Abstract
Purpose
Objective
To investigate the entire human corneal nerve architecture of donors with different durations of insulin-dependent diabetes mellitus (IDDM).
Design
Experimental study.
Participants and Controls
Sixteen fresh human eyes from 8 diabetic donors (aged 43–66 years old, with IDDM for 2–17 years), and 12 eyes from 6 normal donors (aged from 44–67 years old) were obtained from National Disease Research Interchange (NDRI).
Methods
After fixation, corneas were stained with mouse monoclonal anti-β tubulin III antibody and images were acquired to build a whole view of the corneal nerve architecture. The same corneas were used for both whole mount and cross-section examination.
Main outcome measures
Corneal epithelial nerve density was calculated based on the whole mount view of the central area. The number of stromal nerves was calculated by counting the nerve trunks at the corneo-scleral limbus of the entire cornea. Differences between diabetic and normal corneas in epithelial nerve densities and main stromal nerve numbers were compared by paired-samples t test.
Results
The diabetic eyes presented numerous neuropathies in areas where the epithelial nerve bundles emerged. A striking pathologic change was the presence of abundant nerve fiber loops in the stroma. These loops seemed to form by resistance presented by the basement membrane, which may prevent penetration of stromal nerve branches into epithelia. There was no difference in the numbers of main stromal nerve trunks between corneas from diabetic and normal donors, but there was a significant decrease in central epithelial nerve density in the diabetic corneas. We did not find an age effect on this decrease. Instead, it was significantly affected by 5 or more years of IDDM.
Conclusions
This is the first study showing an entire view of the nerve architecture in human diabetic corneas. The decreased epithelial nerve density may result from the abnormalities of stromal nerve architecture and is affected by 5 or more years of IDDM. Although compensation for some nerve regeneration takes place, the alterations in the stromal nerves can explain the poor healing and persistent epithelial defects seen in diabetic patients.
Keywords: corneal sensitivity, diabetic keratopathy, stromal nerves, epithelial nerves, Insulin-dependent diabetes mellitus, immunofluorescence of corneal nerves
Introduction
Human corneal nerves penetrate the cornea in a radial form through the stroma, where they bifurcate and advance to the epithelium, appearing as long bundles, fine branches and nerve terminals1. The cornea is densely innervated and the structure of the corneal nerves is important to maintaining a healthy ocular surface. These nerves modulate the blink response and release neuropeptides, neurotrophins and growth factors that have regenerative properties2–7. Several studies have shown that diabetes alters the structure of corneal nerves8–13. Damage to corneal nerve fibers is responsible for most of the symptoms experienced by diabetic patients with cornea keratopathy; these symptoms include decreased corneal sensitivity, recurrent corneal erosions, delayed corneal epithelial healing, persistent epithelial defects, and neurotrophic corneal ulcers14–18. Changes in corneal nerve morphology have been studied in diabetic rats by light and electron microscopy8. More recently, in vivo confocal microscopy (IVCM) has been used as a non-invasive technique to study corneal nerves affected by different pathologies, including diabetic neuropathy 19. By analysis of corneal nerve fiber density and morphology, it has been reported that there is a reduction of epithelial nerve fiber bundles per image and an increase in nerve tortuosity of diabetic patients compared with control subjects 9–13. However, a more detailed structure of corneal nerves is limited due to technical reasons. For example, the finest nerve fibers (diameter less than 0.5μm) cannot be detected and the stromal nerve density cannot be measured by IVCM because the stromal nerves follow an oblique course in the cornea9,12,13.
We have developed a modified method of immunofluorescence staining and imaging that provides a three dimensional map of the entire architecture of the human corneal nerves in both the epithelium and the stroma1. In the present study, we used this method to examine the nerve structure in human donor corneas with different lengths of IDDM and compared epithelial nerve density and number of stromal nerves with normal corneas.
Materials and Methods
Human Eye Specimens
This study was conducted according to the tenets of the Declaration of Helsinki. Sixteen fresh human eyes from 8 diabetic donors (aged 43–66 years old, with insulin-dependent diabetes mellitus (IDDM) for 2–17 years), and 12 eyes from 6 normal donors (aged from 44–67 years old) were obtained from the National Disease Research Interchange (NDRI). The eyes were kept in a wet chamber and shipped to our laboratory on ice. The donors had no history of eye disease, contact lens wear, ocular surgery, or other systemic diseases that might have affected the corneas. The eyes were examined by slit lamp biomicroscopy and surgical microscopy to exclude possible mechanical damage of corneal central epithelia during eyeball excision.
Tissue preparation, Immunofluorescence Staining and Imaging
The procedure for tissue immunostaining and acquisition of images has been previously described1. After the position of the cornea was defined and marked, the tissue was excised along the sclero-corneal rim and fixed in 4% paraformaldehyde for 24 hours at 4°C. Corneas were carefully washed with 0.1M phosphate buffered saline (PBS) containing 0.1% bovine serum albumin (PBS-BSA) three times for 5 minutes each, placed in a 24-well plate (one cornea/well) and incubated with 10% normal goat serum plus 0.3% Triton X-100 solution in PBS for 1 hour at room temperature to block non-specific binding. This was followed by incubation with a neuronal specific mouse monoclonal anti-βIII-tubulin antibody (Tuj1, MMS-435P, 1:3000, Convance Antibody Services Inc, Berkeley, CA) in 0.1M PBS containing 1.5% normal goat serum plus 0.1% Triton X-100 for 72 hours at 4°C. After thorough washing with PBS-BSA (4 × 15 minutes), the corneas were incubated with the secondary antibody Alexa Fluor® 488 goat anti-mouse IgG (1:1500, Molecular Probes, Eugene, Oregon) for 24 hours at 4°C. Previous studies have shown no staining when omitting the first antibody20, 21. Corneas were then mounted in a bottle cap with a central hole of 12.5 mm interior diameter, which was inversely positioned in a well of a 24-well plate where the bottom had been marked with an “X” in the center of the well. The apex of the cornea was positioned at the intersection of the “X” and covered with 0.1M PBS. Consecutive images, from the center to the periphery as well as from the anterior to the posterior cornea, were acquired in a time-lapse mode with a fluorescence microscope (Nikon Eclipse TE200) equipped with a Photometrics digital camera (CoolSNAP™ HQ) using MetaVue imaging software. The images, recorded on the same plane at adjoining points, were merged using Photoshop imaging software (Adobe, Mountain View, CA) and then pasted onto a Microsoft Office PowerPoint template to build the whole view of the corneal epithelial nerve architecture (as shown in Fig 1 A, E and Fig 2).
Figure 1.
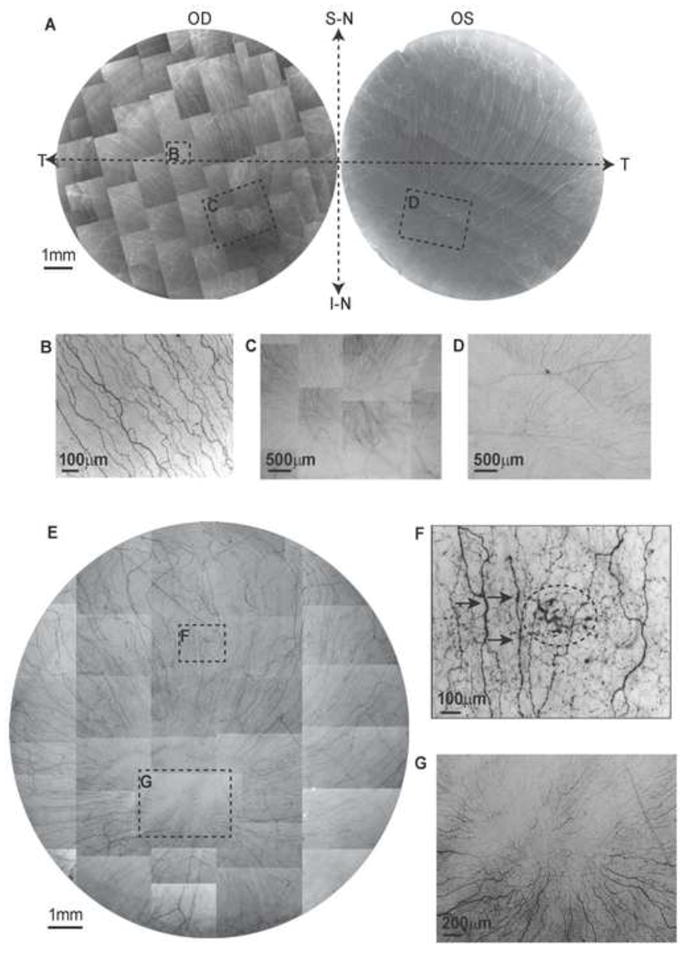
Whole mount view of corneal epithelial nerve distribution in normal and diabetic corneas at early stage of the disease. A) Images were acquired in a time-lapse mode with a Nikon Eclipse TE200 and with a 5X lens in compliance with the natural shape of the cornea from the two eyes of a 64-year-old normal male donor. OD: oculus dexter (right eye); OS: oculus sinister (left eye); S-N: superior nasal quadrant; I-N: inferior nasal quadrant; T: temporal quadrant. The epithelial nerve bundles ran in a radial pattern from the periphery to merge in an area within the inferior-nasal quadrant beyond the corneal apex. Highlighted images showed the detailed architecture of the epithelial nerve distribution in the central (B), and merging areas (C, D). E) Images were taken from the right eye of a 45-year-old male with insulin-dependent diabetes mellitus (IDDM) for two years. Highlighted images show the detailed nerve distribution in the converging area (G). F) Image F, outlined with the small frame, shows the increased thickness in parts of the nerves (arrows) and a sub-epithelial neuropathy (circle).
Figure 2.
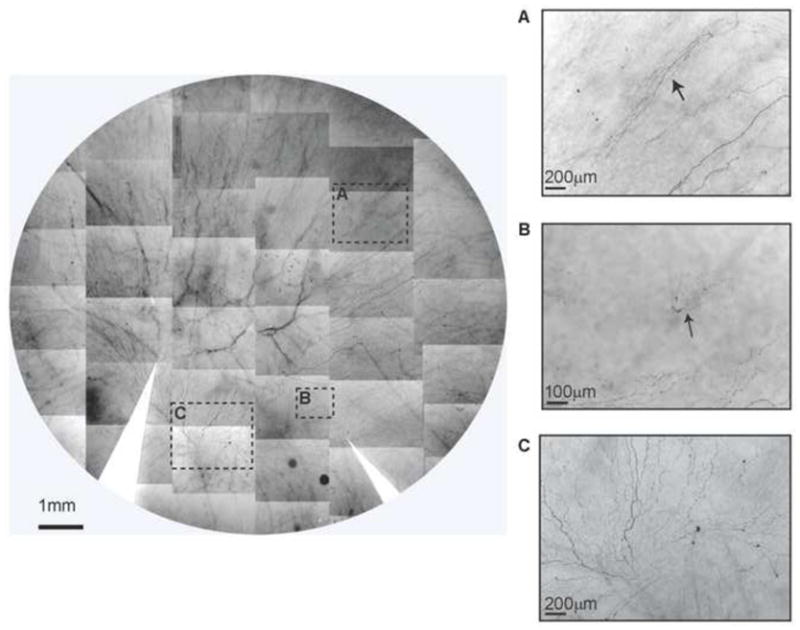
Whole mount view of corneal epithelial nerve distribution in a diabetic cornea after prolonged insulin-dependent diabetes mellitus (IDDM). Images were taken from the left eye of a 58-year-old female with IDDM for 13 years. The montage of images outlined a frame that shows the nerve distribution in detail. The images (A, B) show newly-regenerating epithelial nerve leashes (arrows) with abundant empty spaces without innervations. Image C shows the detailed nerve distribution in the merging area. The long bundles running from the superior quadrants converge in a counter-clock pattern at the inferior quadrant close to the limbal area. Several short nerve bundles, representing newly regenerating nerves, are visible in the lower part.
To obtain a whole image of the stromal nerves, consecutive images were captured in a time lapse mode with a stereoscopic zoom microscope (Nikon SMZ 1500) set at the low magnification of 4 × (objective lens). The images were merged to build a complete view of corneal stromal nerves (as shown in Figs 3A and 6; Fig 4 is available at http://aaojournal.org).
Figure 3.
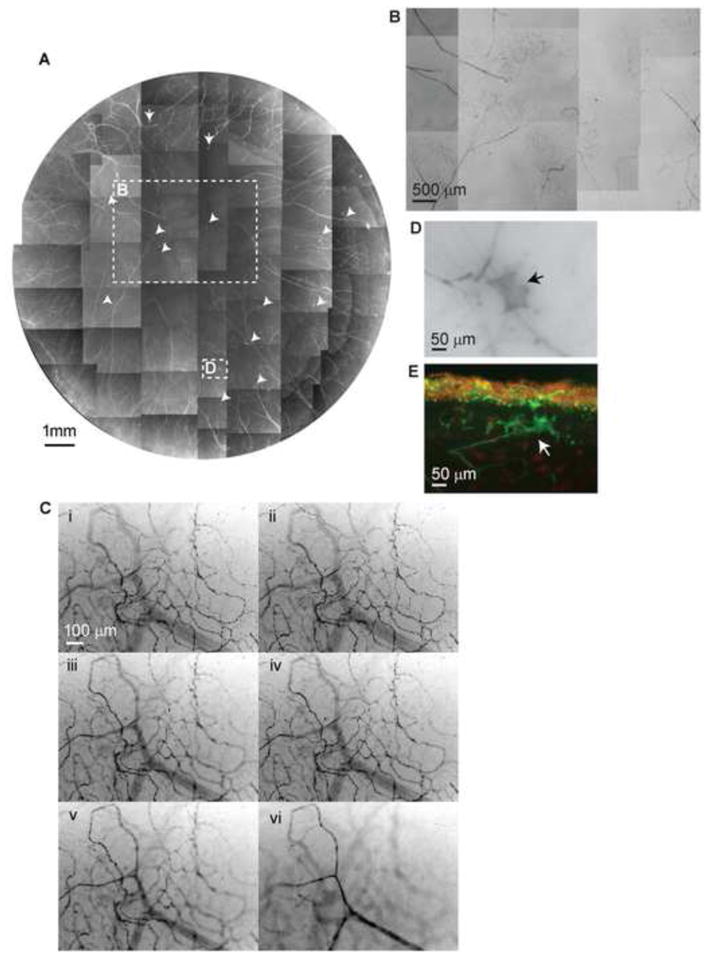
A) Whole mount view of corneal stromal nerve distribution in a diabetic cornea taken from a 43-year-old male with insulin-dependent diabetes mellitus (IDDM) for 5 years. Numerous nerve fiber loops and neuropathic lesions (arrows) are present in the whole cornea. B) Highlighted image shows the nerve fiber loops present in the center. C) Topographic images of the same area show the detailed architecture of corneal nerve loops at different depths. Images were recorded in time-lapse mode from the stroma surface (I) to the middle of the stroma (VI). D) and E) are representative images of diabetic neuropathies in a whole mount and in a transected view.
For transected images of corneal nerves, 20 μm cryostat sections were prepared from the samples after finishing the whole mount examination using the same methods as described previously1, 21.
Data Analysis
Corneal epithelial density is higher in the center cornea and is not affected by gender1. To investigate the distribution of epithelial nerves in diabetic corneas, we compared the nerve density between the diabetic and normal corneas in a 3 mm radius area starting at the apex (the central zone). A total of 16 images per cornea were taken at a magnification of 20x from each of the four quadrants within the central zone. To acquire better contrast, the images were changed to grayscale mode and placed against a white background using Photoshop imaging software. The nerve fibers of each image were carefully drawn with four-pixel lines following the course of each fiber. Percentage of nerve area was quantified for each image using the image analysis program. The number of stromal nerves was counted at the corneo-scleral junction based on whole mount images. Differences between diabetic and normal corneas in corneal epithelial nerve densities and main stromal nerve numbers were compared by paired-samples t test and differences between age groups in central nerve densities were compared by analysis of variance (ANOVA); p<0.05 was considered a statistically significant difference. Quantification of nerve density and number was done by a person unaware from which donor the images were taken.
Results
Epithelial nerve density and structure in diabetic corneas
The main characteristics of the donor corneas are summarized in Table 1 (available at http://aaojournal.org). The average post-mortem time and preservation-in-wet-chamber time, which represents the time interval between death and fixation, were not significantly different between normal and diabetic donors (4.2 ±1.5vs. 3.2 ±1.2, P = 0.19, and 34.8 ±6.7vs. 33.8 ±7.6, p = 0.8, t-test, respectively).
To examine the effect of diabetes on changes in epithelial nerve densities at different ages, we compared the central epithelial nerve densities in corneas of donors from the following age groups: 43–45 years old (4 eyes/6 eyes; normal/diabetes); 54–58 years old (4 eyes/4 eyes); and 63–67 years old (4 eyes/6 eyes) (Fig 5A). A significant decrease (p < 0.01, t-test) was observed in all three age groups of the diabetic donors compared to those of the normal donors. In the normal eyes, there was a gradual decrease in nerve density with aging, but the differences between the groups were not significant (P>0.05). We have previously reported significant decrease of central epithelial nerve density in normal eyes of older donors (75–80 years old)1. Similar results were also detected in the IDDM donors. To test the effect of diabetes duration in epithelial nerve density, we compared the central epithelial nerve densities as a function of time of IDDM. There was a very significant (p < 0.01) decrease in central epithelial nerve density between 2 and 5 years of IDDM (Fig 5B). This decrease was maintained up to 17 years of IDDM.
Figure 5.
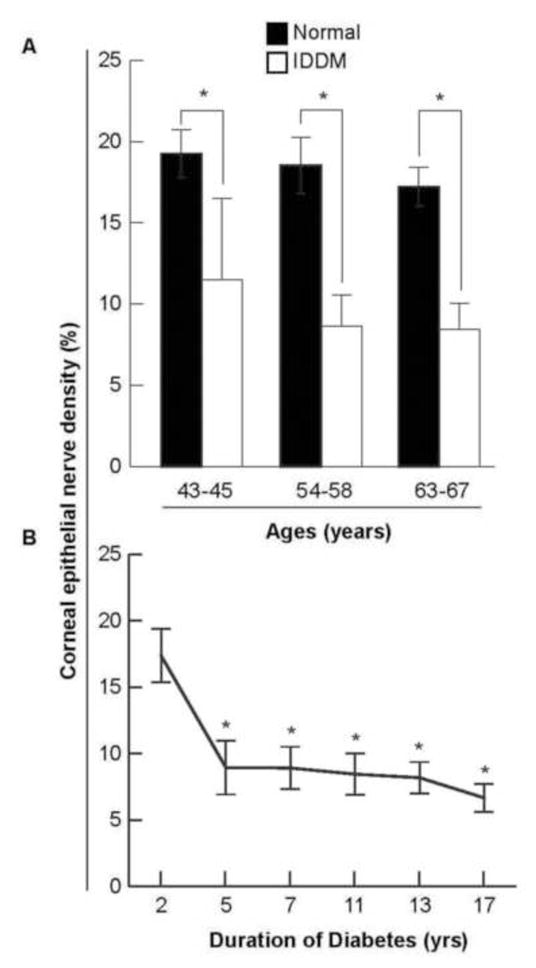
A) Age-paired comparative analysis of corneal epithelial nerve density between normal and diabetic human eyes. The corneal epithelial nerve density was significantly decreased in the diabetic eyes when compared to the normal. Data are expressed as average percent ± standard deviation (SD) of 64 images from 4 corneas in the normal groups and 64–96 images from 4–6 corneas in the diabetic groups (* p< 0.01). B) The effect of insulin-dependent diabetes mellitus (IDDM) duration on corneal epithelial nerve density. A significant decrease (*p < 0.01) was observed in the corneas of patients diagnosed with IDDM for 5 years or more. Data expressed as average percent ± SD, with each time point having 32 images analyzed from two eyes except for 5 years and 7 years time points, both of which have 64 images analyzed from 4 eyes.
Figure 1A shows the whole mount view of the epithelial nerve architecture of the two eyes of a 64-year-old normal male donor. As previously described1, long epithelial nerve bundles originate from the branches of the stromal nerves and run from the periphery to the center, converging into an area within the inferior nasal quadrant (merging area). Highlighted images (2B, C and D) show the details of the epithelial nerve architecture in the center and merging areas. As a comparison, Figure 1E is an image of the whole mount view of the epithelial nerve architecture from the right eye of a 45-year-old male with IDDM for two years. Highlighted images (Fig 1G) show the detailed structure of the epithelial nerve network in the merging area. There is already a moderate decrease in the numbers of long epithelial nerve bundles, and the nerves that course from the periphery to the center show increasing tortuousity with irregular nerve beading when compare to normal corneas. Some parts of the bundles show increased thickness (Fig 1F, arrows). In addition, neuropathic lesions are detected in the sub-epithelial area (Fig 1F, circled area).
In corneas from mid- to long-term diabetics, we observed many areas without epithelial innervation. Around these areas, we often found newly-regenerated epithelial nerves. The image in Figure 2 shows the whole mount view of the epithelial nerve architecture from the left eye of a 58-year-old female with IDDM for 13 years. Highligted images (Fig 2A–C) show the decrease in epithelial nerve density compared to a normal cornea, with many areas devoid of innervation. In the merged area, a few short bundles of newly regenerated nerves are observed (Fig 2C). In Figure 2B, a new regenerated nerve is shown coming from the stroma. In order to re-innervate the epithelium, two types of nerve regeneration occur. One is derived from the underlying stromal nerve branches. As shown in Figure 6 A and B (available at http://aaojournal.ord), which displays the diabetic cornea from the same donor as in Figure 2, the tips of the branches from the stroma penetrate the basement membrane into the epithelia, where they form a new epithelial nerve plexus. This innervation occurs in patches throughout the entire cornea. The second type of epithelial nerve regeneration is done by the neighboring epithelial nerve bundles (Fig 6C, available at http://aaojournal.org), which sprout nerve buds (i) that grow to connect with each other and form a new epithelial nerve network (ii, iii). Nerve terminals appear in these areas (iv) but are less abundant than those in normal corneas1.
Stromal nerve architecture in diabetic corneas
There was no significant difference in the number of main stromal nerve trunks that enter the cornea (34±4.3 vs. 32.7± 3.5, P=0.38, t-test) between normal and diabetic corneas. As previously reported1, the nerves penetrate the stroma in a radial pattern with a structure similar to a tree with thick trunks dividing into branches. The nerves ran toward the center of the stroma and divided into several branches along the course. Some of the branches connected to each other and formed a stromal nerve network. Figure 3A shows a whole mount view of the stromal nerves from the right eye of a 43-year-old male donor with IDDM for 5 years. A remarkable change with respect to healthy corneas is the abundance of nerve fiber loops from the periphery to the center. These loops derive from the tips of stromal nerve branches and appear in the anterior stroma (Fig 3B). The topographic images from the same area in Figure 3C show the architecture of nerve loops at different stromal depths. There are also numerous neuropathies formed in the anterior stroma (Fig 3A, arrows). They are localized in the superficial stroma near the basement membrane (Fig 3 D, E).
A whole mount view of the stromal nerve structure from the two eyes of a 44-year-old female with IDDM for 7 years is shown in Figure 4 (available at http://aaojournal.org). As mentioned previously, no difference was found in the number of main stromal nerve trunks entering into the cornea, but there was an obvious decrease in the number of subdivisions and a shortage of connections between the stromal nerve branches in the diabetic corneas in comparison with the normal corneas1. A massive number of nerve fiber loops are present in the anterior stroma of the right cornea, which is highlighted in the montage of images (Fig 4, inlet A, available at http://aaojournal.org). In the left cornea, where no obvious loops were seen, there was a corneal ulceration present in the inferior quadrant close to the center. Highlighted images show the tortuous stromal nerves present at the bottom of the ulcerating area (Fig 4C, available at http://aaojournal.org), and few epithelial nerves at the edge of the ulcerating area (Fig 4B, available at http://aaojournal.org).
One observed feature of the diabetic stromal nerve architecture was the extensive presence of neuropathic lesions, which were also found in the early- and mid-term diabetic corneas (Figs 3 and 4; Fig 4 is available at http://aaojournal.org). The greater number of lesions affect the distal part of the stromal nerve branches at the place where they penetrate the basement membrane and transit to epithelial nerve bundles (Table 1, available at http://aaojournal.org). They appeared as irregular lesions located at the anterior stroma beneath the basement membrane (Fig 3 D, E). Occasionally we observed that a whole bundle was involved in the nerve degeneration. The whole-mount view in Figure 7 shows the pathologic morphology recorded from a 56-year-old male donor with IDDM for 7 years; the image displays a nerve bundle from the trunk to the branches in the anterior stroma. Segmental demyelination (Fig 7 C, D) was seen in the main stromal nerve trunks, and Schwann cell detachment (Fig 7 A, B) was detected in the distal part of the branches.
Figure 7.
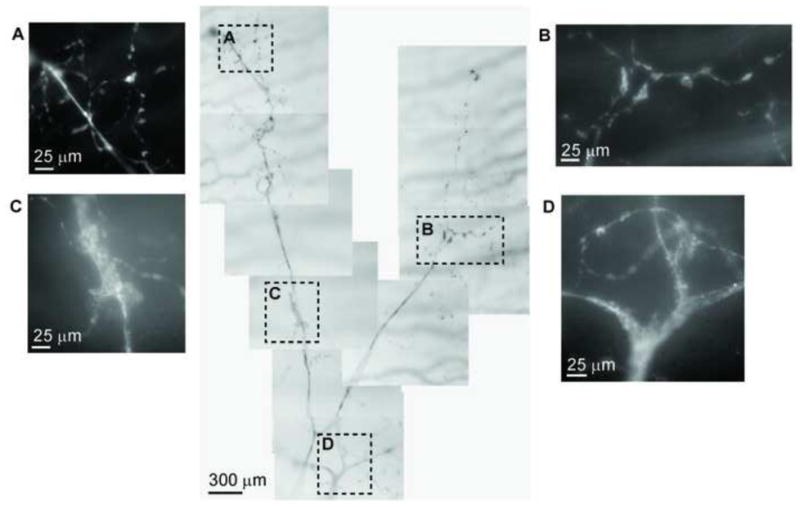
Corneal stromal nerve degeneration. The images reveal the montage of a main stromal nerve trunk together with its branches of a 56-year-old male donor with insulin-dependent diabetes mellitus (IDDM) for 7 years. Highlighted images show the details of nerve degeneration at different parts of the nerve.
Discussion
Studies by electron microscopy and IVCM have shown morphologic alterations in corneal nerves in diabetic patients8–13, 22, but the detailed pathology of the entire corneal nerve architecture remains unclear. Using a modified method of immunofluorescence staining and imaging, we recently provided, for the first time, a three dimensional map of the architecture of the human corneal nerves in both the epithelium and the stroma and demonstrated that aging decreases the density of central epithelial nerves1. In the present study, we used this method to investigate the architecture of corneal nerves in donors with IDDM. To exclude the aging factor as much as possible and evaluate the effect of diabetic condition on corneal innervation, we prepared age-matched diabetic and non-diabetic corneas.
Morphologic changes of epithelial innervation were noted in the diabetic corneas at an early stage of the disease. In comparison with the normal control, the epithelial nerve bundles were obviously thicker and the courses of the nerves from the periphery to the center present more tortuosity in the diabetic cornea. These findings are in agreement with prior studies by IVCM in which there was a significant difference in nerve thickness between the diabetic nerves and normal control12. Thickening of nerve fiber bundles is the result of advanced glycation end products (AGEs) accumulation because diabetic corneas are exposed to increased glucose concentrations23–25. AGE deposition induces a thickened basement membrane and may also play a role in the corneal nerve bundle thickening observed in the early stages of the disease24.
A significant decrease in central corneal epithelial nerve density was found in all corneas with diabetes. There was also a progressive decrease in central epithelial nerve density along with the duration of diabetes from 2 to 17 years. The most significant reduction of nerve fibers was noted between 2 and 5 years of IDDM. One thing to take into consideration is that the analyzed samples recorded the times that the donors were treated with insulin, but the varation in duration of the disease without treatment is not known. This could be why we did not find statistically significant differences between 5 and 17 years of IDDM. Another explanation could be that stromal nerves that penetrate the epithelium are affected earlier in the disease and reach a plateau after a critical period of loss between 2 and 5 years. We found numerous fiber loops in the stroma of a cornea with 5 years of IDDM. This points to the importance of early and controlled treatment in diabetic patients.
Corneal innervation provides important protective and trophic functions for the cornea, and a reduction in nerve density results in delayed wound healing26–27. We found that in diabetic corneas the areas with ulceration have less epithelial innervations (see Fig 4B, available at http://aaojournal.org). The reduction in epithelial nerve fiber density for diabetic corneas might explain slower closure of epithelial defects and the formation of ulcers in the tissue.
Although several IVCM studies have shown a decrease in epithelial nerve density in diabetic corneas, the mechanisms underlying this phenomenon remain unknown. In the present study, quantitative analysis of corneal nerve distribution based on the whole mount images shows that there was no difference in the number of nerve trunks entering the stroma between corneas from diabetic and normal donors, but there was a significant decrease in central epithelial nerve density in the diabetic corneas. We attributed this reduction to the abnormalities of the stromal nerve architecture. As we have reported recently1, central epithelial innervation is supplied by branches of the stromal nerves. These stromal nerves enter the cornea like trunks of a tree that divide into several branches and run towards the anterior stroma, subsequently dividing into smaller branches. The tips of these branches, which are predominantly present within the peripheral area, penetrate the basement membrane into the epithelium and give place to long bundles, which constitute the epithelial nerve network. One of the most striking features in the stromal nerve architecture of diabetic corneas was the presence of abundant nerve fiber loops in the anterior stroma. There are two possible mechanisms that result in the formation of these nerve loops. One is the changes in the composition of components of the extracellular matrix (ECM) in the diabetic corneal stroma. Corneal nerves course the stroma through collagens, proteoglycans and fibronectinin 28–30. These ECM components are known to be up-regulated in diabetes and play an important role in axonal navigation and outgrowth31, 32. Therefore, imbalance in their expression within the environment of navigating axons may disturb the course of nerve outgrowth during nerve regeneration. A second mechanism may consist in the resistance offered by the basement membrane, which may prevent the stromal nerve branches from penetrating into the epithelia. Early studies by electron microscopy and histochemistry have shown alterations in thickness and molecular components of basement membrane in diabetic corneas33–36. It is conceivable, therefore, that increased thickness and altered molecular components in the basement membrane may contribute to increase resistance of nerve fiber penetration, which leads to the formation of nerve fiber loops and a decrease in epithelial bundle density.
The extensive presence of neuropathies in the diabetic stroma is another feature observed in the current study. Previously, we have shown that age-related nerve lesions occur in the peripheral area of normal corneas1. Here, we show that the neuropathic lesions are found extensively in the diabetic cornea. Those neuropathies look like the age-related nerve lesions in appearance, but are not limited to the peripheral area and have little or no relation to the age of the donor. Most of the diabetic neuropathies occurred at the anterior stroma near the basement membrane in the area where the stromal nerve branches will penetrate the epithelium. Therefore, diabetic neuropathies damaged the epithelial nerve bundles at their points of emergence and subsequently led to decreased epithelial innervations. We also observed a few cases where a whole stromal nerve was degenerated.
One interesting finding is the appearance of regenerated nerves, which were found in all the diabetic corneas regardless of the severity or duration of diabetes. According to the regenerating nerve source and site, it can be identified as epithelial nerve regeneration or stromal nerve regeneration. Epithelial nerve regeneration happened when an epithelial bundle was destroyed, which left an area without innervation. In an effort to compensate for the lack of innervation, the surrounding epithelial bundles sprouted fine fibers, which spread in the empty space and contacted with each other to construct a new epithelial nerve network. In diabetic corneas, a less dense array than in healthy corneas of nerve terminals budded from the network to re-innervate the epithelial cells. This explains the decrease in corneal sensitivity observed in diabetic patients 9, 17. In fact, diabetic patients show lower sensitivity than normal subjects to mechanical, chemical, heat and cold stimuli, demonstrating that the disease affects all class of sensory nerves37. Stromal nerve regeneration occurred at the distal part of stromal nerve branches, where the newly-regenerating nerve fibers sprouted and grew upward to form a nerve plexus in the superficial stroma or within the epithelia. New epithelial nerve bundles originated from the nerve plexus to supply the epithelia.
In the current study, there are no available data about the diet and the treatments that the donors followed, but the coexistence of nerve regeneration with neuropathy in all the diabetic corneas suggests the balance between nerve damage and repair may play a critical role in the development of corneal neuropathy. Clinically, reducing nerve damage by optimal glycemic control, by topical application of an aldose reductase inhibitor, or by promoting nerve regeneration should be useful in treating diabetic keratopathy. Growth factors, such as nerve growth factor (NGF), have been shown to regulate corneal nerve repair38. In rabbits, NGF or pigment epithelium- derived factor (PEDF) in combination with docosahexaenoic acid (DHA), a ω-3 fatty acid, stimulate nerve regeneration, restore sensitivity and increase epithelial wound healing after experimental refractive surgery20,21,39,40. We recently discovered that neuroprotectin D1 (NPD1), a docosanoid derived from DHA41, is a very potent nerve regenerative factor in rabbits after nerves are damaged by a stromal dissection (Cortina MS, He J, Russ T, Bazan NG, Bazan HE. Topical Treatment with Neuroprotectin D1 Increases Corneal Nerve Regeneration after Experimental Surgery. Paper presented at: ARVO 2011 Annual Meeting; May 2, 2011). Although there are no studies on diabetic corneas, this could represent an important future treatment to improve regeneration of nerves damaged by this disease.
In summary, by using a recently-developed, modified method of immunofluorescence, we were able for the first time to reveal a detailed map of the entire architecture of diabetic human corneal nerves in both the epithelium and the stroma. The abundant presence of neuropathic lesions and nerve fiber loops in the anterior stroma reduced corneal epithelial innervations either by damaging corneal epithelial nerve bundles at their emergence or by preventing nerve from penetration into epithelia, thereby contributing to the pathomechanisms of diabetic keratopathy.
Supplementary Material
Figure 4 (available at http://aaojournal.org). Whole mount view of corneal stromal nerve distribution in two diabetic corneas taken from a 44-year-old female with insulin-dependent diabetes mellitus (IDDM) for 7 years. There was no significant difference in the numbers of main stromal nerve trunks that entered the cornea in comparison with the normal corneas, but there was a decrease in the number of subdivisions and a shortage of connections between the stromal nerve branches in the diabetic corneas. Abundant nerve fiber loops are present in the anterior stroma of the right cornea as shown in more detail in the montage of images (Inset A). In the diabetic left cornea, where no obvious loops were seen, there was a corneal ulceration present in the inferior quadrant close to the center. Inserted images (B, C) showed the epithelial and stromal nerves surrounding the corneal ulcer (arrows).
Figure 6 (available at http://aaojournal.org). A) Corneal nerve regeneration derived from the stromal nerve branches. Images were acquired from the same donor as described in Figure 2. Topographic images were taken from anterior to posterior in a time lapse mode, showing the architecture of the newly-regenerating nerve plexus from the epithelial layer to the anterior stroma at the planes of the basal cell layer (i), subbasal layer (ii), superficial stroma (iii) and one-third of the anterior stroma (iv). B) Transected view of the new epithelial nerve plexus. C) Corneal nerve regeneration derived from the adjacent epithelial nerve bundles. Images (I) to (III) show the epithelial nerve regeneration at the different stages from the sprout, the new nerves extended to connect with each other to form a new epithelial nerve network. Image (IV) at the high magnification from the frame in (III) shows the details of terminals in the new nerve network.
Acknowledgments
Supported in part by R01EY019465 from the National Eye Institute and by an unrestricted departmental grant from Research to Prevent Blindness, Inc., New York, NY. The content is solely the responsibility of the authors and does not necessarily represent the official views of NEI, the National Institutes of Health, or the Research to Prevent Blindness. The authors thank Stefan Sicinschi (a summer undergraduate student at the Neuroscience Center of Excellence) for his help in calculating the nerve density.
Footnotes
Disclosure: J. He, None; H.E.P. Bazan, None
Financial Disclosure. The authors have no proprietary or commercial interest in any of the material discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.He J, Bazan NG, Bazan HE. Mapping the entire human corneal nerve architecture. Exp Eye Res. 2010;91:513–23. doi: 10.1016/j.exer.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, content and function. Exp Eye Res. 2003;76:521–42. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 3.Riaz SS, Tomlinson DR. Neurotrophic factors in peripheral neuropathies: pharmacological strategies. Prog Neurobiol. 1996;49:125–43. doi: 10.1016/0301-0082(96)00010-x. [DOI] [PubMed] [Google Scholar]
- 4.Kingsley RE, Marfurt CF. Topical substance P and corneal epithelial wound closure in the rabbit. Invest Ophthalmol Vis Sci. 1997;38:388–95. [PubMed] [Google Scholar]
- 5.Jones MA, Marfurt CF. Peptidergic innervation of the rat cornea. Exp Eye Res. 1998;66:421–35. doi: 10.1006/exer.1997.0446. [DOI] [PubMed] [Google Scholar]
- 6.Lambiase A, Bonini S, Micera A, et al. Expression of nerve growth factor receptors on the ocular surface in healthy subjects and during manifestation of inflammatory diseases. Invest Ophthalmol Vis Sci. 1998;39:1272–5. [PubMed] [Google Scholar]
- 7.White DM, Walker S, Brenneman DE, Gozes I. CREB contributes to the increased neurite outgrowth of sensory neurons induced by vasoactive intestinal polypeptide and activity-dependent neurotrophic factor. Brain Res. 2000;868:31–8. doi: 10.1016/s0006-8993(00)02259-9. [DOI] [PubMed] [Google Scholar]
- 8.Ishida N, Rao GN, del Gerro M, Aquavella JV. Corneal nerve alterations in diabetes mellitus. Arch Ophthalmol. 1984;102:1380–4. doi: 10.1001/archopht.1984.01040031122038. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg ME, Tervo TM, Immonen IJ, et al. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41:2915–21. [PubMed] [Google Scholar]
- 10.Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46:683–8. doi: 10.1007/s00125-003-1086-8. [DOI] [PubMed] [Google Scholar]
- 11.Kallinikos P, Berhanu M, O’Donnell C, et al. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci. 2004;45:418–22. doi: 10.1167/iovs.03-0637. [DOI] [PubMed] [Google Scholar]
- 12.Mocan MC, Durukan I, Irkec M, Orhan M. Morphologic alterations of both the stromal and subbasal nerves in the corneas of patients with diabetes. Cornea. 2006;25:769–73. doi: 10.1097/01.ico.0000224640.58848.54. [DOI] [PubMed] [Google Scholar]
- 13.De Cilla S, Ranno S, Carini E, et al. Corneal subbasal nerves changes in patients with diabetic retinopathy: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2009;50:5155–8. doi: 10.1167/iovs.09-3384. [DOI] [PubMed] [Google Scholar]
- 14.Schultz RO, Peters MA, Sobocinski K, et al. Diabetic corneal neuropathy. Trans Am Ophthalmol Soc. 1983;81:107–24. [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen NV, Lund FS. Diabetic polyneuropathy: corneal sensitivity, vibratory perception and Achilles tendon reflex in diabetics. Acta Neurol Scand. 1979;59:15–22. doi: 10.1111/j.1600-0404.1979.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaji Y. Prevention of diabetic keratopathy. Br J Ophthalmol. 2005;89:254–5. doi: 10.1136/bjo.2004.055541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruben ST. Corneal sensation in insulin dependent and non-insulin dependent diabetics with proliferative retinopathy. Acta Ophthalmol (Copenh) 1994;72:576–80. doi: 10.1111/j.1755-3768.1994.tb07182.x. [DOI] [PubMed] [Google Scholar]
- 18.McNamara NA, Brand RJ, Polse KA, Bourne WM. Corneal function during normal and high serum glucose levels in diabetes. Invest Ophthalmol Vis Sci. 1998;39:3–17. [PubMed] [Google Scholar]
- 19.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25:171–7. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esquenazi S, Bazan HE, Bui V, et al. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005;46:3121–7. doi: 10.1167/iovs.05-0241. [DOI] [PubMed] [Google Scholar]
- 21.Cortina MS, He J, Li N, et al. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci. 2010;51:804–10. doi: 10.1167/iovs.09-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehany U, Ishii Y, Lahav M, Rumelt S. Ultrastructural changes in corneas of diabetic patients: an electron-microscopy study. Cornea. 2000;19:534–8. doi: 10.1097/00003226-200007000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Kaji Y, Usui T, Oshika T, et al. Advanced glycation end products in diabetic corneas. Invest Ophthalmol Vis Sci. 2000;41:362–8. [PubMed] [Google Scholar]
- 24.Stitt AW. Advanced glycation: an important pathological event in diabetic and age related ocular disease. Br J Ophthalmol. 2001;85:746–53. doi: 10.1136/bjo.85.6.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato E, Mori F, Igarashi S, et al. Corneal advanced glycation end products increase in patients with proliferative diabetic retinopathy. Diabetes Care. 2001;24:479–82. doi: 10.2337/diacare.24.3.479. [DOI] [PubMed] [Google Scholar]
- 26.Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol. 1980;69:196–201. doi: 10.1016/0014-4886(80)90154-5. [DOI] [PubMed] [Google Scholar]
- 27.Araki K, Ohashi Y, Kinoshita S, et al. Epithelial wound healing in the denervated cornea. Curr Eye Res. 1994;13:203–11. doi: 10.3109/02713689408995778. [DOI] [PubMed] [Google Scholar]
- 28.Birk DE, Fitch JM, Linsenmayer TF. Organization of collagen type I and V in the embryonic chicken cornea. Invest Ophthalmol Vis Sci. 1986;27:1470–7. [PubMed] [Google Scholar]
- 29.He J, Bazan HE. Epidermal growth factor synergism with TGF-beta1 via PI-3 kinase activity in corneal keratocyte differentiation. Invest Ophthalmol Vis Sci. 2008;49:2936–45. doi: 10.1167/iovs.07-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer L, Raslik I, Grone HJ, et al. Small proteoglycans in human diabetic nephropathy: discrepancy between glomerular expression and protein accumulation of decorin, biglycan, lumican, and fibromodulin. FASEB J. 2001;15:559–61. doi: 10.1096/fj.00-0493fje. [DOI] [PubMed] [Google Scholar]
- 31.Grumet M, Flaccus A, Margolis RU. Functional characterization of chondroitin sulfate proteoglycans of brain: interactions with neurons and neural cell adhesion molecules. J Cell Biol. 1993;120:815–24. doi: 10.1083/jcb.120.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tom VJ, Doller CM, Malouf AT, Silver J. Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J Neurosci. 2004;24:9282–90. doi: 10.1523/JNEUROSCI.2120-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor HR, Kimsey RA. Corneal epithelial basement membrane changes in diabetes. Invest Ophthalmol Vis Sci. 1981;20:548–53. [PubMed] [Google Scholar]
- 34.Azar DT, Spurr Michaud SJ, Tisdale AS, Gipson IK. Decreased penetration of anchoring fibrils into the diabetic stroma: a morphometric analysis. Arch Ophthalmol. 1989;107:1520–3. doi: 10.1001/archopht.1989.01070020594047. [DOI] [PubMed] [Google Scholar]
- 35.Azar DT, Spurr Michaud SJ, Tisdale AS, Gipson IK. Altered epithelial-basement membrane interactions in diabetic corneas. Arch Ophthalmol. 1992;110:537–40. doi: 10.1001/archopht.1992.01080160115045. [DOI] [PubMed] [Google Scholar]
- 36.Ljubimov AV, Huang ZS, Huang GH, et al. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J Histochem Cytochem. 1998;46:1033–41. doi: 10.1177/002215549804600907. [DOI] [PubMed] [Google Scholar]
- 37.Neira-Zalentein W, Holopainen JM, Tervo TM, et al. Corneal sensitivity in diabetic patients subjected to retinal laser photocoagulation. Invest Ophthalmol Vis Sci. 2011;52:6043–9. doi: 10.1167/iovs.10-7054. [DOI] [PubMed] [Google Scholar]
- 38.Lambiase A, Rama P, Bonini S, et al. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338:1174–80. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- 39.He J, Bazan HE. Omega-3 fatty acids in dry eye and corneal nerve regeneration after refractive surgery. Prostaglandins Leukot Essent Fatty Acids. 2010;82:319–25. doi: 10.1016/j.plefa.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cortina MS, He J, Li N, et al. Recovery of corneal sensitivity, calcitonin gene-related peptide-positive nerves and increased wound healing induced by pigment epithelial-derived factor plus docosahexaenoic acid after experimental surgery. Arch Ophthalmol. doi: 10.1001/archophthalmol.2011.287. In press. [DOI] [PubMed] [Google Scholar]
- 41.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–6. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 4 (available at http://aaojournal.org). Whole mount view of corneal stromal nerve distribution in two diabetic corneas taken from a 44-year-old female with insulin-dependent diabetes mellitus (IDDM) for 7 years. There was no significant difference in the numbers of main stromal nerve trunks that entered the cornea in comparison with the normal corneas, but there was a decrease in the number of subdivisions and a shortage of connections between the stromal nerve branches in the diabetic corneas. Abundant nerve fiber loops are present in the anterior stroma of the right cornea as shown in more detail in the montage of images (Inset A). In the diabetic left cornea, where no obvious loops were seen, there was a corneal ulceration present in the inferior quadrant close to the center. Inserted images (B, C) showed the epithelial and stromal nerves surrounding the corneal ulcer (arrows).
Figure 6 (available at http://aaojournal.org). A) Corneal nerve regeneration derived from the stromal nerve branches. Images were acquired from the same donor as described in Figure 2. Topographic images were taken from anterior to posterior in a time lapse mode, showing the architecture of the newly-regenerating nerve plexus from the epithelial layer to the anterior stroma at the planes of the basal cell layer (i), subbasal layer (ii), superficial stroma (iii) and one-third of the anterior stroma (iv). B) Transected view of the new epithelial nerve plexus. C) Corneal nerve regeneration derived from the adjacent epithelial nerve bundles. Images (I) to (III) show the epithelial nerve regeneration at the different stages from the sprout, the new nerves extended to connect with each other to form a new epithelial nerve network. Image (IV) at the high magnification from the frame in (III) shows the details of terminals in the new nerve network.


