Abstract
Objectives
To evaluate the added value of diffusion-weighted imaging (DWI) in combination with T2 weighted imaging (T2WI) compared with T2WI alone or positron emission tomography (PET)/CT for detecting viable tumour after neoadjuvant chemoradiation therapy (CRT) in patients with locally advanced rectal cancer.
Methods
50 consecutive patients with locally advanced rectal cancer (≥T3 or lymph node positive) who underwent neoadjuvant CRT and subsequent surgery were enrolled in this retrospective study. All patients underwent 3.0 T rectal MRI and PET/CT after completing CRT. For qualitative analysis, two radiologists independently reviewed T2WI alone and DWI with T2WI over a 1-month interval. One nuclear medicine physician reviewed PET/CT images using a five-point scale. Diagnostic accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for detecting viable tumour were assessed. For quantitative analysis, the apparent diffusion coefficients (ADCs) of the lesions were measured and compared between the viable tumour group and non-viable tumour groups.
Results
For detecting viable tumours, DWI with T2WI improved diagnostic accuracies (Reviewer 1 detected 90%; Reviewer 2, 86%) over T2WI alone (Reviewer 1 detected 76%, p=0.5; Reviewer 2, 64%, p=0.013) or PET/CT (48%, p<0.001). The sensitivity of DWI with T2WI (Reviewer 1 detected 98%; Reviewer 2, 91%) was significantly higher than those of T2WI alone (Reviewer 1 detected 77%; Reviewer 2, 64%) or PET-CT (43%, p<0.05). Only for Reviewer 2 was the NPV of DWI with T2WI (43%) significantly different from that of PET/CT (17%, p<0.05). The specificities and PPVs of DWI with T2WI were not improved over those of T2WI alone or of PET/CT (both p>0.05). The mean ADC of the viable tumour group (0.93×10−3 mm2 sc−1) was significantly lower than that of the non-viable tumour group (1.55×10−3 mm2 sc−1, p<0.0001).
Conclusion
Adding DWI to T2WI is helpful for detecting viable tumours after neoadjuvant CRT compared with T2WI alone or PET/CT in patients with locally advanced rectal cancer.
Neoadjuvant chemoradiation therapy (CRT) has now become the standard of care for clinically staged, locally advanced rectal cancer. This treatment is associated with fewer local recurrences [1] and may also result in improved long-term survival [2]. Post-operative histopathological results are important indicators of prognosis in rectal cancer after CRT [3,4]. Therefore, pre-operative and non-invasive assessment of response to CRT of rectal cancer, and the identification of viable tumours after CRT, are crucial for planning the most beneficial strategies for each individual patient. This could guide the optimisation of the surgical approach, perhaps indicating sphincter-saving surgery in deep-seated tumours or less aggressive resection in initially advanced tumours.
Although MRI is considered the most accurate tool for primary tumour staging in rectal cancer [5-7], this modality has intrinsic limitations in the differentiation of residual viable tumour from surrounding fibrosis after neoadjuvant CRT of rectal cancer [8-10]. With the introduction of higher field-strength MR scanners and the parallel imaging technique for rectal MRI, diffusion-weighted imaging (DWI) has been shown to have several potential benefits for the assessment of tumour localisation and staging [11,12]. The usefulness of DWI at 1.5 T in measuring treatment response has been measured in several clinical studies [13-15]. Recently, 3.0 T MR scanners have become commercially available [16-18]; the increase in signal-to-noise ratio (SNR) provided by these machines offers increased DWI quality and improvements to apparent diffusion coefficient (ADC) maps.
Aside from innovative MRI approaches such as DWI, non-invasive proof of tumour viability can be obtained using fluorine-18 fludeoxyglucose (18F-FDG) positron emission tomography (PET)/CT. Many investigators have reported a substantial decrease in standardised uptake value (SUV) post-CRT in responders compared with non-responders [19-22]. However, only a few studies have compared the abilities of MRI and PET/CT to enable prediction of responses to neoadjuvant CRT [23,24]. There has been no published comparative study of DWI and PET/CT in the evaluation of tumour viability after neoadjuvant CRT in locally advanced rectal cancer.
Thus, the purpose of our study was to evaluate the added value of DWI in combination with T2 weighted imaging (T2WI) compared with T2WI alone or PET/CT for detecting viable tumour after neoadjuvant CRT in patients with locally advanced rectal cancer.
Methods and materials
Patients
The ethics committee of our institute approved this study. Written informed consent was waived owing to the retrospective nature of the analysis.
Between October 2008 and February 2009, 50 consecutive patients (39 men and 11 women; age range, 29–80 years; mean age, 56 years) who satisfied the following inclusion criteria were enrolled in this retrospective study: (1) patients with histopathologically proven locally advanced rectal cancer (≥T3 or regional lymph node involvement) at imaging; (2) patients who underwent post-CRT 3.0 T rectal MRI with DWI and post-CRT 18F-FDG-PET/CT, followed by surgery. Surgery consisted of low anterior resection (n=44), Mile’s operation (n=3), Hartmann’s operation (n=1) or transanal endoscopic microsurgery (n=2). The pre-CRT MRI was performed up to 4 weeks before the beginning of CRT. The post-CRT 3.0 T rectal MRI with DWI and the post-CRT 18F-FDG-PET/CT were obtained 6 weeks after completion of CRT; these examinations were performed on same day or within 2 days of each other. The average interval between post-CRT MRI and surgery was 14 days (range, 6–22 days).
Neoadjuvant chemoradiation therapy
The patients were treated with normofractionated pelvic radiation at a total dose of 45 Gy; a daily fraction of 1.8 Gy was administered 5 days per week for 5 weeks. Chemotherapy was delivered concurrently with radiotherapy on an outpatient basis. It consisted of 5-fiuorouracil (500 mg m–2) per day by bolus infusion on Days 1–3 and on Days 23–25.
Standard of reference and histopathological examination
The standard of reference was based on pathological staging (the TNM staging system) and the Dworak tumour response grading system [25,26]. The response was graded as follows: grade 0, no response; grade 1, dominant tumour mass with obvious fibrosis, vasculopathy or both (minimal response); grade 2, dominant fibrotic changes with a few easy-to-find tumour cells or groups (moderate response); grade 3, few (difficult to find microscopically) tumour cells in fibrotic tissue with or without mucous substance (near complete response); and grade 4, no viable tumour [complete response (CR)].
MR examination
All 50 patients underwent post-CRT 3.0 T rectal MRI with DWI 6 weeks after completion of CRT. Pre-CRT 3.0 T rectal MRI with DWI was obtained in 29 patients. A 3.0 T whole-body system (Intera Achieva 3T; Philips Medical Systems, Best, Netherlands) and a dedicated cardiac sensitivity encoding coil (6-channel) were used for this study. To reduce colonic motility, 20 mg of scopolamine butylbromide (Buscopan; Boehringer Ingelheim GmbH, lngelheim , Germany) was injected intramuscularly 30 min before MRI. The patients were asked to undergo rectal cleansing using rectal suppository laxatives 2–3 h before MR examination. In the MR room, ultrasound transmission gel was administered using a balloon-tipped rectal tube; the rectum was filled until the patient felt a sensation of fullness in the rectum at which point the tube was removed.
First, a sagittal localising image was obtained, for the selection of axial and coronal images, with a T2 weighted turbo spin echo sequence. The oblique axial and coronal T2 weighted turbo spin echo images were obtained orthogonal and parallel to the long axis of the rectal cancer. Finally, an axial T1 weighted turbo field echo sequence was acquired. All sequences were obtained without fat saturation.
Diffusion-weighted MR images were acquired in the oblique axial plane using the single-shot echo planar imaging technique immediately after the conventional rectal MR examination. Diffusion-encoding gradients were applied as a bipolar pair at b-values of 0, 100, 800 and 1000 s mm–2 along the three orthogonal directions of motion-probing gradients. Because high b-value DWI provides better contrast, thereby minimising perfusion effect, yielding greater tissue diffusivity and reducing T2 shine-through effect [27], we used the values of 0 and 1000 s mm–2 to generate the ADC map. Previous studies of DWI for colorectal cancer have used the same b-values [11,12,15]. ADC maps were automatically constructed on a pixel-by-pixel basis. The acquisition time of DWI was within 1 min and 53 s. Our routine MR protocol and sequence parameters are summarised in Table 1.
Table 1. MR sequence parameters.
| Parameter | T2 weighted sagittal, oblique axial and coronal turbo spin-echo sequence | T1 weighted oblique axial turbo field-echo sequence | Single-shot echo planar sequencea |
| Repetition time (ms) | 3689 | 497 | 2820 |
| Echo time (ms) | 80 | 10 | 70 |
| Echo train length | 20 | 3 | 1 |
| Section thickness (mm) | 3 | 3 | 3 |
| Section gap (mm) | 1 | 1 | 1 |
| Matrix size | 800×785 | 400×400 | 104×100 |
| Number of signals acquired | 4 | 2 | 2 |
| Field of view (mm) | 360/360 | 360/360 | 360/360 |
| Acquisition time | 3 min 42 s–4 min 55 s | 2 min 58 sec | 1 min 53 sec |
aThe b-values were 0, 100, 800 and 1000 s mm–2.
18F-FDG PET/CT
All 50 patients underwent post-CRT 18F-FDG PET/CT 6 weeks after completion of CRT. All the patients fasted for at least 6 h prior to the PET study. The 18F-FDG PET/CT scan was performed with a Discovery LS PET/CT scanner (GE Healthcare, Little Chalfont, UK). Whole-body CT scanning from the head to the thigh was performed using a continuous spiral technique on an eight-slice helical CT with a gantry rotation speed of 0.8 s. The CT scan data were collected with the following parameters: 80 mAs, 140 KeV, a 5-mm section width and a table feed of 5 mm per rotation. After the CT scan, an emission scan was performed from the head to the thigh for 4 min per frame at 45 min after the intravenous injection of 370 MBq of 18F-FDG. The attenuation-corrected PET images with the CT data were reconstructed by an ordered subset expectation maximisation algorithm (28 subsets, 2 iterations). The separate CT and PET scan data were accurately coregistered with a Xeleris workstation (GE Healthcare).
Image analysis
For qualitative analysis, two gastrointestinal radiologists, each with at least 5 years’ clinical experience of interpreting rectal MR images, reviewed each of the two image sets, first the T2WI set and then the combined T2WI and DWI set, in different sessions with a month between the two sessions. All images were independently interpreted in a random order and a blinded fashion. These two reviewers were blinded to all other information except the purpose of this study. They were unaware of patient identity or clinical history and had not seen the results of other imaging examinations or histopathological evaluations. At the first reading session, the reviewers independently reviewed both the pre- and post-CRT T2WI and recorded their confidence level using a five-point scale to describe the viable tumour on the post-CRT T2WI. Viable tumour was pre-defined as an area of signal intensity on the T2WI higher than that of the surrounding muscle layer [28]. The five-point scale used to assess viable tumour was as follows: 1, no viable tumour (no identifiable mass and no wall thickening compared with normal rectal wall); 2, probably no viable tumour (identifiable lesion showing iso- or hypointensity compared with that of the muscular layer of the rectum and no wall thickening; 3, possible viable tumour (identifiable lesion showing iso- or hypointensity compared with that of the muscular layer of the rectum with wall thickening but no tumour signal); 4, probable viable tumour (heterogeneous signal intensity with a small portion of tumour signal with or without wall thickening); 5, definite viable tumour [heterogeneous signal intensity with a large portion of (or definite) tumour signal with or without wall thickening].
At the second reading session, the reviewers were required to score their confidence level using the same five-point scale for the combined image set (i.e. the post-CRT rectal T2WI and the post-CRT DWI with ADC map using b-values of both 0 and 1000 s mm–2). Viable tumour was pre-defined as the presence of residual high signal intensity on DWI (b-value, 1000 s mm–2) and reduced ADC on the ADC map in the corresponding tissue. The reference standard for signal intensity was the signal intensity of the unaffected rectal wall (b-value, 1000 s mm–2). When the findings on DWI differed from those on T2WI, reviewers were asked to give priority to their findings on DWI.
For quantitative analysis, a third radiologist with 10 years’ clinical experience of reading rectal MR images measured the post-CRT ADC values. This radiologist had access to pre- and post-CRT MR imaging and to histopathological results so that he could select where to place the region of interest (ROI) within the residual tissue on the ADC map. The mean ADC values of suspected viable tumours were measured on ADC maps obtained using a b-value of 1000 s mm–2. As in the qualitative analyses, viable tumour was pre-defined as the presence of residual high signal intensity on DWI (b-value, 1000 s mm–2) and reduced ADC on the ADC map in the corresponding tissue. By referring to a standardised diagram, circular ROIs were generated on the images obtained using a b-value of 0 s mm–2 for viable tumours in each patient. These ROIs were superimposed on the ADC maps obtained with a b-value of 1000 s mm–2 using MRIcro software version 1.37 [29]. A circular ROI with an area of ≥4 mm2 (i.e. >2 mm in minimum diameter) was placed within three portions of the tumour in a single level in the z-axis to obtain average ADC values of the heterogeneous tumour. When the ROIs were drawn, great care was taken to exclude both intraluminal and extraluminal structures so as to reduce any error in ADC calculations. To minimise the mismatching of the locality of tumours on ADC maps with tissue examined histopathologically, the areas of the tumours on the ADC maps were smaller than those used for histopathology.
For qualitative analysis, the 18F-FDG PET/CT images were interpreted by an experienced nuclear medicine physician. Because there was no comparable pre-CRT PET/CT and the post-CRT viable tumours were too small to be clearly localised on PET/CT, the qualitative analysis of the utility of PET metabolic response for detecting viable tumour was determined by visual inspection of the PET/CT images. The 5-point scale used to assess viable tumour was as follows: 1, no viable tumour (no FDG uptake); 2, probably no viable tumour (linear symmetric physiological uptake only); 3, possible viable tumour (mild asymmetric uptake corresponding to the tumour site); 4, probable viable tumour (moderate asymmetric uptake corresponding to the tumour site); 5, definite viable tumour (high asymmetric uptake corresponding to the tumour site).
Statistical analysis
Diagnostic accuracy, sensitivity and specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for the three reviewers using the assumption that a confidence level of four or higher was positive for the diagnosis of viable tumour. We used the McNemar test to compare the diagnostic accuracy, sensitivity and specificity of T2WI alone, DWI with T2WI and PET/CT. Statistical analyses of differences in the calculated PPVs and NPVs for each observer and each imaging technique were based on a previous report [30]. An analysis of false-positive and false-negative observations was also undertaken. The interobserver agreement between the two reviewers for MR images was calculated with kappa statistics. κ<0.20 indicated poor agreement; κ=0.21–0.40, fair agreement; κ=0.41–0.60, moderate agreement; κ=0.61–0.80, good agreement; and κ>0.81, excellent agreement.
The Mann–Whitney U test was used to compare the mean ADC values of the viable and non-viable tumour groups on the basis of pathology reports. To identify the optimal cut-off value that provided the highest accuracy in distinguishing the viable tumour group from the non-viable tumour group, receiver operating characteristic (ROC) analysis was also applied to the mean ADC values. For all statistical tests, p-values <0.05 were considered statistically significant.
Results
Tumour response
Histopathological examinations of tumour specimens showed a mean tumour size of 2.2 cm (median, 2.0 cm; range, 0.1–6.0 cm). Tumour responses consisted of CR (no viable tumour) (n=6, 12%), near CR (n=13, 26%), moderate response (n=21, 42%) and minimal response (n=10, 20%). One mucinous adenocarcinoma was identified and showed near CR. The pre-CRT local tumour stagings, which were based on MR findings, and the post-CRT pathological tumour stagings are summarised in Table 2.
Table 2. Tumour response to neoadjuvant CRT.
| Number of tumours identified on MRI before neoadjuvant CRT |
||||
| Number of tumours at histopathological evaluation following CRT | T2 | T3 | T4 | |
| T0 | 1 | 5 | 0 | |
| T1 | 2 | 3 | 0 | |
| T2 | 4 | 6 | 1 | |
| T3 | 0 | 19 | 5 | |
| T4 | 0 | 0 | 4 | |
CRT, chemoradiation therapy.
Qualitative analysis
Table 3 shows the diagnostic predictive values for the evaluations of tumour viability for each observer and each technique. The availability of DWI with T2WI (Reviewer 1, 90%; Reviewer 2, 86%) improved the diagnostic accuracy achieved with T2WI alone (Reviewer 1, 76%; Reviewer 2, 64%) for Reviewer 2 but not for Reviewer 1 (Reviewer 1, p=0.5; Reviewer 2, p=0.013). DWI with T2WI gave significantly greater accuracy than PET/CT (48%, p<0.001) (Figure 1). The sensitivities of DWI with T2WI (Reviewer 1, 98%; Reviewer 2, 91%) were significantly higher than those of either T2WI alone (Reviewer 1, 77%; Reviewer 2, 64%) or PET-CT (43%) (p<0.05). Although the NPVs of DWI with T2WI (Reviewer 1, 67%; Reviewer 2, 43%) tended to be higher than those of T2WI alone (Reviewer 1, 29%; Reviewer 2, 20%) or PET/CT (17%), the difference was statistically significant only when the NPV of DWI with T2WI for Reviewer 2 was compared with the PET/CT NPV (p>0.05; except for DWI with T2WI in Reviewer 2 vs PET/CT, p<0.05). The specificities and PPVs of DWI with T2WI were no better than those of T2WI alone or of PET/CT (p>0.05).
Table 3. Comparison of the diagnostic capabilities of T2WI, DWI with T2WI and PET/CT for the evaluation of viable tumour after neoadjuvant chemoradiation therapy in patients with locally advanced rectal cancer.
| Technique | Accuracy, % | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
| T2WI | |||||
| Reviewer 1 | 76 | 77 | 67 | 94 | 29 |
| (38/50) | (34/44) | (4/6) | (34/36) | (4/14) | |
| Reviewer 2 | 64 | 64 | 67 | 93 | 20 |
| (32/50) | (28/44) | (4/6) | (28/30) | (4/20) | |
| T2WI with DWI | |||||
| Reviewer 1 | 90b | 98a,b | 33 | 91 | 67 |
| (45/50) | (43/44) | (2/6) | (43/47) | (2/3) | |
| Reviewer 2 | 86a,b | 91a,b | 50 | 93 | 43b |
| (43/50) | (40/44) | (3/6) | (40/43) | (3/7) | |
| PET/CT | 48 | 43 | 83 | 95 | 17 |
| (24/50) | (19/44) | (5/6) | (19/20) | (5/30) |
DWI, diffusion-weighted imaging; NPV, negative predictive value; PET, positron emission tomography; PPV, positive predictive value; T2WI, T2 weighted imaging.
aSignificant difference with T2WI (p<0.05).
bSignificant difference with PET/CT (p<0.05).
The values in parentheses are raw data.
Figure 1.
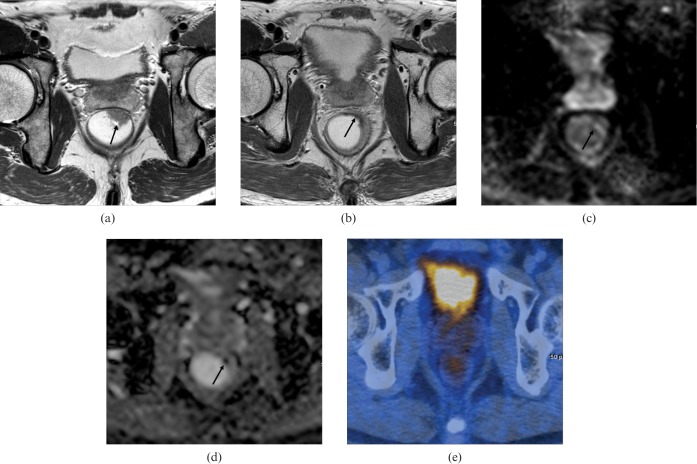
A 50-year-old man with pathologically proven rectal cancer [T2N0 upon histopathology, near complete response to chemoradiation therapy (CRT)]. Correct prediction of the tumour response was made after additional reading of diffusion-weighted imaging (DWI). (a) Pre-CRT T2 weighted axial MRI shows an ulcerofungating mass (arrow) between the 12- and 2-o’clock positions. (b) Post-CRT T2 weighted axial MRI shows slight rectal wall thickening with hypointensity (arrow) in the 2-o’clock position. Both reviewers recorded possible viable tumour. (c) Post-CRT axial DWI (b-value, 1000 s mm−2) shows a hyperintense signal in the corresponding tumour (arrow). (d) Post-CRT apparent diffusion coefficient map shows a hypointense signal in the corresponding tumour (arrow). Both reviewers recorded definite viable tumour. (e) Post-CRT fluorine-18 fluodeoxyglucose (FDG) PET/CT shows no FDG uptake, which was scored as no viable tumour.
In our study, there were seven false-positive and five false-negative findings for the determination of viable tumour on DWI with T2WI. PET/CT gave rise to 1 false-positive and 25 false-negative findings. Five (71%) of the false-positive findings on DWI with T2WI were attributed to lesions with no viable tumour that were shown as hypointensities on the ADC maps; a further two (29%) false-positives were the result of interpretation errors when the reviewer interpreted unaffected rectal wall as viable tumour. Among these false-positive findings, there was one case with abundant mucin pool, which showed as a focal hypointensity on the ADC map and had high asymmetric FDG uptake, but no viable tumour (Figure 2). Both reviewers interpreted a mucinous adenocarcinoma with near CR as viable tumour on DWI with T2WI, whereas all reviewers mistook it as a non-viable tumour when looking at T2WI alone or PET/CT. False-negative findings on DWI with T2WI were attributed to 4 (80%) viable tumours that showed near CR and 1 (20%) viable tumour that showed minimal response with isointensity on the ADC map.
Figure 2.
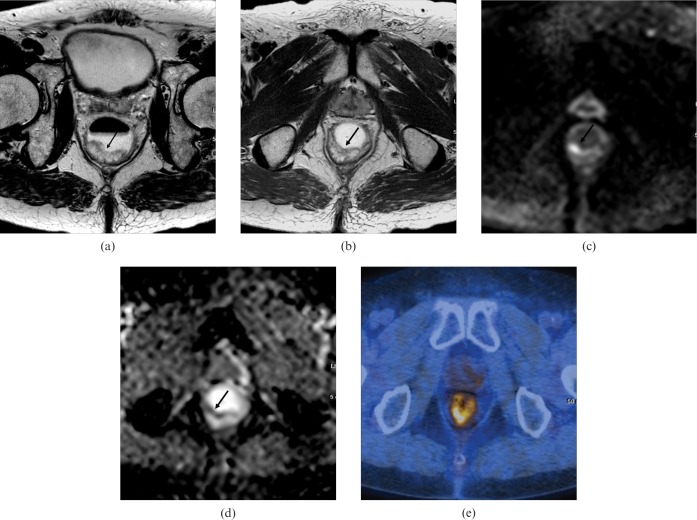
A 73-year-old man with pathologically proven rectal cancer [T0N0 upon histopathology, complete response to chemoradiation therapy (CRT)] with abundant mucin pool. (a) Pre-CRT T2 weighted axial MRI shows a hyperintense polypoid lesion (arrow) in the posterior rectal wall. (b) Post-CRT T2 weighted axial MRI shows a hyperintense polypoid lesion (arrow) with no decrease in tumour size. Both reviewers recorded definite viable tumour. (c) Post-CRT axial DWI (b-value, 1000 s mm−2) shows a focal hyperintensity (arrow) in the corresponding tumour. (d) Post-CRT apparent diffusion coefficient (ADC) map shows a focal hypointensity (arrow) in the corresponding tumour with an ADC value of 1.36×10−3 mm2 s−1. Both reviewers recorded definite viable tumour. (e) Post-CRT fluorine-18 fludeoxyglucose positron emission tomography/CT shows high asymmetric uptake corresponding to the tumour site, which was scored as definite viable tumour.
Among 25 false-negative PET/CT findings, 10 (40%) tumours showed near CR, 12 (48%) showed moderate responses and 3 (12%) showed minimal responses.
In retrospective analysis, all of the reviewers failed to detect one case with a viable tumour measuring 0.3 cm in diameter with near CR on DWI with T2WI or on PET/CT (Figure 3).
Figure 3.
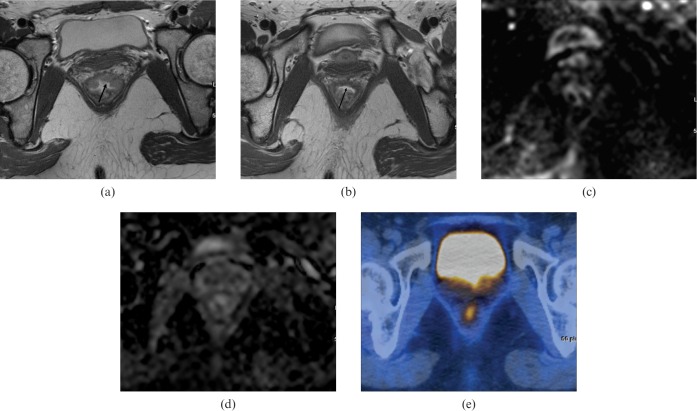
A 33-year-old woman with pathologically proven rectal cancer [T2N0, upon histopathology, near complete response to chemoradiation therapy (CRT)]. (a) Pre-CRT T2 weighted axial MRI shows an ulceroinfiltrative lesion (arrow) in the anterior rectal wall. (b) Post-CRT T2 weighted axial MRI shows a hypointensity (arrow) of the anterior rectal wall with neither wall thickening nor tumour signal. Both reviewers recorded probably no viable tumour. (c) Post-CRT axial diffusion-weighted imaging (b-value, 1000 s mm−2) shows no residual hyperintense signal in the corresponding lesion. (d) Post-CRT apparent diffusion coefficient map shows no abnormal signal intensity in the corresponding lesion. Both reviewers recorded probably no viable tumour. (e) Post-CRT fluorine-18 fluodeoxyglucose positron emission tomography/CT shows mild asymmetric uptake corresponding to the tumour site, which was scored as possible viable tumour.
Interobserver agreement between Reviewers 1 and 2 regarding confidence level was good for the T2WI alone (κ=0.649) and moderate for DWI with T2WI (κ=0.563).
Quantitative analysis
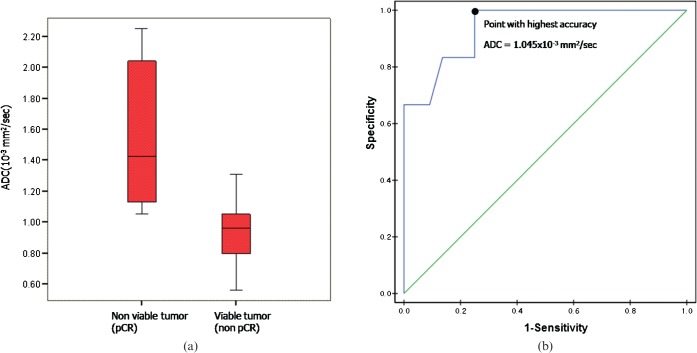
The mean ADC after CRT in the viable tumour group of 0.93×10−3 mm2 s−1 (±0.18×10−3 mm2 s−1, standard deviation) differed significantly from the 1.55×10−3 mm2 s−1 mean in the non-viable tumour group (±0.49×10−3 mm2 s−1, standard deviation) (p<0.0001) (Figure 4). When an ADC of 1.045×10−3 mm2 s−1 was used as the cut-off value for distinguishing between the viable and non-viable tumour groups [the area under the ROC curve (Az), 0.939], the highest accuracy of 78% (39 of 50 cases) was obtained along with a sensitivity of 75% (33 of 44 cases) and a specificity of 100% (6 of 6 cases).
Figure 4.
(a) Box and whisker plot shows the mean apparent diffusion coefficient (ADC) of the viable tumour group (0.93×10−3 mm2 s−1) and that of the non-viable tumour group (1.55×10−3 mm2 s−1) (p<0.0001). The middle line in each box represents the median. The lower and upper boundaries of the boxes represent the lower and upper quartiles (25th and 75th percentiles, respectively). Whiskers indicate the maximum and minimum calculated ADC values. pCR, pathologically complete response (b) Receiver operating characteristic curve to determine an optimal cut-off ADC value to distinguish the viable group from the non-viable group. The optimal cut-off value was 1.045×10−3 mm2 s−1 (Az, 0.939).
Discussion
Our qualitative assessment demonstrated that for detecting viable tumour after neoadjuvant CRT in patients with locally advanced rectal cancer, DWI with T2WI improved diagnostic accuracy, sensitivity and NPV over T2WI alone and over PET/CT.
T2WI has been advocated as an optimal method of staging rectal cancer [5-7,31,32]. Rectal cancer is usually identified as a focal area signal intensity that is higher than that of the rectal muscle layer but lower than that of submucosa [28,32,33]. Alternatively, cancer may be seen as an area of markedly high signal intensity on T2WI, higher than that of the submucosa in parts of the mucous lakes [34]. After neoadjuvant CRT for rectal cancer, however, changes such as rectal muscular wall fibrosis, rectal mucosal oedema and peritoneal fibrosis make the detection of residual viable tumour difficult. Several investigators [9,10] have demonstrated overall tumour staging accuracy following CRT in the range 47–52%, indicating the difficulties of differentiating fibrosis from residual viable tumour with only morphological imaging using T2WI after CRT.
DWI has several potential benefits in the assessment of tumour localisation and staging [11,12]. One report using 1.5 T MRI [15] demonstrated that diagnostic accuracy in evaluating CR after CRT of advanced rectal cancer significantly increased when DWI was added to conventional MR imaging. This finding supports our results using 3.0 T MRI. In our study, T2WI could be used as a reference for tumour location, depicting same axial plane along the z-axis of rectum when DWI was interpreted with T2WI. DWI in combination with T2WI could, therefore, lead to more accurate detection of viable tumour, despite the relatively low spatial resolution and image quality of DWI alone. Furthermore, the increased SNR ratio provided by 3.0-T MR scanners with parallel imaging techniques, which are now commercially available [16-18], makes possible an increase in either the spatial resolution or image quality of ADC maps. This may increase the sensitivity in detecting subtle ADC changes and thus lead to favourable diagnostic predictive values. In our study, we maximised SNR by performing high b-value DWI with multiple excitations and without breath holding at 3.0 T [35].
Functional imaging by 18F-FDG PET has been regarded as a promising modality for the evaluation of tumour response. The use of 18F-FDG PET scans to predict the response of rectal cancer to pre-operative CRT has been investigated before [22,36,37]. On the basis of a previous report [15] and our results, the expected diagnostic accuracies of DWI with T2WI for evaluating tumour viability after CRT of rectal cancer range from 82 to 90%, whereas the diagnostic accuracies of PET/CT in our study and published reports range from 48 to 75% [21,23,38]. We assume that adding DWI to T2WI will provide more accurate functional information than PET/CT for the evaluation of tumour viability after CRT of rectal cancer.
We believe that DWI, which can non-invasively reflect cellularity changes in tumours, and PET/CT, which is capable of imaging tumour cells on the basis of their glucose metabolism, can be used to evaluate accurately tumour response after neoadjuvant CRT of advanced rectal cancer. However, both imaging techniques have inherent limitations, including limited spatial resolution and relatively poor SNR, making it difficult to identify detailed rectal wall layers on high b-value DWI and PET/CT. Thus, the accurate assessment of residual viable tumour after CRT of rectal cancer may be limited, causing crucial false-positive and false-negative results.
In previous randomised studies [39,40], 8–15% of patients who underwent neoadjuvant CRT showed complete response. In our study, 12% (n=6) of patients showed complete response.
In our study, the number of false-positive findings made by the two reviewers of DWI with T2WI were relatively high compared with those made in assessing T2WI alone or PET/CT. The majority (71%) of false-positive findings on DWI with T2WI resulted from the focal hypointensity of the lesion on the ADC map regardless of the absence of viable tumour. All of these focal hypointensities on ADC maps manifested as high signal intensity on DWI (b-value, 1000 s mm−2) in the corresponding tumour. We believe that fibrosis after CRT of rectal cancer may show as a hypointensity on the ADC map that is due to water molecule restriction, and that this may be misinterpreted as a viable tumour.
Mucinous adenocarcinomas are known to have higher signal intensity than ordinary adenocarcinomas on pre-CRT T2WI [34] and to have a tendency to retain high signal intensity after CRT [41]. In our study, a false-positive finding (on both DWI with T2WI and PET/CT, as assessed by all reviewers) resulted in one case with abundant mucin pool without viable tumour. We concur with previous reports suggesting that it is difficult to differentiate viable tumours from abundant mucin pools [41].
In our study, the number of false-negative findings on PET/CT was relatively high compared with those for T2WI alone or for DWI with T2WI. We think that this may be due either to the constitution of the microscopic tumour disease (resulting in tumour FDG uptake below the threshold that can be detected by current PET techniques) or to the low metabolic activity of cancer cells after CRT. Limitations in detecting microscopic tumour disease can be also seen as the main cause of false-negative findings on DWI. Because the tumour microenvironment is both spatially and temporally heterogeneous, DWI with minute voxels cannot be used to determine the tumour response at the level of individual cells.
In our study, the mean ADC of the viable tumour group was significantly lower than that of the non-viable tumour group. When an ADC value of 1.045×10−3 mm2 s−1 was used as the cut-off value for distinguishing the viable tumour group from the non-viable tumour group, the overall accuracy in evaluating tumour viability after CRT of rectal cancer was 78%. Our results reveal relatively low ADC values compared with those in a previous study [15], in which Kim et al [15] suggested that 1.20×10−3 mm2 s−1 be used as the cut-off value for distinguishing the CR group from the non-CR group. This difference might reflect the different study populations and MR scanner field strengths. Differences in pulse sequences and b-value constituents are also likely to play a major role.
Our study had some limitations. First, the retrospective design of our study created an inherent limitation on precisely matching the MR images and histopathological findings. Although standardised histological examination procedures were used and detailed pathology reports were provided by an experienced pathologist, exact matching between the MR images and histopathological findings for the viable tumour may have been suboptimal. Second, the percentage of complete histopathological responders in our study was 12%, a rate similar to that found by others [20,23,24,42-44]. The small number of cases with pathological CR might, however, have led to overestimation of diagnostic accuracy. Third, the increased accuracy observed for the combined image set might have been partially attributable to the readers having gained experience with the grading scheme. Recall bias that could have resulted from the order of evaluation should be considered as a possibility, although we attempted to avoid any recall bias by leaving an interval of 1 month between image review sessions. Finally, pre-CRT PET/CT was not available for enrolled patients. In addition, because viable tumours may be too small to be clearly localised on post-CRT PET/CT, the qualitative analysis of PET metabolic response for detecting viable tumours relied on visual inspection of PET/CT images rather than on the quantitative analysis of the degree of FDG uptake (e.g. by SUV); this might have led to underestimation of the diagnostic accuracy of PET/CT.
In conclusion, adding DWI to T2WI improves the detection of viable tumour after neoadjuvant CRT compared with T2WI alone or PET/CT in patients with locally advanced rectal cancer.
References
- 1.Medical Research Council Rectal Cancer Working Party Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Lancet 1996;348:1605–10 [PubMed] [Google Scholar]
- 2.NIH Consensus Conference Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990;264:1444–50 [PubMed] [Google Scholar]
- 3.Valentini V, Coco C, Picciocchi A, Morganti AG, Trodella L, Ciabattoni A, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys 2002;53:664–74 [DOI] [PubMed] [Google Scholar]
- 4.Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M, et al. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys 2005;62:752–60 [DOI] [PubMed] [Google Scholar]
- 5.Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg 2003;90:355–64 [DOI] [PubMed] [Google Scholar]
- 6.Beets-Tan RG. MRI in rectal cancer: the T stage and circumferential resection margin. Colorectal Dis 2003;5:392–5 [DOI] [PubMed] [Google Scholar]
- 7.Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet 2001;357:497–504 [DOI] [PubMed] [Google Scholar]
- 8.Maretto I, Pomerri F, Pucciarelli S, Mescoli C, Belluco E, Burzi S, et al. The potential of restaging in the prediction of pathologic response after preoperative chemoradiotherapy for rectal cancer. Ann Surg Oncol 2007;14:455–61 [DOI] [PubMed] [Google Scholar]
- 9.Kuo LJ, Chern MC, Tsou MH, Liu MC, Jian JJ, Chen CM, et al. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum 2005;48:23–8 [DOI] [PubMed] [Google Scholar]
- 10.Chen CC, Lee RC, Lin JK, Wang LW, Yang SH. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum 2005;48:722–8 [DOI] [PubMed] [Google Scholar]
- 11.Ichikawa T, Erturk SM, Motosugi U, Sou H, Iino H, Araki T, et al. High-B-value diffusion-weighted MRI in colorectal cancer. AJR Am J Roentgenol 2006;187:181–4 [DOI] [PubMed] [Google Scholar]
- 12.Rao SX, Zeng MS, Chen CZ, Li RC, Zhang SJ, Xu JM, et al. The value of diffusion-weighted imaging in combination with T2-weighted imaging for rectal cancer detection. Eur J Radiol 2008;65:299–303 [DOI] [PubMed] [Google Scholar]
- 13.Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, et al. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 2002;360:307–8 [DOI] [PubMed] [Google Scholar]
- 14.Hein PA, Kremser C, Judmaier W, Griebel J, Pfeiffer KP, Kreczy A, et al. Diffusion-weighted magnetic resonance imaging for monitoring diffusion changes in rectal carcinoma during combined, preoperative chemoradiation: preliminary results of a prospective study. Eur J Radiol 2003;45:214–22 [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Lee JM, Hong SH, Kim GH, Lee JY, Han JK, et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology 2009;253:116–25 [DOI] [PubMed] [Google Scholar]
- 16.Winter L, Bruhn H, Langrehr J, Neuhaus P, Felix R, Hanninen LE. Magnetic resonance imaging in suspected rectal cancer: determining tumor localization, stage, and sphincter-saving resectability at 3-Tesla-sustained high resolution. Acta Radiol 2007;48:379–87 [DOI] [PubMed] [Google Scholar]
- 17.Futterer JJ, Yakar D, Strijk SP, Barentsz JO. Preoperative 3T MR imaging of rectal cancer: local staging accuracy using a two-dimensional and three-dimensional T2-weighted turbo spin echo sequence. Eur J Radiol 2008;65:66–71 [DOI] [PubMed] [Google Scholar]
- 18.Zhang XM, Yu D, Zhang HL, Dai Y, Bi D, Liu Z, et al. 3D dynamic contrast-enhanced MRI of rectal carcinoma at 3T: correlation with microvascular density and vascular endothelial growth factor markers of tumor angiogenesis. J Magn Reson Imaging 2008;27:1309–16 [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg R, Herrmann K, Gertler R, Kunzli B, Essler M, Lordick F, et al. The predictive value of metabolic response to preoperative radiochemotherapy in locally advanced rectal cancer measured by PET/CT. Int J Colorectal Dis 2009;24:191–200 [DOI] [PubMed] [Google Scholar]
- 20.Kalff V, Ware R, Heriot A, Chao M, Drummond E, Hicks RJ. Radiation changes do not interfere with postchemoradiation restaging of patients with rectal cancer by FDG PET/CT before curative surgical therapy. Int J Radiat Oncol Biol Phys 2009;74:60–6 [DOI] [PubMed] [Google Scholar]
- 21.Capirci C, Rubello D, Pasini F, Galeotti F, Bianchini E, Del Favero G, et al. The role of dual-time combined 18-fluorodeoxyglucose positron emission tomography and computed tomography in the staging and restaging workup of locally advanced rectal cancer, treated with preoperative chemoradiation therapy and radical surgery. Int J Radiat Oncol Biol Phys 2009;74:1461–9 [DOI] [PubMed] [Google Scholar]
- 22.Capirci C, Rampin L, Erba PA, Galeotti F, Crepaldi G, Banti E, et al. Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging 2007;34:1583–93 [DOI] [PubMed] [Google Scholar]
- 23.Cho YB, Chun HK, Kim MJ, Choi JY, Park CM, Kim BT, et al. Accuracy of MRI and (18)F-FDG PET/CT for restaging after preoperative concurrent chemoradiotherapy for rectal cancer. World J Surg 2009;33:2688–94 [DOI] [PubMed] [Google Scholar]
- 24.Denecke T, Rau B, Hoffmann KT, Hildebrandt B, Ruf J, Gutberlet M, et al. Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol 2005;15:1658–66 [DOI] [PubMed] [Google Scholar]
- 25.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997;12:19–23 [DOI] [PubMed] [Google Scholar]
- 26.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, et al. AJCC cancer staging manual. 6th ed New York, USA: Springer; 2002 [Google Scholar]
- 27.Meyer JR, Gutierrez A, Mock B, Hebron D, Prager JM, Gorey MT, et al. High-b-value diffusion-weighted MR imaging of suspected brain infarction. AJNR Am J Neuroradiol 2000;21:1821–9 [PMC free article] [PubMed] [Google Scholar]
- 28.Chun HK, Choi D, Kim MJ, Lee J, Yun SH, Kim SH, et al. Preoperative staging of rectal cancer: comparison of 3-T high-field MRI and endorectal sonography. AJR Am J Roentgenol 2006;187:1557–62 [DOI] [PubMed] [Google Scholar]
- 29.Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol 2000;12:191–200 [DOI] [PubMed] [Google Scholar]
- 30.Bennett BM. On comparisons of sensitivity, specificity and predictive value of a number of diagnostic procedures. Biometrics 1972;28:793–800 [PubMed] [Google Scholar]
- 31.Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 2006;333:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beets-Tan RG, Beets GL. Rectal cancer: review with emphasis on MR imaging. Radiology 2004;232:335–46 [DOI] [PubMed] [Google Scholar]
- 33.Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology 1999;211:215–22 [DOI] [PubMed] [Google Scholar]
- 34.Hussain SM, Outwater EK, Siegelman ES. Mucinous versus nonmucinous rectal carcinomas: differentiation with MR imaging. Radiology 1999;213:79–85 [DOI] [PubMed] [Google Scholar]
- 35.Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med 2004;22:275–82 [PubMed] [Google Scholar]
- 36.Paskeviciute B, Bolling T, Brinkmann M, Rudykina G, Ernst I, Stegger L, et al. Impact of (18)F-FDG-PET/CT on staging and irradiation of patients with locally advanced rectal cancer. Strahlenther Onkol 2009;185:260–5 [DOI] [PubMed] [Google Scholar]
- 37.Vliegen RF, Beets-Tan RG, Vanhauten B, Driessen A, Oellers M, Kessels AG, et al. Can an FDG-PET/CT predict tumor clearance of the mesorectal fascia after preoperative chemoradiation of locally advanced rectal cancer? Strahlenther Onkol 2008;184:457–64 [DOI] [PubMed] [Google Scholar]
- 38.Capirci C, Rubello D, Chierichetti F, Crepaldi G, Carpi A, Nicolini A, et al. Restaging after neoadjuvant chemoradiotherapy for rectal adenocarcinoma: role of F18-FDG PET. Biomed Pharmacother 2004;58:451–7 [DOI] [PubMed] [Google Scholar]
- 39.Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40 [DOI] [PubMed] [Google Scholar]
- 41.Allen SD, Padhani AR, Dzik-Jurasz AS, Glynne-Jones R. Rectal carcinoma: MRI with histologic correlation before and after chemoradiation therapy. AJR Am J Roentgenol 2007;188:442–51 [DOI] [PubMed] [Google Scholar]
- 42.Calvo FA, Domper M, Matute R, Martinez-Lazaro R, Arranz JA, Desco M, et al. 18F-FDG positron emission tomography staging and restaging in rectal cancer treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys 2004;58:528–35 [DOI] [PubMed] [Google Scholar]
- 43.Dresen RC, Beets GL, Rutten HJ, Engelen SM, Lahaye MJ, Vliegen RF, et al. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part I. Are we able to predict tumor confined to the rectal wall? Radiology 2009;252:71–80 [DOI] [PubMed] [Google Scholar]
- 44.Kim SH, Lee JM, Park HS, Eun HW, Han JK, Choi BI. Accuracy of MRI for predicting the circumferential resection margin, mesorectal fascia invasion, and tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer. J Magn Reson Imaging 2009;29:1093–101 [DOI] [PubMed] [Google Scholar]