Abstract
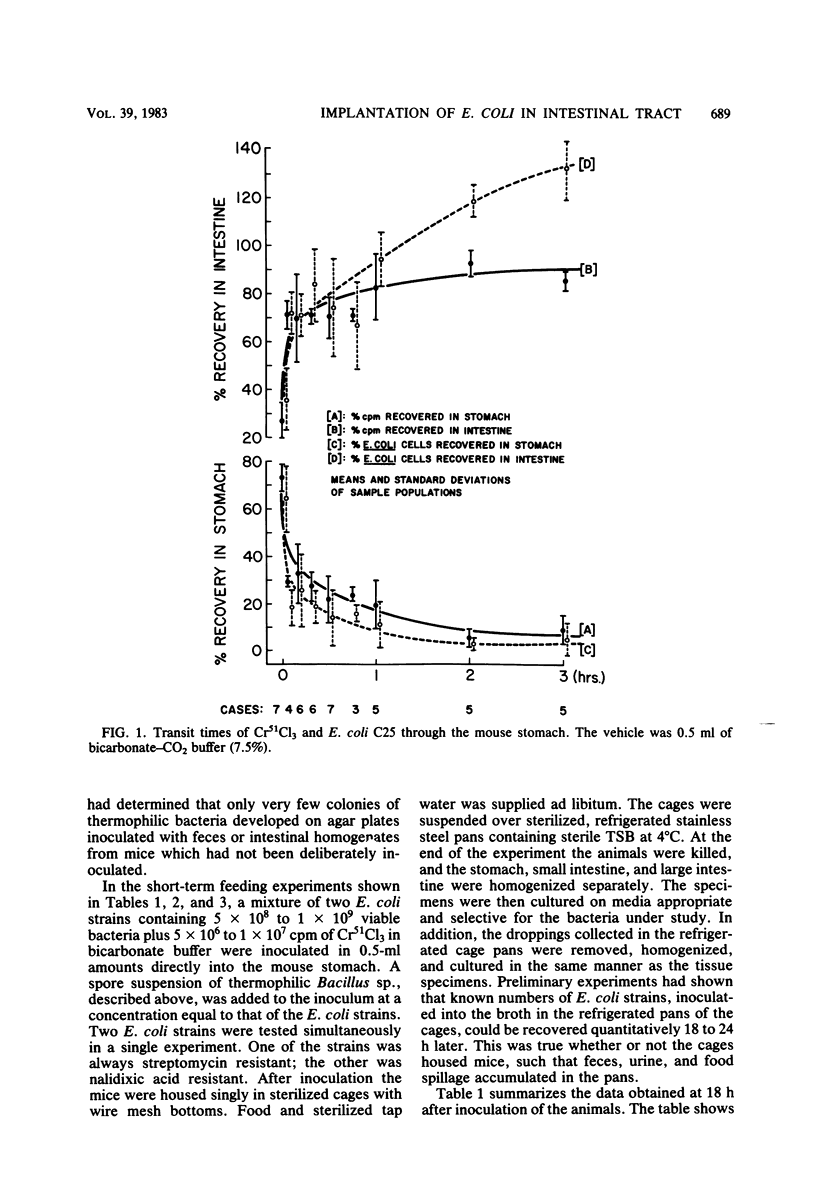
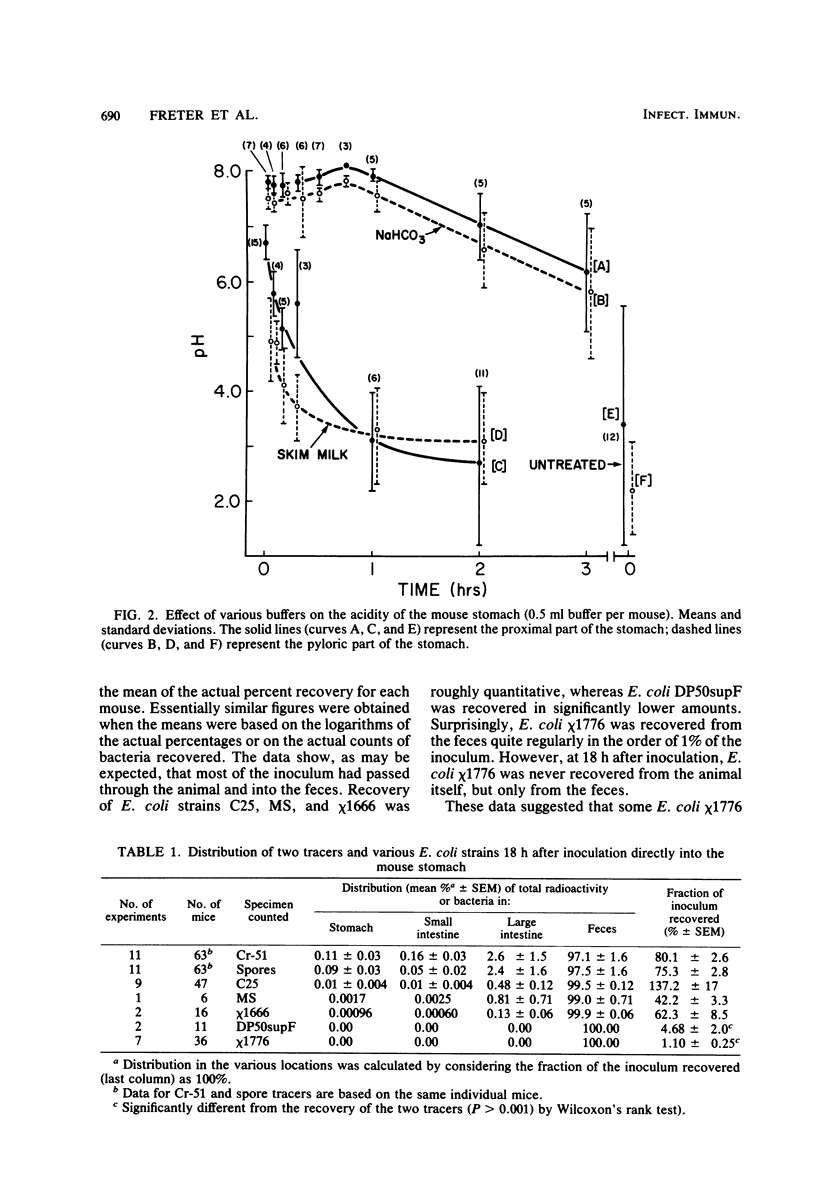
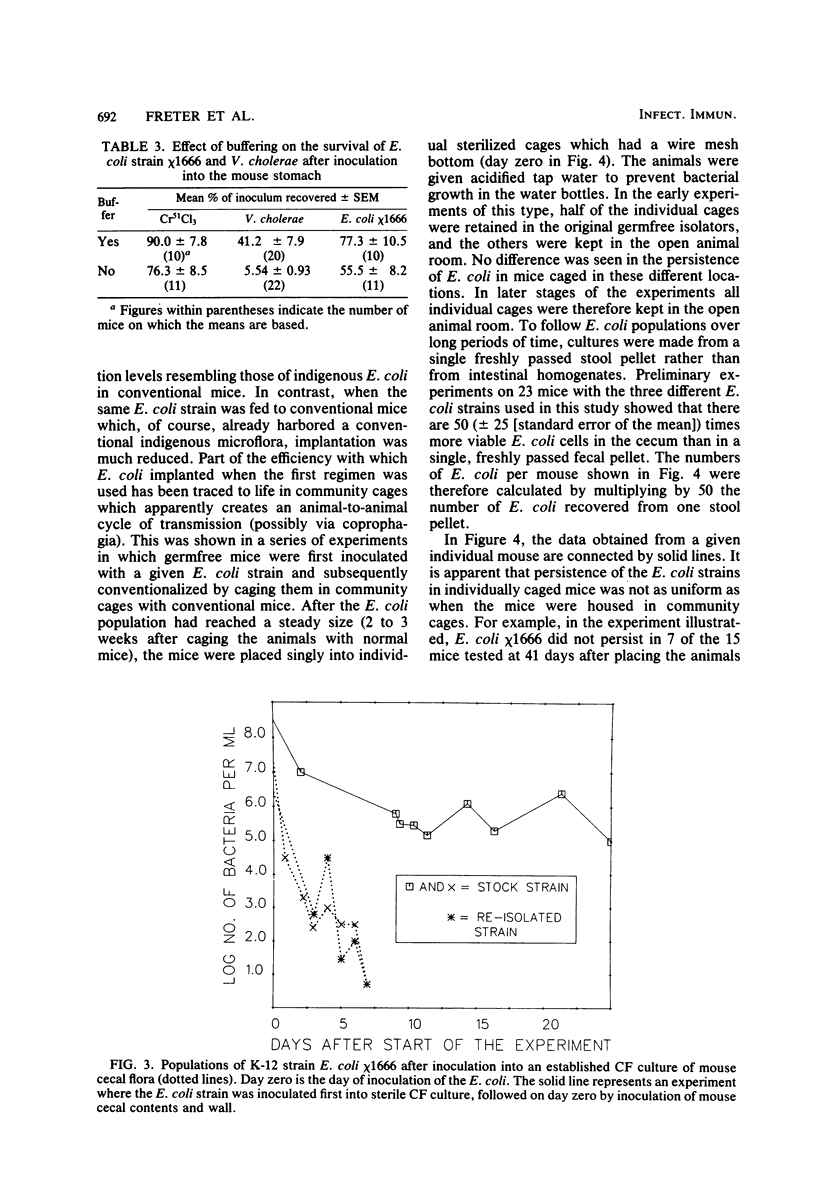
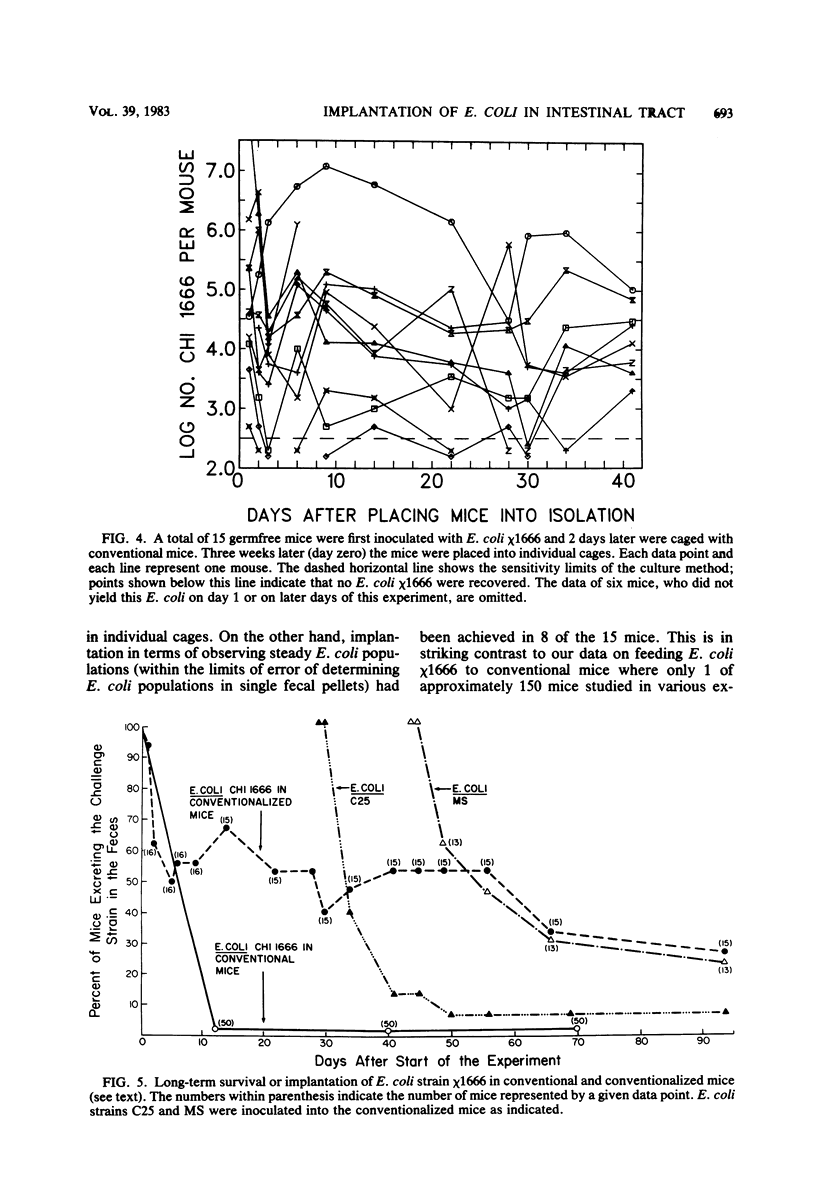
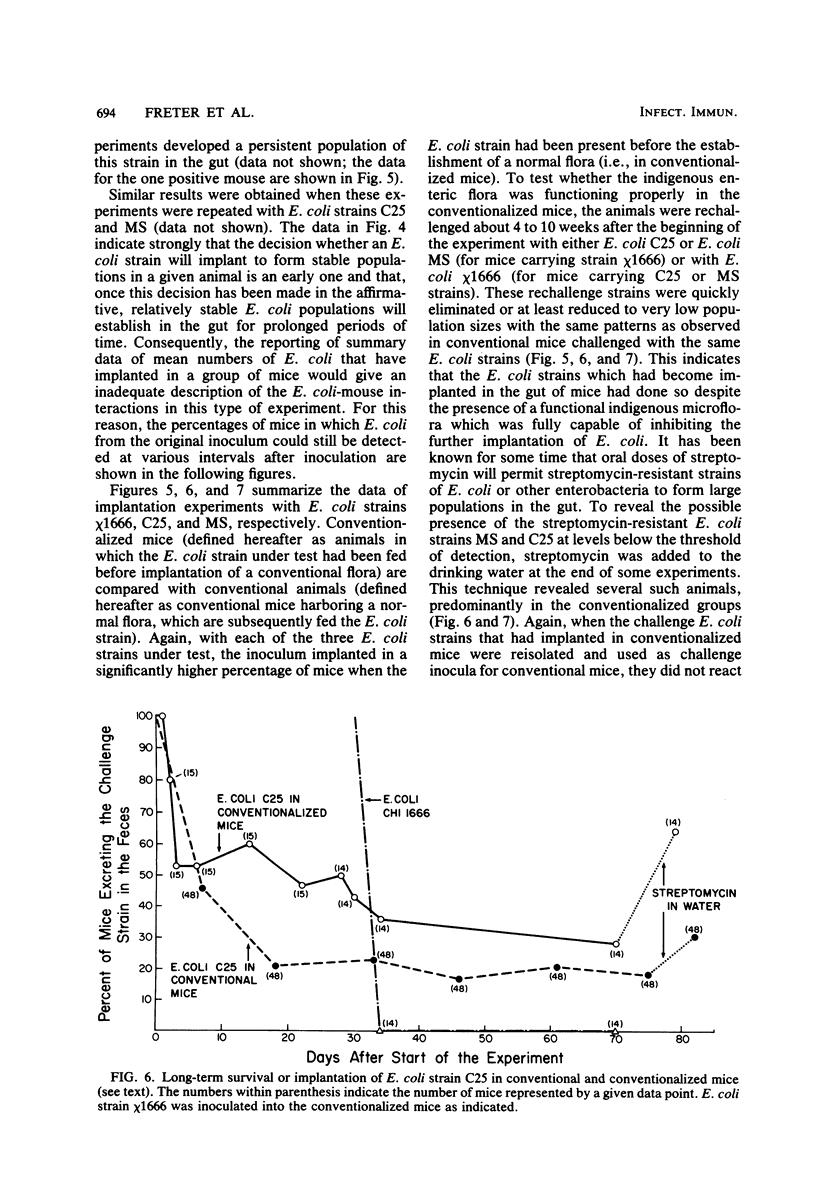
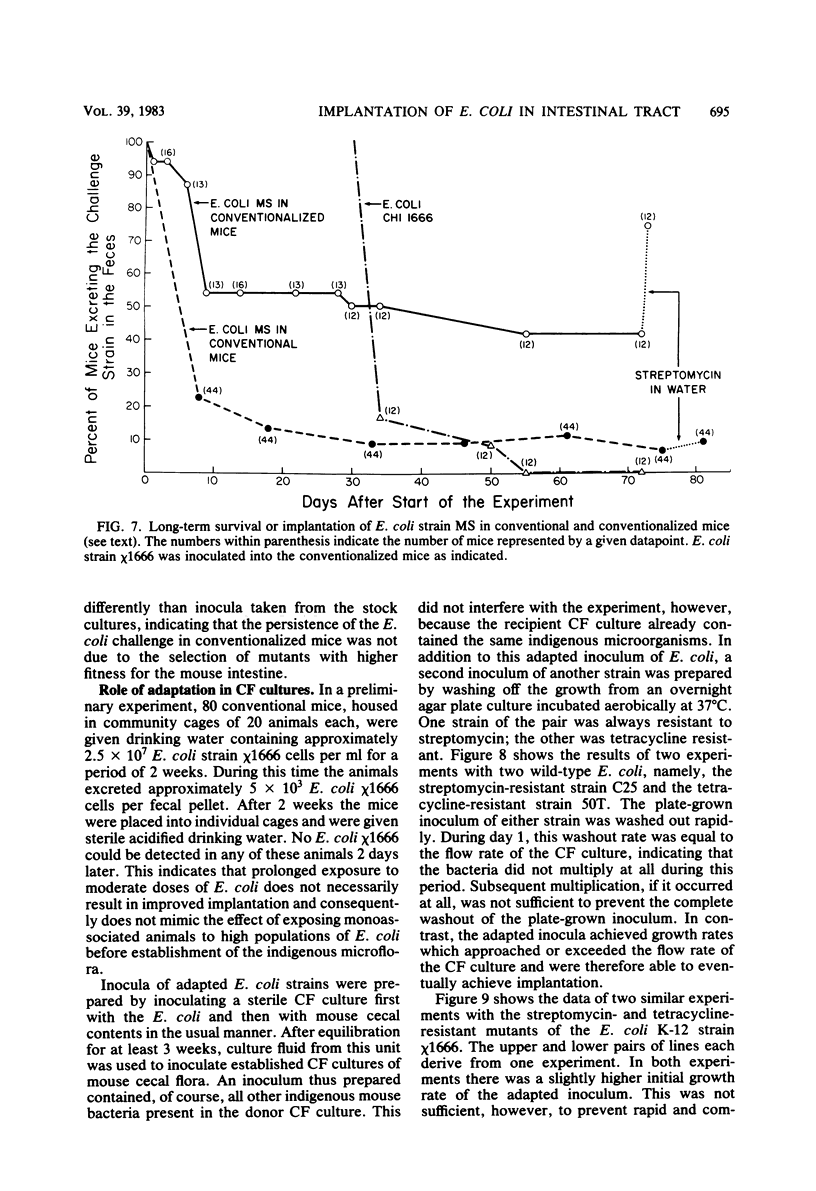
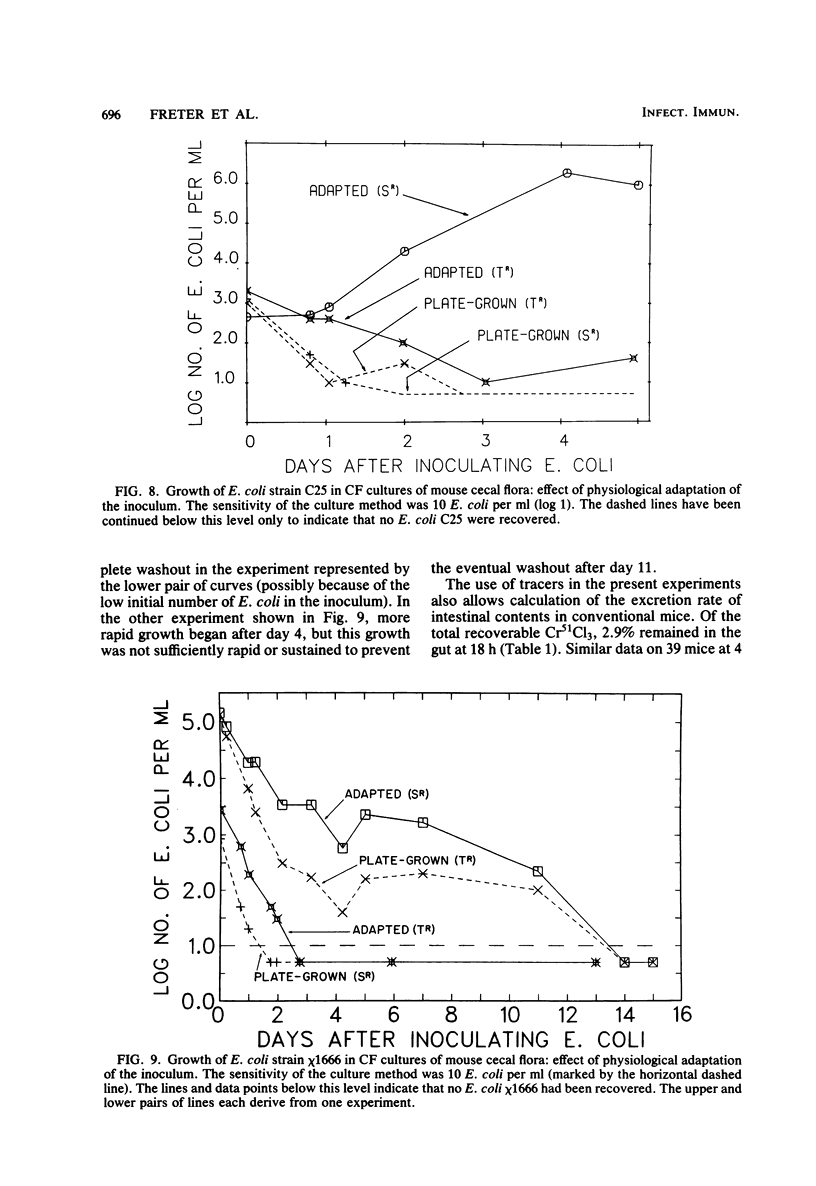
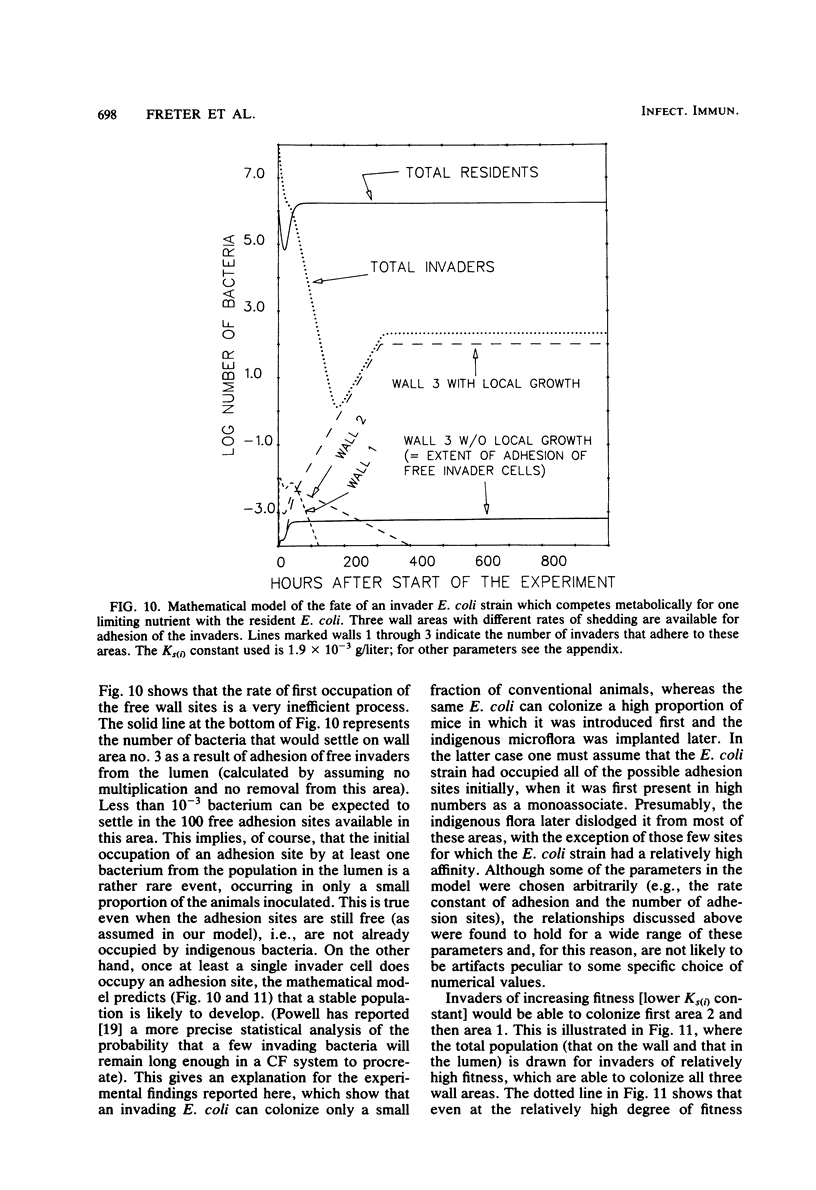
Preliminary experiments established that a 0.5-ml inoculum that is introduced directly into the stomach of mice was cleared rapidly into the small intestine. Bicarbonate buffer, but not skim milk, protected such an inoculum from stomach acid until at least 90% of it had entered the small intestine. Passage and survival of various Escherichia coli strains through the mouse gut were tested by introducing a buffered bacterial inoculum directly into the stomach, together with the following two intestinal tracers: Cr51Cl3 and spores of a thermophilic Bacillus sp. Quantitative recovery of excreted bacteria was accomplished by collecting the feces overnight in a refrigerated cage pan. The data show that wild-type E. coli strains and E. coli K-12 are excreted rapidly (98 to 100% within 18 h) in the feces without overall multiplication or death. E. coli ϰ1776 and DP50supF, i.e., strains certified for recombinant DNA experiments underwent rapid death in vivo, such that their excretion in the feces was reduced to approximately 1.1 and 4.7% of the inoculum, respectively. The acidity of the stomach had little bactericidal effect on the E. coli K-12 strain tested, but significantly reduced the survival of more acidsensitive bacteria (Vibrio cholerae) under these conditions. Long-term implantation of E. coli strains into continuous-flow cultures of mouse cecal flora or into conventional mice was difficult to accomplish. In contrast, when the E. coli strain was first inoculated into sterile continuous-flow cultures or into germfree mice, which were subsequently associated with conventional mouse cecal flora, the E. coli strains persisted in a large proportion of the animals at levels resembling E. coli populations in conventional mice. Metabolic adaptation contributed only partially to the success of an E. coli inoculum that was introduced first. A mathematical model is described which explains this phenomenon on the basis of competition for adhesion sites in which an advantage accrues to the bacterium which occupies those sites first. The mathematical model predicts that two or more bacterial strains that compete in the gut for the same limiting nutrient can coexist, if the metabolically less efficient strains have specific adhesion sites available. The specific rate constant of E. coli growth in monoassociated gnotobiotic mice was 2.0 h−1, whereas the excretion rate in conventional animals was −0.23 h−1. Consequently, limitation of growth must be regarded as the primary mechanism controlling bacterial populations in the large intestine. The beginnings of a general hypothesis of the ecology of the large intestine are proposed, in which the effects of the competitive metabolic interactions described earlier are modified by the effects of bacterial association with the intestinal wall.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams G. D., Bishop J. E. Effect of the normal microbial flora on gastrointestinal motility. Proc Soc Exp Biol Med. 1967 Oct;126(1):301–304. doi: 10.3181/00379727-126-32430. [DOI] [PubMed] [Google Scholar]
- Anderson J. D., Gillespie W. A., Richmond M. H. Chemotherapy and antibiotic-resistance transfer between Enterobacteria in the human gastro-intestinal tract. J Med Microbiol. 1973 Nov;6(4):461–473. doi: 10.1099/00222615-6-4-461. [DOI] [PubMed] [Google Scholar]
- Anthony B. F., Wannamaker L. W. Bacterial interference in experimental burns. J Exp Med. 1967 Feb 1;125(2):319–336. doi: 10.1084/jem.125.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOHNHOFF M., MILLER C. P., MARTIN W. R. RESISTANCE OF THE MOUSE'S INTESTINAL TRACT TO EXPERIMENTAL SALMONELLA INFECTION. I. FACTORS WHICH INTERFERE WITH THE INITIATION OF INFECTION BY ORAL INOCULATION. J Exp Med. 1964 Nov 1;120:805–816. doi: 10.1084/jem.120.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducluzeau R., Bellier M., Raibaud P. Transit Digestif de Divers Inoculums Bactériens Introduits "Per Os" Chez des Souris Axéniques et "Holoxéniques" (Conventionnelles): Effet Antagoniste de la Microflore du Tractus Gastro-Intestinal. Zentralbl Bakteriol Orig. 1970 May;213(4):533–548. [PubMed] [Google Scholar]
- Fordtran J. S., Morawski S. G., Richardson C. T. In vivo and in vitro evaluation of liquid antacids. N Engl J Med. 1973 May 3;288(18):923–928. doi: 10.1056/NEJM197305032881801. [DOI] [PubMed] [Google Scholar]
- Formal S. B., Hornick R. B. Invasive Escherichia coli. J Infect Dis. 1978 May;137(5):641–644. doi: 10.1093/infdis/137.5.641. [DOI] [PubMed] [Google Scholar]
- Fredrickson A. G. Behavior of mixed cultures of microorganisms. Annu Rev Microbiol. 1977;31:63–87. doi: 10.1146/annurev.mi.31.100177.000431. [DOI] [PubMed] [Google Scholar]
- Freter R., Abrams G. D. Function of various intestinal bacteria in converting germfree mice to the normal state. Infect Immun. 1972 Aug;6(2):119–126. doi: 10.1128/iai.6.2.119-126.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Brickner H., Botney M., Cleven D., Aranki A. Mechanisms that control bacterial populations in continuous-flow culture models of mouse large intestinal flora. Infect Immun. 1983 Feb;39(2):676–685. doi: 10.1128/iai.39.2.676-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Stauffer E., Cleven D., Holdeman L. V., Moore W. E. Continuous-flow cultures as in vitro models of the ecology of large intestinal flora. Infect Immun. 1983 Feb;39(2):666–675. doi: 10.1128/iai.39.2.666-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Kapsimalis B. Estimates of the overall rate of growth of the intestinal microflora of hamsters, guinea pigs, and mice. J Bacteriol. 1967 Jan;93(1):510–512. doi: 10.1128/jb.93.1.510-512.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- MEYNELL G. G. Antibacterial mechanisms of the mouse gut. II. The role of Eh and volatile fatty acids in the normal gut. Br J Exp Pathol. 1963 Apr;44:209–219. [PMC free article] [PubMed] [Google Scholar]
- MEYNELL G. G., SUBBAIAH T. V. Antibacterial mechanisms of the mouse gut. I. Kinetics of infection by Salmonella typhi-murium in normal and streptomycin-treated mice studied with abortive transductants. Br J Exp Pathol. 1963 Apr;44:197–208. [PMC free article] [PubMed] [Google Scholar]
- NOVICK A., SZILARD L. Experiments with the Chemostat on spontaneous mutations of bacteria. Proc Natl Acad Sci U S A. 1950 Dec;36(12):708–719. doi: 10.1073/pnas.36.12.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZAWA A., FRETER R. ECOLOGICAL MECHANISM CONTROLLING GROWTH OF ESCHERICHIA COLI IN CONTINUOUS FLOW CULTURES AND IN THE MOUSE INTESTINE. J Infect Dis. 1964 Jun;114:235–242. doi: 10.1093/infdis/114.3.235. [DOI] [PubMed] [Google Scholar]
- POWELL E. O. Criteria for the growth of contaminants and mutants in continuous culture. J Gen Microbiol. 1958 Feb;18(1):259–268. doi: 10.1099/00221287-18-1-259. [DOI] [PubMed] [Google Scholar]
- SEARS H. J., BROWNLEE I. Further observations on the persistence of individual strains of Escherichia coli in the intestinal tract of man. J Bacteriol. 1952 Jan;63(1):47–57. doi: 10.1128/jb.63.1.47-57.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS H. J., BROWNLEE I., UCHIYAMA J. K. Persistence of individual strains of Escherichia coli in the intestinal tract of man. J Bacteriol. 1950 Feb;59(2):293–301. doi: 10.1128/jb.59.2.293-301.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon H. F. Human fecal markers. Am J Clin Nutr. 1979 Dec;32(12):2377–2379. doi: 10.1093/ajcn/32.12.2377. [DOI] [PubMed] [Google Scholar]
- Syed S. A., Abrams G. D., Freter R. Efficiency of various intestinal bacteria in assuming normal functions of enteric flora after association with germ-free mice. Infect Immun. 1970 Oct;2(4):376–386. doi: 10.1128/iai.2.4.376-386.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann B., Knothe H., Doll E. Ubertragung von R-Faktoren in der Darmflora des Menschen. Zentralbl Bakteriol Orig. 1970;213(2):183–193. [PubMed] [Google Scholar]
- ZISCHKA W., PROHASKA E. Uber die Ansiedlung von oral applizierter tetracyclinresistenter Escherichia coli im Darm während der Therapie mit Antibiotica der Tetracyclinreihe. Zentralbl Bakteriol Orig. 1958 Sep;172(5-6):433–436. [PubMed] [Google Scholar]
- van der Waaij D., Berghuis-de Vries J. M., Lekkerkerk Lekkerkerk-v Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 1971 Sep;69(3):405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]



