Abstract
Tendons transfer force from muscle to bone. Specific tendons, including the equine superficial digital flexor tendon (SDFT), also store and return energy. For efficient function, energy-storing tendons need to be more extensible than positional tendons such as the common digital extensor tendon (CDET), and when tested in vitro have a lower modulus and failure stress, but a higher failure strain. It is not known how differences in matrix organization contribute to distinct mechanical properties in functionally different tendons. We investigated the properties of whole tendons, tendon fascicles and the fascicular interface in the high-strain energy-storing SDFT and low-strain positional CDET. Fascicles failed at lower stresses and strains than tendons. The SDFT was more extensible than the CDET, but SDFT fascicles failed at lower strains than CDET fascicles, resulting in large differences between tendon and fascicle failure strain in the SDFT. At physiological loads, the stiffness at the fascicular interface was lower in the SDFT samples, enabling a greater fascicle sliding that could account for differences in tendon and fascicle failure strain. Sliding between fascicles prior to fascicle extension in the SDFT may allow the large extensions required in energy-storing tendons while protecting fascicles from damage.
Keywords: tendon, ageing, collagen, ligament, extracellular matrix
1. Introduction
A tendon's predominant function is to transfer the force generated by muscle contraction to the skeleton, facilitating movement around a joint and positioning the limbs for locomotion. Specific tendons, including the human Achilles tendon and equine superficial digital flexor tendon (SDFT) are able to further decrease the energetic cost of locomotion by acting as energy stores [1]. These tendons are stretched during the stance phase, and recoil during swing; returning the stored potential energy to the system. In vivo, strains of 16 per cent have been recorded in the equine SDFT during galloping exercise [2], and strains in the human Achilles tendon have been shown to exceed 10 per cent during one-legged hopping [3]. Such high levels of extension and subsequent recoil can increase locomotory efficiency by as much as 36 per cent during high-speed locomotion [4]. However, these high strains may also result in damage to these tendons. Injury in the horse model has been examined extensively, and the injury shown to be localized to the tendon core in the mid-metacarpal region of the SDFT [5,6]. It has subsequently been hypothesized that there are differences in the structure and/or properties of this area of the tendon. In contrast, positional tendons such as the human anterior tibialis tendon and the equine common digital extensor tendon (CDET) are relatively inextensible in order to allow efficient transfer of force from muscle to bone and precise placement of the limb, and these tendon types are rarely injured [7,8]. Maximum strains in the equine CDET have been estimated at 2.5 per cent [9], and strains in the human anterior tibialis tendon reach a maximum of 3.1 per cent [10]. Correspondingly, in vitro mechanical testing to failure, comparing the equine SDFT and CDET, has shown that the SDFT has a lower elastic modulus and failure stress than the CDET, but importantly it fails at significantly higher strains [11].
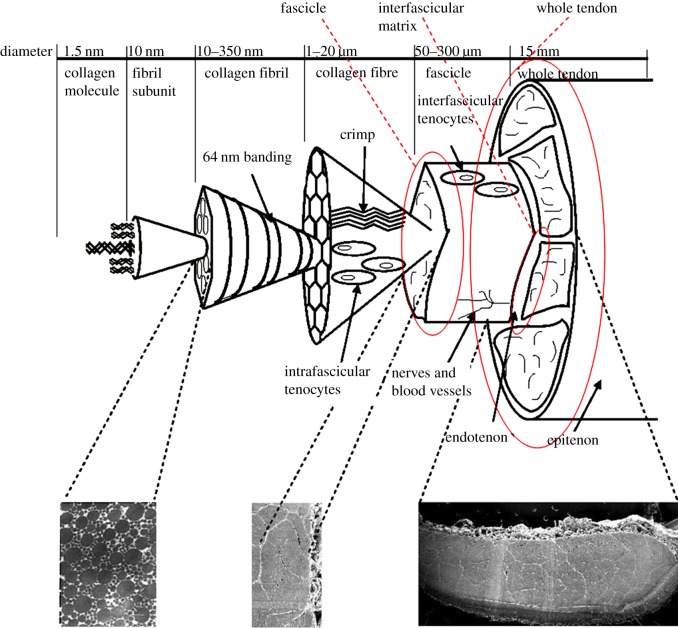
The material properties of tendons are determined by their matrix composition and organization. Tendons contain a high percentage of water, and the remaining dry matter is composed mainly of type I collagen that is arranged in a hierarchical structure [12] (figure 1). Collagen molecules are grouped together in a highly ordered fashion, forming fibrils, fibres and fascicles, with a small amount of proteoglycan-rich matrix between hierarchical levels. The interfascicular matrix (surrounding the fascicles) is a loose connective tissue composed of collagen type III and proteoglycans, and is synthesized and maintained by a small population of interfascicular fibroblasts [14–16]. There is a paucity of data regarding the structure and function of the interfascicular matrix; historically, it has been assumed that it functions simply to bind the tendon fascicles together [15], but recent studies have suggested that it may make a more significant contribution to tendon material properties [17,18].
Figure 1.
Schematic showing the hierarchical structure of tendon. Triple helical collagen molecules are arranged in a highly ordered, quarter-stagger pattern to form fibrils, which are grouped together, forming fibres and fascicles, with proteoglycan-rich matrix interspersing each level to form the whole tendon. The areas outlined in red indicate the levels of the tendon hierarchy investigated in this study. Adapted from Thorpe et al. [13]. (Online version in colour.)
Some studies have shown that differences in material properties exhibited by functionally distinct tendons are accompanied by differences in both matrix composition as well as the arrangement and organization of the matrix components at different levels of the tendon hierarchy [9,19,20]. The SDFT has a greater glycosaminoglycan and water content than the CDET [11]. There are also differences in collagen content, collagen crosslink profile [13,21] and collagen fibril diameter [19] between the SDFT and CDET. However, the precise mechanisms through which compositional changes result in alterations in mechanical properties remain unclear.
As an aligned fibre composite material, the hierarchical structure of tendon enables it to withstand high tensile forces, but results in complex anisotropic and viscoelastic characteristics. The mechanical properties of tendon as a whole are determined by the composition and organization of the matrix at each structural level and the way in which they contribute in response to tensile loading. However, the relationships between structure and function throughout the tendon hierarchy remain unknown. Previous structure–function investigations have focused on the mechanics of the hierarchical collagenous matrix that makes up the majority of the tendon substance [22–24]; few studies have investigated how the interfascicular matrix contributes to the mechanics of the whole tendon. In addition, previous comparisons of equine flexor and extensor tendon mechanics have been restricted to the level of the whole tendon, limiting our understanding of hierarchical contributions to whole tendon mechanics. Previous work directly comparing the mechanical properties of whole tendons and fascicles have reported that tendons have a greater failure stress and modulus than their constituent fascicles [17,18,25,26]. These results are surprising as standard composite models would predict that whole tendon would be less stiff that the fascicles it is composed of [27], and suggest that the interfascicular matrix may somehow contribute to gross tendon mechanical properties.
The aims of this study were to assess the material properties of whole tendons and their constituent fascicles and to determine how these properties differ between the functionally distinct SDFT and CDET. It was hypothesized that differences in the material properties of the SDFT and CDET would be reflected by differences in both fascicle material properties and in the mechanical properties of the interfascicular matrix. Identifying how these different hierarchical levels contribute to the overall mechanical properties of the tendon will increase our understanding of the mechanisms that contribute to tendon injury and aid in the development of appropriate treatment strategies.
2. Material and methods
2.1. Sample collection
The right and left forelimbs were collected from horses aged 3–20 years (n = 17), euthanized at a commercial equine abattoir that had no evidence of previous tendon injury at post-mortem examination. The SDFT and CDET were dissected free from the limbs at the level of the metacarpophalangeal joint, wrapped in tissue paper dampened with phosphate-buffered saline and stored frozen at −20°C wrapped in tin foil. It has previously been shown that one freeze–thaw cycle does not affect tendon mechanical properties [28].
2.2. Protocol for whole tendon testing
On the day of testing, the SDFT and CDET from the left forelimb of each horse were thawed at room temperature, and the cross-sectional area (CSA) measured at the mid-metacarpal level, as both the SDFT and CDET are thinnest in this region [29]. CSA was determined using an alginate paste casting technique that has been shown to be accurate to within 0.8 per cent [30].
For mechanical characterization, tendons were mounted vertically, with the proximal end uppermost, in a servo-hydraulic materials testing machine (Dartec Ltd., Stourbridge, UK) with a 50 kN load cell. Samples were gripped using cryoclamps cooled with liquid CO2 [31], with the clamps set at 10 cm apart. The mid-metacarpal region of the tendon was centred between the clamps, providing a homogeneous length of tendon to be tested. The tendons were pre-loaded to 100 N (SDFT) or 25 N (CDET), which represents a negligible load of approximately 1 per cent of the failure load and allows determination of a resting length. The distance between the two freeze lines was measured to give the effective gauge length.
Tendons were preconditioned to reach a steady state using a protocol adapted from Batson et al. [11]. Tendons underwent 20 preconditioning cycles between 0 per cent and 5.25 per cent strain (approx. 35% of failure strain, assuming a failure strain of 15%) using a sine wave at a frequency of 0.5 Hz. At the end of the preconditioning step, the load was removed so that slack was visible in the tendon. Tendons were then tested to failure at room temperature at a speed of 5 per cent per second.
2.3. Protocol for tendon fascicle testing
Fascicles (length approx. 35 mm) were dissected from the core (n = 6 from each tendon) and periphery (n = 6 from each tendon) of the mid-metacarpal region of the SDFT and CDET from the right forelimb of each horse. The diameter of each fascicle was determined by a non-contact laser micrometer (LSM-501, Mitotuyo, Japan; resolution = 0.5 μm) at multiple points along a 1 cm region in the middle of the fascicle. The smallest diameter recorded was used to calculate CSA, assuming a circular shape. To validate this assumption, the smallest fascicle diameter along the length of fascicles (n = 20) was located using the laser micrometer. Each fascicle was then rotated and the diameter was measured again. This was repeated so four measurements of diameter were obtained for each fascicle. These measures were used to calculate fascicle CSA, and the resulting area compared with that calculated from the initial diameter measurement. Calculating fascicle CSA assuming a circular shape resulted in an overestimation of 4 per cent.
Fascicles were secured in a materials testing machine (Bionix100, MTS, Cirencester, UK, 50 N load cell) by pneumatically driven grips with a serrated surface, exerting a gripping pressure of 3 GPa. The grip-to-grip distance was set to 20 mm. The fascicles were pre-loaded to 0.1 N, which represents a load of approximately 2 per cent of fascicle failure load, and the resulting grip-to-grip distance was taken as the effective gauge length. Fascicles were then preconditioned and returned to slack in the same manner as described for whole tendons, prior to testing to failure at room temperature at a strain rate of 5 per cent per second. During dissection and testing, specimens were kept moist by continually spraying with phosphate-buffered saline solution.
2.4. Calculation of mechanical and material properties of tendon and fascicle
Force and displacement data were continuously recorded at 100 Hz during preconditioning and the test to failure for both tendon and fascicle failure tests. Marks were drawn on the surface of the tendons and fascicles and tests were videoed (Panasonic SDR-550, 30 fps) from which the strain at failure was calculated, for comparison with the machine-derived displacement data. The method used did not result in a significant difference in failure strain; hence, failure strain was calculated from the machine-derived displacement data for all the samples. Engineering stress and strain were calculated using the CSA and effective gauge length for each sample. The displacement at which the initial pre-load was reached was taken as the start point for the test to failure in all specimens. A continuous modulus was calculated across every five data points of the stress–strain curve and smoothed using a five-point moving average filter. From these data, a maximum modulus value was determined.
2.5. Assessment of mechanics at the fascicular interface
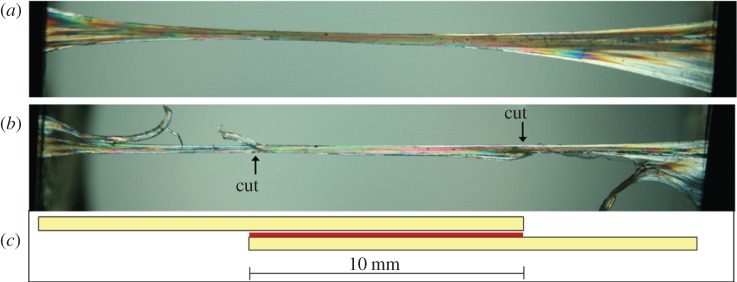
In order to investigate the mechanics of the fascicular interface, groups of two fascicles bound together by their fascicular membrane were dissected from the core (n = 6 from each tendon) or periphery (n = 6 from each tendon) of the mid-metacarpal region of the SDFT and CDET from the right forelimb of each horse. The fascicles were secured into a custom-made dissection rig that was placed under a stereomicroscope fitted with an analyser and rotatable polarizing lens (Leica). This generates elliptically polarized light that enables clear visualization of the individual collagen fascicles. The opposing end of each fascicle was cut transversely, leaving a consistent 10 mm length of intact fascicular membrane (figure 2).
Figure 2.
An illustration of fascicle dissection for testing of the interfascicular membrane. (a) Two intact fascicles bound by fascicular membrane viewed under polarizing light; (b) one end of each fascicle has been cut, leaving a section of intact fascicular membrane of 10 mm length; (c) schematic of fascicle dissection, with fascicles in yellow and interfascicular membrane in red. (Online version in colour.)
After removal from the dissection rig, the intact end of each fascicle was secured in a materials testing machine (Bionix100, MTS), with a grip to grip distance of 20 mm, and the fascicles pulled apart to failure at a speed of 1 mm s−1. An unbalanced test design such as this may lead to some error associated with interface rotation and generation of tension perpendicular to the loading axis. However, it is not possible to use a balanced shear design without causing extensive damage to the samples during preparation. Force and extension data were recorded at 100 Hz during the test, and from these data the point at which the load started to increase steadily was located (approx. 0.02 N) and defined as the test start point. Extension was measured as grip-to-grip displacement. The force and extension at failure were calculated for each sample. In addition, a force–extension curve was drawn for each sample, from which the amount of interface extension (expressed as a percentage of the failure extension) was calculated at different percentages of failure load. The mechanical properties of the interfascicular matrix are likely to be highly dependent on the rate of applied strain; in this study, the test speed was kept constant for all samples, allowing direct comparison of tendon, fascicle and interfascicular mechanics.
2.6. Scanning electron microscopy
A 10 mm transverse section was harvested from the mid-metacarpal region of the SDFT and CDET that had not undergone mechanical testing (n = 2). The sections were snap frozen in hexane and stored at −70°C wrapped in cling film. Each sample was then lyophilized in a freeze drier overnight, mounted onto an aluminium stub, coated with gold palladium and viewed under the scanning electron microscopy (SEM) at a magnification of 120× (Jeol JMS 550OLV).
2.7. Statistical analysis
Statistical significance was determined using linear mixed effects in Splus (v. 8.0, Insightful). In order to account for individual variations, horse (donor) was set as a grouping variable. All data are displayed as mean ± s.d.
3. Results
3.1. Tendon and fascicle material properties
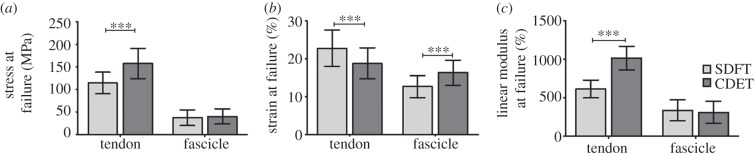
The mechanical properties of the whole tendons, fascicles and the interfascicular matrix are presented in table 1, with representative tendon and fascicle stress–strain curves shown in figure 3. The majority of tendons and fascicles failed in the mid-section, with approximately 20 per cent failing close to the grips. Tendon failure appeared to occur as a combination of fascicle rupture and fascicle pullout. In agreement with previous studies [11], the SDFT as a whole structure had a significantly lower elastic modulus (p < 0.001) and ultimate tensile stress (p < 0.001) than the CDET, while the strain at peak stress was significantly greater (p < 0.001) in the SDFT than in the CDET (figure 4). There were no significant differences in the material properties of fascicles from the core and periphery of either tendon, so these data were combined. The elastic modulus and failure stress did not differ significantly between fascicles from the SDFT and CDET. However, fascicles from the SDFT failed at significantly lower strains (p < 0.001) than those from the CDET.
Table 1.
Mechanical and material properties of the SDFT and CDET at the levels of the whole tendon, individual fascicles and the fascicular interface. Data are presented as mean ± s.d.
| tendon |
fascicle |
fascicular interface |
||||
|---|---|---|---|---|---|---|
| SDFT | CDET | SDFT | CDET | SDFT | CDET | |
| cross-sectional area (mm2) | 89.17 ± 30.80 | 25.58 ± 7.25** | 0.12 ± 0.06 | 0.16 ± 0.09** | — | — |
| force at failure (N) | 9918.55 ± 3318.94 | 3959.34 ± 1161.13** | 3.98 ± 2.11 | 5.58 ± 2.60** | 1.52 ± 0.93 | 1.54 ± 1.05 |
| ext at failure (mm) | — | — | — | — | 2.62 ± 0.70 | 2.95 ± 0.86* |
| ultimate stress (MPa) | 114.56 ± 23.81 | 157.41 ± 33.79** | 37.43 ± 17.57 | 40.21 ± 16.91 | — | — |
| failure strain (%) | 22.73 ± 4.73 | 18.80 ± 4.08** | 12.66 ± 2.88 | 16.38 ± 3.30** | — | — |
| linear modulus (MPa) | 613.80 ± 115.05 | 1012.26 ± 153.60** | 335.82 ± 137.94 | 310.24 ± 142.05 | — | — |
Statistical significance between tendon types: *p < 0.005; **p < 0.001.
Figure 3.
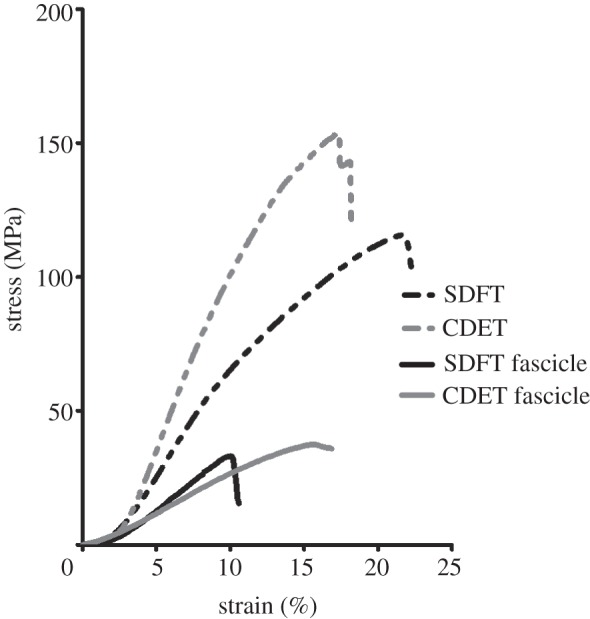
A representative tendon and fascicle stress–strain curves for the SDFT and CDET, illustrating the large differences in elastic modulus, failure stress and strain at failure between the SDFT and CDET. Whole tendons were able to resist higher stresses and extend further than the tendon fascicles.
Figure 4.
Gross tendon and fascicle material properties. Data are shown as mean ± s.d. (a) Failure stress, (b) strain at failure and (c) linear modulus were significantly greater in whole tendons than in fascicles (p > 0.0001). In whole tendons, failure stress and modulus were significantly lower in the SDFT than in the CDET. Failure strain was significantly higher in the SDFT. There was no difference in failure stress or modulus between SDFT and CDET fascicles, and SDFT fascicles failed at lower strains than those from the CDET. Triple asterisks indicate significant difference between tendon types (p < 0.0001).
Elastic modulus, failure stress and failure strain were significantly higher (p < 0.01) in whole tendons than in fascicles, both in the SDFT and in CDET. There was no significant correlation between individual tendon and fascicle material properties.
3.2. Mechanics of the interfascicular matrix
Representative force–extension curves for the fascicular interface are shown in figure 5a, with mean data on interface extension at different levels of applied strain shown in figure 5b. Considering the point of failure, there was no difference in the failure force of the fascicular interface in samples from the SDFT or CDET, while the extension at failure was significantly lower in samples from the SDFT. However, at and below 60 per cent of the force at failure, interfascicular displacement was significantly greater in the SDFT than in the CDET (p < 0.001; figure 5b).
Figure 5.
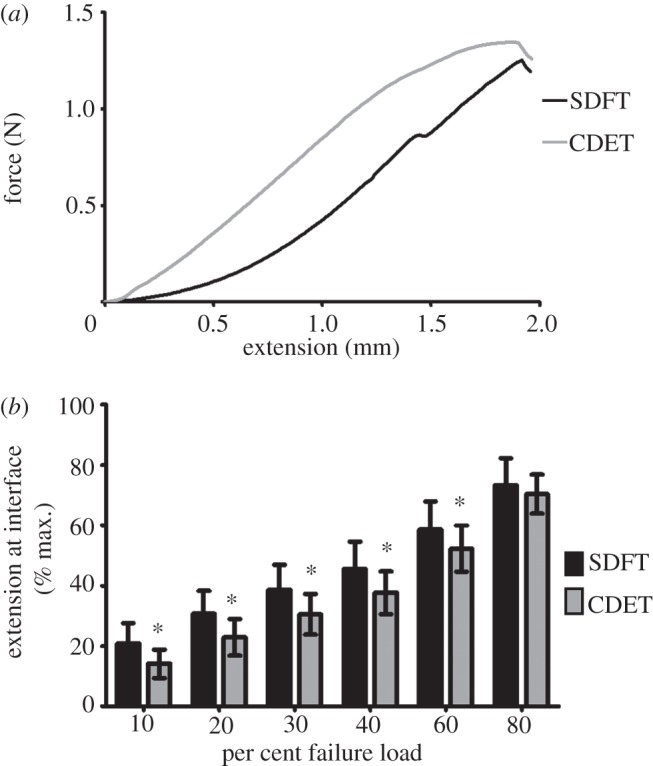
(a) A representative force–extension curve for the fascicular interface in the SDFT and CDET; (b) amount of extension at the fascicular interface at different percentages of failure load. Asterisk indicates significant difference between tendon types (p< 0.0001).
In order to compare the properties of whole tendons and their constituent fascicles, the average difference in strain at failure between paired whole tendons and fascicles was calculated for each tendon type (table 2). From these data, we calculated the displacement required at the fascicular interface to account for the measured differences in tendon and fascicle failure strain. From this, the absolute and relative interfascicular forces at these displacements were determined for both tendons. Failure strain between the SDFT and its fascicles differed by 10 per cent (1 mm), whereas the difference between the CDET and the CDET fascicles was 2.5 per cent (0.25 mm). The interfascicular force required to provide both 0.25 and 1 mm displacement was significantly lower in SDFT samples than in CDET samples. At 1 mm displacement, interfascicular force in the SDFT reached an average of 0.40 N, which corresponds to 28.42 per cent of failure force at the interface.
Table 2.
The average difference in strain at failure between whole tendons and fascicles was calculated for the SDFT and CDET. The resulting values were used to calculate the amount of displacement at the fascicular interface that would be required to account for the differences in tendon and fascicle failure strain. The absolute and relative interfascicular force at the required displacements was calculated for both tendons. Data are presented as mean ± s.d. Values in bold highlight the forces required to account for the mean difference in tendon and fascicle failure strain in the SDFT and CDET.
| mean difference in tendon and fascicle failure strain (%) | required displacement at fascicular interface (mm) | interfascicular force at 0.25 mm displacement |
interfascicular force at 1.00 mm displacement |
|||
|---|---|---|---|---|---|---|
| n | % failure force | n | % failure force | |||
| SDFT | 10 | 1.00 | 0.04 ± 0.03 | 3.17 ± 2.41 | 0.40 ± 0.25 | 28.42 ± 13.93 |
| CDET | 2.5 | 0.25 | 0.09 ± 0.08* | 7.19 ± 5.44* | 0.52 ± 0.31* | 39.79 ± 17.84* |
Statistical significance relative to the SDFT: *p < 0.001.
Of particular interest, when comparing the response of the tendons from each individual animal, the strain at failure measured in the SDFT as a whole showed a significant positive correlation with the percentage extension at the fascicular interface at 30 per cent of failure force (p < 0.01, r = 0.74). There was no correlation between strain at failure in the CDET and displacement at the fascicular interface.
3.3. Fascicle morphology
SEM images of transverse sections of the SDFT and CDET are shown in figure 6. It can be seen that fascicles in both the SDFT and CDET are irregular shapes and sizes. Boundaries between fascicles are clearly defined in the SDFT, with clear spacing between individual fascicles. By contrast, fascicles in the CDET appear much more tightly packed, and it is difficult to identify fascicle boundaries.
Figure 6.
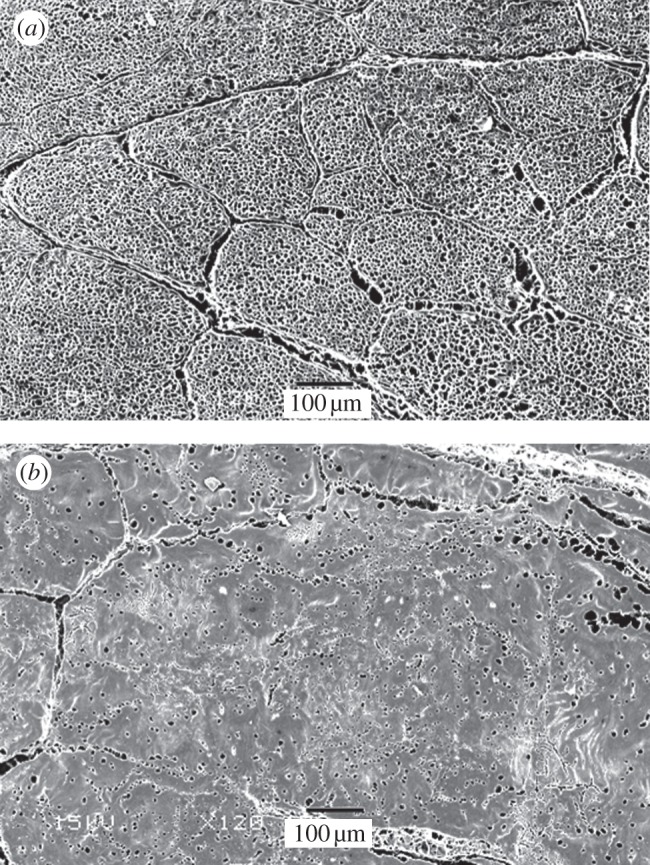
Transverse SEM images of the SDFT (a) and CDET (b) sectioned at the mid-metacarpal level. It is evident that the interfascicular space is larger in the SDFT than in the CDET, and that fascicles are more tightly packed in the CDET.
4. Discussion
The results show significant differences between the material properties of the SDFT and CDET as whole tendons, as previously reported [11,19], reflecting the different functions of these tendons. However, contrary to our hypothesis, these differences are not maintained at the fascicular level. Instead, significant differences in the mechanical properties of the interfascicular matrix between the tendon types appear to account for the differences seen between the tendons at the gross level.
Although we had not set out to compare absolute values for the mechanical behaviour of fascicles compared with the whole tendon, our results are unexpected and require further discussion. Standard composite models of tendon such as the one described by Puxkandl et al. [27] suggest that small-scale levels of organization in tendon are stiffer than large-scale levels, whereas our results show that the whole tendon is a considerably stiffer material than the fascicles that constitute it. Although unexpected, several other studies directly comparing tendon and fascicle mechanics in other animal models have reported similar results [17,18,25,26]. Furthermore, a review of the extensive data regarding either whole tendon or fascicle mechanics strongly indicates a consistent trend for lower moduli in isolated fascicles than whole tendons [32–35].
It is possible that the large differences in whole tendon and fascicle modulus are simply associated with alterations in fascicle diameter associated with fascicle isolation. Indeed, a very recent study reporting an in vitro collagen fibril modulus insufficient to account for the human patellar tendon in vivo modulus implicated removing the tissue from its natural environment [36]. While both the whole tendon and fascicles were tested in vitro in this study, dissecting fascicles free from a tendon may similarly influence material properties. Comparing fascicle CSA calculated from SEM images (figure 6) and laser micrometry measurements indicates that fascicle CSA in situ is approximately half that in vitro. While SEM will also provide artefact, this suggests that fascicle isolation results in swelling. In addition, we have noted that our methods for CSA measurement result in an underestimation of tendon CSA and overestimation of fascicle CSA. Correcting for these factors would bring the average fascicle moduli values in line with that of whole tendon. A separate study by Hirokawa & Hasezaki [17] have shown that isolated tendon fascicles elongate non-uniformly, resulting in a disproportionally low modulus. While we did not observe any obvious non-uniformit in fascicle extension, the isolated fascicle model may not fully reflect how fascicle extension occurs in vivo; if elongation is more uniform, in situ moduli values are likely to be greater. Combined, these factors may account for the unexpectedly low fascicle moduli reported in the current study and by others.
By contrast, failure strain followed a more typical relationship, with whole tendons failing at higher strains than their constitutive fascicles. Elucidating how this is achieved is key to understanding the specialization of tendon mechanics, and so was of particular interest in this study. While it was not possible to use similar gripping methods or specimen length for the very differently sized whole tendons and fascicles, we were able to confirm there was no sample slippage and were also able to ensure that all samples were sufficiently long enough to minimize ‘end’ effects [33]. Previous studies have shown that strain increases at each structural level of the tendon hierarchy [27,37,38], implying considerable influence from the non-collagenous matrix that intersperses each level. Strain distribution within the tendon fascicles is complex and heterogeneous, with a significant degree of sliding occurring between the collagen fibres of any individual fascicle [38]. Further, strain is not uniformly distributed along the length of a fascicle, with differences in the elastic modulus of the fascicle at different sections along its length [17], and areas of stress concentration [39]. For strains in the whole tendon to exceed the strains seen by the collagenous matrix at several levels of the tendon hierarchy, some of the deformation must be dissipated by the non-collagenous matrix surrounding the fibrils, fibres and fascicles.
However, the levels of sliding between fascicles needed to account for our data are surprising. The results do not support our hypothesis, and the difference between the modulus of the SDFT and CDET is not reflected in the fascicles. In fact, the SDFT is more extensible than the CDET, whereas the fascicles from the SDFT failed at lower strains than those from the CDET. This results in an average difference in failure strain of 10 per cent between the SDFT and its fascicles, compared with a 2 per cent difference in the CDET. In order to achieve the strains of up to 16 per cent recorded in vivo [2] without damage to the tendon occurring, deformation of the interfascicular matrix must occur. The potential for the interfascicular matrix to deform and sustain load was investigated by the use of a shear failure model to determine the force required to pull two neighbouring fascicles apart.
Although the total amount of available extension at the fascicular interface was slightly lower in samples from the SDFT, differences in the shape of the force extension curve between tendon types mean that, within the working range, the amount of extension available between SDFT fascicles is significantly greater. Indeed, the data show that the capacity for sliding at the fascicular interface is easily large enough to account for the 10 per cent difference in tendon and fascicle failure strain seen in the SDFT, with the interface able to provide the additional extension at only approximately 30 per cent of its failure load. Furthermore, while there was no relationship between tendon and fascicle failure strain for an individual animal, the amount of extension at the fascicular interface at a 30 per cent of failure load was found to be positively correlated with SDFT failure strain, suggesting that whole tendon material properties are directly influenced by the mechanics at the interface. Interestingly, there was no relationship between interfascicular sliding and failure strain in the CDET, suggesting this mechanism of extension is less important in the positional tendon. It is likely that the low force required to enable interfascicular sliding in the SDFT allows the tendon as a whole to be more extensible than the CDET, despite its constituent fascicles failing at lower strains.
Although interfascicular mechanics have received little attention previously, a study using similar methods to those used here demonstrated that fascicles are able to slide relative to one another and concluded that fascicles are largely independent structures [40]. The study by Haraldsson et al. [40] investigated lateral force transfer at relatively small displacements (up to 0.3 mm, with a test length of 10 mm) in the human Achilles and patella tendons, and recorded maximum interfascicular forces of 0.02 N. In comparison, a displacement of 0.3 mm in the equine SDFT generated an average interfascicular force of 0.07 N. The Achilles tendon also acts as an energy store, and experiences high strains in vivo, so it is possible the extension mechanism is similar in the human Achilles and equine SDFT; however, no studies have directly compared the mechanical properties of the Achilles tendon and its constituent fascicles.
The arrangement of the fascicles in tendon is of great consequence, if the tendon relies on fascicle sliding for extension. It is thought that fascicles run the entire length of the tendon [41], but this has not been confirmed and little is known about the morphology of the myotendinous or osseotendinous junctions. It is possible that fascicles are continuous throughout the mid-section of the tendon, but that not all are anchored firmly at both junction ends, allowing for some degree of interfascicle sliding in vivo. Indeed, the SDFT is 300–400 mm length; therefore, a fascicle traversing the whole tendon length would require an improbable aspect ratio of approximately 1000. While fascicle length remains undetermined, there is no doubt that fascicles are long compared with the critical length required for a composite material. In addition, it is unlikely that all fascicles within a tendon stretch equally, nor that individual fascicles elongate uniformly [17], resulting in interfascicle sliding. The data presented in this study show that it is possible to transfer significant force laterally via the interfascicular matrix prior to interface failure (approx. 1.5 N) with significant amounts of sliding occurring in the matrix with the application of a small amount of load. Taken together, these data suggest a role for the interfascicular matrix in facilitating sliding between fascicles within a tendon, allowing the large extensions required for flexor tendon function without exposing the tendon fascicles to excessive strains.
While it is well established that tendon fascicles are composed predominantly of highly ordered type I collagen, the composition, structure and function of the interfascicular matrix have not been studied in as much detail. The interfascicular matrix is composed of small bundles of type III collagen fibrils, proteoglycans, cells, nerves and capillaries [14,16,40]. Recently, it has been suggested that the glycoprotein lubricin facilitates sliding between fascicles [42,43]. Elastin and elastic-fibre-related proteins may also play a role in fascicle sliding and recoil and there is some evidence to suggest that components of the elastic fibre are localized to the interfascicular matrix [44,45] but their precise function within tendon has yet to be established.
Previous work has shown that the organization and packing of the collagen fibrils differs between the SDFT and CDET. Fibril diameter distribution is bimodal, with a smaller average fibril diameter in the SDFT, allowing for tight packing of the collagen fibrils. By contrast, fibril diameter is larger in the CDET, and has a unimodal distribution [19]. The tight packing of fibrils in the SDFT may explain why fascicles from the SDFT fail at lower strains than those from the CDET. At the fascicle level, this organization is reversed, with more tightly packed fascicles in the CDET and less interfascicular sliding at low loads, resulting in an overall stiffer tendon. The SDFT must be able to withstand extremely high strains in vivo without damage; the combination of stiffer fascicles surrounded by a less stiff interfascicular matrix may allow maximum extension of the tendon with a lower risk of damage to the fascicles. It is possible that the organization of the fascicular and interfascicular matrices in the SDFT enables maximal extension and recoil of the tendon, resulting in greater energy storage and return. However, the mechanisms by which tendons recoil rapidly are poorly understood; this is an area that requires further investigation.
Elucidation of these mechanisms is important for characterizing the mechanisms leading to flexor tendon injury. It is not entirely surprising that energy-storing flexor tendons are injured far more than positional tendons as they are exposed to very high strains during normal loading conditions [7,46,47]. However, factors that predispose certain tendons to tendinopathy are yet to be identified. SDFT injury is often localized to the core region of the tendon, but we did not identify any differences in mechanics of fascicles from the core or peripheral regions. However, our data lead to a hypothesis that energy-storing tendons with a stiffer interfascicular matrix will enable a less interfascicular sliding, eliminating this protective mechanism and leaving the tendon more susceptible to damage at lower strain levels.
5. Conclusions
The data presented in this study illustrate that the different mechanical demands of functionally distinct tendons are met by large differences in gross tendon material properties. While fascicle material properties must influence overall tendon properties, this study provides novel data suggesting that it is the interfascicular matrix that is key in facilitating the high strain characteristics of energy-storing tendons. Full understanding of the mechanisms that govern tendon extension and recoil and the differences that occur between functionally distinct tendons will assist in identification of factors predisposing to tendon injury and aid in the development of appropriate repair strategies that would best recapitulate the unique mechanical characteristics of the flexor tendon.
Acknowledgements
This study was funded by the Horserace Betting Levy Board. The authors thank Rebecca Porter for her assistance with the SEM.
References
- 1.Alexander R. M. 1991. Energy-saving mechanisms in walking and running. J. Exp. Biol. 160, 55–69 [DOI] [PubMed] [Google Scholar]
- 2.Stephens P. R., Nunamaker D. M., Butterweck D. M. 1989. Application of a Hall-effect transducer for measurement of tendon strains in horses. Am. J. Vet. Res. 50, 1089–1095 [PubMed] [Google Scholar]
- 3.Lichtwark G. A., Wilson A. M. 2005. In vivo mechanical properties of the human Achilles tendon during one-legged hopping. J. Exp. Biol. 208, 4715–4725 10.1242/jeb.01950 (doi:10.1242/jeb.01950) [DOI] [PubMed] [Google Scholar]
- 4.Biewener A. A. 1998. Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 120, 73–87 10.1016/S0305-0491(98)00024-8 (doi:10.1016/S0305-0491(98)00024-8) [DOI] [PubMed] [Google Scholar]
- 5.Webbon P. M. 1977. A post mortem study of equine digital flexor tendons. Equine Vet. J. 9, 61–67 10.1111/j.2042-3306.1977.tb03981.x (doi:10.1111/j.2042-3306.1977.tb03981.x) [DOI] [PubMed] [Google Scholar]
- 6.Birch H. L., Bailey A. J., Goodship A. E. 1998. Macroscopic ‘degeneration’ of equine superficial digital flexor tendon is accompanied by a change in extracellular matrix composition. Equine Vet. J. 30, 534–539 10.1111/j.2042-3306.1998.tb04530.x (doi:10.1111/j.2042-3306.1998.tb04530.x) [DOI] [PubMed] [Google Scholar]
- 7.Ely E. R., Avella C. S., Price J. S., Smith R. K., Wood J. L., Verheyen K. L. 2009. Descriptive epidemiology of fracture, tendon and suspensory ligament injuries in National Hunt racehorses in training. Equine Vet. J. 41, 372–378 10.2746/042516409X371224 (doi:10.2746/042516409X371224) [DOI] [PubMed] [Google Scholar]
- 8.Markarian G. G., Kelikian A. S., Brage M., Trainor T., Dias L. 1998. Anterior tibialis tendon ruptures: an outcome analysis of operative versus nonoperative treatment. Foot Ankle. Int. 19, 792–802 [DOI] [PubMed] [Google Scholar]
- 9.Birch H. L., Worboys S., Eissa S., Jackson B., Strassburg S., Clegg P. D. 2008. Matrix metabolism rate differs in functionally distinct tendons. Matrix Biol. 27, 182–189 [DOI] [PubMed] [Google Scholar]
- 10.Maganaris C. N., Paul J. P. 1999. In vivo human tendon mechanical properties. J. Physiol. 521, 307–313 10.1111/j.1469-7793.1999.00307.x (doi:10.1111/j.1469-7793.1999.00307.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batson E. L., Paramour R. J., Smith T. J., Birch H. L., Patterson-Kane J. C., Goodship A. E. 2003. Are the material properties and matrix composition of equine flexor and extensor tendons determined by their functions? Equine Vet. J. 35, 314–318 10.2746/042516403776148327 (doi:10.2746/042516403776148327) [DOI] [PubMed] [Google Scholar]
- 12.Kastelic J., Galeski A., Baer E. 1978. The multicomposite structure of tendon. Connect Tissue Res. 6, 11–23 10.3109/03008207809152283 (doi:10.3109/03008207809152283) [DOI] [PubMed] [Google Scholar]
- 13.Thorpe C. T., Streeter I., Pinchbeck G. L., Goodship A. E., Clegg P. D., Birch H. L. 2010. Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J. Biol. Chem. 285, 15 674–15 681 10.1074/jbc.M109.077503 (doi:10.1074/jbc.M109.077503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlgren L. A., Brower-Toland B. D., Nixon A. J. 2005. Cloning and expression of type III collagen in normal and injured tendons of horses. Am. J. Vet. Res. 66, 266–270 10.2460/ajvr.2005.66.266 (doi:10.2460/ajvr.2005.66.266) [DOI] [PubMed] [Google Scholar]
- 15.Kannus P. 2000. Structure of the tendon connective tissue. Scand. J. Med. Sci. Sports 10, 312–320 10.1034/j.1600-0838.2000.010006312.x (doi:10.1034/j.1600-0838.2000.010006312.x) [DOI] [PubMed] [Google Scholar]
- 16.Fallon J., Blevins F. T., Vogel K., Trotter J. 2002. Functional morphology of the supraspinatus tendon. J. Orthop. Res. 20, 920–926 10.1016/S0736-0266(02)00032-2 (doi:10.1016/S0736-0266(02)00032-2) [DOI] [PubMed] [Google Scholar]
- 17.Hirokawa S., Hasezaki H. 2010. Model analysis to investigate the contribution of ground substance to ligament stiffening. Med. Eng. Phys. 32, 610–616 10.1016/j.medengphy.2010.02.013 (doi:10.1016/j.medengphy.2010.02.013) [DOI] [PubMed] [Google Scholar]
- 18.Hirokawa S., Sakoshita T. 2003. An experimental study of the microstructures and mechanical properties of swine cruciate ligaments. JSME Int. J. Ser. C. 46, 1417–1425 10.1299/jsmec.46.1417 (doi:10.1299/jsmec.46.1417) [DOI] [Google Scholar]
- 19.Birch H. L. 2007. Tendon matrix composition and turnover in relation to functional requirements. Int. J. Exp. Path. 88, 241–248 10.1111/j.1365-2613.2007.00552.x (doi:10.1111/j.1365-2613.2007.00552.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toorani S., Shelton J. C., Screen H. R. C. 2007. The relationship between tendon composition, structure, and mechanical characteristics. Tissue Eng. 13, 393. 10.1089/ten.2006.0082 (doi:10.1089/ten.2006.0082) [DOI] [PubMed] [Google Scholar]
- 21.Birch H. L., Smith T. J., Draper E. R., Bailey A. J., Avery N. C., Goodship A. E. 2006. Collagen crosslink profile relates to tendon material properties. Matrix Biol. 25, S74. 10.1016/j.matbio.2006.08.204 (doi:10.1016/j.matbio.2006.08.204) [DOI] [Google Scholar]
- 22.Gupta H. S., Seto J., Krauss S., Boesecke P., Screen H. R. C. 2010. In situ multi-level analysis of viscoelastic deformation mechanisms in tendon collagen. J. Struct. Biol. 169, 183–191 10.1016/j.jsb.2009.10.002 (doi:10.1016/j.jsb.2009.10.002) [DOI] [PubMed] [Google Scholar]
- 23.Bozec L., Horton M. 2005. Topography and mechanical properties of single molecules of type I collagen using atomic force microscopy. Biophys. J. 88, 4223–4231 10.1529/biophysj.104.055228 (doi:10.1529/biophysj.104.055228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng V. W. T., Screen H. R. C. 2007. The micro-structural strain response of tendon. J. Mater. Sci. 42, 8957–8965 10.1007/s10853-007-1653-3 (doi:10.1007/s10853-007-1653-3) [DOI] [Google Scholar]
- 25.Yamamoto E., Hayashi K., Yamamoto N. 1999. Mechanical properties of collagen fascicles from the rabbit patellar tendon. J. Biomech. Eng. ASME 121, 124–131 10.1115/1.2798033 (doi:10.1115/1.2798033) [DOI] [PubMed] [Google Scholar]
- 26.Toorani S. 2010. The influence of microstructure on the mechanical behaviours of tendons. London, UK: Queen Mary: University of London [Google Scholar]
- 27.Puxkandl R., Zizak I., Paris O., Keckes J., Tesch W., Bernstorff S., Purslow P., Fratzl P. 2002. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Phil. Trans. R. Soc. Lond. B 357, 191–197 10.1098/rstb.2001.1033 (doi:10.1098/rstb.2001.1033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H., Zhang J., Sun K., Zhang X., Tian S. 2011. Effects of repetitive multiple freeze-thaw cycles on the biomechanical properties of human flexor digitorum superficialis and flexor pollicis longus tendons. Clin. Biomech. 26, 419–423 10.1016/j.clinbiomech.2010.12.006 (doi:10.1016/j.clinbiomech.2010.12.006) [DOI] [PubMed] [Google Scholar]
- 29.Birch H. L., Smith T. J., Poulton C., Peiffer D., Goodship A. E. 2002. Do regional variations in flexor tendons predispose to site-specific injuries? Equine Vet. J. Suppl. 34, 288–292 10.1111/j.2042-3306.2002.tb05435.x (doi:10.1111/j.2042-3306.2002.tb05435.x) [DOI] [PubMed] [Google Scholar]
- 30.Goodship A. E., Birch H. L. 2005. Cross sectional area measurement of tendon and ligament in vitro: a simple, rapid, non-destructive technique. J. Biomech. 38, 605–608 10.1016/j.jbiomech.2004.05.003 (doi:10.1016/j.jbiomech.2004.05.003) [DOI] [PubMed] [Google Scholar]
- 31.Riemersa D. J., Schamhardt H. C. 1982. The cryo-jaw, a clamp designed for in vitro rheology studies of horse digital flexor tendons. J. Biomech. 15, 619–620 10.1016/0021-9290(82)90073-2 (doi:10.1016/0021-9290(82)90073-2) [DOI] [PubMed] [Google Scholar]
- 32.Ker R. F., Alexander R. M., Bennett M. B. 1988. Why are mammalian tendons so thick? J. Zool. 216, 309–324 10.1111/j.1469-7998.1988.tb02432.x (doi:10.1111/j.1469-7998.1988.tb02432.x) [DOI] [Google Scholar]
- 33.Legerlotz K., Riley G. P., Screen H. R. 2010. Specimen dimensions influence the measurement of material properties in tendon fascicles. J. Biomech. 43, 2274–2280 10.1016/j.jbiomech.2010.04.040 (doi:10.1016/j.jbiomech.2010.04.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wren T. A. L., Yerby S. A., Beaupré G. S., Carter D. R. 2001. Mechanical properties of the human achilles tendon. Clin. Biomech. 16, 245–251 10.1016/S0268-0033(00)00089-9 (doi:10.1016/S0268-0033(00)00089-9) [DOI] [PubMed] [Google Scholar]
- 35.Komolafe O. A., Doehring T. C. 2010. Fascicle-scale loading and failure behavior of the Achilles tendon. J. Biomech. Eng. 132, 021004. 10.1115/1.4000696 (doi:10.1115/1.4000696) [DOI] [PubMed] [Google Scholar]
- 36.Svensson R. B., Hansen P., Hassenkam T., Haraldsson B. T., Aagaard P., Kovanen V., Krogsgaard M., Kjaer M., Magnusson S. P. 2011. Mechanical properties of human patellar tendon at the hierarchical levels of tendon and fibril. J. Appl. Physiol. 112, 419–426 10.1152/japplphysiol.01172.2011 (doi:10.1152/japplphysiol.01172.2011) [DOI] [PubMed] [Google Scholar]
- 37.Sasaki N., Odajima S. 1996. Elongation mechanism of collagen fibrils and force-strain relations of tendon at each level of structural hierarchy. J. Biomech. 29, 1131–1136 10.1016/0021-9290(96)00024-3 (doi:10.1016/0021-9290(96)00024-3) [DOI] [PubMed] [Google Scholar]
- 38.Screen H. R. C., Bader D. L., Lee D. A., Shelton J. C. 2004. Local strain measurement within tendon. Strain 40, 157–163 10.1111/j.1475-1305.2004.00164.x (doi:10.1111/j.1475-1305.2004.00164.x) [DOI] [Google Scholar]
- 39.Screen H. R. C., Evans S. L. 2009. Measuring strain distributions in the tendon using confocal microscopy and finite elements. J. Strain Anal. Eng. 44, 327–335 10.1243/03093247JSA491 (doi:10.1243/03093247JSA491) [DOI] [Google Scholar]
- 40.Haraldsson B. T., Aagaard P., Qvortrup K., Bojsen-Moller J., Krogsgaard M., Koskinen S., Kjaer M., Magnusson S. P. 2008. Lateral force transmission between human tendon fascicles. Matrix Biol. 27, 86–95 10.1016/j.matbio.2007.09.001 (doi:10.1016/j.matbio.2007.09.001) [DOI] [PubMed] [Google Scholar]
- 41.Basso O., Johnson D. P., Amis A. A. 2001. The anatomy of the patellar tendon. Knee Surg. Sports Traumatol. Arthrosc. 9, 2–5 10.1007/s001670000133 (doi:10.1007/s001670000133) [DOI] [PubMed] [Google Scholar]
- 42.Funakoshi T., Schmid T., Hsu H.-P., Spector M. 2008. Lubricin distribution in the goat infraspinatus tendon: a basis for interfascicular lubrication. J. Bone Joint Surg. Am. 90, 803–814 10.2106/JBJS.G.00627 (doi:10.2106/JBJS.G.00627) [DOI] [PubMed] [Google Scholar]
- 43.Kohrs R. T., Zhao C., Sun Y.-L., Jay G. D., Zhang L., Warman M. L., An K.-N., Amadio P. C. 2011. Tendon fascicle gliding in wild type, heterozygous, and lubricin knockout mice. J. Orthop. Res. 29, 384–389 10.1002/jor.21247 (doi:10.1002/jor.21247) [DOI] [PubMed] [Google Scholar]
- 44.Ritty T. M., Ditsios K., Starcher B. C. 2002. Distribution of the elastic fiber and associated proteins in flexor tendon reflects function. Anat. Rec. 268, 430–440 10.1002/ar.10175 (doi:10.1002/ar.10175) [DOI] [PubMed] [Google Scholar]
- 45.Smith K. D., Vaughan-Thomas A., Spiller D. G., Innes J. F., Clegg P. D., Comerford E. J. 2011. The organisation of elastin and fibrillins 1 and 2 in the cruciate ligament complex. J. Anat. 218, 600–607 10.1111/j.1469-7580.2011.01374.x (doi:10.1111/j.1469-7580.2011.01374.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knobloch K., Yoon U., Vogt P. M. 2008. Acute and overuse injuries correlated to hours of training in master running athletes. Foot Ankle Int. 29, 671–676 10.3113/FAI.2008.0671 (doi:10.3113/FAI.2008.0671) [DOI] [PubMed] [Google Scholar]
- 47.Kausch T., Rütt J. 1998. Subcutaneous rupture of the tibialis anterior tendon: review of the literature and a case report. Arch. Orthop. Trauma Surg. 117, 290–293 10.1007/s004020050250 (doi:10.1007/s004020050250) [DOI] [PubMed] [Google Scholar]