]
- 5.Barriger RB, Forquer JA, Brabham JG, Andolino DL, Shapiro RH, Henderson MA, et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2012;82:457–62 [DOI] [PubMed] [Google Scholar]
- 6.Ong CL, Palma D, Verbakel WF, Slotman BJ, Senan S. Treatment of large stage I-II lung tumors using stereotactic body radiotherapy (SBRT): planning considerations and early toxicity. Radiother Oncol 2010;97:431–6 [DOI] [PubMed] [Google Scholar]
- 7.Takeda A, Kunieda E, Shigematsu N, Hossain DM, Kawase T, Ohashi T, et al. Small lung tumors: long-scan-time CT for planning of hypofractionated stereotactic radiation therapy—initial findings. Radiology 2005;237:295–300 [DOI] [PubMed] [Google Scholar]
- 8.Takeda A, Kunieda E, Sanuki N, Ohashi T, Oku Y, Sudo Y, et al. Dose distribution analysis in stereotactic body radiotherapy using dynamic conformal multiple arc therapy. Int J Radiat Oncol Biol Phys 2009;74:363–9 [DOI] [PubMed] [Google Scholar]
- 9.Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833–9 [DOI] [PubMed] [Google Scholar]
- 10.Norihisa Y, Nagata Y, Takayama K, Matsuo Y, Sakamoto T, Sakamoto M, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys 2008;72:398–403 [DOI] [PubMed] [Google Scholar]
- 11.Van Dyk J, Mah K, Keane TJ. Radiation-induced lung damage: dose-time-fractionation considerations. Radiother Oncol 1989;14:55–69 [DOI] [PubMed] [Google Scholar]
- 12.Uematsu M, Shioda A, Suda A, Fukui T, Ozeki Y, Hama Y, et al. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer: a 5-year experience. Int J Radiat Oncol Biol Phys 2001;51:666–70 [DOI] [PubMed] [Google Scholar]
- 13.Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623–31 [DOI] [PubMed] [Google Scholar]
- 14.Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427–31 [DOI] [PubMed] [Google Scholar]
- 15.Takeda A, Sanuki N, Kunieda E, Ohashi T, Oku Y, Takeda T, et al. Stereotactic body radiotherapy for primary lung cancer at a dose of 50 Gy total in five fractions to the periphery of the planning target volume calculated using a superposition algorithm. Int J Radiat Oncol Biol Phys 2009;73:442–8 [DOI] [PubMed] [Google Scholar]
- 16.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 2005;63:5–24 [DOI] [PubMed] [Google Scholar]
- 17.Claude L, Perol D, Ginestet C, Falchero L, Arpin D, Vincent M, et al. A prospective study on radiation pneumonitis following conformal radiation therapy in non-small-cell lung cancer: clinical and dosimetric factors analysis. Radiother Oncol 2004;71:175–81 [DOI] [PubMed] [Google Scholar]
- 18.Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999;45:323–9 [DOI] [PubMed] [Google Scholar]
- 19.Tsujino K, Hirota S, Endo M, Obayashi K, Kotani Y, Satouchi M, et al. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 2003;55:110–15 [DOI] [PubMed] [Google Scholar]
- 20.Schallenkamp JM, Miller RC, Brinkmann DH, Foote T, Garces YI. Incidence of radiation pneumonitis after thoracic irradiation: dose-volume correlates. Int J Radiat Oncol Biol Phys 2007;67:410–16 [DOI] [PubMed] [Google Scholar]
- 21.Jin H, Tucker SL, Liu HH, Wei X, Yom SS, Wang S, et al. Dose-volume thresholds and smoking status for the risk of treatment-related pneumonitis in inoperable non-small cell lung cancer treated with definitive radiotherapy. Radiother Oncol 2009;91:427–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenkins P, D'Amico K, Benstead K, Elyan S. Radiation pneumonitis following treatment of non-small-cell lung cancer with continuous hyperfractionated accelerated radiotherapy (CHART). Int J Radiat Oncol Biol Phys 2003;56:360–6 [DOI] [PubMed] [Google Scholar]
- 23.Kim TH, Cho KH, Pyo HR, Lee JS, Zo JI, Lee DH, et al. Dose-volumetric parameters for predicting severe radiation pneumonitis after three-dimensional conformal radiation therapy for lung cancer. Radiology 2005;235:208–15 [DOI] [PubMed] [Google Scholar]
- 24.Hernando ML, Marks LB, Bentel GC, Zhou SM, Hollis D, Das SK, et al. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys 2001;51:650–9 [DOI] [PubMed] [Google Scholar]
- 25.Kwa SL, Lebesque JV, Theuws JC, Marks LB, Munley MT, Bentel G, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys 1998;42:1–9 [DOI] [PubMed] [Google Scholar]
- 26.Yorke ED, Jackson A, Rosenzweig KE, Braban L, Leibel SA, Ling CC. Correlation of dosimetric factors and radiation pneumonitis for non-small-cell lung cancer patients in a recently completed dose escalation study. Int J Radiat Oncol Biol Phys 2005;63:672–82 [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Liao Z, Wei X, Liu HH, Tucker SL, Hu CS, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys 2006;66:1399–407 [DOI] [PubMed] [Google Scholar]
- 28.Roberts CM, Foulcher E, Zaunders JJ, Bryant DH, Freund J, Cairns D, et al. Radiation pneumonitis: a possible lymphocyte-mediated hypersensitivity reaction. Ann Intern Med 1993;118:696–700 [DOI] [PubMed] [Google Scholar]
- 29.Gibson PG, Bryant DH, Morgan GW, Yeates M, Fernandez V, Penny R, et al. Radiation-induced lung injury: a hypersensitivity pneumonitis? Ann Intern Med 1988;109:288–91 [DOI] [PubMed] [Google Scholar]
- 30.Kataoka M, Kawamura M, Ueda N, Itoh H, Iio A, Hamamoto K. Diffuse gallium-67 uptake in radiation pneumonitis. Clin Nucl Med 1990;15:707–11 [PubMed] [Google Scholar]
- 31.Kimura T, Matsuura K, Murakami Y, Hashimoto Y, Kenjo M, Kaneyasu Y, et al. CT appearance of radiation injury of the lung and clinical symptoms after stereotactic body radiation therapy (SBRT) for lung cancers: are patients with pulmonary emphysema also candidates for SBRT for lung cancers? Int J Radiat Oncol Biol Phys 2006;66:483–91 [DOI] [PubMed] [Google Scholar]
- 32.Baumann P, Nyman J, Lax I, Friesland S, Hoyer M, Rehn Ericsson S, et al. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta Oncol 2006;45:787–95 [DOI] [PubMed] [Google Scholar]
- 33.Henderson M, McGarry R, Yiannoutsos C, Fakiris A, Hoopes D, Williams M, et al. Baseline pulmonary function as a predictor for survival and decline in pulmonary function over time in patients undergoing stereotactic body radiotherapy for the treatment of stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;72:404–9 [DOI] [PubMed] [Google Scholar]
- 34.Paludan M, Traberg Hansen A, Petersen J, Grau C, Hoyer M. Aggravation of dyspnea in stage I non-small cell lung cancer patients following stereotactic body radiotherapy: is there a dose-volume dependency? Acta Oncol 2006;45:818–22 [DOI] [PubMed] [Google Scholar]
- 35.Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 2000;48:89–94 [DOI] [PubMed] [Google Scholar]


