Abstract
Previously we reported the novel observation that astrocytes ensheath the persistent hyaloid artery, both in the Nuc1 spontaneous mutant rat, and in human PFV (persistent fetal vasculature) disease (Developmental Dynamics 234:36–47, 2005). We now show that astrocytes isolated from both optic nerve and retina of Nuc1 rats migrate faster than wild type astrocytes. Aquaporin 4 (AQP4), the major water channel in astrocytes, has been shown to be important in astrocyte migration. We demonstrate that AQP4 expression is elevated in the astrocytes in PFV conditions, and we hypothesize that this causes the cells to migrate abnormally into the vitreous where they ensheath the hyaloid artery. This abnormal association of astrocytes with the hyaloid artery may impede the normal macrophage-mediated remodeling and regression of the hyaloid system.
Keywords: Aquaporin-4 (AQP4), Astrocytes, βA3/A1-crystallin, Hyaloid vascular system, Migration, Optic Nerve and Retina
INTRODUCTION
Tissue regression is an essential feature of normal development throughout the animal kingdom (Ellis et al., 1991). With respect to the eye, this process is most obvious in the regression of the hyaloid vasculature. Formed during early fetal development, this transient network of intraocular vessels arises from the optic nerve head, extends through the vitreous, and surrounds the developing lens. When vessels begin to appear in the retina, the fetal vasculature normally regresses and disappears completely, prior to birth in humans and within the first few weeks after birth in rodents. This results in an optically clear path between the cornea and the retina (Ito and Yoshioka, 1999). One of the congenital, developmental disorders of the eye, persistent fetal vasculature (PFV), results from the complete or partial failure of this vascular regression (Goldberg, 1997).
We have characterized a spontaneous mutant rat, Nuc1, resulting from a mutation in the βA3/A1-crystallin gene (Sinha et al., 2008). βA3/A1-crystallin is an abundant structural protein of the lens, where it contributes to lens refractivity. It is also present in certain other cells, including astrocytes where its function is unknown (Parthasarathy et al., 2011). The Nuc1 mutation causes lens opacity (cataract) and developmental defects in the remodeling of the retina related to abnormalities in astrocytes (Sinha et al., 2008). The Nuc1 rat also exhibits persistence of the fetal vasculature, similar to the human disease (Zhang et al., 2005). The molecular and cellular mechanisms responsible for the normal regression of the hyaloid vessels are unknown. A possible role for macrophages in the regression process has been postulated (Latker and Kuwabara, 1981; Lang and Bishop, 1993; Lang et al., 1994; Taniguchi et al., 1999; Hose et al., 2005; Lobov et al., 2005).
We have previously made the novel observation that astrocytes, both in the brain and retina, express βA3/A1-crystallin (Sinha et al., 2008). We also showed that in the Nuc1 rat, and in human samples from PFV patients (Zhang et al., 2005), astrocytes abnormally ensheath the hyaloid artery. Therefore, we postulated that these glial cells would be involved in persistence of the hyaloid artery. Although not specifically noted in the published accounts, several other mouse models that exhibit PFV appear to have astrocytes associated with the persistent hyaloid artery. For example, knockout mice lacking both type XV and type XVIII collagen (Hurskainen et al., 2005) and transgenic mice with lens-specific expression of PDGF-A (Reneker and Overbeek, 1996) show presence of astrocytes in association with the retained hyaloid artery.
To understand PFV development, we must first find how the Nuc1 mutation stimulates passage of astrocytes out of the retina. Therefore, we used an in-vitro migration assay to compare Nuc1 and wild type astrocytes. Nuc1 astrocytes moved significantly faster than wild type cells. We also studied AQP4, the major water-selective channel in astrocytes (Nielsen et al., 1997; Rash et al., 2004; Yool, 2007), since it has been shown to be important in astrocyte cell migration (Saadoun et al., 2005; Auguste et al., 2007). In addition we studied macrophages, since they might be involved in vascular regression (Latker and Kuwabara, 1981; Lang and Bishop, 1993; Lang et al., 1994; Taniguchi et al., 1999; Hose et al., 2005; Lobov et al., 2005). Findings reported here allow us to hypothesize that faster migration rates, probably dependent on AQP upregulation, would cause glial ensheathment of the hyaloid vessels.
MATERIALS AND METHODS
Specimen Preparation
Animal experiments were performed using Nuc1 (Sinha et al., 2005) and wild type Sprague Dawley rats in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press) and were approved by the Animal Care and Use Committee, Johns Hopkins University. Experiments involving human tissue conformed to the guidelines set forth in the Declaration of Helsinki for the use of human tissue in research. Paraffin sections of 11 eyes with PFV (5 reported in a previous study; Zhang et al., 2005) were obtained from the W. R. Green Eye Pathology Laboratory, Wilmer Eye Institute. Human samples were fixed in 10% formalin and embedded in paraffin; 5μm sections were cut and either stained with hematoxylin and eosin (H&E) or processed for immunofluorescence.
Real time RT- PCR
Real time RT- PCR was used to determine the expression of AQP4 in wild type and Nuc1 homozygote astrocytes in culture. Total RNA was reverse transcribed using Superscript II Reverse Transcriptase (Invitrogen, Carlsbad, CA). For Real-time PCR analysis, Light Cycler FastStart DNA Master SYBR Green kit (Roche Diagnostics, Indianapolis, IN) and the Light Cycler 480 from Roche Diagnostics were used. Hypoxanthine PhosphoRibosyl Transferase (HPRT) was used as an internal control. SYBR green was incorporated into the reaction mixture to permit measurement of product. The integrity of the PCR product was verified by melting curve analysis. Real-time PCR values were determined by reference to a standard curve that was generated by Real-time PCR amplification of serially diluted cDNAs using AQP4 and HPRT primers. Values obtained for levels of AQP4 were normalized to the levels of HPRT mRNA. Primer sets for rat AQP4 and rat HPRT were taken from published sequences using Primer3 software and are: forward: TGT GAT TCC AAA CGG ACT GA and reverse: CCA CGT CAG GAC AGA AGA CA for AQP4, and forward: CAG GCC AGA CTT TGT TGG AT and reverse: GGC CAC AGG ACT AGA ACG TC for HPRT.
SDS-PAGE and Western Blot Analysis
Astrocytes from primary cultures, were rinsed in PBS, and homogenized in M-PER (Thermo Fisher Scientific, Rockford, IL) with 1% 0.5M EDTA and 1% of a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Samples were incubated on an end over end shaker at 4°C for 30 minutes followed by centrifugation at 13,000 x g for 15 minutes. Protein quantification was performed using the Quick Start Bradford Protein Assay (Bio-Rad Laboratories, Hercules, CA). Approximately 25 μg of protein from the supernatant was mixed with 2x LDS sample buffer (Invitrogen, Carlsbad, CA) and then heated in a boiling water bath for 3 minutes. Each sample was loaded onto a 4–12% Bis-Tris Nu-PAGE gel and run with MES Buffer (Invitrogen, Carlsbad, CA). The gels were stained with colloidal Coomassie brilliant blue. For western blotting, proteins were transferred to nitrocellulose membranes (Invitrogen, Carlsbad, CA) for 90 minutes and then blocked with 3% BSA in TTBS (Tris buffered saline, 0.1% Tween-20) overnight at 4°C. Blots were incubated with either a polyclonal Anti-Aquaporin-4 antibody ((Millipore, Temecula, CA) at a dilution of 1:1000 for 1 hour at room temperature followed by 4 washes of 10 minutes each. As a loading control, parallel samples were probed with a monoclonal anti-actin (1:1000; Sigma Aldrich, St. Louis, MO). Blots were incubated with HRP-conjugated secondary antibodies (Kirkegaard and Perry Laboratories, Gaithersburg, MD) for 1 hour at room temperature at a dilution of 1:20,000 followed by 4 washes of 10 minutes each. ECL western blotting detection reagents (GE Healthcare, Piscataway, NJ) were used for detection with varying exposure times.
Immunofluorescence
The primary antibodies used in this study included mouse monoclonal antibodies to Aquaporin-4 (gift from Dr. Peter Nemeth, University of Pecs, Hungary; 1:200) (Nagy et al., 2002), ED1 (Serotec, Raleigh, NC; 1:300) and rabbit polyclonal antibodies to GFAP (glial fibrillary acidic protein) (DAKO, Carpinteria, CA; 1:1000). Blood vessels were labeled with isolectin-B4 (Vector Labs, Burlingame, CA; 1:200). Frozen sections were incubated with primary antibodies overnight at 4°C, washed with PBS and incubated with goat anti-rabbit/anti-mouse secondary antibodies conjugated to either Cy-2 or Cy-3 (Jackson ImmunoRes, West Grove, PA, 1:200) for 1 hour at room temperature. The sections were finally counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA) and mounted with DAKO fluorescent mounting medium (DAKO, Carpinteria, CA). Fluorescent digital images were taken with a Leica 6000 fluorescent microscope. Confocal microscopy was done on a Zeiss LSM 510.
Electron Microscopy
Eyes from wild type and Nuc1 homozygous rats were fixed and prepared as previously described (Lo et al., 2000; Sinha et al., 2005). Sections (1 μm) were cut with a glass knife, stained with 1% toluidine blue and examined with a light microscope to select areas of interest. Ultrathin sections (80 nm) were cut with a diamond knife, stained with 5% Uranyl acetate followed by Reynold’s lead citrate, and examined in a JEOL 1200EX electron microscope.
Astrocyte Cell culture
Brain astrocytes from P (postnatal) day 2 wild type and Nuc1 homozygous rats were cultured following the method described earlier (Sinha et al., 2008) and used only for quantification of AQP4 expression. For the migration and proliferation studies, astrocytes were isolated from the optic nerve and the retina of P2 wild type and Nuc1 rat pups. In brief, the retina and optic nerve were each dissected from wild type and Nuc1 mutant newborn rats (P2) and enzymatically dissociated using papain solution, as previously described (Barres et al., 1992; Huaiyu and Barres, 1999). Cells were resuspended in DMEM:F12 (1:1) medium, containing 10% heat-inactivated FBS, L-glutamine (2 mM), HEPES buffer (10 mM), sodium pyruvate (1 mM), MEM essential vitamin mixture (1% solution), MEM non-essential aminoacid (1%) solution, 50 IU/ml penicillin and 50 μg/ml streptomycin at 37°C in a humidified 5% CO2 atmosphere. Composition of the cultures was estimated by immunofluorescence staining using DAPI to stain nuclei and rabbit polyclonal antibody anti-GFAP (Dako, Carpinteria, CA), diluted 1:200 to label astrocytes. About 90% of cells in these cultures expressed this marker of astrocytes and astrocytic precursors.
Migration assay
Migration of wild type and Nuc1 astrocytes was analyzed by transwell assay in 24 well plates, using 6.5-mm-diameter Falcon cell culture inserts (8 μm pore size; Becton Dickinson, Franklin Lakes, NJ) precoated with gelatin (0.01%, 1hr, RT). Cells cultured from Nuc1 or wild type retina or optic nerve were trypsinized and resuspended in serum-free medium in the upper chamber of the filter (75,000 cells in 500 μL). 800 μL of medium supplemented with 10% FBS as chemoattractant were added to the lower chamber. After overnight incubation (16h), cells remaining on the upper surface of the filter were removed with a cotton swab. Cells that had migrated to the lower surface were fixed with ethanol (70% v/v) for 5 minutes and nuclei were stained with hematoxylin, destained and photographed. The data are the mean (± standard error of the mean, SEM) of the number of nuclei counted in 8 different High Power Fields (HPF) from three independent experiments.
Cell proliferation assay
Cell proliferation was determined by MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) colorimetric assay performed in 96-well plates (Cory et al., 1991). Cells were seeded at 4 × 103 per well in 10% FBS medium. Cell numbers were determined after 3, 5, and 7 days of incubation. At each time point, 20 μL of MTS solution were added to the well. After 1 h incubation at 37°C the absorbance, which is proportional to the number of viable cells, was measured at λ= 490 nm. Experiments were done in triplicate.
RESULTS
Astrocytes isolated from retina or optic nerve of Nuc1 rats exhibit increased cell migration
We performed transwell migration assays on cells isolated from the developing retina or optic nerve of P2 rat pups. Enrichment of astrocytes in cultured cells isolated from optic nerve and retina was determined by immunofluorescence staining using GFAP as marker for astrocytes. Astrocyte cultures from optic nerve and retina were normally 90% pure, as shown in Figure 1 (top panel). The transwell migration assay showed that cells isolated from Nuc1 retinas displayed a 3-fold increase in migration rate compared to cells obtained from wild type rats (Figure 1, bottom panel). However, only a 2-fold increase was observed in Nuc1 cells obtained from the optic nerve compared to wild type cells (Figure 1, bottom panel).
Figure 1.
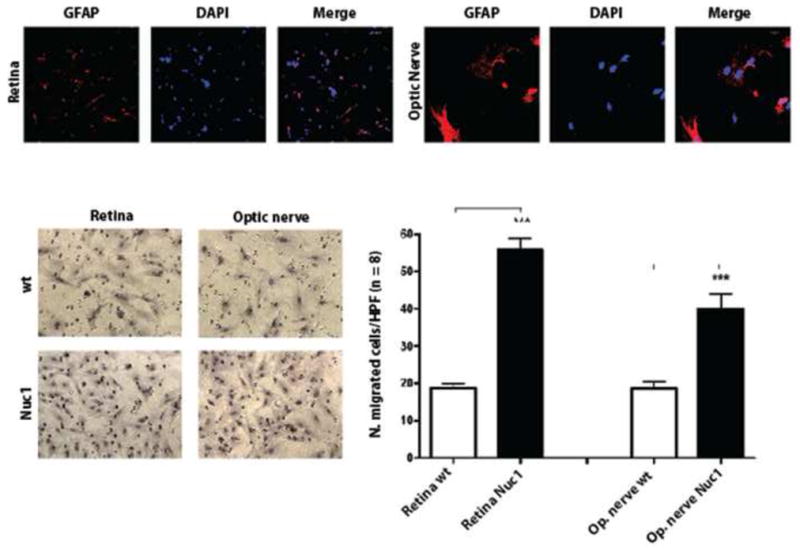
Astrocytes were cultured from optic nerve and retina isolated from P2 rats and confirmed to be GFAP-positive (Top panel). For transwell migration assay, cultured astrocytes were incubated for 16 h in the upper chamber of the filter, which was precoated with gelatin. Filters were then stained with hematoxylin, photographed and the cells migrating to the lower surface of the filter were quantified by cell counting. The Bottom panel, Left shows representative images of cells on the lower surface of membranes after incubation. The data shown in the Bottom panel, Right are the mean (± SEM) of the number of cells counted in 8 different High Power Fields (HPF) from three independent experiments. P-values were calculated between Nuc1 mutant vs. wild type cells using Student t-test (*** P < 0.0002).
Retina or optic nerve astrocytes isolated from Nuc1 rats exhibit increased proliferation
In addition, our data also indicate an increase in the proliferation rate of optic nerve and retinal astrocytes of the Nuc1 mutant rats compared to wild type, as determined by MTS assay (Figure 2). Astrocyte proliferation in both optic nerve and retina cultures showed a significant increase in cell numbers in the Nuc1 mutant compared to wild type by day 5, with further doubling of cell numbers by day 7 in culture. The increase in cell proliferation of Nuc1 astrocytes results from increased cell division and not decreased apoptosis. The data indicate that the increase in Nuc1 astrocyte cell numbers was greater for cells from the optic nerve than from retina (Figure 2).
Figure 2.
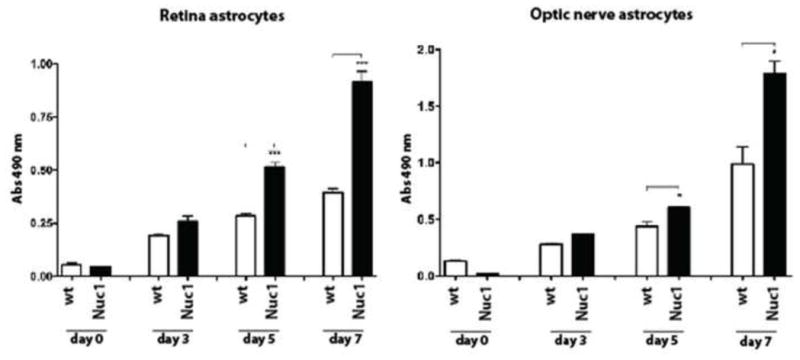
To measure proliferation, the MTS assay was performed at selected times on cultures of astrocytes from retina and optic nerve of wild type and Nuc1 rats. The data represent the mean value of the absorbance at λ = 490 nm, which is proportional to the number of viable cells. Experiments were done in triplicate. P-values were calculated between Nuc1 mutant and wild type cells using Student t-test (* P = 0.03, *** P <0.0007).
AQP4 expression by astrocytes that surround the persistent hyaloid artery in the Nuc1 rat
Astrocytes migrate to the periphery of the retina by postnatal day 8 (P8) in rodents, about the time when involution of the hyaloid artery starts. At P20, the normal regression of the hyaloid artery in the wild type rat was nearly complete with only a small cluster of astrocytes surrounding the residual stump (Figure 3A–C). AQP4 (arrows in Figure 3A) and GFAP (arrows in Figure 3B) expression was confined to the optic nerve head. In the P20 Nuc1 homozygote however, the hyaloid artery was persistent and surrounded by a multi-layer of AQP4-positive astrocytes (Figure 3D–F). It appears that in Nuc1 a higher proportion of astrocytes showed co-localization of AQP4 and GFAP (Figure 3) although the very small number of astrocytes in wild type makes definitive comparison impossible. To show unambiguously that AQP4 expression is in the astrocytes and not in the blood vessels, sections were also double-labeled with isolectin B4, to label endothelial cells (green), and AQP4 (red). There was no overlap of the two markers (Supplemental Figure 1). In order to assess the structure of the retained fetal vasculature in the Nuc1 rat, transmission electron microscopy studies were performed (Supplemental Figure 2). By P14, in the normal rat, regression was well advanced (Supplemental Figure 2B). In contrast, in the Nuc1 eye, the fetal vasculature remained structurally intact and normal in appearance, even at P29 (Supplemental Figure 2C). This is well after the time of complete regression in the normal rat eye.
Figure 3.
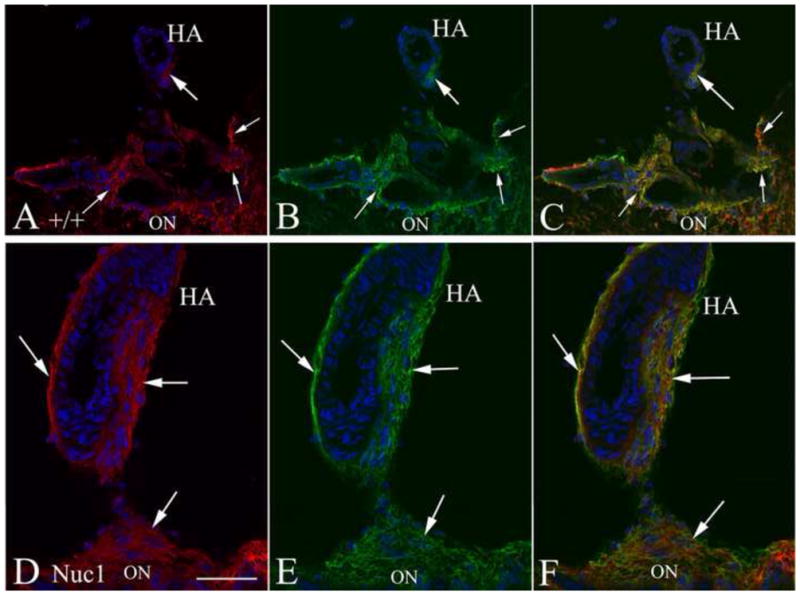
Immunohistochemistry of P20 optic nerve head (ON) and hyaloid artery (HA). Top 3 panels (A–C) are from wild type Sprague-Dawley rats showing only a remnant of the hyaloid artery (HA) remaining. Staining for both AQP4 (A, red) and GFAP (B, green) is evident in the small cluster of cells around the remnant of the HA (thick arrows) and some cells surrounding the vasculature (thin arrows) at the ON head. The merge (C) shows that some astrocytes in the ON head are AQP4-positive (arrows). Lower panels (D–F) show the Nuc1 homozygote with large intact hyaloid artery. Robust staining for both AQP4 (D) and GFAP (E) is present both at the surface of the optic nerve head and surrounding the hyaloid artery (arrows). The merged image (F) indicates the presence of a dense network of AQP4-positive astrocytes ensheathing the hyaloid artery. Nuclei in all panels are labeled with DAPI (blue). Scale bar=50 μm, n=5 wild type, 5 Nuc1 homozygote.
As noted above, macrophages may play a significant role in the normal regression of the hyaloid vasculature. In the 20 day old wild type rat, ED1-positive macrophages closely surround the stump of the regressing hyaloid artery (Figure 4A). In contrast, in the 20 day old Nuc1 eye, the hyaloid artery shows no sign of involution and there are more macrophages along the persistent hyaloid artery (Figure 4B). Our data suggest that the number of macrophages is proportional to the length of the persistent artery.
Figure 4.
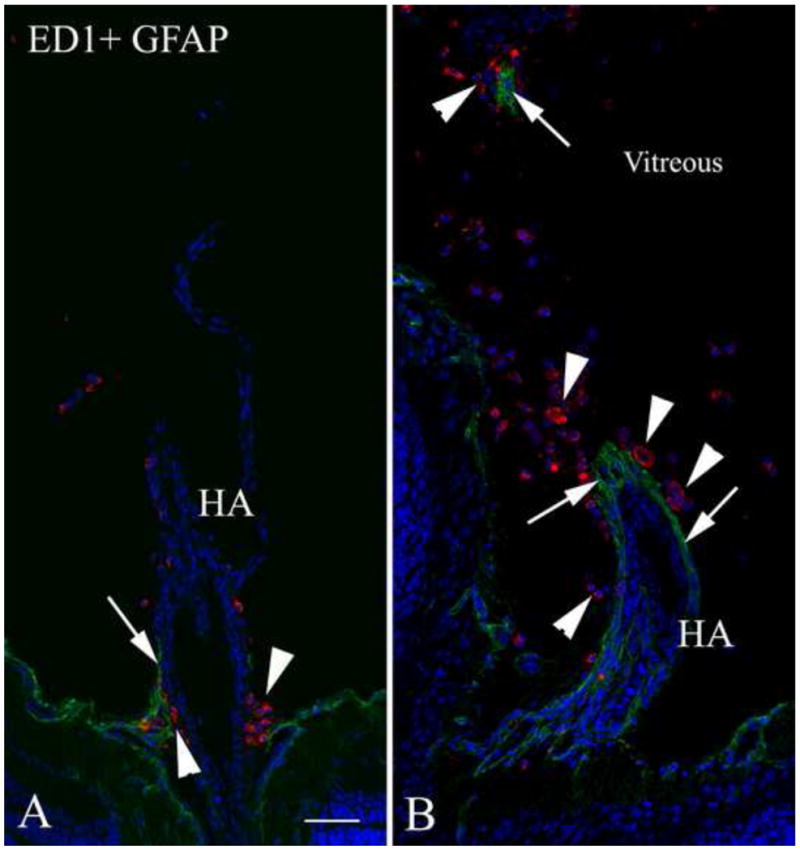
Double labeling with the macrophage marker ED1 (red) and GFAP (green) in 20-day-old wild type and Nuc1 rat eyes. A. In the wild type rat, some GFAP-positive cells (arrow) are seen at the base of the regressing hyaloid artery (HA). Clusters of ED1-positive cells (arrowheads) are also observed at the base of the involuting hyaloid artery. B. In the Nuc1 rat, the persistent hyaloid artery is surrounded by a layer of GFAP-positive astrocytes (arrows), with abundant ED1-positive cells (arrowheads) in the vicinity of the hyaloid artery. A fragment of GFAP-positive cells (arrow) is also observed in the vitreous, presumably from a tangential section of the hyaloid artery, surrounded by ED1-positive cells (arrowhead). Scale bar=50 μm, n=5 wild type, n=5 Nuc1 homozygote.
AQP4 expression levels in Nuc1 and wild type astrocytes
Quantitative RT-PCR analysis indicates that AQP4 mRNA is upregulated in cultured Nuc1 homozygous astrocytes compared to wild type (Figure 5A). The AQP4 protein level is also increased in astrocytes from Nuc1 homozygote rats compared to wild type (Figure 5B). Previous studies have demonstrated that AQP4 splice variants of 30 and 32 kDa are found in rat brain (Nico et al., 2001; Rossi et al., 2010), and it appears that in Nuc1 there is a particular increase in expression of the 30 kDa variant (Figure 5B). Both macroglial cells of the neural retina, astrocytes and Muller cells, express AQP4 under normal conditions (Supplemental Figure 3).
Figure 5.
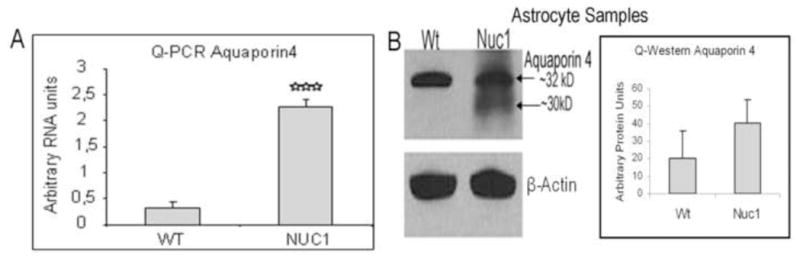
Astrocytes cultured from brains of P2 rat pups show significantly higher expression of AQP4 in Nuc1 homozygous rats compared to wild type (A). Western analysis of astrocyte extracts from wild type and Nuc1 P2 rat pups show a marked increase in AQP4 immunoreactivity in Nuc1 homozygous astrocytes compared to wild type (B). This is consistent with the Quantitative RT-PCR data which show that the mRNA levels for AQP4 were significantly increased in Nuc1 homozygous astrocytes compared to wild type (A).
Human PFV
The hyaloid arteries in all of the eyes were surrounded by astrocytes as reported earlier (Zhang et al., 2005). Figure 6 is a representative tissue section from a 30-year-old female born with multiple ophthalmic abnormalities in her left eye, including persistent fetal vasculature and an imperforate iris, who underwent surgical iris repair at 4 years of age. She developed glaucoma two years later, and the painful left eye was eventually removed due to progressive visual loss despite surgical and pharmacologic therapies. Gross examination of the enucleated globe showed an irregularly shaped and eccentrically placed pupil, a cataractous lens, and persistent fetal hyaloid artery arising from the optic nerve head (arrow, Figure 6A). Such structural abnormalities are not seen in the normal human eye. Microscopic examination confirmed the presence of a central persistent hyaloid artery (arrow, Figure 6B). Interestingly, the hyaloid artery was ensheathed in multilayered fibrillar cells (arrowheads, Figure 6B) similar to those seen in Nuc1 animals. Immunohistochemical analysis of the retained hyaloid artery from this patient revealed that these fibrillar cells were astrocytes, co-expressing AQP4 and GFAP (Figure 7A–C). There was also considerable co-localization of AQP4 immunoreactivity with GFAP in the retina of this patient and all other samples tested (Figure 7D–F).
Figure 6.
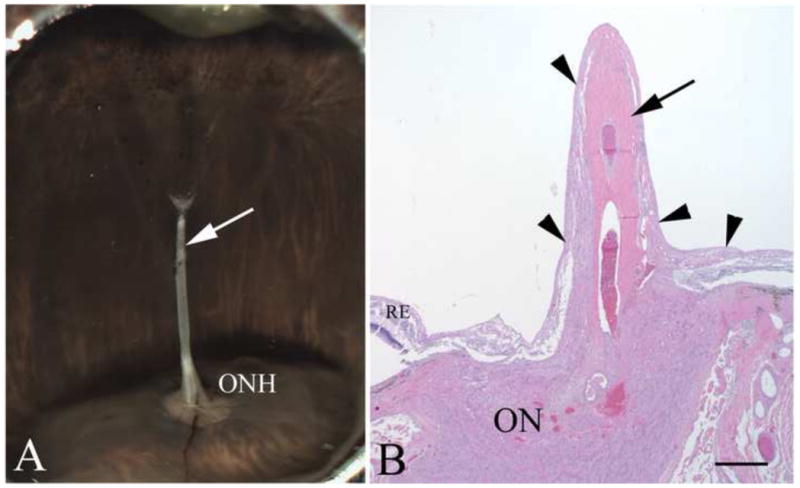
Human PFV: gross morphology and histology. In panel A, the persistent hyaloid artery in an eye from a 30-year old patient is indicated by the arrow and can be seen arising from the optic nerve head (ONH). In panel B, an H&E stained section shows the central persistent hyaloid artery (arrow). The outer layer of hyaloid artery consists of multilayered fibrillar cells (arrowheads). Scale bar=100 μm
Figure 7.
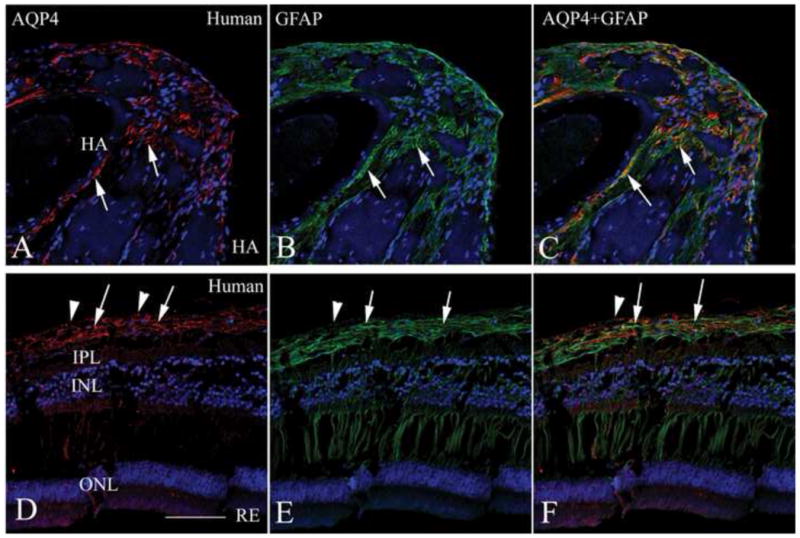
Immunostaining of persistent hyaloid artery and retina from a PFV patient. Paraffin sections from the same eye shown in Figure 6 were stained with AQP4 (A, D, red) and GFAP (B, E, green) with merged images shown in panels C and F. The upper panels show the persistent hyaloid artery. Note the extensive positive staining for both AQP4 (A) and GFAP (B) in the fibrillar cells surrounding the hyaloid artery (arrows). Most of the AQP4 staining co-localizes with GFAP, as seen in the merged image (C), indicating that AQP4-positive astrocytes are closely associated with the persistent hyaloid artery. The lower panels show comparable images for the retina with evidence of AQP4-positive astrocytes being localized primarily in the innermost layer of the retina, near the internal limiting membrane (arrows), where astrocytes are found. However, the innermost layer of the retina (arrowheads) shows positive staining with AQP4, but not with GFAP, possibly representing the Muller cell endfeet, similar to rat retina AQP4 immunolabeling in Supplement figure 3. Scale bar=50 μm.
DISCUSSION
The cellular constituents of the hyaloid vasculature previously described (Zhu et al., 1999) include endothelial cells and pericytes, which constitute the capillaries and larger vessels, as well as hyalocytes (a macrophage population). However, no studies to date have reported cells resembling astrocytes in the normal hyaloid system. Astrocytes originate outside of the retina, arising from the neuroepithelial cells that form the optic stalk, the primordium of the optic nerve (Small et al., 1987). They migrate from the optic nerve head into the inner retina. Astrocytes first appear in the developing rat optic nerve at E (embryonic) day 16 and reach the periphery of the retina by P8 (Miller et al., 1985; Ling et al., 1989). Based on our studies with the Nuc1 spontaneous mutant rat, we reported earlier the presence of astrocytes in association with the retained hyaloid vasculature (Zhang et al., 2005). We now provide direct evidence that Nuc1 astrocytes migrate abnormally along blood vessels in the vitreous from the optic nerve. These astrocytes ensheath the hyaloid artery and we hypothesize that they inhibit normal cellular interactions that are required during the complex process of programmed hyaloid regression. The regulatory mechanisms responsible for normal fetal vascular regression remain obscure, as do the underlying causes for failure of regression. Failure of all or part of this vascular network to regress, a condition called PFV, leads to serious congenital pathologies (Goldberg, 1997). Our studies provide novel evidence that astrocytes are involved in the pathogenesis of PFV.
The fact that astrocytes are not generated in the retina but rather migrate into the retina from the optic nerve (Wantanabe and Raff, 1988), is supported by retroviral lineage-tracing studies showing that, although clones containing various types of retinal neurons and Muller cells are frequently found, clones containing astrocytes are not (Turner and Cepko, 1987; Turner et al., 1990). Cell shape and movement are determined by complex processes regulated by a number of cellular components (Lauffenburger and Horowitz, 1996; Lee et al., 1993). We have previously shown that in Nuc1 homozygous rats the retinal vessels reach the ora serrata at P8, several days earlier than in wild type rats (Gehlbach et al., 2006), suggesting that astrocytes migrate faster in the mutant retina than in wild type, consistent with our in vitro migration assays (Figure 1).
Aquaporin water channels are known to facilitate cell migration, and AQP4 has been shown to be abundant in the end-feet of astrocytes surrounding capillaries throughout the brain (Papadopoulos et al., 2008). Abundant AQP4 labeling has been reported in Muller cells and astrocytes in the normal retina, mostly localized in cell processes (Hamann et al., 1998). It has been elegantly shown that deletion of AQP4 slows astrocyte migration and is associated with delayed glial scar formation (Saadoun et al., 2005). However, the mechanism by which aquaporins facilitate cell migration remains unknown. We determined that the astrocytes associating with the hyaloid artery in the Nuc1 rat express AQP4. Expression of AQP4 co-localizes with GFAP in the Nuc1 homozygous astrocytes that ensheath the hyaloid artery (Figure 3). We also provide evidence, both at the mRNA and protein level that in astrocytes cultured from P2 rat pups, AQP4 is significantly increased in cells from Nuc1 rats compared to wild type (Figure 5A and B). Nuc1 astrocytes express two isoforms of AQP4, 32 and 30 kDa, while only the 32 kDa form is evident in wild type astrocytes. It has been shown that the synthesis of the AQP4 isoforms occurs via a leaky scanning mechanism (Rossi et al., 2010). Interestingly, βA3/A1-crystallin has also been shown to produce βA3 and βA1 proteins from a single βA3/A1 mRNA by leaky ribosomal scanning (Werten et al., 1999).
Retinal astrocytes increase in number until 6 weeks after birth (Raff et al., 1984) and their numbers are not regulated by apoptosis (Raff et al., 1984; Barres et al., 1992). Retinal ganglion cell (RGC) axons, the neurons that carry signals out of the retina to the brain, promote astrocyte proliferation and survival in the developing optic nerve (Burne and Raff, 1997). The first indication of this came from studies on transgenic mice that express a human bcl-2 (survival promoting gene) transgene controlled by a neuron-specific enolase promoter (Martinou et al., 1994). These mice not only had increased numbers of RGC, but astrocytes as well (Raff et al., 1984; Barres et al., 1992). This suggested that the proliferation of optic nerve astrocytes might depend on signals from RGC. We have suggested previously that impaired apoptosis may be the key factor in the defective remodeling of the retinal neurons in Nuc1 homozygous rats (Gehlbach et al. 2006). The increased proliferation of Nuc1 astrocytes (Figure 2) might be attributable to the increased population of RGC in the Nuc1 retina, that results from decreased programmed cell death, as we have reported earlier (Gehlbach et al., 2006).
As we previously demonstrated, astrocytes may abnormally ensheath the hyaloid artery in human PFV disease. As in the Nuc1 rat, these astrocytes also express AQP4 (Figure 7). Taken together, these observations are consistent with the concept that increased expression of AQP4 may induce retinal astrocytes to migrate faster, move beyond their normal limits, associate with the hyaloid artery, and interrupt the cellular interactions that normally occur during the complex process of programmed hyaloid regression, thereby leading to PFV. It is important to note that human PFV disease is a very complex and heterogeneous condition, and multiple factors may be involved in its etiology (Goldberg, 1997). There are several reports of human mutations in βA3/A1-crystallin, all with dominant or semi-dominant cataract phenotypes (Kannabiran et al., 1998; Bateman et al., 2000; Reddy et al., 2004; Ferrini et al., 2004; Qi et al., 2004; Lu et al., 2007; Gu et al., 2010). No PFV has yet been reported for any of these human mutations; however, the patients studied were heterozygotes, whereas persistent hyaloid vasculature is easily detectable and severe only in the homozygous Nuc1 rats.
In the rat, the cells that comprise the hyaloid artery normally disappear by around P20 (Cairns, 1959; Taniguchi et al., 1999). Cell death is a essential feature during this regression process (Terry, 1942; Zhu et al., 1996). Macrophage mediated remodeling is believed to be a major factor in normal hyaloid regression, and interestingly, the number of macrophages diminish in parallel with vessel regression (Jack, 1972; Lang and Bishop, 1993; Lang et al., 1994; Taniguchi et al., 1999; Hose et al., 2005; Lobov et al., 2005). Our data show that in Nuc1 homozygous rats, where regression is inhibited and astrocytes ensheath the hyaloid artery, there are more macrophages surrounding the hyaloid artery than in wild type (Figure 4). It is believed that macrophages target the pericytes, inducing apoptosis, probably by a process requiring cell-to-cell contact (Taniguchi et al., 1999). In the normal eye, loss of pericytes may induce apoptosis in the capillary wall followed by narrowing of the vessels. The resulting cessation of blood flow and withdrawal of growth factors presumably further potentiate apoptosis of endothelial cells. In Nuc1, the astrocytes ensheathing the hyaloid artery may prevent contact between macrophages and pericytes, thereby protecting the pericytes from cell death and blocking involution of the hyaloid artery. Cessation of blood flow is believed to be a major mechanism in hyaloid regression (Lang et al., 1994); this is consistent with the fact that there is usually no vitreous hemorrhage during regression (Taniguchi et al., 1999).
In conclusion, our results indicate that astrocytic ensheathment of the hyaloid artery may lead to interference with the normal cellular interactions required for programmed regression of the hyaloid vasculature, thereby leading to the retention of these vessels. This may be analogous to events in the Nuc1 retina, where remodeling of the vasculature appears to be impaired by the presence of abnormal astrocytes (Sinha et al., 2008). Further investigations are needed to elucidate fully the mechanisms whereby astrocytes may override the strict coordination of cellular interactions during these crucial vascular remodeling processes. Understanding the molecular basis of remodeling during hyaloid regression may lead to development of novel therapeutic approaches for PFV, a potentially blinding disease in an otherwise normal child, for which there are limited treatment options at the present time.
Supplementary Material
AQP4 (red) immunostaining co-labeled with isolectin B4 (green). In both wild type and Nuc 1 20-day-old retinas, isolectin B4-positive ON vessel (arrow in top figure) and hyaloid artery vascular endothelium (arrows in bottom figure) are negative for AQP4 immunostaining. Scale bar=50 μm.
Electron micrographs of hyaloid vasculature from wild type Sprague-Dawley and Nuc1 rat. (A) Cross section of the tunica vasculosa lentis (TVL) at P3 in the wild type indicates anatomically intact vascular elements, including endothelial components with nuclei (N) surrounding an intact capillary lumen. (B) A similar section taken at P14, also in the wild type, shows discontinuity of the capillary lumen (arrows) (indicating disruptions of junctional complexes that ordinarily maintain luminal integrity), and the release of luminal contents into the vitreal space adjacent to the posterior lens capsule. (C) Section through the TVL of a Nuc1 rat at P29, showing a continuous lumen in longitudinal view through a capillary immediately adjacent to the posterior lens capsule as well as a second vascular element containing two red blood cells within the intact lumen above and to the right. Scale bar=5 μm, n=5 wild type, 5 Nuc1 homozygote.
High magnification of AQP4 (red) and GFAP (green) immunostaining in the retina of normal 20-day-old rat. GFAP-positive astrocytes are partially co-localized with AQP4 labeling (arrows in C). In the innermost layer of the retina, GFAP-negative membrane-like structure shows only AQP4 labeling (arrowheads), possibly representing Muller cell endfeet. A small vessel (V) in the inner plexiform layer (IPL) is also positive with AQP4 antibody. Scale bar=50 μm.
Acknowledgments
This work was supported by grants from the National Institutes of Health: EY018636 (DS), EY019037 (DS), EY019037-S (DS), EY05314 (WKL), EY01765 (Wilmer Imaging Core), Intramural Research Program, National Eye Institute (JSZ), Helena Rubinstein Foundation (DS), Research to Prevent Blindness (an unrestricted grant to The Wilmer Eye Institute). We are grateful to Dr. Peter Nemeth, University of Pecs, Hungary, for providing the Aquaporin-4 monoclonal antibody used in this study. We thank Tanya Malpic-Ilanos for brain astrocyte cultures, Rhonda Grebe for confocal microscopy, Vaishnavi Srihar and Katayoon B. Ebrahimi for proliferation and migration assays, and to the Staff Members at Spring Valley Laboratories, Woodbine, MD, for taking care of the experimental animals. We thank Drs. Bhaja Padhi, Gerard A. Lutty and Edward Ratovitski for critical reading and discussion regarding this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auguste KI, Jin S, Uchida K, Yan D, Manley GT, Papadopoulos MC, Verkman AS. Greatly impaired migration of implanted aquaporin-4 deficient astroglial cells in mouse brain toward a site of injury. FASEB J. 2007;21:108–116. doi: 10.1096/fj.06-6848com. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Bateman JB, Geyer DD, Flodman P, Johannes M, Sikela J, Walter N, Moreira AT, Clancy K, Spence MA. A new βA1-crystallin splice junction mutation in autosomal dominant cataract. Invest Ophthalmol Vis Sci. 2000;41:3278–3285. [PubMed] [Google Scholar]
- Burne JF, Raff MC. Retinal ganglion cell axons drive the proliferation of astrocytes in the developing rodent optic nerve. Neuron. 1997;18:223–230. doi: 10.1016/s0896-6273(00)80263-9. [DOI] [PubMed] [Google Scholar]
- Cairns JE. Normal development of the hyaloid and retinal vessels in the rat. Brit J Ophthal. 1959;43:385–393. doi: 10.1136/bjo.43.7.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3(7):207–12. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- Ellis RE, Yuan JY, HoNitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Ferrini W, Schorderet DF, Othenin-Girard P, Uffer S, Heon E, Munier FL. CRYBA3/A1 gene mutation associated with suture-sparing autosomal dominant congenital nuclear cataract: a novel phenotype. Invest Ophthalmol Vis Sci. 2004;45:1436–1441. doi: 10.1167/iovs.03-0760. [DOI] [PubMed] [Google Scholar]
- Gehlbach P, Hose S, Lei B, Zhang C, Cano M, Arora M, Neal R, Barnstable C, Goldberg MF, Zigler S, Sinha D. Developmental abnormalities in the Nuc1 rat retina: a spontaneous mutation that affects neuronal and vascular remodeling and retinal function. Neuroscience. 2006;137:447–61. doi: 10.1016/j.neuroscience.2005.08.084. [DOI] [PubMed] [Google Scholar]
- Goldberg MF. Persistant fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV) LIV Edward Jackson Memorial Lecture. Amer J Ophthalmol. 1997;124:587–626. doi: 10.1016/s0002-9394(14)70899-2. [DOI] [PubMed] [Google Scholar]
- Gu Z, Ji B, Wan C, He G, Zhang J, Zhang M, Feng G, He L, Gao L. A splice site mutation in CRYBA1/A3 causing autosomal dominant posterior polar cataract in a Chinese pedigree. Mol Vis. 2010;16:154–160. [PMC free article] [PubMed] [Google Scholar]
- Hamann S, Zeuthen T, La Cour M, Nagelhus EA, Ottersen OP, Agre P, Nielsen S. Aquaporins in complex tissues: distribution of aquaporins 1–5 in human and rat eye. Am J Physiol Cell Physiol. 1998;274:1332–1345. doi: 10.1152/ajpcell.1998.274.5.C1332. [DOI] [PubMed] [Google Scholar]
- Hose S, Zigler JS, Jr, Sinha D. A novel rat model to study the functions of macrophages during normal development and pathophysiology of the eye. Immunol Lett. 2005;96:299–302. doi: 10.1016/j.imlet.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Huaiyu M, Barres BA. Purification and Characterization of Astrocyte Precursor Cells in the Developing Rat Optic Nerve. J Neuroscience. 1999;19(3):1049–61. doi: 10.1523/JNEUROSCI.19-03-01049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurskainen M, Eklund L, Hägg PO, Fruttiger M, Sormunen R, Ilves M, Pihlajaniemi T. Abnormal maturation of the retinal vasculature in type XVIII collagen/endostatin deficient mice and changes in retinal glial cells due to lack of collagen types XV and XVIII. FASEB J. 2005;19:1564–1566. doi: 10.1096/fj.04-3101fje. [DOI] [PubMed] [Google Scholar]
- Ito M, Yoshioka M. Regression of the hyaloid vessels and papillary membrane of the mouse. Anat Embryol. 1999;200:403–411. doi: 10.1007/s004290050289. [DOI] [PubMed] [Google Scholar]
- Jack RL. Ultrastructural aspects of hyaloid vessel development. Arch Ophthal. 1972;87:427–437. doi: 10.1001/archopht.1972.01000020429013. [DOI] [PubMed] [Google Scholar]
- Kannabiran C, Rogan PK, Olmos L, Basti S, Rao GN, Kaiser-Kupfer M, Hejtmancik JF. Autosomal dominant zonular cataract with sutural opacities is associated with a splice mutation in the βA3/A1-crystallin gene. Mol Vis. 1998;4:18–23. [PubMed] [Google Scholar]
- Lang RA, Bishop MJ. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- Lang R, Lustig M, Francois F, Sellinger M, Plesken H. Apoptosis during macrophage-dependent ocular tissue remodeling. Development. 1994;120:3395–3403. doi: 10.1242/dev.120.12.3395. [DOI] [PubMed] [Google Scholar]
- Latker CH, Kuwabara T. Regression of the tunica vasculosa lentis in the postnatal rat. IOVS. 1981;21:689–699. [PubMed] [Google Scholar]
- Lauffenburger DA, Horowitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lee J, Ishihara A, Jacobsen K. How do cells move along surfaces? Trans Cell. Biol. 1993;3:366–370. doi: 10.1016/0962-8924(93)90084-e. [DOI] [PubMed] [Google Scholar]
- Ling T, Mitrofanis J, Stone J. Origin of retinal astrocytes in the rat: evidence of migration from the optic nerve. J Comp Neurol. 1989;286:345–352. doi: 10.1002/cne.902860305. [DOI] [PubMed] [Google Scholar]
- Lo WK, Shaw AP, Paulsen DF, Mills A. Spatiotemporal distribution of zonulae adherens and associated actin bundles in both epithelium and fiber cells during chicken lens development. Exp Eye Res. 2000;71:45–55. doi: 10.1006/exer.2000.0848. [DOI] [PubMed] [Google Scholar]
- Lobov B, Rao S, Carroll TJ, Vallance J, Ito M, Ondr JK, Kurup S, Glass D, Patel M, Shu W, Morrisey EE, McMahon A, Karsenty G, Lang RA. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Zhao C, Jiao H, Kere J, Tang X, Zhao F, Zhang X, Zhao K, Larsson C. Two Chinese families with pulverulent congenital cataracts and ΔG91 CRYBA1 mutations. Mol Vis. 2007;13:1154–1160. [PubMed] [Google Scholar]
- Martinou J-C, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, Huarte J. Overexpression of bcl-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Miller R, David S, Patel R, Abney E, Raff M. A quantitative immunohistochemical study of macroglial cell development in the rat optic nerve: in vivo evidence for two distinct lineages. Dev Biol. 1985;111:35–41. doi: 10.1016/0012-1606(85)90432-4. [DOI] [PubMed] [Google Scholar]
- Nagy G, Szekeres G, Kvell K, Berki T, Nemeth P. Development and characterization of a monoclonal antibody family against aquaporin 1 (AQP1) and aquaporin 4 (AQP4) Path Oncol Res. 2002;8:115–124. doi: 10.1007/BF03033720. [DOI] [PubMed] [Google Scholar]
- Nico B, Frigeri A, Nicchia GP, Quondamatteo F, Herken R, Errede M, Ribatti D, Svelto M, Roncali L. Role of Aquaporin-4 water channel in the development and integrity of the blood-brain barrier. J Cell Sci. 2001;114:1297–1307. doi: 10.1242/jcs.114.7.1297. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, Saadoun S, Verkman AS. Aquaporins and cell migration. Pflugers Arch Eur J Physiol. 2008;456:693–700. doi: 10.1007/s00424-007-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy G, Ma B, Zhang C, Gongora C, Zigler JS, Jr, Duncan MK, Sinha D. Expression of bA3/A1-crystallin in the developing and adult rat eye. J Mol Histol Epub ahead of print. 2011 doi: 10.1007/s10735-010-9307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Jia H, Huang S, Lin H, Gu J, Su H, Zhang T, Gao Y, Qu L, Li D, Li Y. A deletion mutation in the βA1/A3 crystallin gene (CRYBA1/A3) is associated with autosomal dominant congenital nuclear cataract in a Chinese family. Hum Genet. 2004;114:192–197. doi: 10.1007/s00439-003-1049-7. [DOI] [PubMed] [Google Scholar]
- Raff MC, Abney ER, Miller RH. Two glial cell lineages diverge prenatally in rat optic nerve. Dev Biol. 1984;106:53–60. doi: 10.1016/0012-1606(84)90060-5. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci USA. 1998;95:11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MA, Bateman OA, Chakarova C, Ferris J, Berry V, Lomas E, Sarra R, Smith MA, Moore AT, Bhattacharya SS, Slingsby C. Characterization of the G91del CRYBA1/3-crystallin protein: a cause of human inherited cataract. Hum Mol Gene. 2004;13:945–953. doi: 10.1093/hmg/ddh110. [DOI] [PubMed] [Google Scholar]
- Reneker LW, Overbeek PA. Lens-specific expression of PDGF-A in Transgenic mice results in retinal astrocytic hamartomas. Invest Ophthalmol Vis Sci. 1996;37 (12):2455–2466. [PubMed] [Google Scholar]
- Rossi A, Pisani F, Nicchia GP, Svelto M, Frigeri A. Evidence for a leaky scanning mechanism for the synthesis of the shorter m23 protein isoform of aquaporin-4. J Biol Chem. 2010;7:4562–4569. doi: 10.1074/jbc.M109.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- Sinha D, Hose S, Zhang C, Neal R, Ghosh M, O’Brien TP, Sundin O, Goldberg MF, Robison GW, Russell P, Lo WK, Zigler S. A rat spontaneous mutation affects programmed cell death during the early development of the eye. Exp Eye Res. 2005;80:323–335. doi: 10.1016/j.exer.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Sinha D, Klise A, Sergeev Y, Hose S, Bhutto IA, Hackler L, Jr, Malpic-llanos T, Samtani S, Grebe R, Goldberg MF, Hejtmancik JF, Nath A, Zack DJ, Fariss RN, McLeod DS, Sundin O, Broman KW, Lutty GA, Zigler JS., Jr βA3/A1-Crystallin in astroglial cells regulates retinal vascular remodeling during development. Mol Cell Neurosci. 2008;37:85–95. doi: 10.1016/j.mcn.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small RK, Riddle P, Noble M. Evidence for migration of oligodendrocyte-type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature. 1987;328:155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Kitaoka T, Gong H, Amemiya T. Apoptosis of the hyaloid artery in the rat eye. Ann Anat. 1999;181:555–560. doi: 10.1016/S0940-9602(99)80061-2. [DOI] [PubMed] [Google Scholar]
- Terry TL. Fibroblastic overgrowth of persistent tunica vasculosa lentis in infants born prematurely, III: studies in development and regesssion of hyaloid artery and tunica vasculosa lentis. Am J Ophthalmol. 1942;25:1409–1423. [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990;4:833–845. doi: 10.1016/0896-6273(90)90136-4. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Raff MC. Retinal astrocytes are immigrants from the optic nerve. Nature. 1988;332:834–837. doi: 10.1038/332834a0. [DOI] [PubMed] [Google Scholar]
- Werten PJL, Stege GJJ, de Jong WW. The short 5′ untranslated region of the βA3/A1-crystallin mRNA is responsible for leaky ribosomal scanning. Mol Biol Reports. 1999;26:201–205. doi: 10.1023/a:1007046926233. [DOI] [PubMed] [Google Scholar]
- Yool AJ. Aquaporins: multiple roles in the central nervous system. Neuroscientist. 2007;13:470–484. doi: 10.1177/1073858407303081. [DOI] [PubMed] [Google Scholar]
- Zhang C, Gehlbach P, Gongora C, Cano M, Fariss R, Hose S, Nath A, Green WR, Goldberg MF, Zigler JS, Sinha D. A potential role for β- and γ–crystallins in the vascular remodeling of the eye. Dev Dyn. 2005;234:36–47. doi: 10.1002/dvdy.20494. [DOI] [PubMed] [Google Scholar]
- Zhu M, Penfold PL, Madigan MC, Maslim J, Billson FA. Aspects of apoptosis and vascular regression in the human foetal hyaloid. IOVS. 1996;37(3, Suppl):S131. [Google Scholar]
- Zhu M, Provis JM, Penfold PL. The human hyaloid system: Cellular phenotypes and inter-relationships. Experimental Eye Research. 1999;68:553–563. doi: 10.1006/exer.1998.0632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AQP4 (red) immunostaining co-labeled with isolectin B4 (green). In both wild type and Nuc 1 20-day-old retinas, isolectin B4-positive ON vessel (arrow in top figure) and hyaloid artery vascular endothelium (arrows in bottom figure) are negative for AQP4 immunostaining. Scale bar=50 μm.
Electron micrographs of hyaloid vasculature from wild type Sprague-Dawley and Nuc1 rat. (A) Cross section of the tunica vasculosa lentis (TVL) at P3 in the wild type indicates anatomically intact vascular elements, including endothelial components with nuclei (N) surrounding an intact capillary lumen. (B) A similar section taken at P14, also in the wild type, shows discontinuity of the capillary lumen (arrows) (indicating disruptions of junctional complexes that ordinarily maintain luminal integrity), and the release of luminal contents into the vitreal space adjacent to the posterior lens capsule. (C) Section through the TVL of a Nuc1 rat at P29, showing a continuous lumen in longitudinal view through a capillary immediately adjacent to the posterior lens capsule as well as a second vascular element containing two red blood cells within the intact lumen above and to the right. Scale bar=5 μm, n=5 wild type, 5 Nuc1 homozygote.
High magnification of AQP4 (red) and GFAP (green) immunostaining in the retina of normal 20-day-old rat. GFAP-positive astrocytes are partially co-localized with AQP4 labeling (arrows in C). In the innermost layer of the retina, GFAP-negative membrane-like structure shows only AQP4 labeling (arrowheads), possibly representing Muller cell endfeet. A small vessel (V) in the inner plexiform layer (IPL) is also positive with AQP4 antibody. Scale bar=50 μm.


