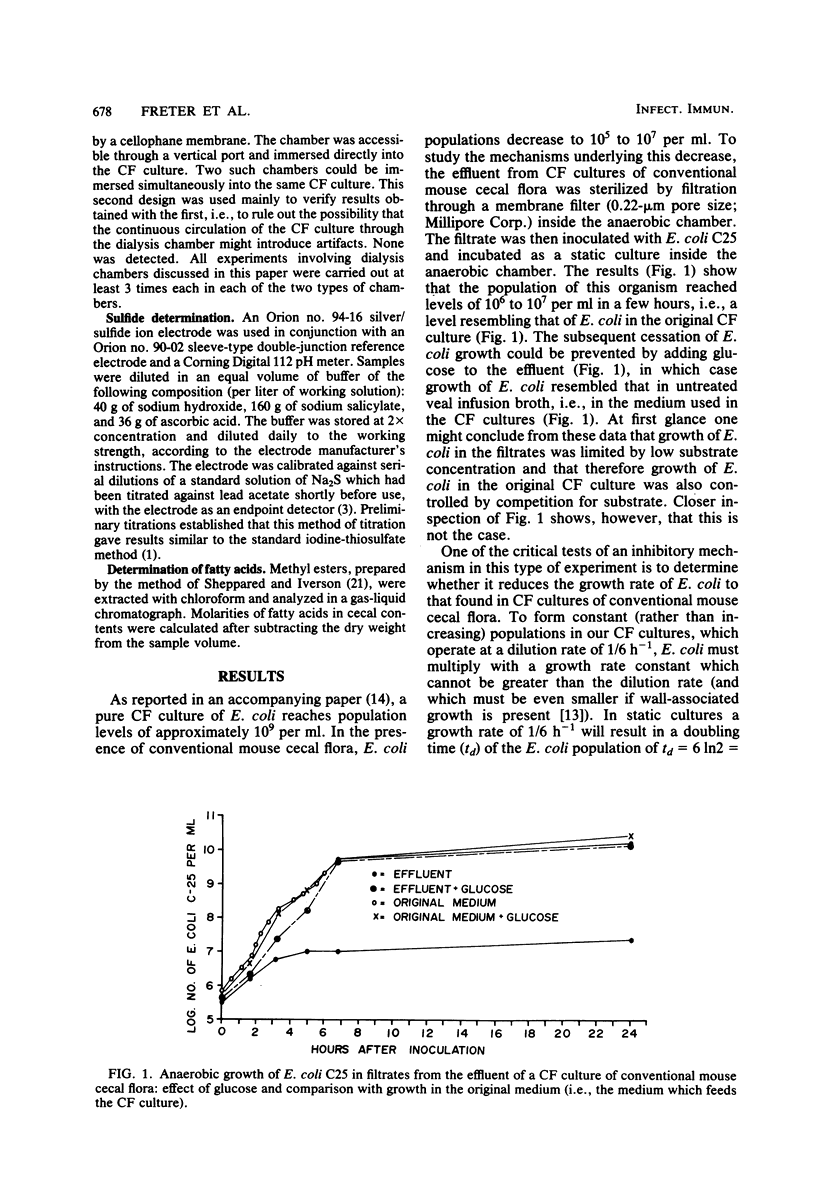
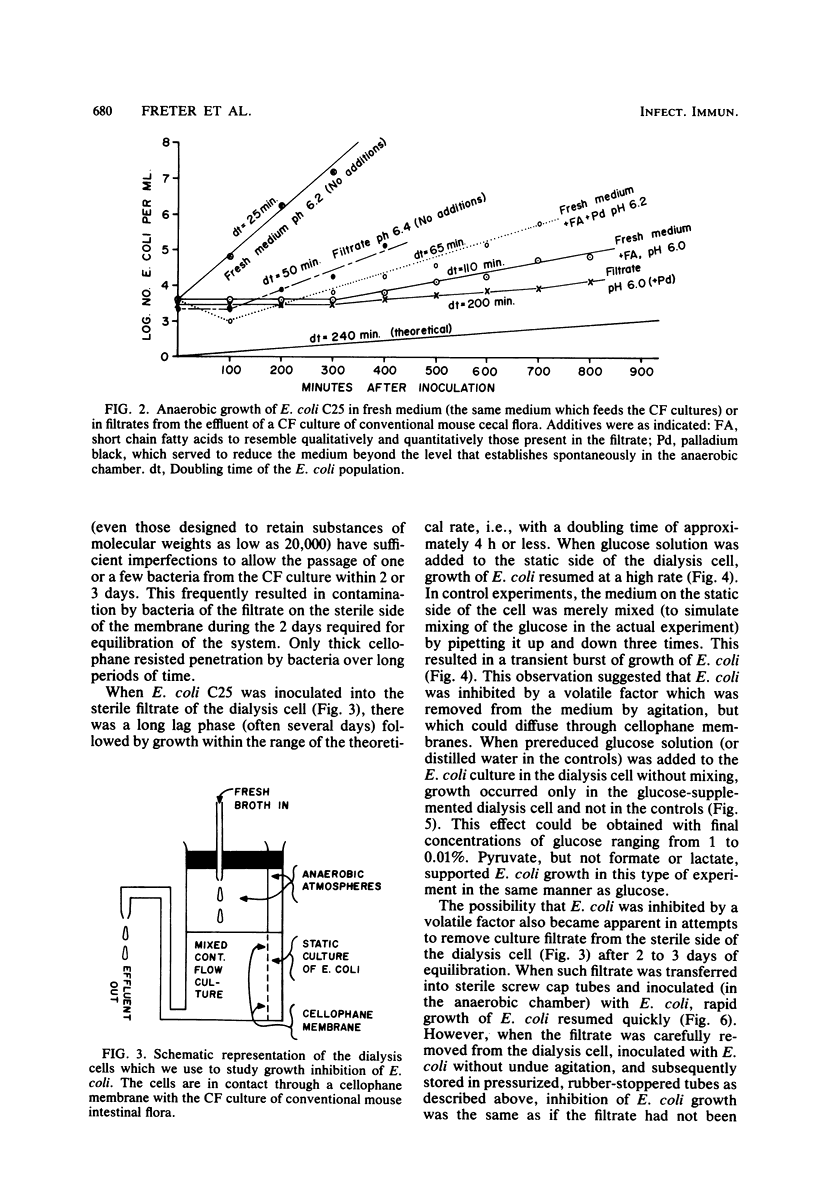
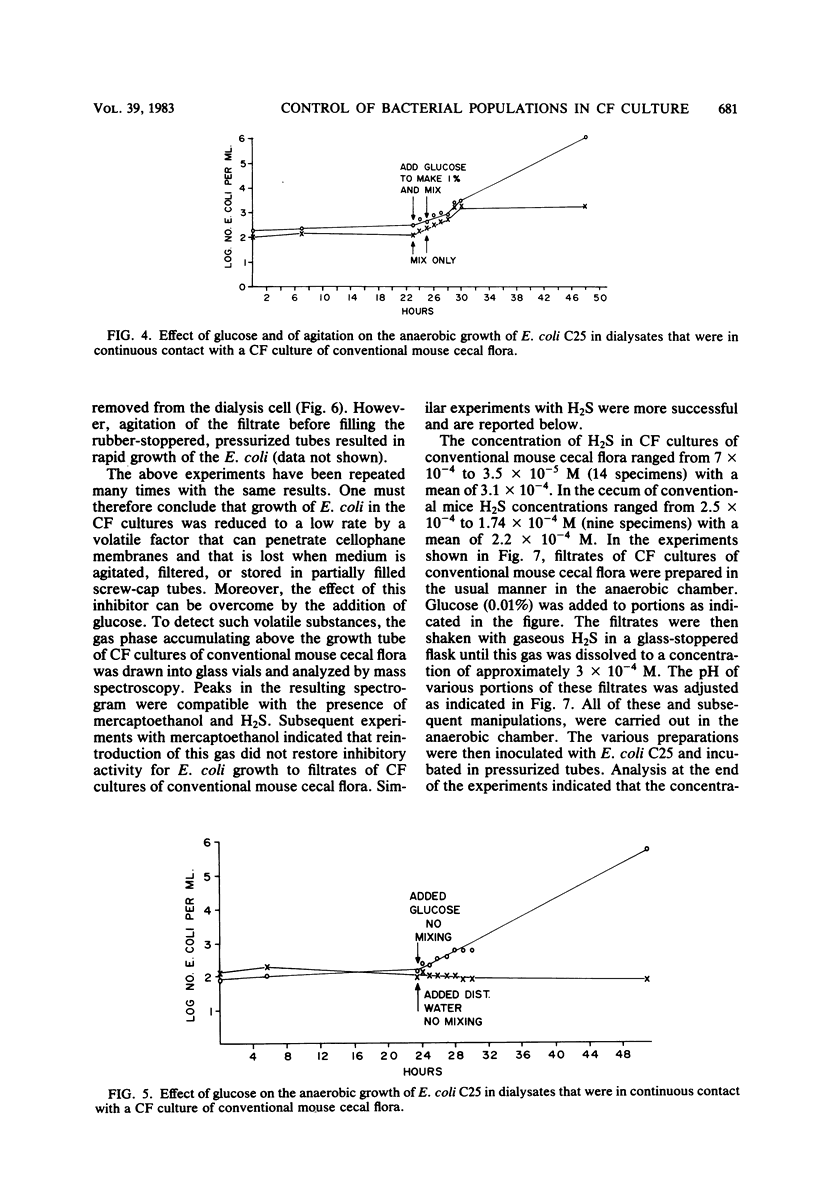
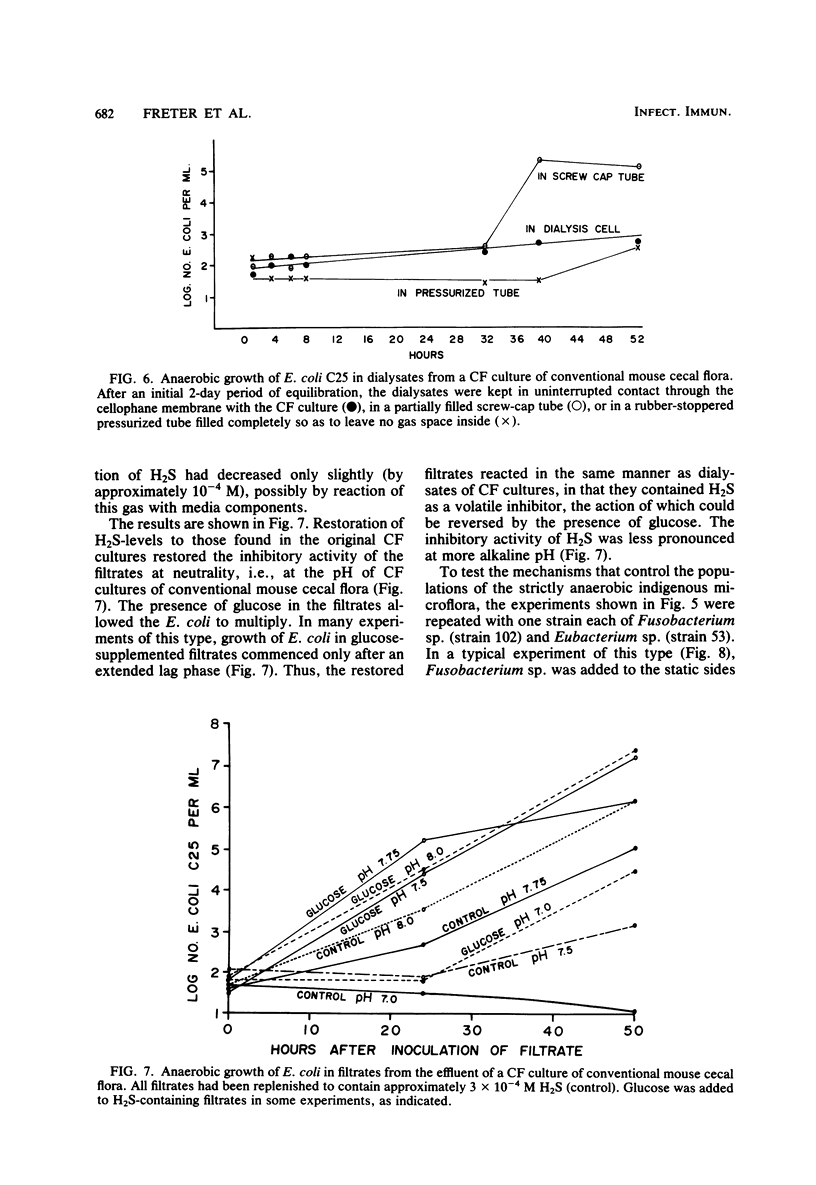
Abstract
A previous study had established that anaerobic continuous-flow (CF) cultures of conventional mouse cecal flora were able to maintain the in vivo ecological balance among the indigenous bacterial species tested. This paper describes experiments designed to determine the mechanisms which control the population sizes of these species in such CF cultures. One strain each of Escherichia coli, Fusobacterium sp., and Eubacterium sp. were studied. Growth of these strains in filtrates of CF cultures was considerably more rapid than in the CF cultures themselves, indicating that the inhibitory activity had been lost in the process of filtration. Growth rates to match those in CF cultures could be obtained, however, by restoring the original levels of H2S in the culture filtrates. The inhibitory effect of H2S in filtrates and in dialysates of CF cultures could be abolished by adding glucose or pyruvate, but not formate or lactate. The fatty acids present in CF cultures matched those in the cecum of conventional mice in both quality and concentration. These acids could not account for the slow rates of growth of the tested strains in CF cultures, but they did cause a marked increase in the initial lag phase of E. coli growth. The results obtained are compatible with the hypothesis that the populations of most indigenous intestinal bacteria are controlled by one or a few nutritional substrates which a given strain can utilize most efficiently in the presence of H2S and at the prevailing conditions of pH and anaerobiosis. This hypothesis consequently implies that the populations of enterobacteria, such as the E. coli strain tested, and those of the predominant anaerobes are controlled by analogous mechanisms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aranki A., Freter R. Use of anaerobic glove boxes for the cultivation of strictly anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1329–1334. doi: 10.1093/ajcn/25.12.1329. [DOI] [PubMed] [Google Scholar]
- Berg R. D. Mechanisms confining indigenous bacteria to the gastrointestinal tract. Am J Clin Nutr. 1980 Nov;33(11 Suppl):2472–2484. doi: 10.1093/ajcn/33.11.2472. [DOI] [PubMed] [Google Scholar]
- FRETER R. Experimental enteric Shigella and Vibrio infections in mice and guinea pigs. J Exp Med. 1956 Sep 1;104(3):411–418. doi: 10.1084/jem.104.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson A. G. Behavior of mixed cultures of microorganisms. Annu Rev Microbiol. 1977;31:63–87. doi: 10.1146/annurev.mi.31.100177.000431. [DOI] [PubMed] [Google Scholar]
- Freter R., Abrams G. D. Function of various intestinal bacteria in converting germfree mice to the normal state. Infect Immun. 1972 Aug;6(2):119–126. doi: 10.1128/iai.6.2.119-126.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Brickner H., Fekete J., Vickerman M. M., Carey K. E. Survival and implantation of Escherichia coli in the intestinal tract. Infect Immun. 1983 Feb;39(2):686–703. doi: 10.1128/iai.39.2.686-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Stauffer E., Cleven D., Holdeman L. V., Moore W. E. Continuous-flow cultures as in vitro models of the ecology of large intestinal flora. Infect Immun. 1983 Feb;39(2):666–675. doi: 10.1128/iai.39.2.666-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison M. E. Effect of colon flora and short-chain fatty acids on growth in vitro of Pseudomonas aeruginsoa and Enterobacteriaceae. Infect Immun. 1973 Jul;8(1):30–35. doi: 10.1128/iai.8.1.30-35.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYNELL G. G., SUBBAIAH T. V. Antibacterial mechanisms of the mouse gut. I. Kinetics of infection by Salmonella typhi-murium in normal and streptomycin-treated mice studied with abortive transductants. Br J Exp Pathol. 1963 Apr;44:197–208. [PMC free article] [PubMed] [Google Scholar]
- McMinn M. T., Crawford J. J. Recovery of anaerobic microorganisms from clinical specimens in prereduced media versus recovery by routine clinical laboratory methods. Appl Microbiol. 1970 Feb;19(2):207–213. doi: 10.1128/am.19.2.207-213.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Syed S. A., Abrams G. D., Freter R. Efficiency of various intestinal bacteria in assuming normal functions of enteric flora after association with germ-free mice. Infect Immun. 1970 Oct;2(4):376–386. doi: 10.1128/iai.2.4.376-386.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkheimer G., Schulz F. H. The phenomenon of persorption. Digestion. 1968;1(4):213–218. doi: 10.1159/000196856. [DOI] [PubMed] [Google Scholar]
- Wostmann B. S., Pleasants J. R., Bealmear P. Dietary stimulation of immune mechanisms. Fed Proc. 1971 Nov-Dec;30(6):1779–1784. [PubMed] [Google Scholar]
- van der Waaij D., Berghuis-de Vries J. M., Lekkerkerk Lekkerkerk-v Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 1971 Sep;69(3):405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]


