Abstract
Globally, more than 1000 tonnes of titanium (Ti) is implanted into patients in the form of biomedical devices on an annual basis. Ti is perceived to be ‘biocompatible’ owing to the presence of a robust passive oxide film (approx. 4 nm thick) at the metal surface. However, surface deterioration can lead to the release of Ti ions, and particles can arise as the result of wear and/or corrosion processes. This surface deterioration can result in peri-implant inflammation, leading to the premature loss of the implanted device or the requirement for surgical revision. Soft tissues surrounding commercially pure cranial anchorage devices (bone-anchored hearing aid) were investigated using synchrotron X-ray micro-fluorescence spectroscopy and X-ray absorption near edge structure. Here, we present the first experimental evidence that minimal load-bearing Ti implants, which are not subjected to macroscopic wear processes, can release Ti debris into the surrounding soft tissue. As such debris has been shown to be pro-inflammatory, we propose that such distributions of Ti are likely to effect to the service life of the device.
Keywords: titanium, crevice corrosion, microfocus spectroscopy
1. Introduction
Metallic prostheses, fixation and anchorage devices are used extensively for orthopaedic, craniofacial and dental rehabilitation, and are generally associated with predictable biological responses following their initial implantation. However, the development of peri-implant inflammation can result in the premature loss of the implanted device or the requirement for revision/rescue surgery [1]. Both scenarios can impact on patients’ well-being and economically on the health service provider. In the USA, it has been reported that the direct cost of such revision surgery now exceeds $100 million per annum and is increasing due to an ageing population [2]. In the orthopaedic context, failures of replaced articulating joints are frequently aseptic, and it has been recognized that a key contributory factor is the degradation of the implant surface(s), which leads to ion leaching and the accumulation of metal debris in the peri-prosthetic milieu [3–6]. Despite the concerns being raised regarding the likely biological effects of metal debris and ions generated through wear and/or corrosion processes, the exact mechanisms underpinning their release in vivo and their subsequent involvement in peri-implant inflammation are not fully understood.
More than 1000 tonnes of titanium (Ti) is implanted into patients in the form of biomedical devices globally on an annual basis [7]. Cranial anchorage devices and dental implants are manufactured from ‘commercially pure’ grades (II–IV) of Ti (CPTi), whereas orthopaedic components are most commonly fabricated from the grade V alloy, Ti 6Al 4V. Ti in these forms is highly reactive, and therefore its perceived ‘biocompatibility’ is attributed to the presence of a robust passive oxide film (approx. 4 nm thick) at the metal surface. However, surface deterioration can lead to the release of Ti ions, and particles can arise as the result of wear and/or corrosion processes [8], whereas wear processes are an inevitable consequence of employing metals to replace load-bearing articulating surfaces as found in artificial joints, and the mechanisms underpinning the in vivo corrosion of Ti are complex and inadequately characterized [9]. Gross corrosion of the intra-medullary stem of cemented ‘Furlong’ Ti hip replacements has been demonstrated and resulted in cortical hypertrophy, systemic distribution of Ti ions, pain and aseptic loosening [9]. Similarly, Ti intra-medullary fixation implants unexposed to significant wear have been associated with elevated serum Ti levels over prolonged time-periods, suggesting corrosive deterioration [10]. Metallurgical simulations have demonstrated that in tight crevices, such as between components of modular Ti prosthetic devices, moisture and relative micro-motion of component surfaces can result in the dissolution of Ti ions, local acidification and the degradation of the protective oxide film from the metal, exposing it to the corrosive solution [8]. Intuitively, the process that is understood as crevice corrosion may occur in all Ti devices where peri-implant crevices exist, including dental and cranial anchorage implants. The potential sequelae are persistent Ti ion leaching or Ti particle release into the peri-prosthetic environment irrespective of whether there is significant associated loading or wear.
Investigations at a cellular and molecular level have demonstrated that Ti particles can stimulate the host-inflammatory response contributing to the pathology of peri-prosthetic bone loss [11]. Interactions of Ti particles with macrophages, osteoblasts, bone-marrow-derived mesenchymal stem cells (MSCs), fibroblasts, endothelial cells and T-cells are believed to contribute to the production of pro-inflammatory and pro-osteoclastogenic cytokines [11–13]. Moreover, Ti particles have been shown to initiate apoptosis, increase production of reactive oxygen species and induce oxidative stress-related genes [11]. Notably, data demonstrate that Ti particles can alter cell function and phenotype with differential effects regulated by Ti particle size [14]. Lymphocyte populations, including T-cells, respond to Ti particles by activation of the specific CD69+ and CCR4+ sub-populations and by increasing their secretion of the cytokine receptor activator of nuclear factor kappa-B ligand, which indirectly locally inhibits osteoblast function and promotes bone loss by enhanced osteoclastogenesis [15]. Ti particle exposure of multipotent MSCs results in particulate endocytosis, reducing their rate of cell-proliferation and cell-substrate adhesion and increasing the rate of apoptosis, which further impairs innate repair mechanisms [16].
The aim of the current investigation was to test the null hypothesis that there is no association between CPTi implants unexposed to significant macroscopic wear and the accumulation of Ti in the peri-implant tissues. Given the evidence to suggest that the accumulation of Ti in peri-implant tissues can significantly modify inflammatory and regenerative processes, the objective is clinically important as frequently such devices fail owing to peri-implant inflammatory processes. A cranial anchorage device, the bone-anchored hearing aid (BAHA), was used as an experimental model. BAHAs are a well-established treatment for conductive or mixed hearing impairment. A CPTi implant is inserted into the outer table of the post-auricular skull and connected via a modular percutaneous CPTi abutment to a sound processor. However, relatively few patients (1–3%) extrude the BAHA device [17–19], and up to 33 per cent suffer from peri-implant skin inflammation, which becomes hypertrophic and requires revision surgery [18].
2. Methodology
Peri-implant inflamed skin tissue was obtained from consenting competent adults undergoing scheduled revision surgery associated with a BAHA at University Hospital Birmingham NHS Foundation Trust. The inflamed tissue was surgically retrieved from around the percutaneous grade IV commercially pure Ti implant (Baha, Cochlear Ltd, Sydney, Australia) using a full thickness circumferential excision (figure 1). Implants had been inserted into the outer table of the skull, post-auricularly, more than 3 years previously and were used to anchor an external sound processor as part of the BAHA system to treat unilateral sensorineural hearing loss. Skin was retrieved from around implants that remained firm and well osseointegrated but associated with a hypertrophic reaction surrounding the Ti implant and necessitating skin reduction surgery. Orientated tissue was immediately snap-frozen in liquid nitrogen in order to preserve the chemical state and location of any Ti. Frozen tissue sections of 4–6 μm thickness were prepared using a cryotome with a ceramic knife and mounted on ultra-pure fused silica microscope slides (less than 10 ppb Ti; Spectrosil 2000, Heraeus Quarzglas GmbH & Co., Hanau, Germany). Spurious contamination with Ti during sample preparation was controlled for by processing further epithelial tissue with no known exposure or adjacency to Ti in an identical manner. To comply with experimental facility protocols regarding exposure to unfixed human tissue, the tissue sections were covered in a 25 μm polyimide film (Kapton, Dupont, Wilmington, NC, USA), which was secured at the periphery of the glass slide with epoxy resin.
Figure 1.
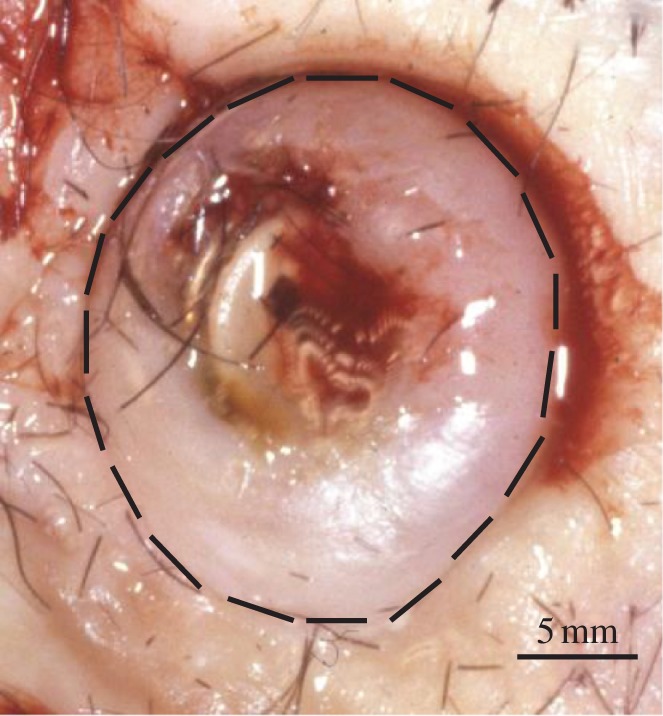
A clinical photograph of peri-implant soft tissue overgrowth associated with a CPTi BAHA fixture. Up to 33% of patients suffer from peri-implant skin inflammation, which can necessitate implant removal. Interrupted line indicates circumferential incision line for tissue explanation.
Synchrotron micro-focus X-ray fluorescence measurements were undertaken using a four-element Si drifts detector on the I18-Beamline at the Diamond Light Source (Oxfordshire, UK) using an incident energy of 5.7 keV. Tissue sections were initially mapped using a 50 μm resolution to identify areas of interest. Subsequently, the incident beam was focused to give a spot size 5 µm (H) by 3.4 µm (W), and the sample was mounted at a 45° angle to the incident beam, thus resulting in a beam footprint of 5 × 5 µm providing a resolution at a length scale similar to that of individual cells. Ti K-edge X-ray absorption near edge structure (XANES) spectra was acquired, in fluorescence mode and at room temperature, from regions where Ti was detected to determine its chemical state. In addition to the explanted human tissue samples, spectra were also collected in transmission mode from Ti standards, including Ti foil (metallic) and several containing Ti4+ ions in different coordination environments: cristobalite (fourfold), Na2TiSiO5 (fivefold) and anatase, rutile and CaTiSiO5 (all sixfold). Reference standards were compared with experimental measurements using Athena software in order to determine speciation [20].
3. Results
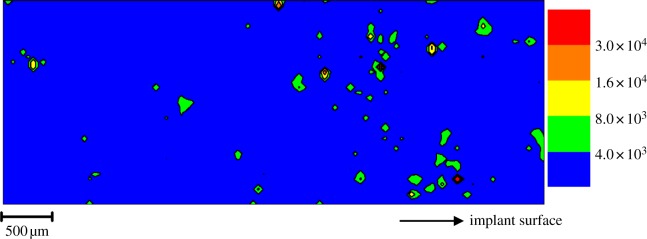
Synchrotron X-ray fluorescence mapping of thin (4–6 μm) sections of the inflamed skin tissue recovered from adjacent to percutaneous CPTi implants using a 5 μm X-ray beam demonstrated a widespread scattered distribution of Ti (figure 2 and electronic supplementary material, figure S2; data, http://dx.doi.org/10.5061/dryad.r5rh1). The distribution was heterogeneous in scale with fine fragments varying from 5 μm (highest resolution of detection) to 150 μm. Orientation of the tissue prior to snap-freezing allowed location of the distribution relative to the adjacent Ti implant surface and demonstrated that the Ti was scattered radially and distant to the tissue-implant interface (greater than 25 μm) and deep to the skin surface (greater than 2000 μm). Preliminary investigation of tissue sections, cut adjacent to the above mapped sections and stained with haematoxylin and eosin, by conventional optical microscopy revealed no discernible relationship between the underlying tissue architecture and Ti distribution (see electronic supplementary material figure S1). A comparison of the XANES spectra of the Ti standards with spectra taken from areas of high, medium and low relative Ti fluorescence intensity, as identified by X-ray fluorescence mapping, revealed that the Ti was present in a number of forms. Metallic particles were detected; however, there was a considerable distribution of oxide species (figure 3) with sixfold coordination states akin to anatase and rutile being identified. No Ti was detected by X-ray fluorescence mapping performed on the control tissue, which had no known Ti exposure and had been collected, stored and prepared according to an identical experimental protocol.
Figure 2.
An X-ray fluorescence map (5300 × 2000 µm) of the associated distribution of Ti in peri-implant soft tissue (6 µm thickness) taken at greater than 25 µm from a CPTi BAHA. Legend refers to increasing Ti fluorescence values. Eleven per cent of the tissue volume exhibited Ti levels in excess of the fitted background.
Figure 3.
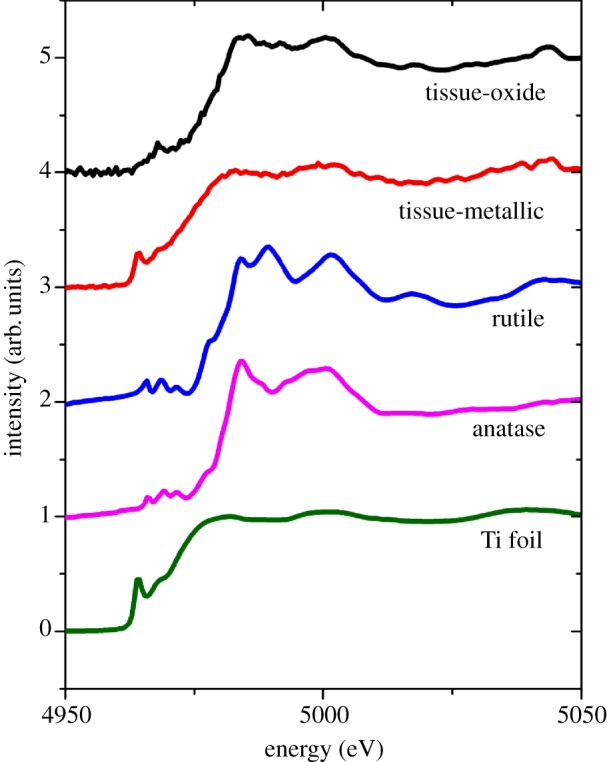
XANES spectra for Ti controls and experimental traces demonstrating both metallic and oxide species. ‘Tissue-metallic’ spectra were taken from the highest intensity feature (coloured red in figure 2) at 700 µm from implant surface.
4. Conclusions
Previously, investigators have used microscopy, optical spectroscopy and mass spectrometry techniques to confirm the existence of Ti in peri-prosthetic orthopaedic soft tissues [21,22]. The techniques have limited resolution or an inability to relate the quantity, size and chemical speciation of Ti to cellular and sub-cellular structures that would be required to inform biological investigations. Accordingly, synchrotron X-ray micro-focus spectroscopy was used in the current investigation and conferred the ability to detect trace distributions of Ti in thin tissue sections at a high resolution [23]. This investigation has demonstrated, for the first time, a scattered and heterogeneous distribution of Ti in inflamed tissues taken from around failing skin-penetrating Ti implants. The tissue taken was adjacent to a CPTi device that was not exposed to obvious macroscopic wear or loading in service. Furthermore, the location of the distributed Ti, which was deep with respect to the skin surface, suggests that wear processes are unlikely to be a major contributor. Debris from implant insertion is highly unlikely to lead to the observed widespread distribution of fine fragments of both oxide and metal. In the absence of obvious macroscopic wear or loading processes, we propose that the Ti in the tissue results from micro-motion and localized corrosion in surface crevices [24]. The mechanism also accounts for systemically distributed Ti that has been detected in serum in ionic states and bound to proteins [10]. Our results provide primary evidence that the expected passive surface of skin-penetrating Ti implants can deteriorate in clinical service, leading to the accumulation of Ti debris in the surrounding tissue. The quantities, size and speciation of Ti debris reflect stimuli previously demonstrated to be pro-inflammatory in nature. Our results emphasize the need to understand further both the physical and the chemical mechanisms leading to the dispersal of Ti species in tissue around implants and their potential to exacerbate inflammation. Similar processes are likely to contribute to the failure of other metal implants in soft tissues where macroscopic wear is not considered to be a risk.
Acknowledgements
Ethical approval was obtained through the United Kingdom National Research Ethics Service, prior to the commencement of the study (REC 08/H1203/128).
Owen Addison is a recipient of Clinician Scientist Award (NIHR/CS/010/001) from the National Institute for Health Research.
References
- 1.Cadosch D., Chan E., Gautschi O. P., Filqueira L. 2009. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening current concepts. J. Biomed. Mater. Res. A 15, 1252–1262 10.1002/jbm.a.32625 (doi:10.1002/jbm.a.32625) [DOI] [PubMed] [Google Scholar]
- 2.Bozic K. J., Kurtz S. M., Lau E., Ong K., Vail T. P., Berry D. G. 2009. The epidemiology of revision total hip arthroplasty in the USA. J. Bone Joint Surg. Am. 91, 128–133 10.2106/JBJS.H.00155 (doi:10.2106/JBJS.H.00155) [DOI] [PubMed] [Google Scholar]
- 3.Revell J. R. 2008. The combined role of wear particles, macrophages and lymphocytes in the loosening of total joint prostheses. J. R. Soc. Interface 6, 1263–1278 10.1098/rsif.2008.0142 (doi:10.1098/rsif.2008.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korovessis P., Petsinis G., Repanti M., Repantis T. 2006. Metallosis after contemporary metal on metal total hip arthroplasty. Five to nine year follow up. J. Bone Joint Surg. Am. 88, 1183–1191 10.2106/JBJS.D.02916 (doi:10.2106/JBJS.D.02916) [DOI] [PubMed] [Google Scholar]
- 5.Ingham E., Fisher J. 2000. Biological reactions to wear debris in total joint replacement. Proc. Inst. Mech. Eng. H 214, 21–37 10.1243/0954411001535219 (doi:10.1243/0954411001535219) [DOI] [PubMed] [Google Scholar]
- 6.COM/06/S1 2006. Biological effects of metal wear debris generated from hip implants: genotoxicity. London, UK: Medicines and Healthcare products Regulatory Agency; See http://www.mhra.gov.uk/home/groups/dts-bi/documents/websiteresources/con2024175.pdf [Google Scholar]
- 7.Kalorama Information 2009. Implant-based dental reconstruction: World dental implant and bone graft market, 3rd edn, Rockville, MD: Kalorama Information [Google Scholar]
- 8.Baldwin L., Hunt J. 2006. Host inflammatory response to NiCr, CoCr, and Ti in a soft tissue implantation model. J. Biomed. Mater. Res. A 79, 574–581 10.1002/jbm.a.30856 (doi:10.1002/jbm.a.30856) [DOI] [PubMed] [Google Scholar]
- 9.Hallam P., Haddad F., Cobb J. 2004. Pain in a well-fixed titanium hip implant. J. Bone Joint Surg. Br. 86, 27–30 [PubMed] [Google Scholar]
- 10.Nuevo-Ordóñez Y., Montes-Bayón M., Blanco-González E., Paz-Aparicio J., Raimundez J. D., Tejerina J. M., Peña M. A., Sanz-Medel A. 2011. Titanium release in serum of patients with different bone fixation implants and its interaction with serum biomolecules at physiological levels. Anal. Bioanal. Chem. 401, 2747–2754 10.1007/s00216-011-5232-8 (doi:10.1007/s00216-011-5232-8) [DOI] [PubMed] [Google Scholar]
- 11.Møller P., et al. 2010. Role of oxidative damage in toxicity of particulates. Free Radic. Res. 44, 1–46 10.3109/10715760903300691 (doi:10.3109/10715760903300691) [DOI] [PubMed] [Google Scholar]
- 12.Hallab N. J., Anderson S., Caicedo M., Brasher A., Mikecz K., Jacobs J. J. 2005. Effects of soluble metals on human peri-implant cells. J. Biomed. Mater. Res. A 74, 124–140 10.1002/jbm.a.30345 (doi:10.1002/jbm.a.30345) [DOI] [PubMed] [Google Scholar]
- 13.Cadosch D., Meagher J., Gautschi O. P., Filqeira L. 2009. Uptake and intracellular distribution of various metal ions in human monocyte-derived dendritic cells detected by Newport Green DCF diacetate ester. J. Neurosci. Methods 30, 182–187 10.1016/j.jneumeth.2008.12.008 (doi:10.1016/j.jneumeth.2008.12.008) [DOI] [PubMed] [Google Scholar]
- 14.Choi M. G., Koh H. S., Kluess D., O'Connor D., Mathur A., Truskey G. A., Rubin J., Zhou D. X., Sung K. L. 2005. Effects of titanium particle size on osteoblast function in vitro and in vivo. Proc. Natl Acad. Sci. USA 102, 4578–4583 10.1073/pnas.0500693102 (doi:10.1073/pnas.0500693102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cadosch D., Sutanto M., Chan E., Mhawi A., Gautschi O. P., von Katterfeld B., Simmen H. P., Filgueira L. 2010. Titanium uptake, induction of RANK-L expression, and enhanced proliferation of human T-lymphocytes. J. Orthop. Res. 28, 341–347 10.1002/jor.21013 (doi:10.1002/jor.21013) [DOI] [PubMed] [Google Scholar]
- 16.Okafor C. C., Haleem-Smith H., Laqueriere P., Manner P. A., Tuan R. S. 2006. Particulate endocytosis mediates biological responses of human mesenchymal stem cells to titanium wear debris. J. Orthop. Res. 24, 461–473 10.1002/jor.20075 (doi:10.1002/jor.20075) [DOI] [PubMed] [Google Scholar]
- 17.House J. W., Kutz J. W. 2007. Bone anchored hearing aids: incidence and management of post-operative complications. Otol. Neurotol. 28, 213–217 10.1097/MAO.0b013e31802c74c4 (doi:10.1097/MAO.0b013e31802c74c4) [DOI] [PubMed] [Google Scholar]
- 18.Gillett D., Fairley J. W., Chandrashaker T. S., Bean A., Gonzalez J. 2006. Bone-anchored hearing aids: results of the first eight years of a programme in a district general hospital, assessed by the Glasgow benefit inventory. J. Laryngol. Otol. 120, 537–542 10.1017/S0022215106001277 (doi:10.1017/S0022215106001277) [DOI] [PubMed] [Google Scholar]
- 19.Grant M. M., Monksfield P., Proops D., Brine M., Addison O., Sammons R. L., Matthews J. B., Reid A., Chapple I. L. C. 2010. Fluid exudates from inflamed bone anchored hearing aids demonstrate elevated levels of cytokines and biomarkers of tissue and bone metabolism. Otol. Neurotol. 31, 433–439 10.1097/MAO.0b013e3181cddb78 (doi:10.1097/MAO.0b013e3181cddb78) [DOI] [PubMed] [Google Scholar]
- 20.Ravel B., Newville M. 2005. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 12, 537–541 . (doi:10.1107/S0909049505012719) [DOI] [PubMed] [Google Scholar]
- 21.Ichinose S., Muneta T., Sekiya I., Itoh S., Aoki H., Tagami M. 2003. The study of metal ion release and cytotoxicity in Co-Cr-Mo and Ti-Al-V alloy in total knee prosthesis. J. Mat. Sci. 14, 79–86 10.1023/A:1021557605458 (doi:10.1023/A:1021557605458) [DOI] [PubMed] [Google Scholar]
- 22.Sarmiento-González A., Marchante-Gayón J. M., Tejerina-Lobo J. M., Paz-Jiménez J., Sanz-Medel A. 2008. High-resolution ICP-MS determination of Ti, V, Cr, Co, Ni, and Mo in human blood and urine of patients implanted with a hip or knee prosthesis. Anal. Bioanal. Chem. 391, 2583–2589 10.1007/s00216-008-2188-4 (doi:10.1007/s00216-008-2188-4) [DOI] [PubMed] [Google Scholar]
- 23.Mosselmans J. F. W., et al. 2009. I18: the microfocus spectroscopy beamline at the Diamond Light Source. J. Synchrotron Rad. 16, 1–7 10.1107/S0909049508028872 (doi:10.1107/S0909049508028872) [DOI] [PubMed] [Google Scholar]
- 24.Griess J. C. 1968. Crevice corrosion of titanium in aqueous salt solutions. Corrosion 24, 96–109 [Google Scholar]