Abstract
Inducing apoptosis in cancer cells is an effective strategy for cancer therapy. The cationic α-helix forming KLAKLAKKLAKLAK peptide (KLAK) has been known to induce apoptosis by disrupting the mitochondria. In the present study, we have designed a thermally targeted KLAK peptide by genetically engineering the KLAK sequence to the carboxy terminus of the heat responsive biopolymer elastin-like polypeptide (ELP). The cellular internalization of ELP-KLAK was made possible by engineering a cell penetrating peptide sequence (SynB1) to the amino terminus of ELP. The SynB1-ELP1-KLAK fusion polypeptide was cytotoxic against both estrogen receptor positive and negative human breast cancer cell lines. The potency of SynB1-ELP1-KLAK was further enhanced when mild hyperthermia was added to the treatment. In response to hyperthermia, SynB1-ELP1-KLAK selectively triggered apoptosis, which was associated with disruption of the mitochondria. The thermally responsive SynB1-ELP-KLAK polypeptide can have improved tumor targeting by the application of mild hyperthermia. Furthermore, the pharmacokinetic properties of ELP can prevent degradation of KLAK in vivo, and the use of SynB1 can mediate tumor cell uptake, thereby augmenting the effect of KLAK.
Keywords: Elastin-like polypeptides, proapoptotic peptides, thermal targeting, drug delivery, breast cancer
Introduction
Peptides that modulate cancer cell specific molecular pathways have a great potential as anticancer therapeutics. Since inhibition of apoptosis is a major mechanism by which cancer cells evade cell killing, peptides that induce apoptosis specifically in cancer cells can have enormous therapeutic values. Based on rapidly emerging information on protein-protein interactions and surface dynamics, proapoptotic peptides can be rationally designed to inhibit the activity of anti-apoptotic proteins or promote the activity of pro-apoptotic proteins in the apoptosis pathway (Raucher et al. 2009). In vivo validation of peptides in general, however, have been mainly limited by poor pharmacokinetics and weak tumor penetration (Raucher et al. 2009). The thermally responsive elastin-like polypeptide (ELP) can potentially address these issues.
Previous studies have shown that fusing cancer cell inhibitory peptides to ELP leads to improvement in their anticancer effect especially in response to hyperthermia, mainly because hyperthermia triggered phase transition of ELP leads to enhanced uptake of ELP inside the cells (Bidwell and Raucher 2005; Massodi et al. 2009; Bidwell et al. 2010; Massodi et al. 2010). ELPs are derived from human tropoelastin. They are recombinant polypeptides composed of a Val-Pro-Gly-Xaa-Gly motif, where Xaa is the “guest reside”, and can be any amino acid except proline. ELPs are temperature sensitive because they undergo inverse phase transition at a unique temperature known as transition temperature (Tt), below which they are soluble in aqueous solution, and above which they desolvate and aggregate from solution (Yamaoka et al. 2003). This process is completely reversible and the thermal transition process does not change the stability of the protein (Chilkoti et al. 2002). The molecular composition of Xaa, the number of pentapeptide repeats, cosolutes, and concentration affect the transition temperature of ELP (reviewed in (MacEwan and Chilkoti 2010)). For clinical applications of ELP, these parameters were manipulated to generate an ELP variant (ELP1) that undergoes phase transition between 39 – 42 °C. The efficacy of ELP1 as a promising drug carrier has been observed for the delivery of small molecule drugs (Dreher et al. 2003; Furgeson et al. 2006; Bidwell et al. 2007; Bidwell et al. 2007; MacKay et al. 2009; Moktan et al. 2010) as well as cell inhibitory therapeutic peptides (Bidwell and Raucher 2005; Massodi et al. 2009; Bidwell et al. 2010; Massodi et al. 2010).
Motivated by the results of these work, we combined the thermal targeting utilities of ELP1 with the cell killing function of a proapoptotic peptide, KLAKLAKKLAKLAK (abbreviated KLAK).The de novo KLAK peptide adopts an amphipathic α-helical conformation in amphipathic media (Javadpour et al. 1996). Cellular internalization of KLAK peptide is necessary for its apoptotic activity (Ellerby et al. 1999). Therefore, it is typically fused to cell penetrating peptides, receptor ligands, or antibodies to facilitate cellular uptake (Ellerby et al. 1999; Mai et al. 2001; Fantin et al. 2005; Rege et al. 2007; Kwon et al. 2008). Once internalized, KLAK induces mitochondrial membrane disruption associated apoptosis (Ellerby et al. 1999).
In this study, the amino terminus of ELP1 was modified with the cell penetrating peptide, SynB1. SynB1 is derived from the antimicrobial peptides called protegrins and are known to mediate cellular uptake of its cargo via endocytotic mechanisms (Rousselle et al. 2000). The carboxy terminus of ELP was modified with the KLAK peptide sequence. The fusion polypeptide SynB1-ELP1-KLAK exhibited cytotoxic effect in both estrogen receptor (ER) positive and negative human breast cancer cell lines, which was further enhanced by the application of hyperthermia. Cytotoxicity of SynB1-ELP1-KLAK correlated with the cellular uptake of the polypeptide, with over 3-fold enhancement in internalization seen when hyperthermia was added to the treatment. Once internalized, SynB1-ELP1-KLAK was found to localize in the cytoplasm. Finally, the cytotxicity of SynB1-ELP1-KLAK was associated with mitochondrial membrane disruption and apoptosis induction, which were significantly enhanced by hyperthermia. The advantage of combining KLAK with ELP is that it can not only protect peptides from premature degradation in vivo, but it can also increase the tumor specificity of these peptides. At ~60 kDa in size, the ELP macromolecules can passively target to the tumor region via the enhanced permeability and retention effect (Maeda et al. 1992). Furthermore, the thermally responsive ELP can also be actively targeted to the tumor region by the application of hyperthermia focused at the tumor (Bidwell and Raucher 2010).
Materials and Methods
Synthesis and Characterization of SynB1-ELP-KLAK
The SynB1-ELP-KLAK construct was generated in the pET25b+ vector (Novagen) with slight modifications from a previously described procedure (Bidwell et al. 2010). Briefly, a 5’ phosphorylated DNA cassette encoding amino acids of SynB1-SfiI-KLAK was inserted within the NdeI and BamHI restriction sites of the pET vector. Then, the ELP insert was excised from pUC19 plasmids and ligated with a previously SfiI digested SynB1-SfiI-KLAK vector as described (Bidwell and Raucher 2005). Plasmids were transformed into XL1-Blue competent cells (Stratagene), and successful candidates were selected by DNA sequencing. The polypeptides used in this study are outlined in Table 1. The proteins were then expressed in E. coli BLR (DE3) competent cells (Novagen) using a hyperexpression protocol and purified by inverse thermal cycling as previously described (Meyer and Chilkoti 1999; Bidwell and Raucher 2005). The purity of the protein was assessed on a 7.5% SDS-PAGE gel using copper staining according to the manufacturer’s protocol (Biorad).
Table 1.
Polypeptides used in this study
| Polypeptide Name |
Polypeptide Sequence | Molecular Weight (kDa) |
|---|---|---|
| SynB1-ELP1- KLAK |
RGGRLSYSRRRFSTSTGRG-CGPGVG-(VPGX†G)150- VPGWPGSG-KLAKLAKKLAKLAK |
63.2 |
| SynB1-ELP2- KLAK |
RGGRLSYSRRRFSTSTGRG-CGPGVG-(VPGX‡G)160- VPGWPGSG-KLAKLAKKLAKLAK |
64.5 |
| SynB1-ELP1 |
RGGRLSYSRRRFSTSTGRG-GPG-(VPGX‡G)150- WPGSGGC |
61.8 |
| SynB1-ELP2 |
RGGRLSYSRRRFSTSTGRG-GPG-(VPGX‡G)160- WPGSGGC |
63.4 |
X is present in a V:G:A ratio of 5:3:2. Tt of ELP1 ~39 – 42 °C.
X is present in a V:G:A ratio of 1:7:8. Tt of ELP2 >> 37 °C.
SynB1 sequence is underlined.
KLAK sequence is italicized.
The thermal properties of ELP modified with SynB1 and/or KLAK were characterized by monitoring the turbidity of the protein solutions at different concentrations in response to increase in temperature. Protein solutions were prepared in 10% serum to simulate experimental conditions, because co-solutes can influence the transition temperature of ELP. Samples were heated from 20 to 80 °C at a rate of 1 °C/min using the thermal function of a multi-cell holder UV-visible spectrophotometer (Cary 100, Varian Instruments), and the turbidity at the UV-Vis range of 350 nm was collected as a function of temperature. Data was converted to % of maximum for each sample, and the Tt was defined as the temperature at which the turbidity of the solution was at half maximum. Furthermore, to evaluate the concentration dependence of ELP on Tt, the Tt from each sample was plotted against ELP concentration, and the resulting curve was fitted to a logarithmic equation.
Cell Culture
MCF-7 cells (American Type Culture Collection, ATCC) was grown and maintained as previously described (Bidwell and Raucher 2005). MDA-MB-231 cells (ATCC) were cultured in DMEM medium (Mediatech) suppletemented with 10% fetal bovine serum (Atlanta Biologicals) and 1% Penicillin-Streptomycin/Amphotericin B (Mediatech). The murine breast cancer E0771 cells (from Dr. F.M. Sirotnak, Memorial Sloan-Kettering Cancer Center, New York, NY) were grown in RPMI medium (Mediatech) supplemented with 10% fetal bovine serum, 1% Penicillin-Streptomycin/Amphotericin B, and 2 g/L of NaHCO3. All cell cultures were maintained at 37 °C atmosphere with 95% humidity and 5% CO2. Hyperthermia treatment was performed in a 42 °C incubator with 95% humidity and 5% CO2.
Cell Proliferation
The cell inhibitory effect of SynB1-ELP-KLAK was evaluated in both estrogen receptor (ER) positive (MCF-7) and negative (MDA-MB-231) human breast cancer cell lines. The ER+ murine breast cancer cell line E0771 was also included in this assay.
MCF-7 (10,000 cells/mL), MDA-MB-231 (8000 cells/mL) and E0771 (4000 cells/mL) were plated in 24-well tissue culture plates, and incubated for 24 h at 37 °C. On day 0 cells were either exposed to media only or media containing various concentrations of SynB1-ELP1-KLAK for 1 h at 37 or 42 °C. In addition, MCF-7 cells were also exposed to the control polypeptides SynB1-ELP2-KLAK, SynB1-ELP1, or SynB1-ELP2 at 80 µM for 1 h at 37 or 42 °C. Treatments were removed and replaced with 1 mL of fresh media. Cells were incubated at 37 °C, and the treatment was repeated again on day 3. On day 6, 72 h after the second treatment, cells were collected by trypsinization, washed in 1 mL phosphate buffered saline (PBS), and counted using Coulter Counter (Beckman Coulter).
Cellular Uptake
2×105 MCF-7 cells were plated in 6-well tissue culture plates. Cells were allowed to grow for 24 h at 37 °C. The next day cells were treated with 40 µM fluorescein-labeled ELP1, SynB1-ELP1-KLAK, or SynB1-ELP2-KLAK for 1 h at 37 or 42 °C. Polypeptide labeling with fluorescein is described elsewhere (Bidwell and Raucher 2005). After treatment, cells were washed with PBS, and collected using a non-enzymatic cell dissociation buffer (Invitrogen). Total mean fluorescence was measured using the Gallios Flow Cytometer (Beckman Coulter). Cell debris was excluded from the forward vs. side scatter plot. Mean fluorescence of each treatment was corrected for the labeling efficiency of the respective polypeptide, and normalized to autofluorescence from untreated cells. In order to distinguish extracellular attachment of the polypeptides from cellular internalization, 10–15 µL of tyrpan blue was added to the cell samples to quench the extracellular flourescein signal (Hed et al. 1987; Raucher and Chilkoti 2001). Trypan blue exposed cells were then analyzed by flow cytometry.
Laser Scanning Confocal Microscopy
The subcellular localization of SynB1-ELP-KLAK was verified by confocal microscopy. Briefly, MCF-7 cells were plated at ~50% confluence on cover slips. Cells incubated at 37 °C for 24 h were exposed to 40 µM of rhodamine-labeled SynB1-ELP1-KLAK at 37 or 42 °C for 1 h. Polypeptide labeling with rhodamine is described elsewhere (Bidwell and Raucher 2005). After treatment, cells were allowed to grow at 37 °C. 72 h after treatment cells were washed with PBS, incubated with 40 nM Mito Tracker® Green (Invitrogen) for 30 min at 37 °C, washed with PBS, and mounted on glass slides. Live cells were then imaged using Leica TCS SP2 laser scanning confocal microscope (Leica).
Apoptosis Assay
MCF-7 cells were plated at 50,000 cells/well in 6-well plates. Cells were left untreated or treated with 80 µM SynB1-ELP1-KLAK, SynB1-ELP2-KLAK, or SynB1-ELP1 at 37 or 42 °C for 1 h on day 0. Protein solutions were removed and cells were incubated at 37 °C in fresh media for 72 h. The treatment was repeated again on day 3. On day 6 both floating and adherent cells were harvested, washed in annexin buffer, and exposed to 2 µg/ml of propidium iodide (PI) and 5 µL of FITC annexin V reagent to determine the necrotic and the apoptotic cells, respectively. The Vybrant® Apoptosis Kit #3 (Invitrogen) was used for this assay. FITC and PI signals were measured using FL1 and FL3 channels, respectively in the Gallios Flow Cytometer and analyzed by Kaluza V1.1 software (Beckman Coulter). Fluorescence was estimated for a sample of 10, 000 cells. Forward versus side scatter gating was used to remove cell debris from the analysis. Data was normalized to untreated cells at 37 °C.
Mitochondrial Membrane Disruption
Since the KLAK peptide induces apoptosis by disrupting the mitochondrial membrane, we evaluated the effect of SynB1-ELP-KLAK on mitochondrial morphology by fluorescence microscopy. MCF-7 cells were plated at ~50% confluence on cover slips. Cells were left untreated or treated with 80 µM SynB1-ELP1-KLAK or SynB1-ELP1 at for 1 h at 37 or 42 °C on day 0 and day 3. After 1 h treatment, protein was removed and cells were allowed to grow at 37 °C in fresh media. On day 6, after removal of media, cells were washed with PBS, and exposed to 50 nM MitoTracker Red (Invitrogen) for 40 min at 37 °C. Cells were washed and exposed to 1 µg/ml of Hoechst 33342 for 10 min at room temperature. Cells were washed and fixed with 4% paraformaldehyde for 10 min at room temperature. After a final washing step, cells were imaged using an epifluorescent microscope. The mitochondrial dye selectively stains the mitochondria allowing to visualize any changes in mitochondrial structure due to the treatment. Hoechst 33342 staining was used to visualize the cell nuclei.
Statistical Analyses
Differences among untreated controls and polypeptide treatment both at 37 °C and 42 °C were analyzed using a one-way ANOVA and a post-hoc Bonferroni multiple comparison with a 95% confidence interval.
Results
Thermal Characteristics of SynB1-ELP-KLAK
The polypeptides listed in Table 1 were purified by inverse thermal cycling (Bidwell and Raucher 2005) and ran on SDS-PAGE to evaluate purity before use. The polypeptides are seen as a single band on a representative gel indicating that the inverse thermal cycling technique yields highly purified proteins (Figure 1A). To evaluate the effect of addition of SynB1 and KLAK to ELP on its Tt, the turbidity of various concentrations of SynB1-ELP1-KLAK was monitored as a function of temperature at the UV-vis wavelength of 350 nm. Protein samples were prepared in 10% serum to account for co-solutes present in the media that could influence the Tt. A % of maximum plotted against temperature curve gave the Tt, which is defined as the temperature at which turbidity of a given sample reaches 50% of the maximum. The Tt of 20 – 100 µM SynB1-ELP1-KLAK ranged from 39 to 42 °C (Figure 1B), which is ideal for thermal targeting. The increase in turbidity of the ELP solution with respect to temperature is consistent with ELP aggregate formation as determined by differential light scattering elsewhere (Raucher and Chilkoti 2001).The concentration dependence of ELP on Tt is broad as shown by the logarithmic fit of the Tt versus concentration curve (Figure 1C). Since the curve asymptotes at y = 37 °C, potentially higher concentrations of SynB1-ELP1-KLAK can be used without resulting in aggregation of the polypeptide at physiological temperature. In contrast, SynB1-ELP2-KLAK, which is based on the thermally insensitive ELP variant (ELP2), has a Tt of 66.7 °C at 80 µM (Figure 1D). Therefore, SynB1-ELP2-KLAK is an effective control to parse the non-specific effects of hyperthermia on ELP performance. The Tt of 80 µM SynB1-ELP1 was 37.5 °C and that of 80 µM SynB1-ELP2 was 61.8 °C (data not shown) indicating that addition of KLAK does shift the Tt of the ELP system, although this does not drastically change the overall Tt.
Figure 1. Thermal Characterization of SynB1-ELP-KLAK.

SDS-PAGE (7.5%) visualized by copper staining of 10, 20 and 30 µg of SynB1-ELP1-KLAK and SynB1-ELP2-KLAK, and 20 µg of SynB1-ELP1 and SynB1-ELP2 shows a single band for each polypeptide indicating that inverse thermal cycling yields proteins with high level of purity (A). The turbidity of SynB1-ELP1-KLAK at indicated concentrations was monitored as a function of temperature at the UV-vis range (B). Turbidity values are plotted as % of maximum. The transition temperature (Tt) is defined as the temperature at which turbidity is 50% of the maximum. The Tt values from (B) were plotted against the protein concentration and fitted using a logarithmic equation to determine the optimum concentration range in which Tt lies between 37 and 42 °C; R2 = 0.93 (C). The turbidity profile of equimolar concentrations of thermally responsive SynB1-ELP1-KLAK and thermally insensitive SynB1-ELP2-KLAK was compared at 80 µM (D).
Cytotoxic Effect of SynB1-ELP-KLAK in Breast Cancer Cells
The anti-proliferative effect of SynB1-ELP1-KLAK was evaluated in the ER+ MCF-7 and the ER- MDA-MB-231 human breast cancer cell lines. The ER+ murine breast cancer cell line E0771 was also included in this assay. SynB1-ELP1-KLAK killed approximately 20 – 50 % of breast cancer cells at the highest concentration tested at 37 °C. However, the killing effect was much more pronounced at 42 °C resulting in approximately 65 to 90% cell death (Figures 2A, B and C). It is important to note here that hyperthermia only did not have any effect on cell proliferation. At the same time the control polypeptides, SynB1-ELP2-KLAK, SynB1-ELP1 and SynB1-ELP2 had little or no effect on MCF-7 cell proliferation at 37 or 42 °C compared to SynB1-ELP1-KLAK at the same concentration at 42 °C (Figure 2D). These results indicate that heat triggered phase transition is necessary for the enhancement of the cytotoxic effect of SynB1-ELP1-KLAK in breast cancer cells.
Figure 2. Cytotoxic Effect of SynB1-ELP-KLAK in Breast Cancer Cells.
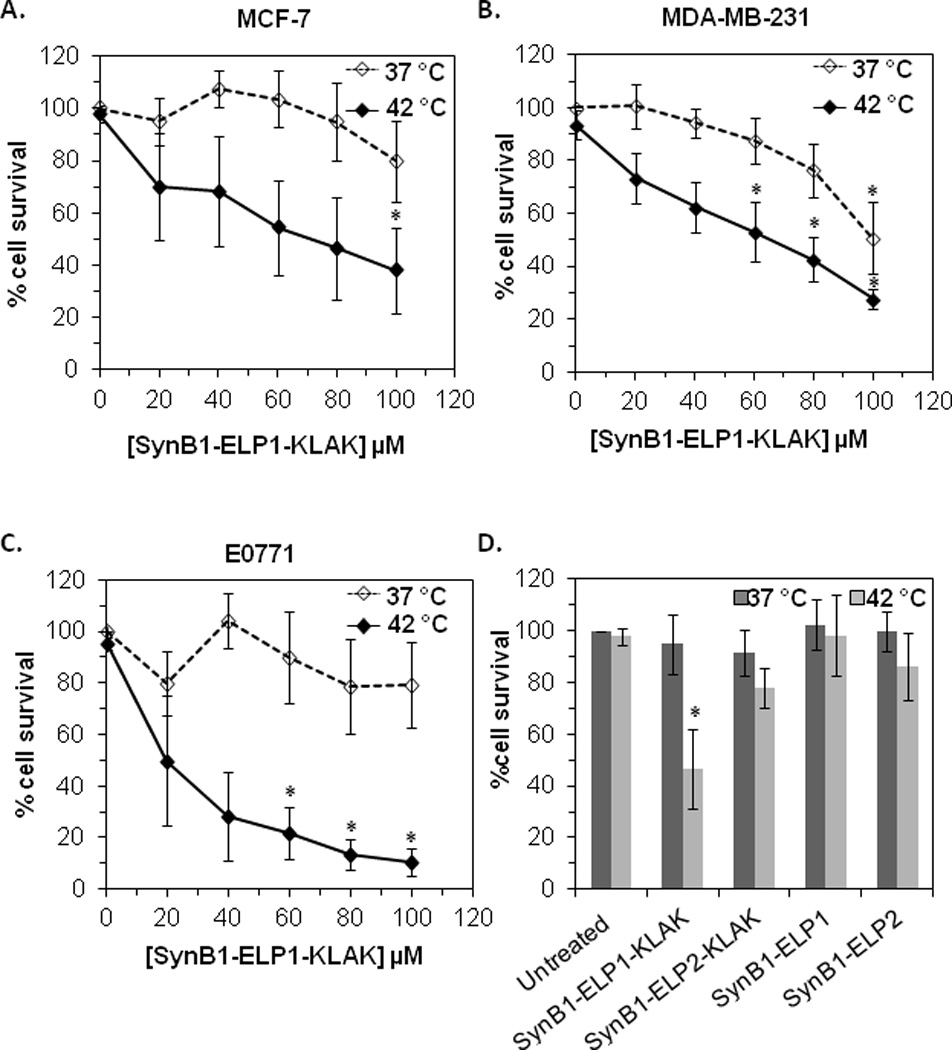
The cytotoxic effect of SynB1-ELP1-KLAK was evaluated in (A) MCF-7, (B) MDA-MB-231 and (C) E0771 cells at the indicated concentrations. MCF-7 cells were also treated with the control polypeptides - SynB1-ELP2-KLAK, SynB1-ELP1 or SynB1-ELP2 at 80 µM and compared with the effect at the same concentration of SynB1-ELP1-KLAK (D). Cell counts were taken using a Coulter Counter. % survival is calculated with respect to untreated cells at 37 °C. Data represents mean ± S.E.M of at least three independent experiments. * indicates p<0.001 for treatment vs. untreated control at 37 °C.
Cellular Uptake of SynB1-ELP-KLAK
To determine if the cytotoxic effect of SynB1-ELP-KLAK in combination with hyperthermia is due to enhanced cellular internalization of the polypeptide, we evaluated the cellular uptake of SynB1-ELP1-KLAK by flow cytometric analysis of the cells’ mean fluorescence intensity. As shown in Figure 3A, uptake of ELP1 alone is poor at 37 °C and treatment at 42 °C does not improve the uptake. In contrast, addition of the cell penetrating moiety SynB1 to ELP1-KLAK improves the uptake of the polypeptide by nearly 1.6-fold compared to ELP1 alone at 37 °C. When the treatment was carried out at 42 °C, SynB1-ELP1-KLAK uptake was significantly higher by nearly 2.5-fold compared to ELP1 uptake. At the same time, SynB1-ELP2-KLAK displayed limited cellular uptake and showed no significant difference relative to ELP1 following treatment at 37 or 42 °C. Furthermore, the treatment at 42 °C did not enhance the uptake of SynB1-ELP2-KLAK relative to treatment at 37 °C. These data indicate that the SynB1cell penetrating motif on SynB1-ELP1-KLAK is necessary for cellular uptake, and that hyperthermia further improves this uptake by triggering phase transition of the polypeptides and not by any non-specific effects. In order to compare cellular internalization versus extracellular attachment of SynB1-ELP1-KLAK-fluorescein, fluorescence outside the cells was quenched by using trypan blue (Hed et al. 1987). When the treatment was carried out at 37 °C, SynB1-ELP1-KLAK internalization was significantly higher by 2-fold than ELP1 alone. At 42 °C, the difference in uptake between SynB1-ELP1-KLAK and ELP1 alone was even higher at nearly 3-fold (Figure 3A). These data are consistent with the previous data and strongly suggest that SynB1 mediates the intracellular uptake of the SynB1-ELP1-KLAK polypeptide, and that hyperthermia enhances this effect. A representative plot of the cells incubated with fluorescein-labeled ELP1, SynB1-ELP1-KLAK, or SynB1-ELP2-KLAK polypeptides is shown in Figure 3B. Since the raw data is uncorrected for labeling efficiency of each polypeptide, the distinction between total and internal fluorescence is not so obvious. Regardless, the highest mean fluorescence is only observed with SynB1-ELP1-KLAK treatment (Figure 3B).
Figure 3. Cellular Uptake and Subcellular Distribution of SynB1-ELP-KLAK.
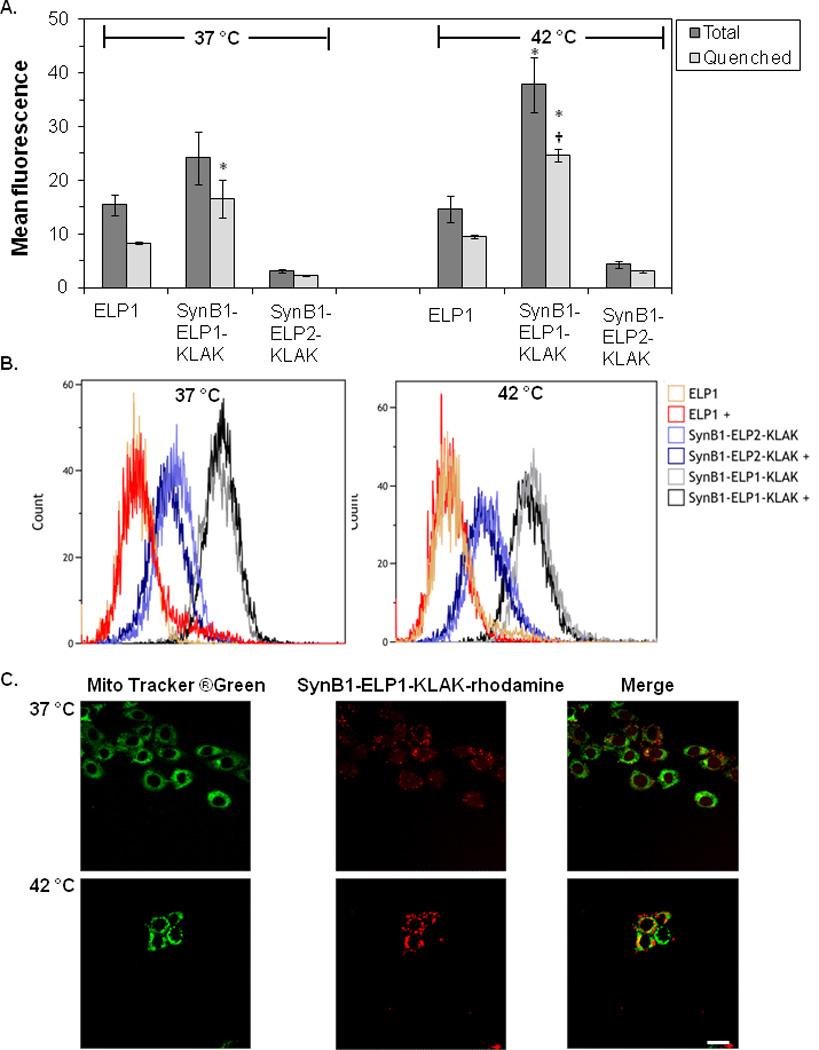
The uptake of fluoresceinlabeled SynB1-ELP1-KLAK polypeptide was compared to that of the ELP1 polypeptide that lacked the cell penetrating SynB1 peptide. Total and quenched fluorescein signal was measured using a flow cytometer. Mean ± S.E.M of three independent experiments. * indicates p<0.001 for treatment vs. ELP1 control at 37 °C. † indicates p<0.001 for the same treatment at 37 °C vs. 42 °C (A). A representative histogram of the cells treated with the indicated fluorescein-labeled polypeptides is shown in overlay. + indicates the addition of trypan blue to a subset of samples to quench the extracellular signal (B). Confocal scans of rhodamine-labeled SynB1-ELP1-KLAK treated cells show that the polypeptide localizes exclusively in the cytoplasm. Scale bar = 20 µm (C).
Subcellular localization of SynB1-ELP-KLAK
The subcellular distribution of SynB1-ELP-KLAK following cellular internalization was evaluated next. MCF-7 cells treated with rhodamine labeled SynB1-ELP1-KLAK were costained with Mito Tracker® Green to mark the cytoplasm. Cells were then imaged using confocal microscopy. At 37 °C, SynB1-ELP1-KLAK showed distinct cytoplasmic and membrane staining as indicated by punctate marks inside and outside the cells (Figure 3C). At 42 °C, SynB1-ELP1-KLAK also displayed cytoplasmic stanining, but mainly as aggregates due to the phase transition of ELP1 at this temperature (Figure 3C). These data further verify that following cellular internalization of SynB1-ELP1-KLAK, it effectively localizes in the cytoplasm. Furthermore, since the use of Mito Tracker® Green required imaging of live cells, the aggregates seen after SynB1-ELP1-KLAK treatment are not a product of fixation artifacts, rather they are due to hyperthermia mediated phase transition of SynB1-ELP1-KLAK.
Induction of Apoptosis by SynB1-ELP-KLAK
Previous studies have demonstrated that once internalized, KLAK triggers apoptosis in a variety of cells (Ellerby et al. 1999; Mai et al. 2001; Fantin et al. 2005; Rege et al. 2007; Kwon et al. 2008; Standley et al. 2010). Hence, we evaluated the apoptotic potential of SynB1-ELP1-KLAK and determined whether hyperthermia enhances this potential. Induction of apoptosis by the control polypeptide SynB1-ELP1 or SynB1-ELP1-KLAK at 37 °C was at basal levels (Figure 4). The apoptotic effect of SynB1-ELP1 remained similar to that of the untreated cells even at 42 °C. However, there was a 6-fold increase in apoptotic induction in cells treated with SynB1-ELP1-KLAK at 42 °C compared to the same treatment at 37 °C (Figure 4). These data suggest that hyperthermia mediated phase transition of SynB1-ELP1-KLAK enhances its apoptotic effect.
Figure 4. Induction of Apoptosis by SynB1-ELP-KLAK.

Annexin assay was performed to evaluate the induction of apoptosis by SynB1-ELP1-KLAK in MCF-7 cells. Flow cytometry analyses of the treated cells suggest that hyperthermia significantly enhances the apoptotic effect of SynB1-ELP1-KLAK relative to the unheated treatment. Mean ± S.E.M. * indicates p<0.001 for treatment at 42 °C vs. untreated control at 37 °C. † indicates p<0.001 for the same treatment at 37 °C vs. 42 °C.
Mitochondrial Membrane Disruption by SynB1-ELP-KLAK
Previous studies have shown that the α-helical KLAK peptide mediated apoptosis is associated with mitochondrial membrane disruption (Ellerby et al. 1999; Standley et al. 2010). To evaluate if SynB1-ELP mediated delivery of the KLAK peptide retains this function, we treated MCF-7 cells with SynB1-ELP1 or SynB1-ELP1-KLAK at 37 or 42 °C. The alteration in mitochondrial and nuclear morphology in response to the treatment was assessed by the Mitotraker Red dye and Hoecsht 33342, respectively. The extended lace-like network of normal mitochondria found in untreated cells and SynB1-ELP1 treated control cells were replaced by condensed clump structures in SynB1-ELP1-KLAK treated cells, which is similar to the observations made by Ellerby et al. (1999) (Figure 5). Most notably, this alteration in mitochondrial structure was only observed under hyperthermia condition, and not when cells were treated at 37 °C (Figure 5), which is consistent with the results from previous assays. Similarly, treatment with the control polypeptide, SynB1-ELP1 either at 37 or 42 °C, did not change the mitochondrial morphology, again indicating that the membrane disrupting effect is unique to SynB1-ELP1-KLAK.
Figure 5. Disruption of Mitochondria Structure by SynB1-ELP-KLAK.
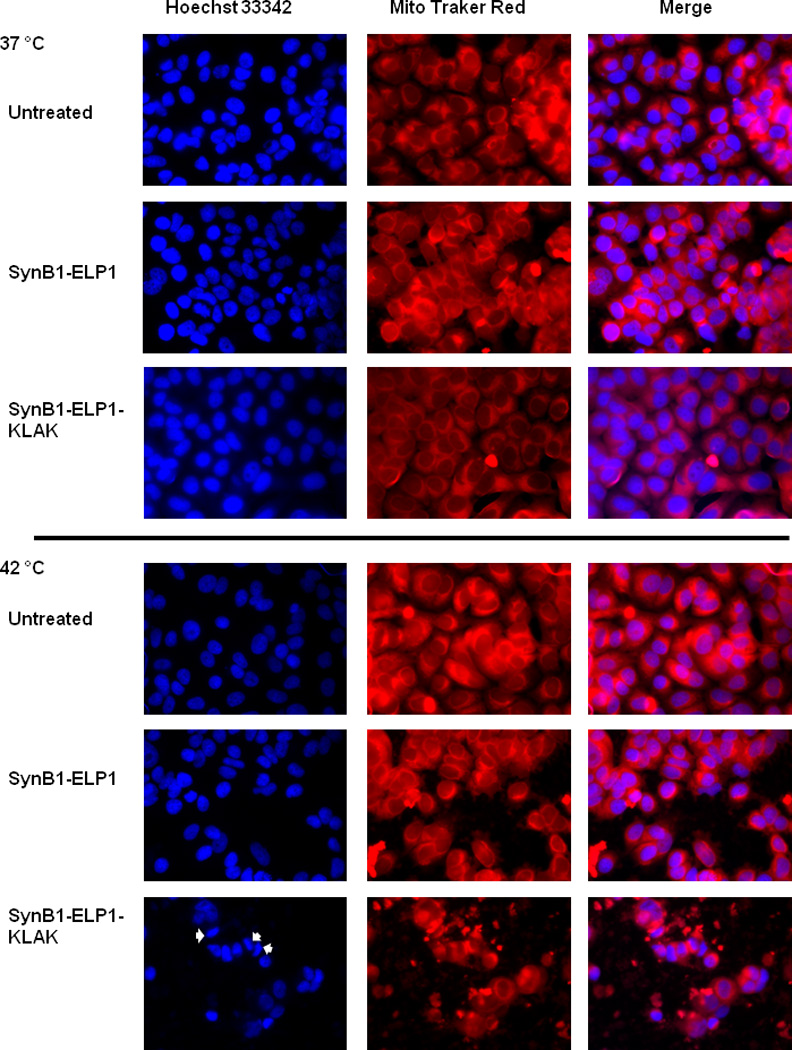
To evaluate the effect of SynB1-ELP1-KLAK on mitochondria, MCF-7 cells were exposed to the indicated polypeptides at 80 µM. Cells were stained with Mito Tracker® Red and Hoecsht 33342 to image the mitochondria and nuclei, respectively. Condensed nuclei indicating apoptotic cells are evident following SynB1-ELP1-KLAK treatment (arrows).
Discussion
The field of targeted cancer therapeutics can benefit significantly from rationally designed therapeutic peptides that modulate aberrant protein function in cancer cells. As our insight into protein structure, protein sequences, and protein-protein interactions continues to grow, a library of peptides can be designed with exact specifications to inhibit or simulate protein-protein interactions in cancer cells (Raucher et al. 2009). However, the use of peptides as therapeutics is riddled with physiological challenges, mainly in vivo degradation and tumor permeability (Raucher et al. 2009). Although the use of non-natural amino acid is a viable solution to the plasma stability problem, it does not address the tumor penetration issue. The use of cell penetrating macromolecular carriers can address both these major issues in the further development of therapeutic peptides.
Our previous studies with the thermally responsive biodegradable polymer, elastin-like polypeptide (ELP) have shown that ELP functionalized with a cMyc inhibitory peptide, a p21 mimetic peptide, or a peptide that inhibits interaction of Sm core proteins with the survival of motor neuron proteins has potent activity against cancer cells (Bidwell and Raucher 2005; Bidwell et al. 2010; Massodi et al. 2010). Furthermore, hyperthermia-mediated aggregation of ELP resulted in an enhanced cell inhibitory effect of these peptides (Bidwell and Raucher 2005; Bidwell et al. 2010; Massodi et al. 2010), indicating that ELP has a potential application in peptide therapeutics. Therefore, in this study we used the ELP vector to deliver the proapoptotic peptide, KLAKLAKKLAKLAK (abbreviated KLAK). Although earlier studies with KLAK have demonstrated the effectiveness of this peptide when combined with cell penetrating moieties (Ellerby et al. 1999; Mai et al. 2001; Fantin et al. 2005; Rege et al. 2007; Kwon et al. 2008), KLAK fused to ELP has several advantages over these strategies – (1) ELP sequence can be easily modified with a cell penetrating peptide (CPP) sequence. ELPs affixed with CPPs can not only extravasate into tumor tissues, but can also localize in the cancer cells (Bidwell and Raucher 2010), (2) ELP terminal plasma half-life is ~8.7 h (Liu et al. 2006), therefore it can impart plasma stability to short peptides like KLAK, and (3) ELP has increased tumor targeting because it can be both actively and passively targeted to the tumor by the application of hyperthermia and through the enhanced permeability and retention effect, respectively (Raucher et al. 2008).
In the present study, we have demonstrated that the SynB1-ELP1-KLAK fusion polypeptide undergoes inverse phase transition within the thermal targeting range of 39–42 °C (Figure 1B), which is ideal for the use of mild hyperthermia without increasing the incidence of edema and necrosis in healthy tissue surrounding a heated tumor (Liu et al. 2006). The thermally insensitive variant SynB1-ELP2-KLAK undergoes phase transition at T >> 42 °C (Figure 1D). Therefore, it serves as an effective control to parse the non-specific effects of hyperthermia. In the proliferation assays, SynB1-ELP1-KLAK treatment at 37 °C had marginal cytotoxitc effect on estrogen receptor positive MCF-7 and estrogen receptor negative MDA-MB-231 human breast cancer cell proliferation as well as estrogen receptor positive E0771 murine breast cancer cell proliferation (Figures 2A, B and C). However, the same treatment at 42 °C caused a concentration-dependent decrease in cell proliferation irrespective of the receptor status of the cell lines (Figures 2A, B and C). Application of hyperthermia on the thermally unresponsive control SynB1-ELP2-KLAK had marginal effect on MCF-7 cell proliferation. Furthermore, the polypeptides lacking the KLAK peptide – SynB1-ELP1 and SynB1-ELP2 did not have any effect on MCF-7 cell proliferation at 37 or 42 °C (Figure D). This suggests that the cell inhibitory effect of SynB1-ELP1-KLAK is enhanced by hyperthermia-induced phase transition of ELP1. In order to determine if the enhanced cytotoxicity of SynB1-ELP1-KLAK observed at 42 °C is due to enhanced uptake, we investigated the cellular uptake of SynB1-ELP1-KLAK at 37 and 42 °C and compared it to ELP1 alone. Indeed, SynB1-ELP1-KLAK showed significantly higher cellular uptake relative to ELP1 alone confirming that the cell penetrating moiety SynB1 is necessary to facilitate uptake. As shown by the total vs. quenched fluorescence data, the uptake of SynB1-ELP1-KLAK is associated with the actual internalization of the polypeptide, and not mere attachment of the polypeptide on the cell surface. Moreover, uptake of SynB1-ELP1-KLAK was enhanced when hyperthermia was added to the treatment (Figures 3A and 3B). This is because hyperthermia-induced phase transition of ELP1 causes the polypeptides to aggregate in response to increase in temperature from ~7 nm monomeric particles at 37 °C to ~100 nm particles at 42 °C. (Raucher and Chilkoti 2001). As a result, the aggregates pool out of the media, attach to the cell surface and get endocytosed over time because of the SynB1 peptide (Rousselle et al. 2000). Since ELP1 phase transition is a reversible process, once hyperthermia is removed, the polypeptides re-dissolve and are free to distribute to the subcellular target site. Therefore, the purpose of hyperthermia is to increase the local concentration of the polypeptide that is delivered inside the cell (Bidwell and Raucher 2005; Bidwell et al. 2007; Bidwell et al. 2009). This is further supported by the data that hyperthermia had no effect on the uptake of the thermally insensitive control SynB1-ELP2-KLAK, which does not undergo phase transition at 42 °C (Figure 3A). Since SynB1 is known to mediate cellular uptake via endocytosis (Rousselle et al. 2000), the subcellular distribution of SynB1-ELP1-KLAK following its endocytosis was examined by confocal microscopy. The confocal images confirm that SynB1-ELP1-KLAK effectively localizes in the cytoplasm (Figure 3C). This is especially important because the KLAK peptide has a cytoplasmic target. Since fluorescence intensity of the 42 °C sample was higher and the gain had to be adjusted before image acquisition, the images in Figure 3C are not directly quantitative.
To determine the mechanism of cytototxicity of SynB1-ELP1-KLAK, we investigated its role in mediating apoptosis. Data from the annexin assay confirmed that the cytotoxic effect of SynB1-ELP1-KLAK was due to the significantly higher induction of apoptosis at 42 °C compared to 37 °C treatment, which was consistent with the results from the cell proliferation assays (Figure 4). SynB1-ELP1-KLAK was also found to disrupt the mitochondrial structure at 42 ° C (Figure 5). These results suggest that the cytotoxic effect of SynB1-ELP1-KLAK may be a result of apoptotic cell death through the disruption of the mitochondria. This observation is consistent with previous studies which show that the KLAK peptide selectively triggers apoptosis (Ellerby et al. 1999; Mai et al. 2001; Fantin et al. 2005; Rege et al. 2007; Kwon et al. 2008). It is also consistent with the findings of Ellerby et al. (1999) who showed that the toxicity of the KLAK peptide is mainly due to the disruption of the mitochondrial membrane.
By designing the SynB1-ELP-KLAK fusion polypeptide, we have created an anticancer agent that in response to hyperthermia has high affinity for breast cancer cells. Hyperthermia is a clinically accepted method routinely used in the treatment of glioblastoma, head and neck cancer, breast cancer, cancer of the gastrointestinal or urogenital tract, and sarcoma (reviewed in (Dewhirst et al. 1997; Falk and Issels 2001; Takahashi et al. 2002)). It is accomplished using microwave, radio-frequency, and high-intensity focused ultrasound (HIFU) that allows precise heating of deep-seated tissues. Therefore, devices to generate hyperthermia for use with ELP therapeutics are already at hand in current clinical settings. Further studies with SynB1-ELP1-KLAK include investigating its therapeutic potential in vivo. We expect that the thermal targeting features combined with the pharmacokinetic properties of ELP will potentiate the antitumor effect of KLAK. This can result in a potent peptide therapeutic, and solidify the role of ELP as a platform to produce more effective peptide-based drugs.
Acknowledgements
This work was supported by grants from the National Science Foundation (CBET-0931041) and National Institute of Health (1R21CA137418-01A2 and 1R21CA139589-01). We would like to thank Ms. Rebecca Singleterry for technical assistance.
References
- Bidwell GL, 3rd, Davis AN, et al. A thermally targeted elastin-like polypeptide-doxorubicin conjugate overcomes drug resistance. Invest New Drugs. 2007;25(4):313–326. doi: 10.1007/s10637-007-9053-8. [DOI] [PubMed] [Google Scholar]
- Bidwell GL, 3rd, Davis AN, et al. Targeting a c-Myc inhibitory polypeptide to specific intracellular compartments using cell penetrating peptides. J Control Release. 2009;135(1):2–10. doi: 10.1016/j.jconrel.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Bidwell GL, 3rd, Fokt I, et al. Development of elastin-like polypeptide for thermally targeted delivery of doxorubicin. Biochem Pharmacol. 2007;73(5):620–631. doi: 10.1016/j.bcp.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Bidwell GL, 3rd, Raucher D. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol Cancer Ther. 2005;4(7):1076–1085. doi: 10.1158/1535-7163.MCT-04-0253. [DOI] [PubMed] [Google Scholar]
- Bidwell GL, 3rd, Raucher D. Cell penetrating elastin-like polypeptides for therapeutic peptide delivery. Adv Drug Deliv Rev. 2010;62(15):1486–1496. doi: 10.1016/j.addr.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell GL, 3rd, Whittom AA, et al. A thermally targeted peptide inhibitor of symmetrical dimethylation inhibits cancer-cell proliferation. Peptides. 2010;31(5):834–841. doi: 10.1016/j.peptides.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilkoti A, Dreher MR, et al. Design of thermally responsive, recombinant polypeptide carriers for targeted drug delivery. Adv Drug Deliv Rev. 2002;54(8):1093–1111. doi: 10.1016/s0169-409x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Dewhirst MW, Prosnitz L, et al. Hyperthermic treatment of malignant diseases: current status and a view toward the future. Semin. Oncol. 1997;24(6):616–625. [PubMed] [Google Scholar]
- Dreher MR, Raucher D, et al. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy. J Control Release. 2003;91(1–2):31–43. doi: 10.1016/s0168-3659(03)00216-5. [DOI] [PubMed] [Google Scholar]
- Ellerby HM, Arap W, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med. 1999;5(9):1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia. 2001;17(1):1–18. doi: 10.1080/02656730150201552. [DOI] [PubMed] [Google Scholar]
- Fantin VR, Berardi MJ, et al. A bifunctional targeted peptide that blocks HER-2 tyrosine kinase and disables mitochondrial function in HER-2-positive carcinoma cells. Cancer Res. 2005;65(15):6891–6900. doi: 10.1158/0008-5472.CAN-05-0395. [DOI] [PubMed] [Google Scholar]
- Furgeson DY, Dreher MR, et al. Structural optimization of a "smart" doxorubicin-polypeptide conjugate for thermally targeted delivery to solid tumors. J Control Release. 2006;110(2):362–369. doi: 10.1016/j.jconrel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Hed J, Hallden G, et al. The use of fluorescence quenching in flow cytofluorometry to measure the attachment and ingestion phases in phagocytosis in peripheral blood without prior cell separation. J. Immunol. Methods. 1987;101(1):119–125. doi: 10.1016/0022-1759(87)90224-9. [DOI] [PubMed] [Google Scholar]
- Javadpour MM, Juban MM, et al. De novo antimicrobial peptides with low mammalian cell toxicity. J Med Chem. 1996;39(16):3107–3113. doi: 10.1021/jm9509410. [DOI] [PubMed] [Google Scholar]
- Kwon MK, Nam JO, et al. Antitumor effect of a transducible fusogenic peptide releasing multiple proapoptotic peptides by caspase-3. Mol Cancer Ther. 2008;7(6):1514–1522. doi: 10.1158/1535-7163.MCT-07-2009. [DOI] [PubMed] [Google Scholar]
- Liu W, Dreher MR, et al. Tumor accumulation, degradation and pharmacokinetics of elastin-like polypeptides in nude mice. J Control Release. 2006 doi: 10.1016/j.jconrel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Liu W, Dreher MR, et al. Tumor accumulation, degradation and pharmacokinetics of elastin-like polypeptides in nude mice. J Control Release. 2006;116(2):170–178. doi: 10.1016/j.jconrel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- MacEwan SR, Chilkoti A. Elastin-like polypeptides: biomedical applications of tunable biopolymers. Biopolymers. 2010;94(1):60–77. doi: 10.1002/bip.21327. [DOI] [PubMed] [Google Scholar]
- MacKay JA, Chen M, et al. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat Mater. 2009;8(12):993–999. doi: 10.1038/nmat2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Seymour LW, et al. Conjugates of anticancer agents and polymers: advantages of macromolecular therapeutics in vivo. Bioconjug. Chem. 1992;3(5):351–362. doi: 10.1021/bc00017a001. [DOI] [PubMed] [Google Scholar]
- Mai JC, Mi Z, et al. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 2001;61(21):7709–7712. [PubMed] [Google Scholar]
- Massodi I, Moktan S, et al. Inhibition of ovarian cancer cell proliferation by a cell cycle inhibitory peptide fused to a thermally responsive polypeptide carrier. Int J Cancer. 2010;126(2):533–544. doi: 10.1002/ijc.24725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massodi I, Thomas E, et al. Application of thermally responsive elastin-like polypeptide fused to a lactoferrin-derived peptide for treatment of pancreatic cancer. Molecules. 2009;14(6):1999–2015. doi: 10.3390/molecules14061999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer DE, Chilkoti A. Purification of Recombinant Proteins by Fusion with Thermally Responsive Polypeptides. Nature Biotechnology. 1999;17:1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- Moktan S, Ryppa C, et al. A thermally responsive biopolymer conjugated to an acid-sensitive derivative of paclitaxel stabilizes microtubules, arrests cell cycle, and induces apoptosis. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9560-x. [DOI] [PubMed] [Google Scholar]
- Raucher D, Chilkoti A. Enhanced uptake of a thermally responsive polypeptide by tumor cells in response to its hyperthermia-mediated phase transition. Cancer Res. 2001;61(19):7163–770. [PubMed] [Google Scholar]
- Raucher D, Massodi I, et al. Thermally targeted delivery of chemotherapeutics and anti-cancer peptides by elastin-like polypeptide. Expert Opin Drug Deliv. 2008;5(3):353–369. doi: 10.1517/17425247.5.3.353. [DOI] [PubMed] [Google Scholar]
- Raucher D, Moktan S, et al. Therapeutic peptides for cancer therapy. Part II - cell cycle inhibitory peptides and apoptosis-inducing peptides. Expert Opin Drug Deliv. 2009;6(10):1049–1064. doi: 10.1517/17425240903158909. [DOI] [PubMed] [Google Scholar]
- Rege K, Patel SJ, et al. Amphipathic peptide-based fusion peptides and immunoconjugates for the targeted ablation of prostate cancer cells. Cancer Res. 2007;67(13):6368–6375. doi: 10.1158/0008-5472.CAN-06-3658. [DOI] [PubMed] [Google Scholar]
- Rousselle C, Clair P, et al. New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy. Mol Pharmacol. 2000;57(4):679–686. doi: 10.1124/mol.57.4.679. [DOI] [PubMed] [Google Scholar]
- Standley SM, Toft DJ, et al. Induction of cancer cell death by self-assembling nanostructures incorporating a cytotoxic peptide. Cancer Res. 2010;70(8):3020–3026. doi: 10.1158/0008-5472.CAN-09-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I, Emi Y, et al. Clinical application of hyperthermia combined with anticancer drugs for the treatment of solid tumors. Surgery. 2002;131(1 Suppl):S78–S84. doi: 10.1067/msy.2002.119308. [DOI] [PubMed] [Google Scholar]
- Yamaoka T, Tamura T, et al. Mechanism for the phase transition of a genetically engineered elastin model peptide (VPGIG)40 in aqueous solution. Biomacromolecules. 2003;4(6):1680–1685. doi: 10.1021/bm034120l. [DOI] [PubMed] [Google Scholar]


