Abstract
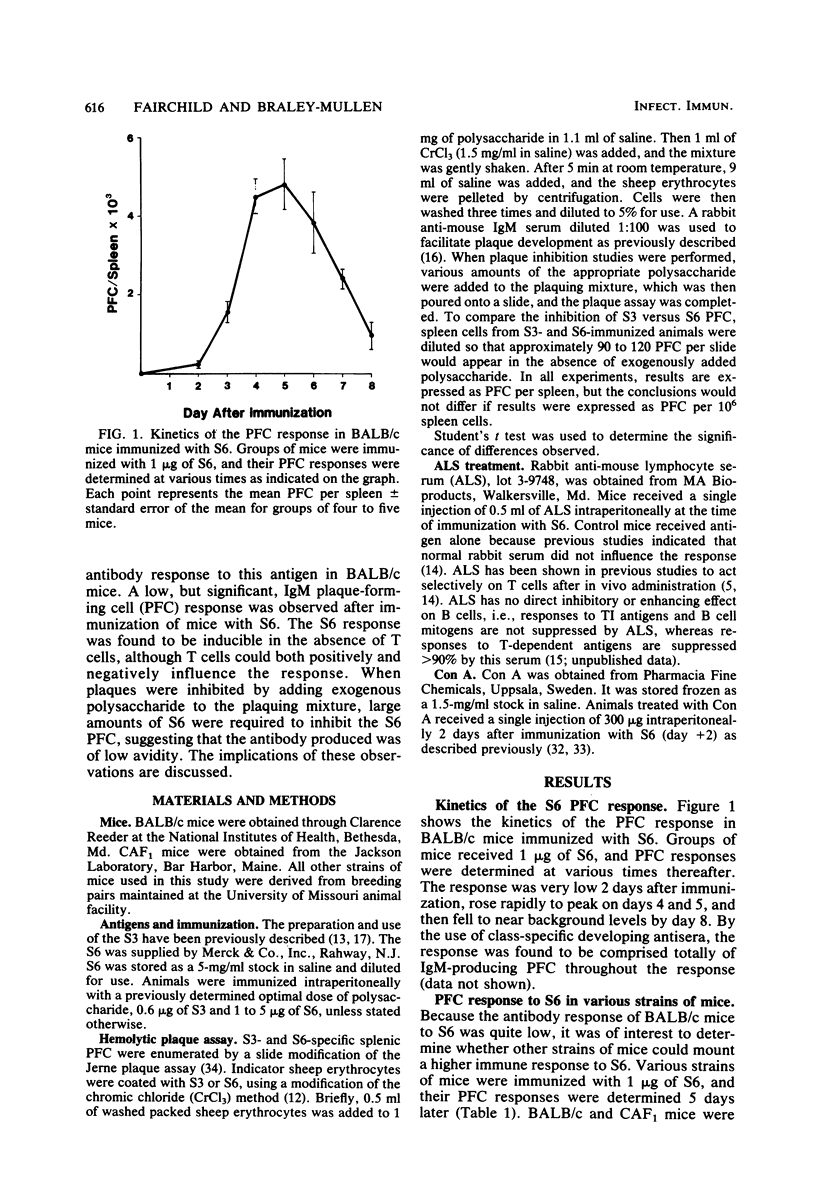
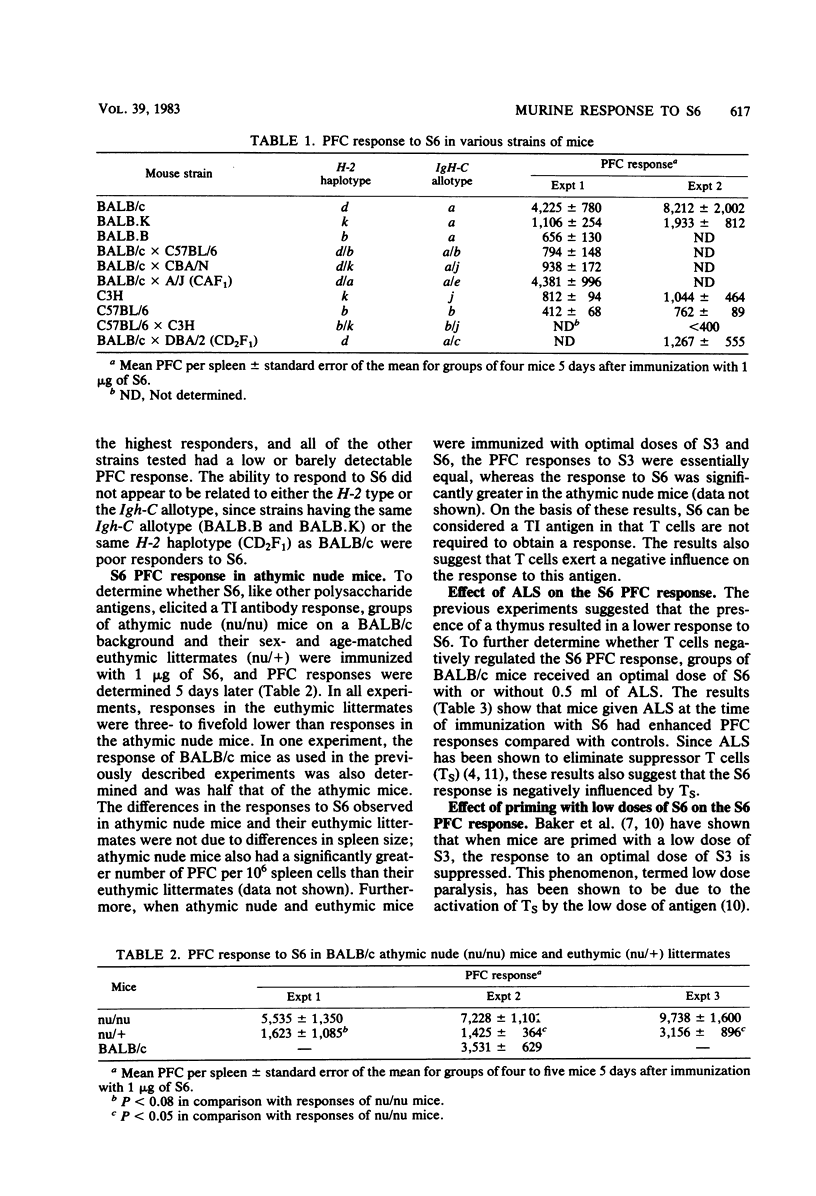
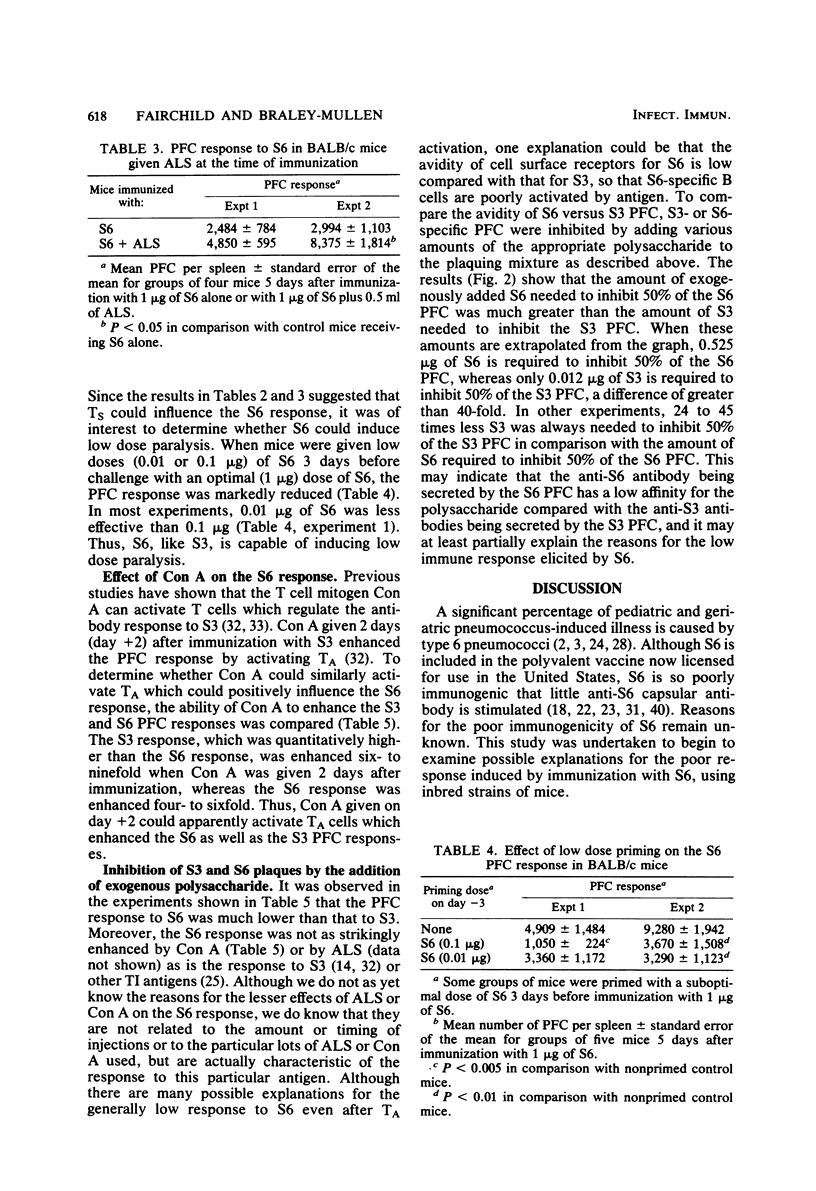
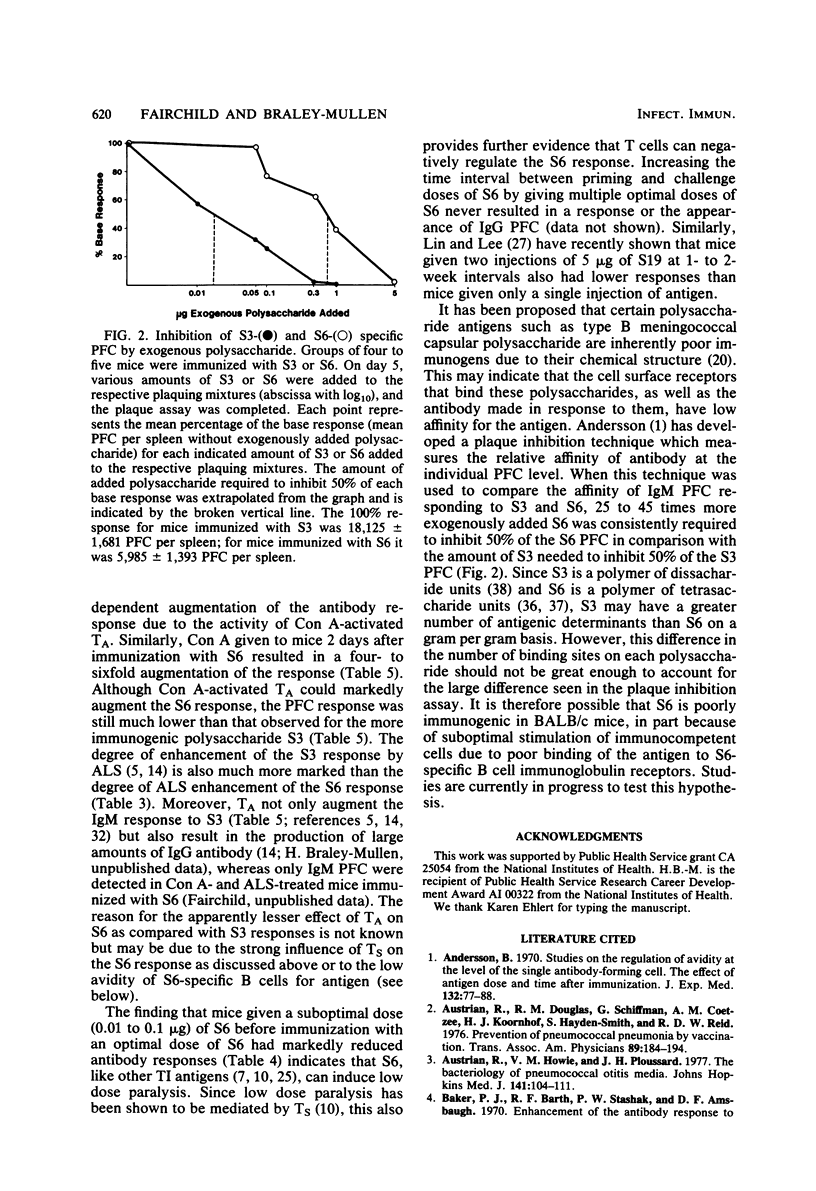
The antibody response to type 6 (Danish type 6A) pneumococcal capsular polysaccharide (S6) was determined in various strains of mice. S6 elicited a very low plaque-forming cell (PFC) response in most strains of mice. BALB/c mice responded better than most other strains, and the response to S6 was further characterized in BALB/c mice. Immunization with 1 to 5 micrograms of S6 induced a plaque-forming cell response of short duration which consisted totally of immunoglobulin M-producing PFC. Athymic nude mice produced three- to fivefold more PFC in response to S6 than their euthymic littermates, suggesting that thymus-derived (T) cells negatively influence the S6 response. Regulation of the response by T cells was further suggested by the ability of antilymphocyte serum to enhance the S6 PFC response and by the suppressed response induced by priming with a low dose of S6 3 days before immunization with an optimal dose of S6 (low dose paralysis). When concanavalin A was given 2 days after immunization with S6, the response was enhanced two- to sevenfold, suggesting that the response is also positively influenced by T amplifier cells. When the avidity of the antibody produced by S6-specific PFC was measured by a plaque inhibition test, the avidity of the anti-S6 antibody was found to be very low. These results suggest that S6 is a poor immunogen because the affinity of the S6-specific antibody and B cell surface receptors is low.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B. Studies on the regulation of avidity at the level of the single antibody-forming cell. The effect of antigen dose and time after immunization. J Exp Med. 1970 Jul 1;132(1):77–88. doi: 10.1084/jem.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austrian R., Douglas R. M., Schiffman G., Coetzee A. M., Koornhof H. J., Hayden-Smith S., Reid R. D. Prevention of pneumococcal pneumonia by vaccination. Trans Assoc Am Physicians. 1976;89:184–194. [PubMed] [Google Scholar]
- Austrian R., Howie V. M., Ploussard J. H. The bacteriology of pneumococcal otitis media. Johns Hopkins Med J. 1977 Sep;141(3):104–111. [PubMed] [Google Scholar]
- Baker P. H., Stashak P. W. Quantitative and qualitative studies on the primary antibody response to pneumococcal polysaccharides at ehe cellular level. J Immunol. 1969 Dec;103(6):1342–1348. [PubMed] [Google Scholar]
- Baker P. J., Barth R. F., Stashak P. W., Amsbaugh D. F. Enhancement of the antibody response to type 3 pneumococcal polysaccharide in mice treated with antilymphocyte serum. J Immunol. 1970 May;104(5):1313–1315. [PubMed] [Google Scholar]
- Baker P. J., Reed N. D., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. I. Nature of regulatory cells. J Exp Med. 1973 Jun 1;137(6):1431–1441. doi: 10.1084/jem.137.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B., Barth R. F. Evidence for the existence of two functionally distinct types of cells which regulate the antibody response to type 3 pneumococcal polysaccharide. J Immunol. 1970 Dec;105(6):1581–1583. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. II. Studies on the relative rate of antibody synthesis and release by antibody-producing cells. Immunology. 1971 Apr;20(4):481–492. [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. II. Mode of action of thymic-derived suppressor cells. J Immunol. 1974 Jan;112(1):404–409. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. IV. Role of suppressor T cells in the development of low-dose paralysis. J Immunol. 1974 Jun;112(6):2020–2027. [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Prescott B. Use of erythrocytes sensitized with purified pneumococcal polysaccharides for the assay of antibody and antibody-producing cells. Appl Microbiol. 1969 Mar;17(3):422–426. doi: 10.1128/am.17.3.422-426.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H., Chase G. R., Sharp G. C., Freeman M. J. Genetic control of the immune response of mice to type III pneumococcal polysaccharide. Cell Immunol. 1974 Feb;10(2):280–286. doi: 10.1016/0008-8749(74)90119-1. [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H. Regulatory role of T cells in IgG antibody formation and immune memory to type III Pneumococcal polysaccharide. J Immunol. 1974 Dec;113(6):1909–1920. [PubMed] [Google Scholar]
- Braley-Mullen H. Secondary IgG responses to type 3 pneumococcal polysaccharide. III. T cell requirement for development of B memory cells. Eur J Immunol. 1977 Nov;7(11):775–781. doi: 10.1002/eji.1830071106. [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H., Sharp G. C. Conversion of type III pneumococcal polysaccharide low responders to high responders by immunization with a thymus-dependent form of antigen. Cell Immunol. 1974 Apr;12(1):49–60. doi: 10.1016/0008-8749(74)90055-0. [DOI] [PubMed] [Google Scholar]
- Braley H. C., Freeman M. J. Strain differences in the antibody plaque-forming cell responses of inbred mice to pneumococcal polysaccharide. Cell Immunol. 1971 Feb;2(1):73–81. doi: 10.1016/0008-8749(71)90026-8. [DOI] [PubMed] [Google Scholar]
- Cowan M. J., Ammann A. J., Wara D. W., Howie V. M., Schultz L., Doyle N., Kaplan M. Pneumococcal polysaccharide immunization in infants and children. Pediatrics. 1978 Nov;62(5):721–727. [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J Immunol. 1981 Sep;127(3):1011–1018. [PubMed] [Google Scholar]
- Jones J. M., Amsbaugh D. F., Stashak P. W., Prescott B., Baker P. J., Alling D. W. Kinetics of the antibody response to type III pneumococcal polysaccharide. I. Evidence that suppressor cells function by inhibiting the recruitment and proliferation of antibody-producing cells. J Immunol. 1976 Mar;116(3):647–656. [PubMed] [Google Scholar]
- Karma P., Luotonen J., Timonen M., Pöntynen S., Pukander J., Herva E., Grönroos P., Leinonen M., Sibakov M., Mäkelä P. H. Efficacy of pneumococcal vaccination against recurrent otitis media. Preliminary results of a field trial in Finland. Ann Otol Rhinol Laryngol Suppl. 1980 May-Jun;89(3 Pt 2):357–362. doi: 10.1177/00034894800890s383. [DOI] [PubMed] [Google Scholar]
- Klein J. O. The epidemiology of pneumococcal disease in infants and children. Rev Infect Dis. 1981 Mar-Apr;3(2):246–253. doi: 10.1093/clinids/3.2.246. [DOI] [PubMed] [Google Scholar]
- Lake J. P., Reed N. D. Regulation of the immune response to polyvinylpyrrolidone:effect of antilymphocyte serum on the response of normal and nude mice. Cell Immunol. 1976 Feb;21(2):364–370. doi: 10.1016/0008-8749(76)90065-4. [DOI] [PubMed] [Google Scholar]
- Lee C. J., Malik F. G., Robbins J. B. The regulation of the immune response of mice to Haemophilus influenzae type b capsular polysaccharide. Immunology. 1978 Jan;34(1):149–156. [PMC free article] [PubMed] [Google Scholar]
- Lin K. T., Lee C. J. Immune response of neonates to pneumococcal polysaccharide-protein conjugate. Immunology. 1982 Jun;46(2):333–342. [PMC free article] [PubMed] [Google Scholar]
- Luotonen J., Herva E., Karma P., Timonen M., Leinonen M., Mäkelä P. H. The bacteriology of acute otitis media in children with special reference to Streptococcus pneumoniae as studied by bacteriological and antigen detection methods. Scand J Infect Dis. 1981;13(3):177–183. doi: 10.3109/inf.1981.13.issue-3.04. [DOI] [PubMed] [Google Scholar]
- Markham R. B., Reed N. D., Stashak P. W., Prescott B., Amsbaugh D. F., Baker P. J. Effect of concanavalin A on lymphocyte interactions involved in the antibody response to type III pneumococcal polysaccharide. II. Ability of suppressor T cells to act on both B cells and amplified T cells to limit the magnitude of the antibody response. J Immunol. 1977 Sep;119(3):1163–1168. [PubMed] [Google Scholar]
- Markham R. B., Stashak P. W., Prescott B., Amsbaugh D. F., Baker P. J. Effect of concanavalin A on lymphocyte interactions involved in the antibody response to type III pneumococcal polysaccharide I. Comparison of the suppression induced by con A and low dose paralysis. J Immunol. 1977 Mar;118(3):952–956. [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson M. A., Kruss D. M., Wasil R. E., Metzger W. I. Capsular types and outcome of bacteremic pneumococcal disease in the antibiotic era. Arch Intern Med. 1974 Sep;134(3):505–510. [PubMed] [Google Scholar]
- Mäkelä O., Pasanen V. J., Sarvas H., Lehtonen M. A gene of the immunoglobulin H-chain cluster controls the murine antibody response to pneumococcal polysaccharide type 14. Scand J Immunol. 1980;12(2):155–158. doi: 10.1111/j.1365-3083.1980.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Sibakov M., Herva E., Henrichsen J., Luotonen J., Timonen M., Leinonen M., Koskela M., Pukander J., Pöntynen S. Pneumococcal vaccine and otitis media. Lancet. 1980 Sep 13;2(8194):547–551. doi: 10.1016/s0140-6736(80)91989-3. [DOI] [PubMed] [Google Scholar]
- Weibel R. E., Vella P. P., McLean A. A., Woodhour A. F., Davidson W. L., Hilleman M. R. Studies in human subjects of polyvalent pneumococcal vaccines (39894). Proc Soc Exp Biol Med. 1977 Oct;156(1):144–150. doi: 10.3181/00379727-156-39894. [DOI] [PubMed] [Google Scholar]



