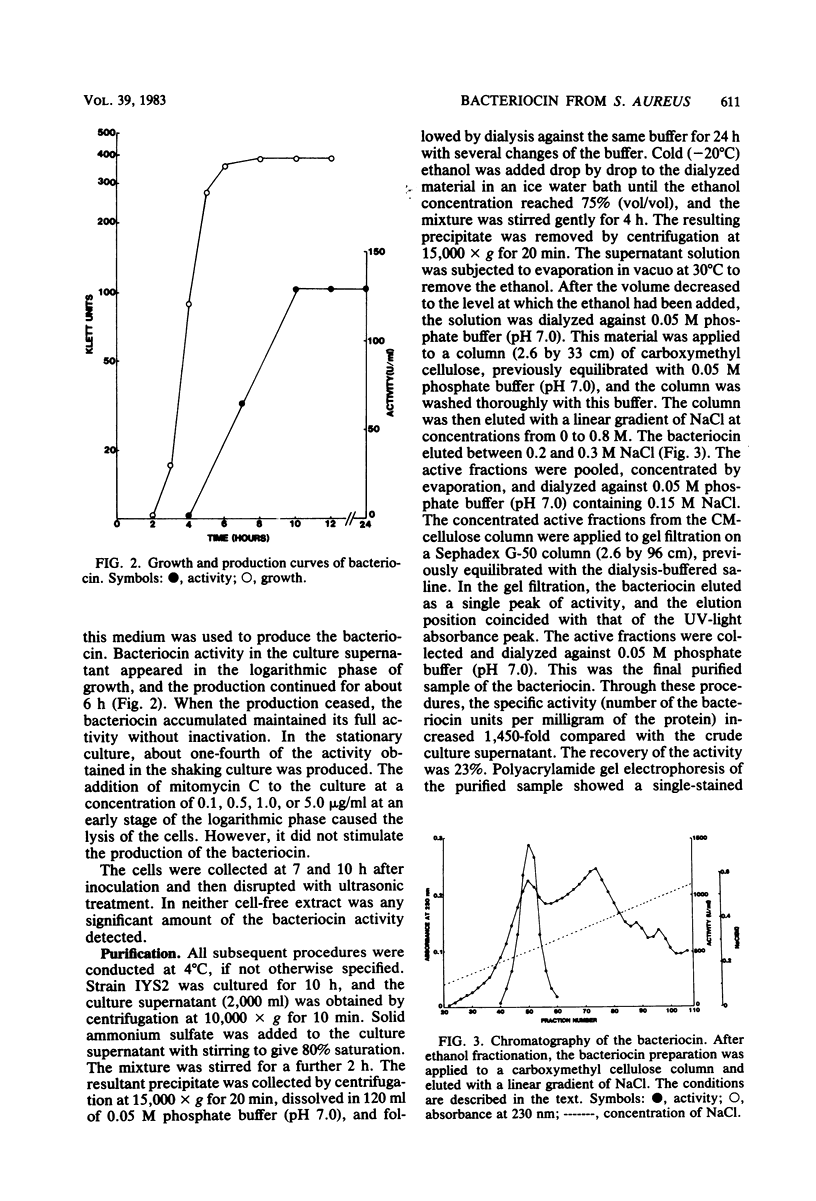
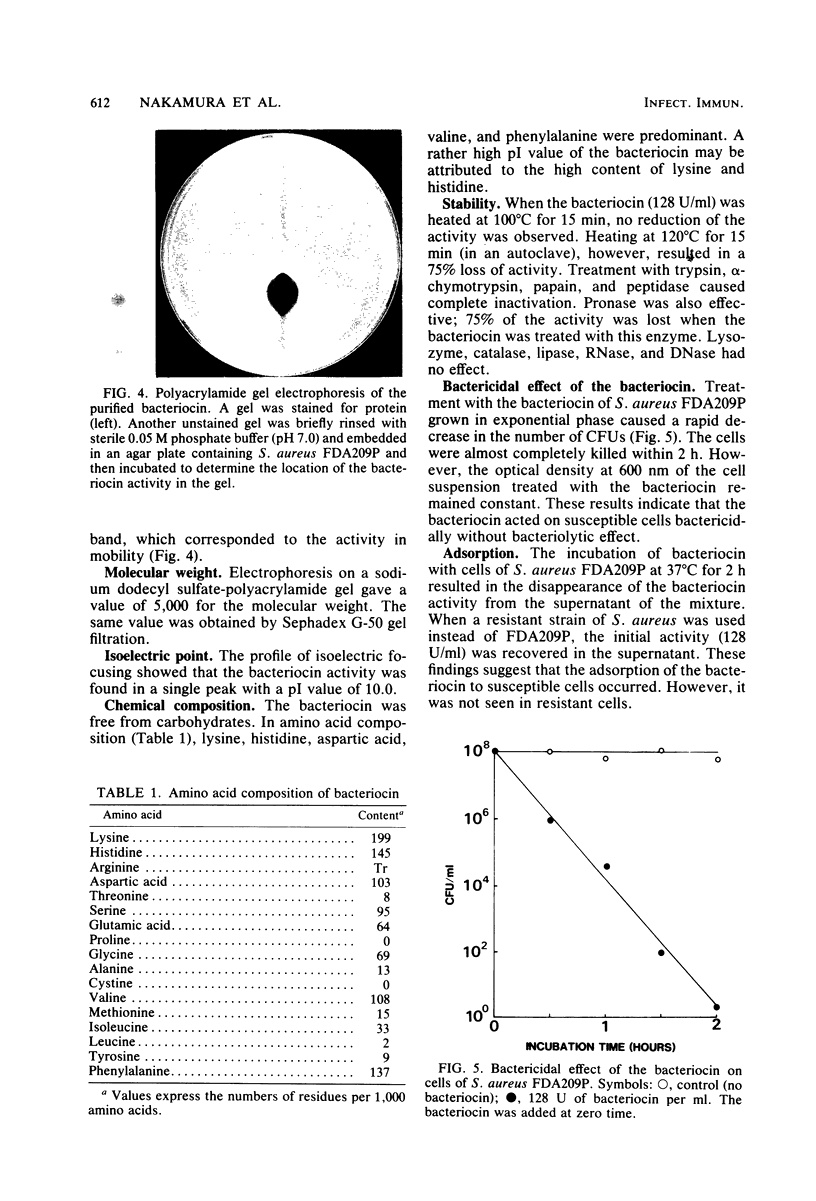
Abstract
Staphylococci from samples of human saliva were isolated on staphylococcal-selective agar plates. These strains were tested for the inhibition of the growth of Staphylococcus aureus FDA209P. The frequency of inhibitory strains among all of the staphylococcal isolates was 5.2%. Strain IYS2, which formed the biggest inhibitory zone against the growth of the indicator strain, was used as the producer of bacteriocin. IYS2 was identified to be S. aureus, based on its biological properties. The bacteriocin was purified by sequential procedures, including ammonium sulfate precipitation, fractionation with ethanol, ion-exchange chromatography, and gel filtration. Its molecular weight was determined to be 5,000. The isoelectric point was 10.0. In amino acid composition, lysine, histidine, aspartic acid, valine, and phenylalanine were predominant. The bacteriocin was heat stable but inactivated by proteases or peptidase. The bacteriocin had a bactericidal effect on susceptible cells. An analysis of the inhibitory spectrum among typical oral indigenous bacteria showed that Streptococcus salivarius, Propionibacterium acnes, Corynebacterium parvulum, and Actinomyces israelii were susceptible to the bacteriocin. Streptococcus mutans, Streptococcus sanguis, Streptococcus mitis, Actinomyces viscosus, Actinomyces naeslundii, Fusobacterium nucleatum, Bacterionema matruchotii, and Bacteroides melaninogenicus were resistant. The majority of S. aureus tested were susceptible, and all Staphylococcus epidermidis strains tested were resistant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aly R., Maibach H. I., Shinefield H. R., Mandel A., Strauss W. G. Bacterial interference among strains of Staphylococcus aureus in man. J Infect Dis. 1974 Jun;129(6):720–724. doi: 10.1093/infdis/129.6.720. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani A. S., Wannamaker L. W. Kinetic studies on the interaction of bacteriophage type 71 staphylococcal bacteriocin with susceptible bacteria. J Bacteriol. 1973 May;114(2):738–742. doi: 10.1128/jb.114.2.738-742.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura S., Makino T., Hayashi T. [Effect of mitomycin C on the production of staphylokinase, nuclease and hemolysin by Staphylococcus aureus]. Nihon Saikingaku Zasshi. 1970 Jun;25(5):316–320. doi: 10.3412/jsb.25.316. [DOI] [PubMed] [Google Scholar]
- Fujimura S., Nakamura T. Purification and properties of a bacteriocin-like substance (acnecin) of oral Propionibacterium acnes. Antimicrob Agents Chemother. 1978 Dec;14(6):893–898. doi: 10.1128/aac.14.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura S., Nakamura T. Sanguicin, a bacteriocin of oral Streptococcus sanguis. Antimicrob Agents Chemother. 1979 Sep;16(3):262–265. doi: 10.1128/aac.16.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujumura S., Makino T., Hayashi T. Stimulation of staphylokinase production by mitomycin C. Naturwissenschaften. 1970 Aug;57(8):394–395. doi: 10.1007/BF00599983. [DOI] [PubMed] [Google Scholar]
- Hale E. M., Hinsdill R. D. Biological activity of staphylococcin 162: bacteriocin from Staphylococcus aureus. Antimicrob Agents Chemother. 1975 Jan;7(1):74–81. doi: 10.1128/aac.7.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale E. M., Hinsdill R. D. Characterization of a bacteriocin from Staphylococcus aureus strain 462. Antimicrob Agents Chemother. 1973 Dec;4(6):634–640. doi: 10.1128/aac.4.6.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Ooshima T. Production and properties of bacteriocins (mutacins) from Streptococcus mutans. Arch Oral Biol. 1975 Oct;20(10):641–648. doi: 10.1016/0003-9969(75)90131-4. [DOI] [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Hsu C., Wiseman G. M. Antibacterial substances from staphylococci. Can J Microbiol. 1967 Aug;13(8):947–955. doi: 10.1139/m67-127. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D. Mode of action of a Staphylococcus epidermidis bacteriocin. Antimicrob Agents Chemother. 1972 Dec;2(6):456–463. doi: 10.1128/aac.2.6.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Vogels G. D., de Windt F. Production and purification of a Staphylococcus epidermidis bacteriocin. J Bacteriol. 1972 Oct;112(1):235–242. doi: 10.1128/jb.112.1.235-242.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nakamura T., Fujimura S., Obata N., Yamazaki N. Bacteriocin-like substance (melaninocin) from oral Bacteroides melaninogenicus. Infect Immun. 1981 Jan;31(1):28–32. doi: 10.1128/iai.31.1.28-32.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Suginaka Y., Obata T., Obata N., Yamazaki N. Bacteriocin-like activities of human dental plaque flora against oral anaerobic microorganisms. Bull Tokyo Dent Coll. 1977 Nov;18(4):217–219. [PubMed] [Google Scholar]
- Paul D., Slade H. D. Production and properties of an extracellular bacteriocin from Streptococcus mutans bacteriocidal for group A and other streptococci. Infect Immun. 1975 Dec;12(6):1375–1385. doi: 10.1128/iai.12.6.1375-1385.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A. H. Bacteriocinogeny and the properties of some bacteriocins of Streptococcus mutans. Arch Oral Biol. 1976;21(2):99–104. doi: 10.1016/0003-9969(76)90079-0. [DOI] [PubMed] [Google Scholar]
- Rogers A. H., van der Hoeven J. S., Mikx F. H. Effect of bacteriocin production by Streptococcus mutans on the plaque of gnotobiotic rats. Infect Immun. 1979 Mar;23(3):571–576. doi: 10.1128/iai.23.3.571-576.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWYER S. J., MACDONALD J. B., GIBBONS R. J. Biochemical characteristics of Bacteroides melaninogenicus. A study of thirty-one strains. Arch Oral Biol. 1962 Nov-Dec;7:685–691. doi: 10.1016/0003-9969(62)90117-6. [DOI] [PubMed] [Google Scholar]
- Sahl H. G., Brandis H. Production, purification and chemical properties of an antistaphylococcal agent produced by Staphylococcus epidermidis. J Gen Microbiol. 1981 Dec;127(2):377–384. doi: 10.1099/00221287-127-2-377. [DOI] [PubMed] [Google Scholar]
- Schlegel R., Slade H. D. Alteration of macromolecular synthesis and membrane permeability by a Streptococcus sanguis bacteriocin. J Gen Microbiol. 1974 Mar;81(1):275–277. doi: 10.1099/00221287-81-1-275. [DOI] [PubMed] [Google Scholar]
- Schlegel R., Slade H. D. Properties of a Streptococcus sanguis (group H) bacteriocin and its separation from the competence factor of transformation. J Bacteriol. 1973 Aug;115(2):655–661. doi: 10.1128/jb.115.2.655-661.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinefield H. R., Ribble J. C., Boris M. Bacterial interference between strains of Staphylococcus aureus, 1960 to 1970. Am J Dis Child. 1971 Feb;121(2):148–152. doi: 10.1001/archpedi.1971.02100130102013. [DOI] [PubMed] [Google Scholar]
- Vesterberg O., Wadström T., Vesterberg K., Svensson H., Malmgren B. Studies on extracellular PROTEINS FROM Staphylococcus aureus. I. Separation and characterization of enzymes and toxins by isoelectric focusing. Biochim Biophys Acta. 1967 Apr 11;133(3):435–445. doi: 10.1016/0005-2795(67)90547-8. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weerkamp A., Bongaerts-Larik L., Vogels G. D. Bacteriocins as factors in the in vitro interaction between oral streptococci in plaque. Infect Immun. 1977 Jun;16(3):773–780. doi: 10.1128/iai.16.3.773-780.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]





