Abstract
Objectives
The aim of this prospective study was to elucidate the efficacy of contrast-enhanced three-dimensional (3D) ultrasound with Sonazoid® (GF Healthcare, Oslo, Norway) as a non-invasive tool to discriminate idiopathic portal hypertension (IPH) from cirrhosis by demonstration of portal vein structure.
Methods
There were 16 patients: 11 with biopsy-proven cirrhosis and 5 with biopsy-proven IPH. Intrahepatic right portal vein images were taken by 3D ultrasound from 1 min after the injection of Sonazoid (0.0075 ml kg–1). Portal vein appearances were compared between 3D ultrasound and percutaneous transhepatic portography (PTP) by four independent reviewers. Sensitivity, specificity and area under the receiver operating characteristic curve (Az) of the images were used for the diagnosis of cirrhosis/IPH, and interimaging, inter-reviewer and interoperator agreement were examined.
Results
Sensitivity, specificity and Az of PTP for the diagnosis of cirrhosis/IPH were 63.6%/100%, 100% and 0.9 (0.71–1.0) by Reviewer I and 90.9%/100%, 100% and 1.0 by Reviewer III, respectively. Similarly, sensitivity, specificity and Az of 3D ultrasound for diagnosis of cirrhosis/IPH were 54.5%/80%, 100% and 0.96 (0.84–1.0) by Reviewer II and 72.7%/80%, 100% and 0.97 (0.9–1.0) by Reviewer IV, respectively. Diagnostic agreement between PTP and 3D ultrasound was good between Reviewers I and II (κ=0.793) and good between Reviewers III and IV (κ=0.732). Inter-reviewer agreement was good between Reviewers I and III for PTP diagnosis (κ=0.735), and good between Reviewers II and IV for 3D ultrasound diagnosis (κ=0.792). Interoperator agreement of diagnostic results was good (κ=0.740).
Conclusion
Non-invasive visualisation of intrahepatic portal vein structure by contrast-enhanced 3D ultrasound with Sonazoid may have the potential to discriminate IPH from cirrhosis.
Diagnosis of diffuse liver disease is a difficult but important issue in the appropriate management of patients, and cirrhosis should be correctly diagnosed because of the high risk of development of hepatocellular carcinoma [1-4]. Idiopathic portal hypertension (IPH) is also a disorder featuring chronic liver disease, resulting in oesophageal varices, hypersplenism and ascites [5,6]. IPH has different clinical aspects from cirrhosis including lower mortality from variceal rupture, better survival rate and reduced incidence of developing hepatocellular carcinoma [7,8]. However, differential diagnosis between these two liver diseases is sometimes complicated because of some common presentations caused by portal hypertension [9-12].
An earlier study [13] reported that IPH has a unique vascular structure of the portal vein, paucity of medium-sized portal branches, irregular and often obtuse-angled division of the peripheral branches, their occasional abrupt interruptions, an avascular area beneath the liver surface, non-opacification of some of the large intrahepatic portal branches and of their periphery, and an increase in the very fine vasculature around large intrahepatic portal branches. It is considered that these findings reflect intrahepatic portal vein occlusion, which is believed to be the pathophysiology of IPH. Although such vascular findings may be helpful to discriminate IPH from cirrhosis, obtaining portal vein images requires interventional techniques that are affected by invasive procedures and radiation exposure.
Significant recent advances in digital technology have led to ultrasound being used to demonstrate three-dimensional (3D) vascular images in the liver [14,15]. Furthermore, the detection rate of peripheral blood flow has improved with the application of microbubble ultrasound contrast agents [16,17]. Contrast harmonic imaging has the advantages of fewer artefacts, less dependence on the angle between the ultrasound beam and the vessel and an improved signal-to-noise ratio in comparison with Doppler sonography [18-20]. In addition, images under low mechanical index (MI) could cancel out most of the tissue signals to enable clear visualisation of the vascular structure [21]. On the basis of this information, we designed the present study to examine the possibility of using the newly developed ultrasound contrast agent Sonazoid® (GE Healthcare, Oslo, Norway) to demonstrate the intrahepatic portal vein structure by comparison with its angiographic appearance. The purpose of this study was to elucidate the efficacy of contrast-enhanced 3D ultrasound with Sonazoid under a low MI setting as a non-invasive tool to discriminate IPH from cirrhosis by demonstration of the intrahepatic portal vein structure.
Methods and materials
Patients
From February 2008 to December 2009, this prospective study, approved by the hospital's ethics committee, was performed at Chiba University Hospital (Chiba, Japan) after obtaining informed written consent from all the patients. Inclusion criteria were: (1) patients suspected of having cirrhosis or IPH admitted to our hospital for endoscopic treatment of oesophageal varices diagnosed by endoscopy; (2) effective treatment by endoscopic sclerotherapy, confirmed by endoscopic ultrasonography (Sonoprobe System SP701; Fujinon, Tokyo, Japan); (3) patients scheduled for definitive diagnosis of liver disease by biopsy; and (4) patients scheduled to undergo examination of the portal haemodynamics by percutaneous transhepatic portography (PTP) and hepatic venography to inform subsequent clinical management. All participants who fulfilled the inclusion criteria underwent contrast-enhanced ultrasound examination prior to liver biopsy and angiographic examinations.
The subjects were 11 patients with cirrhosis (age range, 38–75 years; mean±standard deviation, 61±12 years; males, 6; females, 5) and 5 patients with IPH (age range, 49–78 years; mean±standard deviation, 60±12 years; males, 3; females, 2). Treatment of oesophageal varices was elective in 13 patients and prophylactic in 3. Histological diagnosis was made in all 16 patients by liver biopsy, in which the following results were found: non-cirrhotic specimens in 5; cirrhosis in 11, with an aetiology of hepatitis C virus in 4, alcohol abuse in 2, autoimmune disease in 2, hepatitis B virus in 1, non-alcoholic steatohepatitis in 1 and cryptogenic disease in 1. In the patients with cirrhosis, the degree of liver function reserve, as classified by the Child–Pugh scoring system, was A in 7 and B in 4.
Diagnosis of IPH was based on blood tests, hepatic venography and histology from biopsy specimens in all patients according to the general rules for the study of portal hypertension, with the following criteria: (1) evidence of portal hypertension (oesophageal varices, hypersplenism or ascites); (2) patent portal veins and hepatic veins on Doppler ultrasound at the time of diagnosis; (3) no cirrhotic appearance on the liver biopsy sample; and (4) exclusion of other conditions causing cirrhosis (chronic viral hepatitis, alcoholic liver disease, non-alcoholic steatohepatitis, autoimmune hepatitis, granulomatous liver disease, congenital hepatic fibrosis, Wilson's disease, Budd–Chiari syndrome, haematological disorder and parasitic disease) [22]. Subjects with focal hepatic lesions and/or portal vein thrombosis found by ultrasound examination, or with egg allergy, a contraindication for the use of Sonazoid, were not included in this study.
Non-contrast ultrasound examination
All ultrasound examinations were performed using SSA-790A (Aplio-XG; Toshiba, Tokyo, Japan), with pre-examination fasting for at least 5 h. Gain was adjusted to the optimal level, and the dynamic range was set at 50 dB. At first, non-contrast greyscale ultrasound (tissue harmonic imaging, 2.5/5.0 Hz) was performed with a 3.75 MHz convex probe, for screening of focal hepatic diseases. Then, observation by colour Doppler ultrasound was carried out for the screening of portal vein thrombosis or vascular abnormalities such as intrahepatic arterioportal, arteriovenous or portal–hepatic communication, or reversed flow in the portal vein.
Setting for contrast-enhanced 3D ultrasound examination
After non-contrast ultrasound examination, the settings were changed for contrast-enhanced 3D ultrasound. We used a PVT382MV (3.5 MHz; Toshiba), a specialised transducer for 3D ultrasound with a mechanical auto-sweep scan system. Observation was performed in the harmonic mode with microflow imaging, which was reported as the real-time temporal maximum intensity projection imaging mode based on the additive synthesis of a series of frames for the improvement of spatial continuity between branches of vessels [23]. Depth and region of interest were adjusted to include the whole area of the right lobe of the liver on the sonogram. The viewing angle was set at 40° and the focal point was set 10 cm below the skin surface. The volume rate was 1 Hz at these settings.
Acquisition of contrast enhancement
A microbubble contrast agent, Sonazoid (median diameter of 2–3 μm), was manually injected at a dose of 0.0075 ml kg–1 followed by a flush with 3.0 ml of normal saline solution via the cubital vein. The acquisition of contrast-enhanced images of the intrahepatic portal vein was conducted by observation under right intercostal scan from 1 min after injection of the agent, because the phase just after the injection had been considered an arterial dominant phase in previous studies [16,24].
Initially, ultrasound transmission at the maximum MI level (MI 0.77) was carried out for 5 s to destroy the microbubbles within the scan plane, as existing microbubbles in the liver were thought to prevent demonstration of peripheral vessels by a fresh inflow of microbubbles. Just after this clean sweep of microbubbles, contrast-enhanced images of the intrahepatic portal vein induced by an inflow of microbubbles were acquired with the auto-sweep scan under possible breath-holding for about 5–6 s. The MI used for this acquisition was 0.25, a low MI level that offers stable observation of the inflow behaviour of microbubbles [24]. The acquired volume images were immediately saved onto the hard disk of the ultrasound equipment. The ultrasound examination was performed by MT, with injection of the agent carried out by HI. In addition, six of the subjects, five with cirrhosis and one with IPH, underwent a second contrast-enhanced 3D ultrasound examination within 1 week by HI, with injection of the agent by MT, to examine the interoperator agreement. Both operators are hepatologists, with over 6 years experience of ultrasound examination at the time of the initial case.
Complications were assessed by clinical symptoms during and after the ultrasound examination, and blood pressure and pulse rate were measured before and after the ultrasound examination.
Image conditioning
Image conditioning for 3D vascular images was performed by MT on the display of the same ultrasound system after completion of all the examinations for each patient. The filter was adjusted to avoid excessive noise, and appropriate brightness and contrast levels were selected to achieve clear vascular images. Finally, rendered stereoscopic vascular images rotated to the corresponding angiographic image were digitally stored on the hard disk and moved to an offline personal computer for the blind review process. All the images taken by MT went through the review process as the results of 3D ultrasound, and the second images taken by HI were used only to examine the interoperator agreement.
Angiographic examination
PTP was performed by means of ultrasound-guided catheterisation into the portal vein. Portograms were taken during the injection of iodinated contrast medium (30 ml, 6 ml s–1, Omnipaque300; Daiichi, Tokyo, Japan) into the splenic hilum by means of a mechanical injection system (Mark V ProVis®; Medrad, Warrendale, PA). Hepatic venography with pressure measurement was carried out in accordance with a previous report [25]. All angiographic procedures were performed by HM in this study.
Review process of portal vein images on PTP and 3D ultrasound
The vascular structures of the intrahepatic portal vein were reviewed regarding the presence or absence of the following findings based on an earlier report [13]. Six findings were proposed for IPH: (a) paucity of medium-sized portal branches, (b) irregular and often obtuse-angled division of the peripheral branches, (c) occasional abrupt interruptions of the peripheral branches, (d) an avascular area beneath the liver surface, (e) non-opacification of some of the large intrahepatic portal branches and of their periphery and (f) an increase in the very fine vasculature around large intrahepatic portal branches. Also, six findings were proposed for cirrhosis: (a) some winding or distortion in the large intrahepatic portal branches, (b) peripheral vessels were more or less straight with minimal winding, (c) peripheral vessels were divided at regular acute angles in most places, (d) no crossing over or abrupt cut-off of the vessels, (e) contrast medium reached the periphery without stopping short of the liver capsule or leaving an avascular area beneath the liver surface and (f) occasional small irregularities along the portal branches suggesting compression by regenerating nodules. The presence or absence of each finding was blindly reviewed on PTP images and 3D ultrasound images by the reviewers and the results were scored as follows: +1 for the presence of an IPH finding, –1 for the presence of a cirrhosis finding and 0 for the absence of findings. Diagnosis of liver disease was made based on the scoring of each image: as IPH by a total of more than +3, and as cirrhosis by a total of less than –3 (Table 1). Four independent reviewers with more than 9 years experience as consultants in hepatology and radiology participated in our study: reviewers I (HY) and III (SK) for PTP images and reviewers II (HM) and IV (HO) for 3D ultrasound images. All were blinded to information relating to the patients, and inter-reviewer variability was also examined for reading the results of portal vein images. The second ultrasound images taken by HI were assessed by reviewer II for evaluation of interoperator agreement, because the quality of 3D ultrasound images might be dependent on the operator's technique.
Table 1. Reviewed and final diagnosis of the subjects.
| Case | Diagnosis by reviewer |
Final diagnosis | |||
| I |
II |
III |
IV |
||
| PTP | 3D ultrasound | PTP | 3D ultrasound | ||
| 1 | Cirrhosis −5 | Cirrhosis −4 | Cirrhosis −5 | Cirrhosis −4 | Cirrhosis |
| 2 | IPH +6 | IPH +5 | IPH +6 | IPH +5 | IPH |
| 3 | Cirrhosis −4 | – −3 | Cirrhosis −4 | – −3 | Cirrhosis |
| 4 | Cirrhosis −6 | Cirrhosis −5 | Cirrhosis −5 | Cirrhosis −4 | Cirrhosis |
| 5 | – −3 | – −3 | Cirrhosis −4 | – −3 | Cirrhosis |
| 6 | Cirrhosis −6 | Cirrhosis −5 | Cirrhosis −5 | Cirrhosis −4 | Cirrhosis |
| 7 | Cirrhosis −4 | Cirrhosis −4 | – −3 | Cirrhosis −5 | Cirrhosis |
| 8 | – −3 | Cirrhosis −4 | Cirrhosis −5 | Cirrhosis −4 | Cirrhosis |
| 9 | IPH +5 | IPH +4 | IPH +5 | IPH +4 | IPH |
| 10 | IPH +4 | IPH +5 | IPH +4 | − +3 | IPH |
| 11 | IPH +5 | IPH +5 | IPH +5 | IPH +5 | IPH |
| 12 | IPH +4 | – +3 | IPH +4 | IPH +4 | IPH |
| 13 | Cirrhosis −4 | – −3 | Cirrhosis −5 | Cirrhosis −4 | Cirrhosis |
| 14 | – −3 | – −3 | Cirrhosis −4 | – −3 | Cirrhosis |
| 15 | Cirrhosis −5 | Cirrhosis −5 | Cirrhosis −5 | Cirrhosis −5 | Cirrhosis |
| 16 | – −3 | – −3 | Cirrhosis −4 | Cirrhosis −4 | Cirrhosis |
3D, three-dimensional; IPH, idiopathic portal hypertension; PTP, percutaneous transhepatic portography; –, no diagnosis achieved.
Numbers indicate scores obtained by the reviewers. Diagnosis of liver disease was made by the scores of each image: IPH by a total of more than +3, and cirrhosis by a total of less than –3. Final diagnosis was made by biopsy result for cirrhosis, and blood tests, hepatic venography and biopsy result for IPH.
Statistical analysis
All data were expressed as the mean±standard deviation or percentage. Sensitivity and specificity of PTP and 3D ultrasound for diagnosis of cirrhosis/IPH were obtained by true positive/(true positive + false negative) and true negative/(false positive + true negative), respectively. In this study, sensitivity was defined as the proportion of cirrhosis/IPH patients who were correctly identified as having cirrhosis/IPH, and specificity was defined as the proportion of patients without cirrhosis/IPH who were correctly identified as not having cirrhosis/IPH. The receiver operating characteristic (ROC) curve was provided for each reviewer and the area under the ROC curve (Az) with a 95% confidence interval was calculated in each imaging modality to compare the diagnostic performance. Inter-reviewer (Reviewer I vs III on PTP, Reviewer II vs IV on 3D ultrasound) and interoperator (MT vs HI) agreement was assessed by κ-value calculation. Agreement of diagnostic results between PTP and 3D ultrasound was also examined between Reviewers I and II and between Reviewers III and IV by κ-value calculation. The agreement grade was defined as <0.2 for poor, 0.2–0.4 for moderate, 0.4–0.6 for fair, 0.6–0.8 for good and 0.8–1.0 for excellent. Statistical analysis was performed using SPSS® [v.11.0J for Windows; IBM Corporation (formerly SPSS Inc.), Armonk, NY].
Results
Intrahepatic portal vein appearances by PTP and 3D ultrasound
Vascular findings on three of the multiplanar reconstruction images (upper left, horizontal image; lower left, coronal image; upper right, sagittal image; Figures 1b and 2b) provided only fragmented and sectional information, which was not comparable with angiographic findings (Figures 1 and 2). However, rendered stereoscopic images (lower right; Figures 1b and 2b) showed a dendritically expanded intrahepatic portal vein. The intrahepatic portal vein was demonstrated as having six or seven branches by PTP and five or six branches by 3D ultrasound, with clear delineation. No cases required exclusion because of inappropriate blurred images due to insufficient breath-holding. Although the reviewed vascular findings were almost similar between 3D ultrasound and PTP, the number of positively reviewed findings on 3D ultrasound images was less than half of that on the PTP images in terms of two findings: (1) “contrast medium reached the periphery without stopping short of the liver capsule or leaving an avascular area beneath the liver surface” had a score of 21 on the PTP images and a score of 3 on the 3D ultrasound images and (2) “an avascular area beneath the liver surface” had a score of 10 on the PTP images and a score of zero on the 3D ultrasound images (Table 2). Neither portal vein thrombosis nor vascular abnormalities were found in the subjects, and no complications were observed during or after the contrast-enhanced ultrasound examinations.
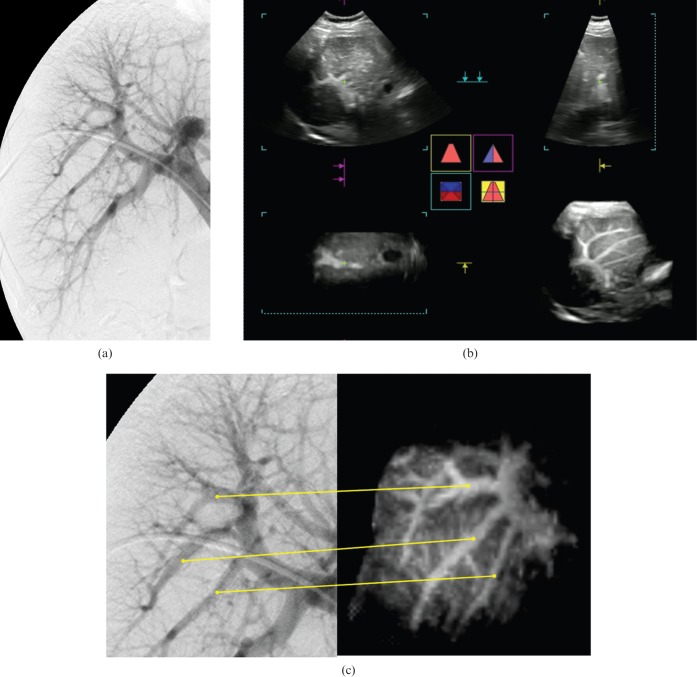
Figure 1.
A 76-year-old female with hepatitis C-related cirrhosis (case 6). (a) Portogram obtained by percutaneous transhepatic portography (PTP). This portogram had positive findings of some winding or distortion in the large intrahepatic portal branches, peripheral vessels were more or less straight with minimal winding, peripheral vessels were divided at regular acute angles in most places, there was no crossing over or abrupt cut-off of the vessels, contrast medium reached the periphery without stopping short of the liver capsule or leaving an avascular area beneath the liver surface, and there were occasional small irregularities along the portal branches suggesting compression by regenerating nodules with no finding suggestive of idiopathic portal hypertension (IPH), according to the review results by Reviewer I. This patient was diagnosed as having cirrhosis by a score of –6 on the PTP image. (b) Multiplanar reconstruction image. Three plane images (upper left, horizontal image; lower left, coronal image; upper right, sagittal image) provided only fragmented information of vascular findings, which were hard to compare with angiographic images. The lower right image shows dendritically expanded intrahepatic portal vein appearances. This rendered stereoscopic image was rotated to correspond with the angiographic image and used as the contrast-enhanced three-dimensional (3D) ultrasound sonogram. (c) Portograms obtained by PTP and contrast-enhanced 3D ultrasound with Sonazoid® (GF Healthcare, Oslo, Norway). Corresponding portal veins between the PTP image (left side) and the 3D ultrasound image (right side) are indicated by the lines. Contrast-enhanced 3D ultrasound with Sonazoid had positive findings of some winding or distortion in the large intrahepatic portal branches, peripheral vessels were more or less straight with minimal winding, peripheral vessels were divided at regular acute angles in most places and there was no crossing over or abrupt cut-off of the vessels with no finding suggestive of IPH according to the review results by Reviewer IV. This patient was also diagnosed as having cirrhosis by a score of –4 on the 3D ultrasound image.
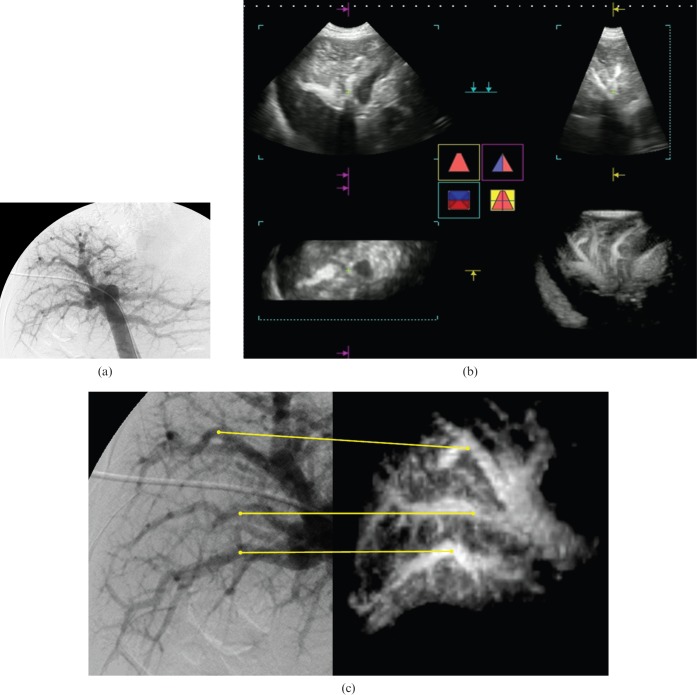
Figure 2.
A 55-year-old male with idiopathic portal hypertension (IPH) (case 9). (a) Portogram obtained by percutaneous transhepatic portography (PTP). This portogram had positive findings of paucity of medium-sized portal branches, irregular and often obtuse-angled division of the peripheral branches, occasional abrupt interruptions of the peripheral branches, an avascular area beneath the liver surface and an increase in the very fine vasculature around the large intrahepatic portal branches with no finding suggestive of cirrhosis, according to the review results by reviewer III. This patient was diagnosed as having IPH by a score of +5 on the PTP image. (b) Multiplanar reconstruction image. Three plane images (upper left, horizontal image; lower left, coronal image; upper right, sagittal image) provided only fragmented information of vascular findings, which were hard to compare with the angiographic images. The lower right image shows dendritically expanded intrahepatic portal vein appearances. This rendered stereoscopic image was rotated to correspond with the angiographic image and used as the contrast-enhanced three-dimensional (3D) ultrasound sonogram. (c) Portograms obtained by PTP and contrast-enhanced 3D ultrasound with Sonazoid® (GF Healthcare, Oslo, Norway). Corresponding portal veins between the PTP image (left side) and the 3D ultrasound image (right side) are indicated by the lines. Contrast-enhanced 3D ultrasound with Sonazoid had positive findings of paucity of medium-sized portal branches, irregular and often obtuse-angled division of the peripheral branches, occasional abrupt interruptions of the peripheral branches and an increase in the very fine vasculature around the large intrahepatic portal branches with no finding suggestive of cirrhosis, according to the review results by Reviewer II. This patient was also diagnosed as having IPH by a score of +4 on the 3D ultrasound image.
Table 2. Vascular reading signs found by PTP and 3D ultrasound. The number of positive findings obtained by four reviewers: two for PTP and two for 3D ultrasound.
| PTP |
3D ultrasound |
|||||||
| Cirrhosisa |
IPHa |
Cirrhosisa |
IPHa |
|||||
| Reviewer I | Reviewer III | Reviewer I | Reviewer III | Reviewer II | Reviewer IV | Reviewer II | Reviewer IV | |
| A | 8 (72.7%) | 11 (100%) | 0 | 0 | 11 (100%) | 11 (100%) | 0 | 0 |
| a | 0 | 0 | 5 (100%) | 5 (100%) | 0 | 0 | 5 (100%) | 5 (100%) |
| B | 3 (27.3%) | 8 (72.7%) | 0 | 0 | 2 (18.2%) | 2 (18.2%) | 0 | 0 |
| b | 0 | 0 | 2 (40%) | 4 (80%) | 0 | 0 | 4 (80%) | 4 (80%) |
| C | 9 (81.8%) | 2 (18.2%) | 0 | 0 | 11 (100%) | 11 (100%) | 0 | 0 |
| c | 0 | 0 | 3 (60%) | 2 (40%) | 0 | 0 | 3 (60%) | 3 (60%) |
| D | 11 (100%) | 11 (100%) | 0 | 0 | 11 (100%) | 9 (81.8%) | 0 | 0 |
| d | 0 | 0 | 5 (100%) | 5 (100%) | 0 | 0 | 0 | 0 |
| E | 10 (90.9%) | 11 (100%) | 0 | 0 | 2 (18.2%) | 1 (9.1%) | 0 | 0 |
| e | 0 | 0 | 4 (80%) | 4 (80%) | 0 | 0 | 5 (100%) | 5 (100%) |
| F | 5 (45.5%) | 6 (54.5%) | 0 | 0 | 6 (54.5%) | 9 (81.8%) | 0 | 0 |
| f | 0 | 0 | 5 (100%) | 4 (80%) | 0 | 0 | 5 (100%) | 5 (100%) |
IPH, idiopathic portal hypertension; PTP, percutaneous transhepatic portography; 3D, three-dimensional.
A, some winding or distortion in large intrahepatic portal branches; B, peripheral vessels were more or less straight with minimal winding; C, peripheral vessels were divided at regular acute angles in most places; D, there was no crossing over or abrupt cut-off of the vessels; E, contrast medium reached the periphery without stopping short of the liver capsule or leaving an avascular area beneath the liver surface; F, occasional small irregularities along portal branches suggesting compression by regenerating nodules; a, paucity of medium-sized portal branches; b, irregular and often obtuse-angled division of peripheral branches; c, occasional abrupt interruptions of peripheral branches; d, an avascular area beneath the liver surface; e, non-opacification of some of the large intrahepatic portal branches and of their periphery; f, increase in the very fine vasculature around large intrahepatic portal branches.
aFinal diagnosis.
Comparison of diagnostic ability for cirrhosis/IPH between PTP and 3D ultrasound
The sensitivity, specificity and Az value of PTP for the diagnosis of cirrhosis/IPH were 63.6%/100%, 100% and 0.9 (0.71–1.0) by Reviewer I and 90.9%/100%, 100% and 1.0 by Reviewer III, respectively. Similarly, the sensitivity, specificity and Az value of 3D ultrasound for diagnosis of cirrhosis/IPH were 54.5%/80%, 100% and 0.96 (0.84–1.0) by Reviewer II and 72.7%/80%, 100% and 0.97 (0.9–1.0) by Reviewer IV, respectively. Agreement of the diagnostic results between PTP and 3D ultrasound was good between reviewers I and II (κ=0.793) and good between reviewers III and IV (κ=0.732). Inter-reviewer agreement was good between Reviewers I and II for PTP diagnosis (κ=0.735), and good between Reviewers III and IV for 3D ultrasound diagnosis (κ=0.792). Interoperator agreement of the diagnostic results was good between MT and HI (κ=0.740).
Discussion
The search for minimally invasive procedures to differentiate diffuse liver diseases is an ongoing challenge for clinicians, and our technique may be the first reported for the non-invasive visualisation of 3D intrahepatic portal vein images. The difference in vascular structure in the intrahepatic portal vein was clearly depicted between cirrhosis and IPH with sufficient inter-reviewer and interoperator agreement, although the latter was examined in only 6 of the 16 subjects. Contrast-enhanced 3D ultrasound with Sonazoid might reasonably replace the angiographic procedure for the evaluation of intrahepatic portal vein structure.
The use of 3D ultrasound has become popular in gastroenterology as well as in cardiology in the last decade [14,15]. There are two ways to present 3D images: rendered stereoscopy and multiplanar reconstruction. The latter is frequently applied for the characterisation of hepatic tumours, because it can provide a detailed structure of the tumour and the blood flow pattern within it [26-28]. The former was used in our study, because we aimed to detect the outer appearance of the intrahepatic portal veins over a large area. Rendered stereoscopic imaging could demonstrate the dendritically expanded structure of the intrahepatic portal vein better than any plane image provided by the multiplanar reconstruction method, which presented fragmented and sectional information. This may also be true for conventional two-dimensional representations, which might be inferior to 3D imaging to evaluate vascular findings, although we did not compare the findings between these two imaging methods in a narrow sense. The method of 3D image presentation is determined according to the purpose, and rendered stereoscopic imaging should be recommended to evaluate the portal vein appearance.
The advantage of our technique may be the use of microflow imaging, which helps to establish the spatial continuity between portal vein branches. This mode is based on integrating the path of moving microbubbles to depict the vascular morphology, and improvement in the detailed visualisation of the vascular structure in hepatic tumours was noted in a preliminary study [23]. As 3D ultrasound with maximum intensity projection acquired the trajectories of microbubble flows into the liver, dendritically expanded intrahepatic portal vein images were dramatically demonstrated. At this point, the adverse effect of motion is the most problematic factor for this technique, and patient selection for good breath-holding might be required to obtain excellent images [23]. Although no subject was excluded in this study owing to poor breath-holding, an autocorrection system for the blurring of images caused by motion would probably be an advantage for wider acceptance of our technique.
Six findings that were considered as typical vascular abnormalities for cirrhosis or IPH were used for the diagnosis in reference to the previous literature [13]. Fundamentally, however, their diagnosis should be based on histological results, and intrahepatic vascular appearance reflects the histological change of the liver. In fact, as angiographic abnormalities of the intrahepatic portal vein depend on the progress of liver disease [13], diagnosis of cirrhosis/IPH does not necessarily require all of the six abnormal findings. In this study, the authors adopted a scoring system whereby four or more findings indicated a positive diagnosis, although sensitivity and specificity were maximised when we adopted a scoring system whereby three or more findings indicated a positive diagnosis. However, the validity of this scoring system lacks evidence because a diagnosis based on intrahepatic vascular findings remains to be determined.
There were two major discrepancies in the vascular findings between 3D ultrasound and PTP: (1) “contrast medium reached the periphery without stopping short of the liver capsule or leaving an avascular area beneath the liver surface” for cirrhosis and (2) “an avascular area beneath the liver surface” for IPH. Although these two findings were frequent on PTP images, they were quite rare or absent on 3D ultrasound images. This might be explained by the difference in difficulty of recognition of the liver surface between PTP and 3D ultrasound. The outline of the liver surface was easily visible on PTP images taken as radiographs, whereas it was hardly visible by 3D ultrasound; the 3D ultrasound images were focused on vascular enhancement, because the signal from the tissue needed to be reduced as much as possible on the sonograms to demonstrate vascular images to the periphery. In addition, insufficient demonstration of peripheral vessels might result in poor diagnostic ability by 3D ultrasound in comparison with an angiogram. The authors speculate that demonstration of peripheral vessels may depend on the flow velocity as the vascular images on 3D ultrasound were provided by microbubbles with physiological movement. As the vascular images on an angiogram are obtained by contrast material injected by power injector, the peripheral vessels should be depicted more clearly than those on 3D ultrasound images. Improvement of 3D ultrasound image processing to visualise both vessels and liver surface may resolve this problem in the future.
Pressure measurement by means of catheterisation in the hepatic vein is a well-established technique to discriminate IPH from cirrhosis [25]. In addition, the vascular structure of the hepatic vein also reflects differences between IPH and cirrhosis, with the former demonstrating a weeping willow appearance. However, we did not focus on demonstration of the hepatic vein, as contrast enhancement in the hepatic vein was not strong enough for the extraction of selective vascular images. The reason for this might be that there was a relatively small amount of microbubbles in the hepatic vein, probably because of the accumulation of Sonazoid in the liver. The control of phase for image acquisition and/or regulation of images might be required for the application of 3D ultrasound in the evaluation of hepatic vein images with Sonazoid.
There were some limitations in our study. First, the intrahepatic portal vein demonstrated on 3D ultrasound was a limited part of the portal venous system, whereas PTP detected the intrahepatic portal vein in both the right and left lobes of the liver with the extrahepatic portal vein simultaneously. As any abnormality of the portal vein structure may appear heterogeneously in the liver, a wider viewing angle by improvement of the transducer may allow more informative investigation by 3D ultrasound. Second, Sonazoid has a characteristic property of accumulation in the liver, which is the major difference from SonoVue® (Bracco, Mlan, Italy) and Definity® (Bristol-Myers Squibb, North Billerica, MA), which are so-called “blood pool agents” [16,29,32]. In fact, our recent study has shown that intensity analysis of liver parenchymal enhancement caused by the accumulation of Sonazoid was effective for the diagnosis of IPH [33]. However, our technique in the present study may not take full advantage of Sonazoid because we did observe the vascular-phase images produced by circulating microbubbles but not the late-phase images produced by accumulated microbubbles. SonoVue and Definity may also be effective for obtaining 3D vascular images by a similar technique. Third, our study did not include liver diseases other than cirrhosis and IPH, although portal vein findings in chronic hepatitis and other liver diseases were of interest. As we used the portal vein image obtained by PTP as the standard, our study did not include patients who were not candidates for PTP. It remains to be determined whether our technique can diagnose chronic hepatitis with various grades of fibrosis or liver disease of other aetiologies. Finally, we diagnosed liver disease with positive results equal to or more than four of six findings. There was, in a narrow sense, no scientific evidence to support the scoring adopted in this study. However, a previous study [13] reported that one or more of the characteristic vascular findings was evident on the portograms in the majority of patients with IPH. Therefore, diagnosis of cirrhosis/IPH may not necessarily require all vascular features, although the adequacy of our scoring system has not been proven.
In conclusion, although further study with a larger patient population may be needed to confirm our results, contrast-enhanced 3D ultrasound with Sonazoid under a low MI setting may have the potential to discriminate IPH from cirrhosis by demonstration of the intrahepatic portal vein structure non-invasively and effectively.
References
- 1.Bolondi L. Screening for hepatocellular carcinoma in cirrhosis. J Hepatol 2003;39:1076–84 [DOI] [PubMed] [Google Scholar]
- 2.Collier J, Sherman M. Screening for hepatocellular carcinoma. Hepatology 1998;27:273–8 [DOI] [PubMed] [Google Scholar]
- 3.Bosch J, Abraldes JG, Groszmann R. Current management of portal hypertension. J Hepatol 2003;38:S54–68 [DOI] [PubMed] [Google Scholar]
- 4.Lubel JS, Angus PW. Modern management of portal hypertension. Intern Med J 2005;35:45–9 [DOI] [PubMed] [Google Scholar]
- 5.Hillaire S, Valla DC, Lebrec D. Non cirrhotic portal hypertension. Clin Liver Dis 1997;1:1223–40 [DOI] [PubMed] [Google Scholar]
- 6.Benhamou JP, Valla DC. Intrahepatic portal hypertension. Bircher J, Benhamou JP, McIntyre N, et al., Oxford textbook of clinical hepatology. 2nd edn Oxford, UK: Oxford University Press; 1999. pp. 661–70 [Google Scholar]
- 7.Okuda K, Obata H. Idiopathic portal hypertension (hepatoportal sclerosis). Okuda K, Benhamou JP, Portal hypertension. Clinical and physiological aspects. Tokyo, Japan: Springer; 1992. pp. 271–87 [Google Scholar]
- 8.Sarin SK, Kapoor D. Non-cirrhotic portal fibrosis: current concepts and management. J Gastroenterol Hepatol 2002;17:526–34 [DOI] [PubMed] [Google Scholar]
- 9.Hillaire S, Bonte E, Denninger M-H, Casadevall N, Cadranel JF, Lebrec D, et al. Idiopathic non-cirrhotic intrahepatic portal hypertension in the West: a re-evaluation in 28 patients. Gut 2002;51:275–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohnishi K, Saito M, Sato S, Terabayashi H, Iida S, Nomura F, et al. Portal hemodynamics in idiopathic portal hypertension (Banti's syndrome). Comparison with chronic persistent hepatitis and normal subjects. Gastroenterology 1987;92:751–8 [DOI] [PubMed] [Google Scholar]
- 11.Dhiman RK, Chawla Y, Vasishta RK, Kakkar N, Dilawari JB, Trehan MS, et al. Non-cirrhotic portal fibrosis (idiopathic portal hypertension): experience with 151 patients and a review of the literature. J Gastroenterol Hepatol 2002;17:6–16 [DOI] [PubMed] [Google Scholar]
- 12.Okuda K, Kono K, Ohnishi K, Kimura K, Omata M, Koen H, et al. Clinical study of eighty-six cases of idiopathic portal hypertension and comparison with cirrhosis with splenomegaly. Gastroenterology 1984;86:600–10 [PubMed] [Google Scholar]
- 13.Futagawa S, Fukazawa M, Horisawa M, Musha H, Ito T, Sugiura M, et al. Portographic liver changes in idiopathic noncirrhotic portal hypertension. AJR Am J Roentgenol 1980;134:917–23 [DOI] [PubMed] [Google Scholar]
- 14.Nelson TR, Pretorius DH. Three-dimensional ultrasound imaging. Ultrasound Med Biol 1998;24:1243–70 [DOI] [PubMed] [Google Scholar]
- 15.Downey DB, Fenster A, Williams JC. Clinical utility of three-dimensional US. Radiographics 2000;20:559–71 [DOI] [PubMed] [Google Scholar]
- 16.Goldberg BB. Ultrasound contrast agents. London, UK: Mastin Dunitz; 1997 [Google Scholar]
- 17.Burns PN, Wilson SR, Simpson DH. Pulse inversion imaging of liver blood flow: improved method for characterizing focal masses with microbubble contrast. Invest Radiol 2000;35:58–71 [DOI] [PubMed] [Google Scholar]
- 18.Mitchell DG. Color Doppler imaging: principles, limitations, and artifacts. Radiology 1990;177:1–10 [DOI] [PubMed] [Google Scholar]
- 19.Foley WD, Erickson SJ. Color Doppler flow imaging. AJR Am J Roentgenol 1991;156:3–13 [DOI] [PubMed] [Google Scholar]
- 20.Rubin JM, Bude RO, Carson PL, Bree RL, Adler RS. Power Doppler US: a potentially useful alternative to mean frequency-based color Doppler sonography. Radiology 1994;190:853–6 [DOI] [PubMed] [Google Scholar]
- 21.Maruyama H, Matsutani S, Saisho H, Mine Y, Yuki H, Miyata K. Extra-low acoustic power harmonic images of the liver with Perflutren: novel imaging for real-time observation of liver perfusion. J Ultrasound Med 2003;22:931–8 [DOI] [PubMed] [Google Scholar]
- 22.The Japan Society for Portal Hypertension The general rules for study of portal hypertension. 2nd edn Tokyo, Japan: The Japan Society for Portal Hypertension; 2004. pp. 91–2 [Google Scholar]
- 23.Wilson SR, Jang HJ, Kim TK, Iijima H, Kamiyama N, Burns PN. Real-time temporal maximum-intensity-projection imaging of hepatic lesions with contrast-enhanced sonography. AJR Am J Roentgenol 2008;190:691–5 [DOI] [PubMed] [Google Scholar]
- 24.Maruyama H, Takahashi M, Ishibashi H, Okugawa H, Okabe S, Yoshikawa M, et al. Ultrasound-guided treatments under low acoustic power contrast harmonic imaging for hepatocellular carcinomas undetected by B-mode ultrasonography. Liver Int 2009;29:708–14 [DOI] [PubMed] [Google Scholar]
- 25.Futagawa S, Fukazawa M, Musha H, Isomatsu T, Koyama K, Ito T, et al. Hepatic venography in noncirrhotic idiopathic portal hypertension. Radiology 1981;141:303–9 [DOI] [PubMed] [Google Scholar]
- 26.Ohto M, Kato H, Tsujii H, Maruyama H, Matsutani S, Yamagata H, et al. Vascular flow patterns of hepatic tumors in contrast-enhanced 3-dimensional fusion ultrasonography using plane shift and opacity control modes. J Ultrasound Med 2005;24:49–57 [DOI] [PubMed] [Google Scholar]
- 27.Luo W, Numata K, Morimoto M, Kondo M, Takebayashi S, Okada M, et al. Focal liver tumors: characterization with 3D perflubutane microbubble contrast agent-enhanced US versus 3D contrast-enhanced multidetector CT. Radiology 2009;251:287–95 [DOI] [PubMed] [Google Scholar]
- 28.Luo W, Numata K, Morimoto M, Nozaki A, Nagano Y, Sugimori K, et al. Three-dimensional contrast-enhanced sonography of vascular patterns of focal liver tumors: pilot study of visualization methods. AJR Am J Roentgenol 2009;192:165–73 [DOI] [PubMed] [Google Scholar]
- 29.Marelli C. Preliminary experience with NC100100, a new ultrasound contrast agent for intravenous injection. Eur Radiol 1999;9:S343–6 [DOI] [PubMed] [Google Scholar]
- 30.Watanabe R, Matsumura M, Munemasa T, Fujimaki M, Suematsu M. Mechanism of hepatic parenchyma-specific contrast of microbubble-based contrast agent for ultrasonography: microscopic studies in rat liver. Invest Radiol 2007;42:643–51 [DOI] [PubMed] [Google Scholar]
- 31.Morel DR, Schwieger I, Hohn L, Terrettaz J, Llull JB, Cornioley YA, et al. Human pharmacokinetics and safety evaluation of SonoVue, a new contrast agent for ultrasound imaging. Invest Radiol 2000;35:80–5 [DOI] [PubMed] [Google Scholar]
- 32.Jang HJ, Kim TK, Burns PN, Wilson SR. Enhancement patterns of hepatocellular carcinoma at contrast enhanced US: comparison with histologic differentiation. Radiology 2007;244:898–906 [DOI] [PubMed] [Google Scholar]
- 33.Maruyama H, Ishibashi H, Takahashi M, Imazeki F, Yokosuka O. Effect of signal intensity from the accumulated microbubbles in the liver for differentiation of idiopathic portal hypertension from liver cirrhosis. Radiology 2009;252:587–94 [DOI] [PubMed] [Google Scholar]