Abstract
Context
Because of epilepsy’s common occurrence, the narrow therapeutic and safety margins of antiepileptic medications, and the recognized complications of medication nonadherence in adults with epilepsy, identifying the rates, patterns, and predictors of nonadherence in children with epilepsy is imperative. The onset and evolution of antiepileptic drug nonadherence in children with newly diagnosed epilepsy remains unknown.
Objectives
To identify and characterize trajectories of adherence in children with newly diagnosed epilepsy over the first 6 months of therapy and to determine sociodemographic and epilepsy-specific predictors of adherence trajectories.
Design, Setting, and Patients
Prospective, longitudinal observational study of antiepileptic drug adherence in a consecutive cohort of 124 children (2–12 years old) with newly diagnosed epilepsy at Cincinnati Children’s Hospital Medical Center. Patients were recruited from April 2006 through March 2009, and final data collection occurred in September 2009.
Main Outcome Measure
Objective adherence measured using electronic monitors.
Results
Fifty-eight percent of children with newly diagnosed epilepsy demonstrated persistent nonadherence during the first 6 months of therapy. Group-based trajectory models identified 5 differential adherence patterns (Bayesian information criterion=−23611.8): severe early nonadherence (13%; 95% confidence interval [CI], 8%–20%), severe delayed nonadherence (7%; 95% CI, 3%–12%), moderate nonadherence (13%; 95% CI, 8%–20%), mild nonadherence (26%; 95% CI, 19%–34%), and near-perfect adherence (42%; 95% CI, 33%–50%). The adherence pattern of most patients was established by the first month of therapy. Socioeconomic status was the sole predictor of adherence trajectory group status ( [n = 115]; P < .001; partial r2 = 0.25), with lower socioeconomic status associated with higher nonadherence.
Conclusion
Five trajectory patterns were identified that captured the spectrum of nonadherence to antiepileptic drugs among children with newly diagnosed epilepsy; the patterns were significantly associated with socioeconomic status.
Epilepsy, a disorder of recurrent unprovoked seizures, affects 325 000 children younger than 15 years in the United States. Antiepileptic drugs have variable efficacy and the potential for both short- and long-term toxic effects. Nonadherence rates (defined as not taking antiepileptic medications as prescribed) in children with epilepsy are between 12% and 35% based on cross-sectional studies using self-report.1 Although unknown in pediatric epilepsy, in other chronic diseases, nonadherence demonstrates both intrapatient and interpatient variability over time, suggesting that it is a dynamic behavior.2,3 As such, nonadherence in children with epilepsy presents a potential ongoing challenge for achieving a key therapeutic goal of no seizures.
In adults with epilepsy, nonadherence has been associated with increased morbidity (eg, continued seizures),4 elevated mortality,5–8 and higher health care costs.9 It remains unclear whether nonadherence in children has similar consequences since it is fundamentally different, including involvement of parents, siblings, and peers as well as developmental processes (eg, tantrums, puberty). Delineating the dynamic nature of nonadherence patterns in children, along with associated predictors, logically precedes examination of whether the consequences of nonadherence in children are similar to those in adults.
Identifying differential trajectories of adherence in pediatric epilepsy is important to determine subgroups at the highest risk of nonadherence, critical periods for adherence intervention, and minimal adherence thresholds to optimize antiepileptic drug efficacy. Because nonadherence is a dynamic process,2,3 evidence-based adherence intervention10 needs to be targeted to those at highest risk and during the period of greatest need. For example, children with new-onset epilepsy and their caregivers may be quite adherent initially,11 but adherence may falter if seizures are under good control. Furthermore, in contrast to other diseases,12,13 the target threshold for adherence in pediatric epilepsy has yet to be identified.
The purpose of the current study is to identify and characterize adherence trajectories for children with new-onset epilepsy using objective electronic monitors and to identify sociodemographic and epilepsy-specific predictors of adherence trajectories. It was hypothesized that a minimum of 3 distinct adherence trajectory subgroups (eg, high, medium, and low) would be identified and that these trajectories would be predicted from patient-specific factors such as child age, socioeconomic status (SES), parent marital status, sex, and epilepsy type based on prior literature.3,11,14 Treatment factors, including specific seizure type and frequency, initial and total number of antiepileptic medications, frequency of adverse events, and who witnessed the first seizure (ie, parent or nonparent), were also hypothesized to affect adherence trajectories.
METHODS
Participants and Procedures
Study participants included a consecutive cohort of children with epilepsy and their primary caregivers seen at the new-onset seizure clinic at Cincinnati Children’s Hospital Medical Center from April 2006 to March 2009. Study inclusion criteria included (1) children 2 to 12 years of age; (2) new diagnosis of epilepsy and initiation of antiepileptic drug therapy; and (3) absence of significant developmental disorders (eg, autism, Down syndrome) or comorbid chronic illnesses requiring daily medication (eg, diabetes). For all study patients, epilepsy was diagnosed by a pediatric epileptologist based on history, examination, and electroencephalogram. In the new-onset seizure clinic, patients with localization-related epilepsy received carbamazepine; patients with all other types of epilepsy received valproic acid. Both medications were dosed on a twice-daily schedule.
After obtaining written informed consent from the caregiver and assent from the child, caregivers completed a demographics questionnaire and were given a cap and bottle to begin electronically monitoring adherence to their prescribed treatment. Caregivers were made aware that the cap monitored adherence to the prescribed antiepileptic drug and that these data would not be shared with the child’s clinicians. Electronic monitoring was used for all formulations (solid and liquid) of antiepileptic medication. Families were asked to remove pills or liquid from the bottle only at the time of dosing.
As a part of routine clinical care, patients returned to clinic approximately 1 month after diagnosis and every 3 months thereafter for follow-up appointments. Seizure frequency and spontaneously reported adverse events were recorded at every clinic visit; a change in medication occurred if inadequate seizure control or intolerable adverse events were noted. Information about their child’s epilepsy, antiepileptic medications, and prognosis were provided in both oral and written forms to the family by a pediatric epileptologist (first visit) or by a pediatric epilepsy nurse practitioner (subsequent visits). Caregivers received a $20 gift card at each study visit. The protocol and consent forms were approved by the Cincinnati Children’s Hospital Institutional Review Board.
Measures
Demographic Background Questionnaire
Primary caregivers completed a background questionnaire documenting the child’s age, sex, race/ethnicity, caregiver marital status, and caregiver level of education and occupation. Race and ethnicity were defined based on the National Institutes of Health categories (race: white, black/African American, Asian, American Indian or Alaska native, native Hawaiian or other Pacific islander, or biracial/multiracial; ethnicity: Hispanic or non-Hispanic), and caregivers selected responses from these defined categories. Race/ethnicity was assessed to describe the cohort. The revised Duncan score,15 an occupation-based measure of SES,16 was calculated for each family. For 2-caregiver households, the higher Duncan score, which ranged from 15 (representing unemployed) to 97 (representing occupations such as physicians), was used. Higher Duncan scores reflect higher SES.
Epilepsy and Treatment
Seizure type, seizure frequency, initial and total number of antiepileptic medications, and frequency of adverse events were recorded prospectively. Seizures were classified 2 ways: (1) using the International League Against Epilepsy standard classification (partial, generalized, or unclassified seizures) and (2) according to whether the seizure had a visible motor component at the time of diagnosis. Seizures described by the family as containing stiffening, jerking, shaking, or twitching were considered convulsive; all others were non-convulsive. Similar to other studies,17,18 seizure frequency was dichotomized to seizure-free or not seizure-free from the initiation of treatment to the 6-month treatment mark, given the cohort’s heterogeneous nature of seizure types and frequencies (ranging from multiple a day to monthly). Frequency of adverse events was defined as the number of adverse events spontaneously reported by caregivers at the 1-month follow-up visit.
Electronic Monitoring
The Medication Event Monitoring Systems Track-Cap (Aardex Group, Sion, Switzerland) is an electronic monitoring system that measures the dosing histories of patients prescribed oral medications. The cap contains a microelectronic circuit to register the dates and times the bottle is opened and closed. The device stores times and dates for up to 3518 events for a period of 36 months and the data can be transferred to a Windows-based computer. Data from the electronic monitors were downloaded at all follow-up clinic visits. Electronic monitoring data were assumed to be an accurate proxy for patients taking the correct medication dose. Caregivers were also given the opportunity to reveal days the electronic monitor was not used (eg, vacation) to ensure that the most accurate representation of adherence behaviors was collected. These nonmonitored periods were not used in analyses. For purposes of the current study, daily adherence rates were used in analyses for the first 6 months of antiepileptic drug therapy, with the last participant completing the clinic visit in September 2009. Daily adherence rates were 0, 50%, or 100% based on a twice-daily dosing schedule for most antiepileptic drugs.
Statistical Analysis
Adherence to antiepileptic drug treatment over time was described using group-based trajectory modeling (GBTM).19 Group-based trajectory modeling is appropriate when the goal is to use trajectory subgroups to identify and characterize differential patterns of individual change over time in the population of interest. When using GBTM, each individual is assumed to belong to 1 and only 1 group wherein each group has its own distinct trajectory.19 In this study, 3-, 4-, 5-, and 6-group solutions based on quadratic trajectories and a censored normal probability distribution for the percentage of adherence were compared to identify the number of groups that best characterized the data. The final model was selected based on a combination of the Bayesian information criterion (BIC; wherein the value closest to 0 indicates the best-fitting model) and estimated trajectory group proportions that were sufficiently large (eg, >0.05).20 The GBTM was estimated using SAS software, version 9.1 (SAS Institute Inc, Cary, North Carolina) and the PROC TRAJ macro (http://www.andrew.cmu.edu/user/bjones), a closed-source module developed specifically for use with SAS software.
After determining the most appropriate GBTM, group status for each individual was obtained to identify relevant predictors of adherence trajectory groups. An individual was assigned to the trajectory group in which he or she was most likely to be, as determined by the group posterior probabilities from the final model. A multinomial logistic regression model was specified to identify predictors of the adherence trajectory groups. The outcome variable was adherence trajectory group membership, and the testable predictors were child age, family SES, sex, caregiver marital status, seizure type and frequency, initial and total number of antiepileptic medications, frequency of adverse events, and who first observed the child’s seizure (eg, parent or someone else).
The logistic regression model was estimated using PROC LOGISTIC in SAS version 9.1. Partial r2 was calculated for statistically significant predictors using values.21,22 corrects for the fact that standard R2 measures generally have an upper limit of less than 1 for discrete variables and, consequently, allows for straightforward interpretation of the partial r2 in the present context. Statistical significance was defined as P < .05. Study participants were part of a larger longitudinal investigation of factors underlying variability in antiepileptic drug adherence in pediatric epilepsy; the larger study was determined to be adequately powered with a sample size of at least 93. As a result, power and sample size calculations for this study were not performed.
RESULTS
Participants
One hundred thirty consecutive children with new-onset epilepsy and their caregivers met study inclusion/exclusion criteria. Five children and their caregivers declined participation because of busy schedules and lack of interest. One child and parent, who provided written consent, were later found to not meet study eligibility criteria and were thus excluded. The final sample included 124 children (mean age, 7.2 [SD, 2.9] years; range, 2–12 years); 64% were male. The sample’s race composition was 75.8% white, 16.9% black, 6.5% biracial/multiracial, and 0.8% Asian; 3% of the sample was Hispanic. Forty-eight percent of the cohort was diagnosed as having idiopathic localization-related epilepsy, 19% idiopathic generalized epilepsy, 15% idiopathic unclassified epilepsy, 8% cryptogenic localization-related epilepsy, 5% cryptogenic generalized epilepsy, 5% symptomatic localization-related epilepsy, and 0.8% symptomatic generalized epilepsy. No children experienced prolonged seizures or status epilepticus during the study period. Carbamazepine was initially prescribed to 60% and valproic acid to 40%. Four patients (3%) were prescribed liquid medications and had successful electronic monitoring.
Primary caregivers of children with new-onset epilepsy were predominately mothers/stepmothers (85%) as well as fathers (13%) and other legal guardians (eg, aunts; 2%). Twenty percent of primary caregivers were single, 64% were married, and 16% were divorced, separated, or widowed. The mean family revised Duncan score, a measure of SES, was 52.39 (SD, 20.4). This score reflects occupations such as office supervisor, mail carrier, firefighter, and police officer.
Determining Adherence Trajectories
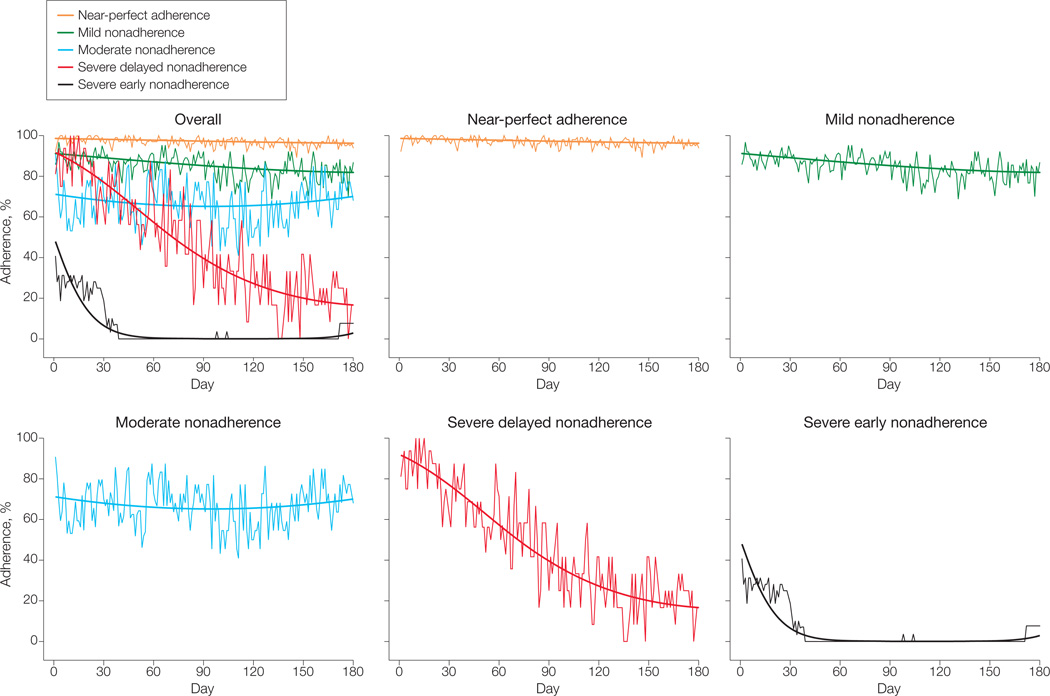
The BIC values and estimated group proportions for the 3-, 4-, 5-, and 6-groupGBTMmodels were used to determine the best GBTM model (Table 1). A 7-group model was tested but failed to converge. Results for the 5- and 6-group models were similar, with the primary difference being that group 1 in the 5-group model was essentially separated into groups 1 and 2 in the 6-group model. The 6-group model provided the best statistical fit based on the BIC; however, the extremely small proportion (<0.05) in group 2 was considered too small to be clinically useful or statistically stable.20 Consequently, the 5-group model was selected as the final model. Final model estimates for each adherence trajectory group are shown in Table 2. Additional diagnostic criteria for judging the adequacy of a GBTM are presented in Table 3 and demonstrate that the 5-group model performed well based on the Nagin criteria.19 The Figure illustrates each of these trajectories along with the averaged raw group data at each point. The 5 groups, based on severity and course of nonadherence, are severe early nonadherence (n = 16; 13%; 95% confidence interval [CI], 8%–20%), severe delayed nonadherence (n = 8; 7%; 95% CI, 3%–12%), moderate nonadherence (n = 16; 13%; 95% CI, 8%–20%), mild nonadherence (n = 32; 26%; 95% CI, 19%–34%), and near-perfect adherence (n = 52; 42%; 95% CI, 33%–50%). Table 4 presents descriptive data by adherence trajectory group.
Table 1.
Bayesian Information Criterion (BIC) Values and Predicted Group Proportions for Group-Based Trajectory Models for 3-, 4-, 5-, and 6-Group Adherence Trajectory Solutions
| Model | No. of Groups |
BIC | Predicted Group Proportions | |||||
|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |||
| 1 | 3 | −24159.1 | 0.13 | 0.34 | 0.54 | |||
| 2 | 4 | −23758.8 | 0.13 | 0.09 | 0.30 | 0.48 | ||
| 3 | 5 | −23611.8 | 0.13 | 0.07 | 0.13 | 0.26 | 0.42 | |
| 4 | 6 | −23307.4 | 0.09 | 0.03 | 0.14 | 0.07 | 0.26 | 0.42 |
Table 2.
Final 5-Group Group-Based Trajectory Model for Antiepileptic Drug Adherence
| Group | Estimate (95% Confidence Interval) |
t | P Value | |
|---|---|---|---|---|
| Severe early nonadherence | ||||
| Intercept | 50.54 (32.10 to 68.98) | 5.37 | <.001 | |
| Time | −6.518 (−7.347 to −5.689) | −15.42 | <.001 | |
| Time2 | 0.0297 (0.0266 to 0.0328) | 12.64 | <.001 | |
| Severe delayed nonadherence | ||||
| Intercept | 205.84 (182.81 to 228.87) | 17.51 | <.001 | |
| Time | −2.651 (−3.237 to −2.065) | −8.87 | <.001 | |
| Time2 | 0.0066 (0.0035 to 0.0097) | 4.15 | <.001 | |
| Moderate nonadherence | ||||
| Intercept | 112.28 (97.68 to 126.88) | 15.06 | <.001 | |
| Time | −0.399 (−0.787 to −0.011) | −2.02 | .04 | |
| Time2 | 0.0021 (0.0000 to 0.0043) | 1.98 | <.05 | |
| Mild nonadherence | ||||
| Intercept | 200.73 (187.85 to 213.61) | 30.55 | <.001 | |
| Time | −0.491 (−0.195 to −0.787) | −3.26 | .001 | |
| Time2 | 0.0012 (−0.0004 to 0.0028) | 1.48 | .14 | |
| Near-perfect adherence | ||||
| Intercept | 294.16 (277.97 to 310.35) | 35.60 | <.001 | |
| Time | −0.420 (−0.777 to −0.0633) | −2.30 | .02 | |
| Time2 | 0.0009 (−0.0011 to 0.0029) | 0.95 | .34 | |
Table 3.
Diagnostics for Group-Based Trajectory Model
| Group | Model Estimate of Group Probability (95% CI)a |
Proportion Classified in Groupb |
Average Posterior Probabilityc |
Odds Correct Classificationd |
|---|---|---|---|---|
| Severe early nonadherence | 0.13 (0.08–0.20) | 0.13 | >0.999 | 22 500 |
| Severe delayed nonadherence | 0.07 (0.03–0.12) | 0.07 | 0.999 | 14 370 |
| Moderate nonadherence | 0.13 (0.08–0.20) | 0.13 | 0.992 | 837 |
| Mild nonadherence | 0.26 (0.19–0.34) | 0.26 | 0.990 | 285 |
| Near-perfect adherence | 0.42 (0.33–0.50) | 0.42 | 0.995 | 276 |
95% confidence intervals (CIs) based on parametric bootstrap method.19
Proportion classified in group is based on the maximum posterior probability rule. The values of the proportion classified in the group should be similar to the model estimates of group probabilities in the second column.
Average posterior probability is obtained by averaging the posterior probabilities for a given group for all individuals placed in this group by the maximum posterior probability rule. Acceptable values for this criterion are 0.7 or greater for all groups.19
Acceptable values of the odds correct classification are 5.0 or greater for all groups.19
Figure.
Six-Month Adherence Trajectories of Children With New-Onset Epilepsy
Smooth curves represent model-based group trajectories.
Table 4.
Participant Characteristics by Adherence Trajectory Group
| Characteristics | Adherence Trajectory Groupa | |||||
|---|---|---|---|---|---|---|
| Severe Early Nonadherence (n = 16) |
Severe Delayed Nonadherence (n = 8) |
Moderate Nonadherence (n = 16) |
Mild Nonadherence (n = 32) |
Near-Perfect Adherence (n = 52) |
||
| Age, mean (SD), y | 6.9 (2.6) | 8.2 (2.8) | 6.8 (2.8) | 7.0 (3.1) | 7.4 (3.0) | |
| Family Duncan score, mean (SD)b | 42.4 (17.8) | 31.3 (16.7) | 37.1 (12.0) | 56.4 (19.0) | 60.8 (18.9) | |
| Female | 8 (50) | 4 (50) | 4 (25) | 12 (37.5) | 20 (38.5) | |
| Race/ethnicity | ||||||
| Non-Hispanic white | 10 (62.5) | 6 (75) | 10 (62.5) | 22 (68.75) | 45 (86.5) | |
| Hispanic white | 0 | 0 | 0 | 0 | 1 (1.9) | |
| Black | 6 (37.5) | 1 (12.5) | 4 (25) | 6 (18.75) | 4 (7.7) | |
| Biracial/multiracial | 0 | 1 (12.5) | 2 (12.5) | 4 (12.5) | 1 (1.9) | |
| Asian | 0 | 0 | 0 | 0 | 1 (1.9) | |
| Caregiver marital status | ||||||
| Single | 6 (37.5) | 2 (25) | 5 (31.25) | 6 (18.8) | 6 (11.5) | |
| Married | 8 (50) | 2 (25) | 7 (43.75) | 23 (71.9) | 39 (75.0) | |
| Divorced/separated/widowed | 2 (12.5) | 4 (50) | 4 (25) | 3 (9.4) | 7 (13.4) | |
| Initial antiepileptic drug | ||||||
| Carbamazepine | 11 (69) | 3 (37.5) | 9 (56) | 22 (69) | 30 (58) | |
| Valproic acid | 5 (31) | 5 (62.5) | 7 (44) | 10 (31) | 22 (42) | |
| No. of antiepileptic drugs prescribed, mean (SD) | 1.3 (0.48) | 1.1 (0.35) | 1.2 (0.40) | 1.3 (0.55) | 1.1 (0.34) | |
| Monotherapy | 16 (100) | 8 (100) | 16 (100) | 30 (94) | 51 (98) | |
| Twice-daily dosingc | 16 (100) | 8 (100) | 16 (100) | 30 (93.8) | 52 (100) | |
| Seizure type | ||||||
| Localization-related | 11 (68.75) | 3 (37.5) | 10 (62.5) | 22 (68.75) | 28 (54) | |
| Generalized | 2 (12.5) | 1 (12.5) | 3 (18.8) | 8 (25.0) | 16 (31) | |
| Unclassified | 3 (18.75) | 4 (50) | 3 (18.8) | 2 (6.25) | 8 (15) | |
| Convulsive seizures at diagnosis | 12 (75) | 5 (62.5) | 12 (75) | 18 (56) | 34 (65) | |
| No. of seizures prior to diagnosis, mean (SD) | 3.9 (4.8) | 2.7 (0.8) | 3.7 (2.9) | 3.8 (3.9) | 3.1 (1.2) | |
| Total adverse events at 1-mo follow-up, mean (SD) | 8.1 (9.2) | 8.1 (8.3) | 7.9 (5.1) | 7.2 (6.7) | 6.0 (5.5) | |
| Seizure activity over 6 mo | 5 (42) | 5 (62.5) | 10 (62.5) | 19 (59) | 33 (63.5) | |
| Parents observed first seizure | 11 (69) | 2 (25) | 12 (75) | 24 (75) | 35 (67) | |
| Adherence over 6 mo | 5.5 (22.0) | 49.1 (42.1) | 66.8 (36.6) | 84.7 (27.7) | 96.8 (13.0) | |
Data are expressed as No. (%) unless otherwise specified.
Compared with the near-perfect adherence group, family socioeconomic status was statistically significantly different in the severe early nonadherence group (P = .01), the severe delayed nonadherence group (P = .01), and the moderate nonadherence group (P < .001).
Represents twice-daily dosing after children completed titration schedules and were receiving a maintenance dose.
Predictors of Adherence Trajectories
The results of the multinomial logistic regression model for determining predictors of adherence trajectory groups are shown in Table 5. Because of the high correlation (Cramer V = 0.95) between initial antiepileptic drug and seizure type (ie, partial, generalized, unclassified), only the initial antiepileptic drug was retained in the model. No significant differences were noted between adherence trajectories for child age, sex, caregiver marital status, convulsive seizures at diagnosis, seizure frequency, initial and total number of antiepileptic medications, total number of adverse events, or who witnessed the first seizure. However, results revealed that family SES was the only significant predictor of adherence trajectory group status ( [n = 115]; P < .001; partial r2 = 0.25). Specifically, higher SES was associated with higher adherence trajectories. The model also yielded the following odds ratios (ORs) for a 10-unit increase in family SES using the near-perfect adherence trajectory group as the reference group compared with the other 4 adherence trajectory groups: severe early nonadherence, OR, 0.48 (95% CI, 0.27–0.87); P = .02; severe delayed nonadherence, OR, 0.30 (95% CI, 0.13–0.71); P = .01; moderate nonadherence, OR, 0.43 (95% CI, 0.26–0.69); P < .001; and mild nonadherence, OR, 0.91 (95% CI, 0.68–1.21); P = .52.
Table 5.
Multinomial Logistic Regression: Predictors of Adherence Trajectory Group Statusa
| Variable |
P Value |
||
|---|---|---|---|
| Child age, y | 2.63 | .62 | |
| Family socioeconomic status | 19.27 | <.001 | |
| Sex (reference: male) | 3.13 | .54 | |
| Caregiver marital status (married vs not married) | 3.54 | .47 | |
| Initial antiepileptic drug | 4.00 | .41 | |
| No. of drugs in 6 mo (1 vs >1) | 4.57 | .33 | |
| Convulsive seizures at diagnosis (yes vs no) | 6.11 | .19 | |
| Total No. of adverse events at 1-mo follow-up visit | 3.74 | .44 | |
| Seizure absence/presence during 6-mo study | 1.77 | .78 | |
| Parent observed first seizure (vs nonparent) | 4.92 | .30 |
R2 = 0.46; max-rescaled R2 = 0.49.
COMMENT
Nonadherence is a common and previously underrecognized problem for children with newly diagnosed epilepsy. Prior cross-sectional studies examining adherence in pediatric epilepsy reported nonadherence rates between 12% and 35% using self-report. These studies had multiple methodological problems including reporting mean adherence rates across the entire cohort; lack of rigorous, well-validated, objective measures of adherence14,17,23–25; lack of prospective, longitudinal designs23–25; and lack of a newly diagnosed homogenous, consecutive cohort of young children with epilepsy.14,23–25 These issues prevented rigorous and systematic examination of the variability and individual differences in adherence behaviors necessary to develop and implement evidence-based adherence interventions.
Given the results of our prior study demonstrating nonadherence rates of approximately 20% in the first month of therapy,11 the current results showing almost 60% of the cohort as nonadherent in the first 6 months were surprising. Socioeconomic status was the only significant predictor of nonadherence and may help identify patients at highest risk. Given that nonadherence is frequent, may compromise the benefits of drug therapy, may complicate interpretation of clinical response, and can be addressed through evidence-based interventions,10 clinicians should consider routinely assessing adherence to antiepileptic drug therapy in all children with epilepsy. Self-report measures of adherence have recently been developed for children with epilepsy26,27 and could be used in routine clinical care.
In the current study, children demonstrated significant intrapatient and interpatient variability based on objective adherence data. Five distinct groups were identified. The severe early nonadherence group reflects children who took between one-quarter and one-half of their antiepileptic drug doses in the first month of therapy and then became completely nonadherent over time. This suggests “volitional” nonadherence,28–30 wherein parents may have actively decided that their children should not take antiepileptic drugs based on reasoned decisions. Potential reasons cited in the larger literature include denial that the child has epilepsy, being seizure-free, believing that the risks of antiepileptic drugs outweigh those of seizures, fearing intolerable adverse effects, or having financial constraints. However, our data suggest that seizure frequency and adverse events played no role in determining adherence trajectories.
In contrast, the severe delayed nonadherence group initially had high adherence (90%) that gradually declined over time, with the group taking only about 20% of their antiepileptic drug doses 6 months after initiating treatment. While this group represents the smallest percentage of patients (7%) and demonstrated significant variability, this pattern may reflect caregivers who occasionally missed giving antiepileptic drug doses with no major health consequence (eg, seizure) and, thus, made decisions to discontinue antiepileptic drugs. These 2 groups represent children with epilepsy and their families who are most in need of adherence interventions focused on discussing the family’s beliefs regarding epilepsy and antiepileptic drugs and providing education about treatment misconceptions.
The moderate nonadherence group exhibited significant variability over time, with average adherence at about 70% (eg, missing 4 of 14 doses in any given week). Several factors may contribute to this pattern of adherence. For example, forgetting is the primary barrier to adherence across several pediatric populations,31,32 including pediatric epilepsy.14,24,27 The high variability in adherence may also have reflected families missing antiepileptic drug doses in blocked periods of time, such as when families go on vacation or when competing activities occur (eg, week-end sports). These families would benefit from problem-solving regarding barriers to adherence and instituting general behavioral and organizational strategies that are efficacious.10
The mild nonadherence group, conversely, demonstrated lower variability in nonadherence, with stable adherence rates at about 85%. One goal of epilepsy therapy is to attain seizure freedom for at least 2 years. Given the pharmacokinetic properties of antiepileptic drugs, including marked interpatient and intrapatient variability in blood levels and the unpredictable nature of seizures, it is unknown whether 85% adherence is sufficient to maintain the therapeutic benefit of antiepileptic drugs for this period. The target adherence threshold (ie, the minimal adherence rate necessary for symptom management) is disease-specific and undetermined for most diseases. It ranges from 80% in adult hypertension treatment12 to 95% for human immunodeficiency virus therapy13 and is unknown for adult or pediatric epilepsy. However, even mild nonadherence may have clinically important implications. In addition to the potential for continued seizures, drug toxic effects can develop if doses are increased or drugs added unnecessarily. Adherence interventions for the mild nonadherence group could potentially be delivered within the context of routine clinic visits compared with more intensive outpatient behavioral intervention. For example, psychosocial services within the Cincinnati Children’s Hospital’s new-onset seizure clinic provide brief problem-solving interventions for forgetting to take antiepileptic drugs when away from home (eg, visiting friends, grandparents), such as setting cell phone alarms or placing a few doses in the caregiver’s bag or purse.
The near-perfect adherence group, which represents less than half of the cohort (42%), demonstrated extremely stable patterns of high adherence during the first 6 months of antiepileptic drug therapy. Similar high-adherence subgroups have been identified in 2 other chronic disease populations.2,3 Families in the near-perfect adherence group have anecdotally reported incorporating antiepileptic drug administration into well-defined family routines,33,34 such as brushing teeth or eating meals. Furthermore, these families also likely have fewer barriers to adherence and, thus, are better able to manage epilepsy and its treatment.27,32 Families in the near-perfect adherence group are exemplars within clinical practice, need no intervention, and could serve as models for other families who are having difficulties administering antiepileptic drugs on a daily basis.
No seizure-related variables, including seizure type, seizure frequency, and frequency of adverse events differentiated adherence trajectory groups. Family SES was the only significant patient-specific predictor of adherence trajectories. Children with higher SES were more likely to demonstrate adherence trajectories characterized by better adherence. These results are similar to prior work in pediatric epilepsy11 and other reports in liver transplantation35 suggesting a positive association between adherence and SES. While it is not possible for clinicians to change the socioeconomic situation of families, this finding suggests the need to recognize that lack of financial resources places children with epilepsy at risk of nonadherence. Given the often intrinsic link between SES and education, it is plausible that limited financial resources have implications for both tangible (eg, inability to pay for medications) and intangible (eg, parental supervision36) aspects that contribute to poor adherence. Thus, proactive adherence promotion efforts are particularly salient for families who are economically disadvantaged.
To our knowledge, this study is the first to examine adherence trajectories for children with epilepsy; however, several limitations are noted with implications for future research. First, while research suggests the use of large sample sizes (eg, ≥200) for GBTM, others have successfully used sample sizes consistent with ours.2,37,38 An important area for future research is a confirmatory analysis of the trajectories identified.
Second, these data represent a consecutive cohort of children between 2 and 12 years of age with newly diagnosed epilepsy; thus, results may not be generalizable to adolescent and adult samples or to individuals with recurrent seizures or treatment-resistant epilepsy. Future studies should include a larger cohort of youth with epilepsy, including adolescents, to elucidate developmental differences in adherence and examine adherence patterns for youth with treatment-resistant epilepsy. In addition, it is possible that we found no differences in seizure activity and adverse events by adherence trajectories because of the heterogeneous nature of our sample and lack of validated tools to assess adverse events in children with epilepsy and quantify seizure activity.
Third, it is plausible that adherence behaviors may have been influenced by the monitoring itself (ie, reactivity). However, adherence research has demonstrated that reactivity is negligent or short-lived, with adherence behaviors returning to baseline shortly after monitoring is initiated.39–41
Fourth, the current study examined only sociodemographic and medical factors affecting adherence trajectories. We are currently examining psychosocial factors that contribute to adherence trajectories. Such factors, including internalizing (eg, anxiety and depression) and externalizing (eg, oppositional behaviors, inattention) disorders, epilepsy-related stigma, and knowledge about epilepsy may affect adherence and shed further light on families who are at the highest risk of nonadherence.
Finally, we were unable to examine the effect of adherence trajectories on health outcomes, including seizures and health-related quality of life. Six months of therapy is too short to rigorously determine the efficacy or effectiveness of antiepileptic drug therapy in a cohort of children with a variety of seizure types and baseline seizure frequencies,42,43 let alone the effect of differential nonadherence trajectories on ultimate seizure control. However, the rate of nonadherence over the course of the first 6 months of therapy is concerning and suggests a need for intervention studies that aim to optimize adherence early in the course of therapy.
Acknowledgments
Funding/Support: This research was funded by grant K23HD057333 from the National Institutes of Health awarded to Dr Modi.
Role of the Sponsor: The study sponsors had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Drs Modi and Rausch had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Modi, Glauser.
Acquisition of data: Modi.
Analysis and interpretation of data: Modi, Rausch, Glauser.
Drafting of the manuscript: Modi, Rausch, Glauser.
Critical revision of the manuscript for important intellectual content: Modi, Rausch, Glauser.
Statistical analysis: Modi, Rausch, Glauser.
Obtained funding: Modi.
Administrative, technical, or material support: Modi, Glauser.
Study supervision: Modi, Glauser.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Modi reports that she has been a consultant for Novartis Pharmaceuticals Inc, which has an interest in antiepileptic drugs. Dr Glauser reports that he is an advisor to, a speaker for, and has received research grants from companies with interests in antiepileptic drugs, including ucb Pharma, Eisai, Upsher-Smith, Lundbeck, Sunovion, Supernus, and Questcor. No other disclosures were reported.
Additional Contributions: We thank the research assistants, undergraduate and graduate students, predoctoral interns, and postdoctoral fellows at Cincinnati Children’s Hospital Medical Center for recruiting study patients and data collection. We also thank the new-onset seizure team at Cincinnati Children’s Hospital for their support of the study.
REFERENCES
- 1.Modi AC, Guilfoyle SM. Adherence to antiepileptic drug therapy across the developmental life-span. In: Pinikahana J, Walker C, editors. Society, Behaviour and Epilepsy. New York, NY: Nova Science Publishers Inc; 2011. pp. 175–205. [Google Scholar]
- 2.Modi AC, Cassedy AE, Quittner AL, et al. Trajectories of adherence to airway clearance therapy for patients with cystic fibrosis. J Pediatr Psychol. 2010;35(9):1028–1037. doi: 10.1093/jpepsy/jsq015. [DOI] [PubMed] [Google Scholar]
- 3.Glass TR, Battegay M, Cavassini M, et al. Longitudinal analysis of patterns and predictors of changes in self-reported adherence to antiretroviral therapy: Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2010;54(2):197–203. doi: 10.1097/QAI.0b013e3181ca48bf. [DOI] [PubMed] [Google Scholar]
- 4.Bassili A, Omar T, Zaki A, Abdel-Fattah M, Tognoni G. Egyptian-Italian Collaborative Group on Paediatric Chronic Diseases. Pattern of diagnostic and therapeutic care of childhood epilepsy in Alexandria, Egypt. Int J Qual Health Care. 2002;14(4):277–284. doi: 10.1093/intqhc/14.4.277. [DOI] [PubMed] [Google Scholar]
- 5.Lund L. Anticonvulsant effect of diphenylhydantoin relative to plasma levels: a prospective 3-year study in ambulant patients with generalized epileptic seizures. Arch Neurol. 1974;31(5):289–294. doi: 10.1001/archneur.1974.00490410037002. [DOI] [PubMed] [Google Scholar]
- 6.Leestma JE, Walczak T, Hughes JR, Kalelkar MB, Teas SS. A prospective study on sudden unexpected death in epilepsy. Ann Neurol. 1989;26(2):195–203. doi: 10.1002/ana.410260203. [DOI] [PubMed] [Google Scholar]
- 7.Neuspiel DR, Kuller LH. Sudden and unexpected natural death in childhood and adolescence. JAMA. 1985;254(10):1321–1325. [PubMed] [Google Scholar]
- 8.Faught E, Duh MS, Weiner JR, Guérin A, Cunnington MC. Nonadherence to antiepileptic drugs and increased mortality: findings from the RANSOM Study. Neurology. 2008;71(20):1572–1578. doi: 10.1212/01.wnl.0000319693.10338.b9. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger AB, Manjunath R, Candrilli SD, Davis KL. Prevalence and cost of nonadherence to antiepileptic drugs in elderly patients with epilepsy. Epilepsy Behav. 2009;14(2):324–329. doi: 10.1016/j.yebeh.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590–611. doi: 10.1093/jpepsy/jsm128. [DOI] [PubMed] [Google Scholar]
- 11.Modi AC, Morita DA, Glauser TA. One-month adherence in children with new-onset epilepsy: white-coat compliance does not occur. Pediatrics. 2008;121(4):e961–e966. doi: 10.1542/peds.2007-1690. [DOI] [PubMed] [Google Scholar]
- 12.Haynes RB, Sackett DL, Gibson ES, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet. 1976;1(7972):1265–1268. doi: 10.1016/s0140-6736(76)91737-2. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134(10):968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 14.Al-Faris EA, Abdulghani HM, Mahdi AH, Salih MA, Al-Kordi AG. Compliance with appointments and medications in a pediatric neurology clinic at a University Hospital in Riyadh, Saudi Arabia. Saudi Med J. 2002;23(8):969–974. [PubMed] [Google Scholar]
- 15.Stevens G, Featherman DL. A revised socioeconomic index of occupational status. Soc Sci Res. 1981;10:364–395. [Google Scholar]
- 16.Mueller CW, Parcel TL. Measures of socioeconomic status: alternatives and recommendations. Child Dev. 1981;52:13–20. [Google Scholar]
- 17.Mitchell WG, Scheier LM, Baker SA. Adherence to treatment in children with epilepsy: who follows “doctor’s orders”? Epilepsia. 2000;41(12):1616–1625. doi: 10.1111/j.1499-1654.2000.001616.x. [DOI] [PubMed] [Google Scholar]
- 18.Modi AC, Ingerski LM, Rausch JR, Glauser TA. Treatment factors affecting longitudinal quality of life in new onset pediatric epilepsy. J Pediatr Psychol. doi: 10.1093/jpepsy/jsq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagin D. Group-Based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- 20.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 21.Cohen J, Cohen P, West S, Aiken L. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3rd ed. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- 22.Nagelkerke N. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–692. [Google Scholar]
- 23.Asadi-Pooya AA. Drug compliance of children and adolescents with epilepsy. Seizure. 2005;14(6):393–395. doi: 10.1016/j.seizure.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Asato MR, Manjunath R, Sheth RD, et al. Adolescent and caregiver experiences with epilepsy. J Child Neurol. 2009;24(5):562–571. doi: 10.1177/0883073809332396. [DOI] [PubMed] [Google Scholar]
- 25.Kyngäs H. Compliance with health regimens of adolescents with epilepsy. Seizure. 2000;9(8):598–604. doi: 10.1053/seiz.2000.0470. [DOI] [PubMed] [Google Scholar]
- 26.Modi AC, Guilfoyle SM, Morita DA, Glauser TA. Development and reliability of a correction factor for parent-reported adherence to pediatric antiepileptic drug therapy. Epilepsia. 2011;52(2):370–376. doi: 10.1111/j.1528-1167.2010.02789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Modi AC, Monahan S, Daniels D, Glauser TA. Development and validation of the Pediatric Epilepsy Medication Self-Management Questionnaire. Epilepsy Behav. 2010;18(1–2):94–99. doi: 10.1016/j.yebeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams CD, Dreyer ML, Dinakar C, Portnoy JM. Pediatric asthma: a look at adherence from the patient and family perspective. Curr Allergy Asthma Rep. 2004;4(6):425–432. doi: 10.1007/s11882-004-0007-3. [DOI] [PubMed] [Google Scholar]
- 29.Schurman JV, Cushing CC, Carpenter E, Christenson K. Volitional and accidental nonadherence to pediatric inflammatory bowel disease treatment plans: initial investigation of associations with quality of life and disease activity. J Pediatr Psychol. 2011;36(1):116–125. doi: 10.1093/jpepsy/jsq046. [DOI] [PubMed] [Google Scholar]
- 30.Graves MM, Adams CD, Bender JA, Simon S, Portnoy AJ. Volitional nonadherence in pediatric asthma: parental report of motivating factors. Curr Allergy Asthma Rep. 2007;7(6):427–432. doi: 10.1007/s11882-007-0065-4. [DOI] [PubMed] [Google Scholar]
- 31.Modi AC, Crosby LE, Guilfoyle SM, Lemanek KL, Witherspoon D, Mitchell MJ. Barriers to treatment adherence for pediatric patients with sickle cell disease and their families. Child Health Care. 2009;38(2):107–122. [Google Scholar]
- 32.Modi AC, Quittner AL. Barriers to treatment adherence for children with cystic fibrosis and asthma: what gets in the way? J Pediatr Psychol. 2006;31(8):846–858. doi: 10.1093/jpepsy/jsj096. [DOI] [PubMed] [Google Scholar]
- 33.Irvine L, Crombie IK, Alder EM, Neville RG, Clark RA. What predicts poor collection of medication among children with asthma? a case-control study. Eur Respir J. 2002;20(6):1464–1469. doi: 10.1183/09031936.02.00302102. [DOI] [PubMed] [Google Scholar]
- 34.Fiese BH, Wamboldt FS, Anbar RD. Family asthma management routines: connections to medical adherence and quality of life. J Pediatr. 2005;146(2):171–176. doi: 10.1016/j.jpeds.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 35.Berquist RK, Berquist WE, Esquivel CO, Cox KL, Wayman KI, Litt IF. Adolescent nonadherence: prevalence and consequences in liver transplant recipients. Pediatr Transplant. 2006;10(3):304–310. doi: 10.1111/j.1399-3046.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 36.Schwebel DC, Gaines J. Pediatric unintentional injury: behavioral risk factors and implications for prevention. J Dev Behav Pediatr. 2007;28(3):245–254. doi: 10.1097/01.DBP.0000268561.80204.2a. [DOI] [PubMed] [Google Scholar]
- 37.Conklin CA, Perkins KA, Sheidow AJ, Jones BL, Levine MD, Marcus MD. The return to smoking--1-year relapse trajectories among female smokers. Nicotine Tob Res. 2005;7(4):533–540. doi: 10.1080/14622200500185371. [DOI] [PubMed] [Google Scholar]
- 38.Mulvaney S, Lambert EW, Garber J, Walker LS. Trajectories of symptoms and impairment for pediatric patients with functional abdominal pain: a 5-year longitudinal study. J Am Acad Child Adolesc Psychiatry. 2006;45(6):737–744. doi: 10.1097/10.chi.0000214192.57993.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsui D, Hermann C, Klein J, Berkovitch M, Olivieri N, Koren G. Critical comparison of novel and existing methods of compliance assessment during a clinical trial of an oral iron chelator. J Clin Pharmacol. 1994;34(9):944–949. doi: 10.1002/j.1552-4604.1994.tb04009.x. [DOI] [PubMed] [Google Scholar]
- 40.Pai AL, Gray E, Kurivial K, Ross J, Schoborg D, Goebel J. The Allocation of Treatment Responsibility scale: a novel tool for assessing patient and caregiver management of pediatric medical treatment regimens. Pediatr Transplant. 2010;14(8):993–999. doi: 10.1111/j.1399-3046.2010.01391.x. [DOI] [PubMed] [Google Scholar]
- 41.Riekert KA, Rand CS. Electronic monitoring of medication adherence: when is high-tech best? J Clin Psychol Med Settings. 2002;9(1):25–34. [Google Scholar]
- 42.Glauser T, Ben-Menachem E, Bourgeois B, et al. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006;47(7):1094–1120. doi: 10.1111/j.1528-1167.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 43.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]