SUMMARY
Evolutionary modification has produced a spectrum of animal defense traits to escape predation, including the ability to autotomize body parts to elude capture1,2. Following autotomy, the missing part is either replaced through regeneration (e.g. urodeles, lizards, arthropods, crustaceans) or is permanently lost (mammals). While most autotomy involves the loss of appendages (e.g. leg, cheliped, antennae, tail), skin autotomy can occur in certain taxa of scincid and gekkonid lizards3. Here we report the first demonstration of skin autotomy in Mammalia (African spiny mice, Acomys). Mechanical testing revealed a propensity for skin to tear under very low tension and the absence of a fracture plane. Following skin loss, rapid wound contraction was followed by hair follicle regeneration in dorsal skin wounds. Surprisingly, we found regenerative capacity in Acomys extended to ear holes where they exhibited complete regeneration of hair follicles, sebaceous glands, dermis, and cartilage. Salamanders capable of limb regeneration form a blastema (a mass of lineage-restricted progenitor cells4) following limb loss, and our findings suggest that ear tissue regeneration in Acomys may proceed through assembly of a similar structure. This study underscores the importance of investigating regenerative phenomena outside of traditional model organisms and suggests that mammals may retain a higher capacity for regeneration than previously believed. As re-emergent interest in regenerative medicine seeks to isolate molecular pathways controlling tissue regeneration in mammals, Acomys may prove useful in identifying mechanisms to promote regeneration in lieu of fibrosis and scarring.
Among mammals, autotomy appears to have evolved several times, but is taxonomically sparse. Documented autotomy is typically restricted to the tail and occurs through loss of the tail sheath (false autotomy) or through breakage across the vertebra (true autotomy)2,5. In addition to tail autotomy, casual reference has been made to mammalian species with weak or fragile skin, although whether these animals are capable of skin autotomy remains unknown. Thus, we first sought to investigate anecdotal evidence that two species of African spiny mouse (Acomys kempi and Acomys percivali) readily shed portions of their skin as a predator escape behavior.
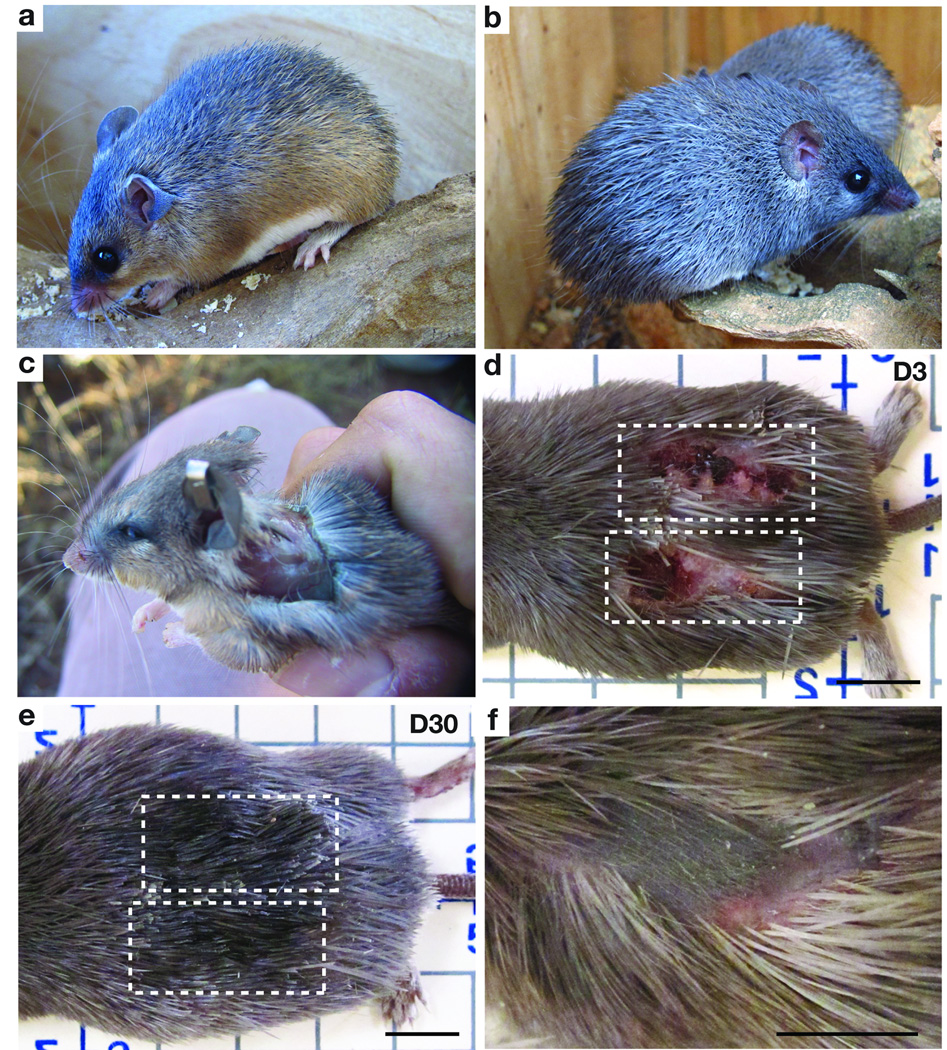
To test the hypothesis that A. kempi and A. percivali are capable of skin autotomy, we live-trapped individuals on rocky outcroppings (kopjes) in central Kenya. In addition to guard hairs, species in the genus Acomys are notable for the presence of spine-like hairs on the dorsum (Fig. 1a, b). Handling both species in the field confirmed that vigorous movement often led to tearing of the skin. Tearing resulted in large open wounds or skin loss ranging from small pieces, to areas approximating 60% of the total dorsal surface area (Fig. 1c). In addition to integumentary loss, both species exhibited autotomy of the tail sheath as previously reported for other Acomys species and individuals were often captured with missing tails2. Among captive individuals, we observed severe skin wounds to heal quickly, and rapid re-growth of spiny hairs totally obscured the wounded area (Fig. 1d, e). Field-captured individuals showed similar healing and, in some cases, patterned hair follicles in anagen (i.e. growth phase) that appeared to have regenerated in wounded areas (Fig. 1f).
Figure 1. A. kempi and A. percivali exhibit skin autotomy and subsequent rapid healing.
(a–b) A. kempi (a) and A. percivali (b) possess stiff, spine-like hairs on the dorsum. (c) A. kempi following loss of dorsal skin. (d–e) Scab formation following full thickness skin injury visible at D3 (d). The same wounds in (d) are no longer visible at D30 and new spiny hairs cover the damaged area (e). (f) Healing wound in field-caught specimen showing new hair follicles within the wound bed. Scale bars = 1 cm.
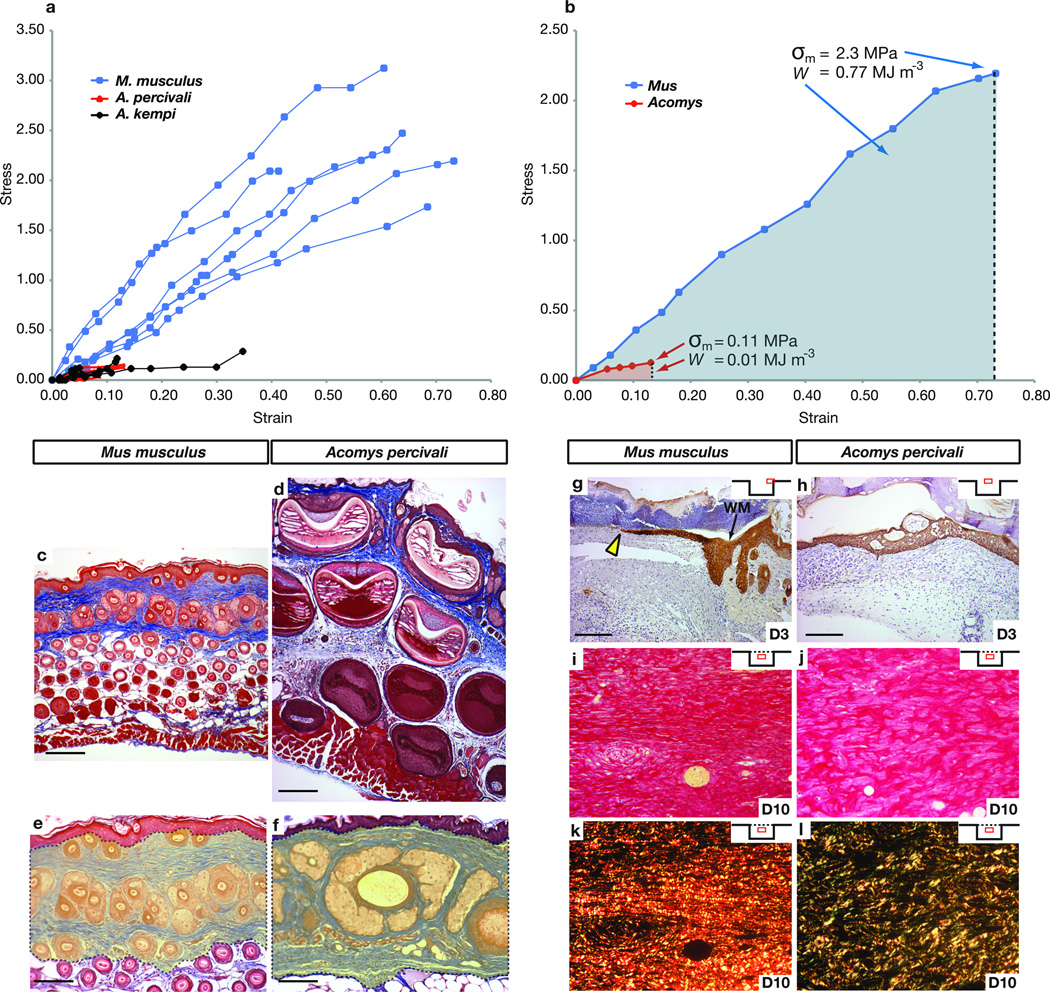
To evaluate how Acomys skin tears so easily, we asked whether the mechanical properties of Acomys skin might underlie its observed weakness. Based on experiments investigating skin autotomy in geckos3, weak skin (i.e. skin possessing uniform structural properties that fails or breaks under relatively low induced loading) can be differentiated from fragile skin (i.e. skin possessing specific morphological characterizations such as a fracture plane that allows the outer layers to be released). To assess skin weakness, we compared mechanical properties of Acomys and Mus skin. During mechanical loading, Mus skin displayed elastic properties prior to breaking whereas Acomys skin was brittle and began tearing shortly after load was applied (Fig. 2a). We derived stress-strain curves from dorsal skin to determine the mean tensile strength (σm) and found that Mus skin was 20 times stronger than Acomys skin (2.3 MPa ±0.19 and 0.11 MPa ±0.03) (Fig. 2a, b). Lastly, calculating mean toughness (W), nearly 77 times more energy was required to break Mus skin relative to Acomys skin (Fig. 2b). These results demonstrate that Acomys possess skin that tears (or breaks) easily in response to low applied tension and provide a mechanical basis for the weakness of their skin.
Figure 2. Acomys skin is weak, tears easily, and during repair develops a porous extracellular matrix rich in collagen type III.
(a–b) Stress-strain curves for Mus n=6, A. kempi n=5, A. percivali n=5, depicted up to the failure strain (a) and for one individual (b) approximating the real mean tensile strength (σm) and mean toughness (W) (represented as shaded areas). (c–d) Masson’s Trichrome staining of unwounded back skin from M. musculus (c) and A. percivali (d). (e–f) Percent adnexa (e.g. hair follicles and associated glands) in the dermis (yellow shading) of Mus (e) and A. percivali (f). (g) Cytokeratin stained keratinocytes (yellow arrow) just beginning to migrate in small wounds at D3 in Mus. (h) Completely re-epithelialized wounds in Acomys at D3. Time after injury in days. WM = wound margin. Insets show relative wound position of the pictured tissue. (i–l) Picrosirius red staining of small wounds in Mus (i, k) and A. percivali (j, l). Bifringence of picrosirius stain (k, l) differentiates thick collagen type I fibers (red/orange) from thin collagen type III fibers (green). Collagen fibers in Mus are predominantly type I, densely packed and run parallel to the epidermis (k). Collagen fibers in A. percivali are more porous with a greater proportion of collagen type III (l). Scale bars = 100µm.
To evaluate whether structural properties of Acomys skin contributed to its mechanical weakness, we examined cellular features of A. percivali skin and found it was anatomically comparable to that of Mus and other rodents, albeit with much larger hair follicles (Fig. 2c, d). We found no evidence of a fracture plane, which is the mechanism of skin autonomy in geckos and skinks3. Examining elastin fibers, which enhance skin elasticity, we found all three species possessed a similar distribution and abundance of elastin in the dermis and beneath the panniculus carnosus (Fig. S1a–f). We tested if larger hair follicles in Acomys skin reduced the total dermal area occupied by connective tissue by examining the proportion of adnexa (e.g. follicles and associated glands) within the dermis and found it was greater in A. percivali (55.61% ±4.28) compared to M. musculus (43.65% ±4.62) (t=1.9, P=0.043) (Fig. 2e, f). These findings suggest that although the basic tissue structure of Acomys skin is similar to Mus, the space occupied by adnexa in the dermis reduces the absolute connective tissue content, potentially contributing to the decreased elasticity and lower tensile strength when the skin is placed under tension6. The lack of a fracture plane underscores this finding and supports an inherent structural difference underlying the observed weakness of Acomys skin.
Given its inherent structural weakness and propensity to tear, we assessed the ability of Acomys to heal skin wounds using small (4mm) and large (1.5cm), full thickness excisional (FTE) wounds. In wounds of both sizes scab formation and hemostasis was rapid, and in large wounds, contributed to a 64% ±3.1 reduction in wound area 24 hrs after injury (Fig. S2a). During scar-free healing in terrestrial salamanders7 and mammalian fetuses8, the wound bed is re-epithelialized within several days, while a 4 mm wound in adult rat skin takes between 5–7 days to re-epithelialize9. In Acomys, we found that five out of six 4mm wounds had completely re-epithelialized by Day 3 post injury (D3), whereas Mus wounds failed to re-epithelialize this quickly (Fig. 2g, h). Following re-epithelialization, loose-skinned mammals (e.g. rodents, rabbits, etc.) rely primarily on contraction to heal their wounds10. Similarly, we observed high contraction rates, which accounted for 95% of wound closure after 17 days (Fig. S2a–c). In contrast to scarring, where collagen fibers organize into a dense network parallel to the epidermis, during scar-free healing collagen fibers assume a pattern similar to unwounded dermis10. Examining the extracellular matrix (ECM) at D10, we observed scarring in Mus whereas in Acomys, collagen fibrils were less densely packed and contained a more porous structure (Fig. 2i, j). Using picrosirius red we found collagen type I predominated the wound bed at D10 in Mus, whereas collagen type III was in greater abundance in Acomys (Fig. 2k, l). This difference was even more pronounced in 1.5 cm wounds (Fig. S3a–b’). Together, these data show that rapid re-epithelialization and wound edge contraction greatly reduces the size of open skin tears in Acomys. Our findings, that wound ECM (1) is deposited slowly, (2) has a porous configuration, and (3) is dominated by type III collagen, suggest that this composition favors regeneration over fibrosis during skin repair in Acomys.
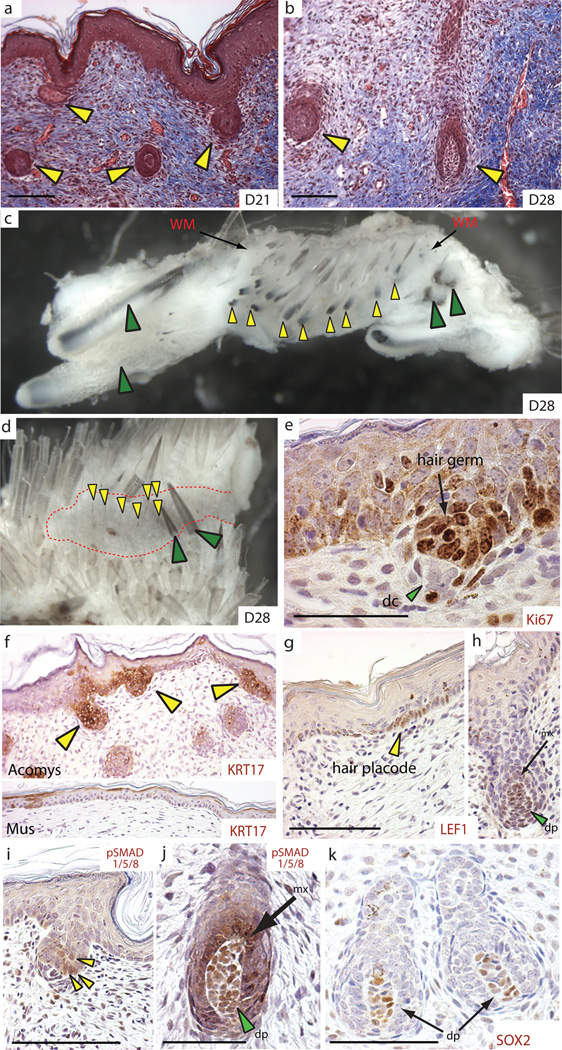
To test the regenerative capacity of the wound environment we sampled large healing wounds for evidence of hair follicle neogenesis and dermal regeneration. In association with the more porous ECM, we observed folliculargenesis of normal pelage hairs and large spiny hairs in the wound bed between D21 and D28 and we could distinguish old, large follicles near the wound margins from newly regenerated follicles within the wound bed (Fig. 3a–d and Fig. S3c–e). New follicles appeared to regenerate throughout the uncontracted portion of the wound bed not just in the central region (Fig. 3c and Fig. S3e) and we observed regenerating hair follicles in various stages of development (Fig. 3a–m and Fig. S4a–c). A localized and highly proliferative population of epidermal cells drives hair follicle development and we observed a similar phenomenon during follicle regeneration (Fig. 3e and Fig. S4a–c). To investigate if embryonic signaling networks used during hair follicle development were deployed during hair follicle regeneration, we examined Keratin-17 (Krt17); which is diffusely expressed within the epidermis during skin development and becomes progressively restricted to developing hair follicles11. Following re-epithelialization, KRT17 was highly enriched throughout the neoepidermis overlying the wound bed at D14 and as new hair follicles formed in the wound bed, KRT17 became restricted to follicular epithelium (Fig. 3f and Fig. S5). During wound repair in Mus, we found KRT17 was also highly upregulated in the re-epithelialized epidermis at D14 (Fig. S5) and although KRT17 localized to some basal keratinocytes in Mus epidermis at D21, these sites failed to aggregate into placodes or new hair follicles such that KRT17 was completely absent from the new epidermis by D26 (Fig. 3f). The disappearance of KRT17 from basal keratinocytes in Mus, together with our observation of continued localization in new placodes and hair follicles in Acomys, suggests the underlying dermal signals required to induce placode formation in Mus are lacking.
Figure 3. Acomys exhibit de novo hair follicle regeneration in wounded skin.
(a–d) Hair follicles regenerating in A. percivali (yellow arrows) between D21 and D28 in large skin wounds. Days are post injury. New hair follicles (yellow arrows) are present throughout the wound bed (red dotted area) at D28 (c–d). Green arrows indicate old follicles. WM = wound margin. (e–k) Regenerating hair follicles express proteins associated with development and differentiation; Ki67 labels proliferating hair germ (e), Keratin-17 (yellow arrows) in Acomys, but is absent in Mus at D26 (f), nuclear localized LEF1 in follicle placodes (g) and later in dermal papilla cells (dp) and surrounding matrix cells (mx) (h), phosphorylated SMAD 1/5/8 (as a readout of Bmp-signaling) in epidermal hair germ cells (i) and later in dermal papilla cells (dp) and matrix cells (mx) of regenerating follicles (j), and Sox2 in dermal papilla cells (k). Scale bars = 100 µm, except (e) = 50 µm.
Although the precise signal for placode formation remains obscure, there is an absolute requirement for Wnt-signaling during normal follicle formation12. Nuclear localization of LEF1 protein has been used as a readout of this inductive signaling13. We detected nuclear accumulation of LEF1 in regenerating epidermal placodes, condensing dermal fibroblasts beneath the hair germ, and in dermal papilla and matrix cells (Fig. 3g, h and Fig. S6a). We also detected nuclear LEF1 staining at low levels in some non-placode basal keratinocytes, whereas we did not detect nuclear LEF1 in the epidermis during wound healing in Mus, suggesting epidermal Wnt-activation in Acomys may partially underlie our observation of hair follicle regeneration (Fig. S6b, c).
Regulation of canonical Bmp-signaling also plays a role during hair follicle induction and differentiation of follicular progenitor populations into the mature hair follicle (reviewed in14). Phosphorylation of SMADs 1, 5, and 8 (pSMAD1/5/8) is a robust readout of canonical Bmp signaling. We detected pSMAD1/5/8 at low levels during follicle induction and later at higher levels in dermal papilla and matrix cells undergoing differentiation in the hair bulb (Fig. 3i, j). Additionally, we detected SOX2 positive dermal papilla in some regenerating hair follicles, which is consistent with its role in specifying various hair types during mouse hair follicle development15 (Fig. 3k). Taken together, these results demonstrate that regenerating hair follicles in Acomys progress through defined stages of hair follicle development, exhibit high rates of proliferation, and re-deploy molecular pathways utilized during embryonic hair follicle development to regenerate new hair follicles.
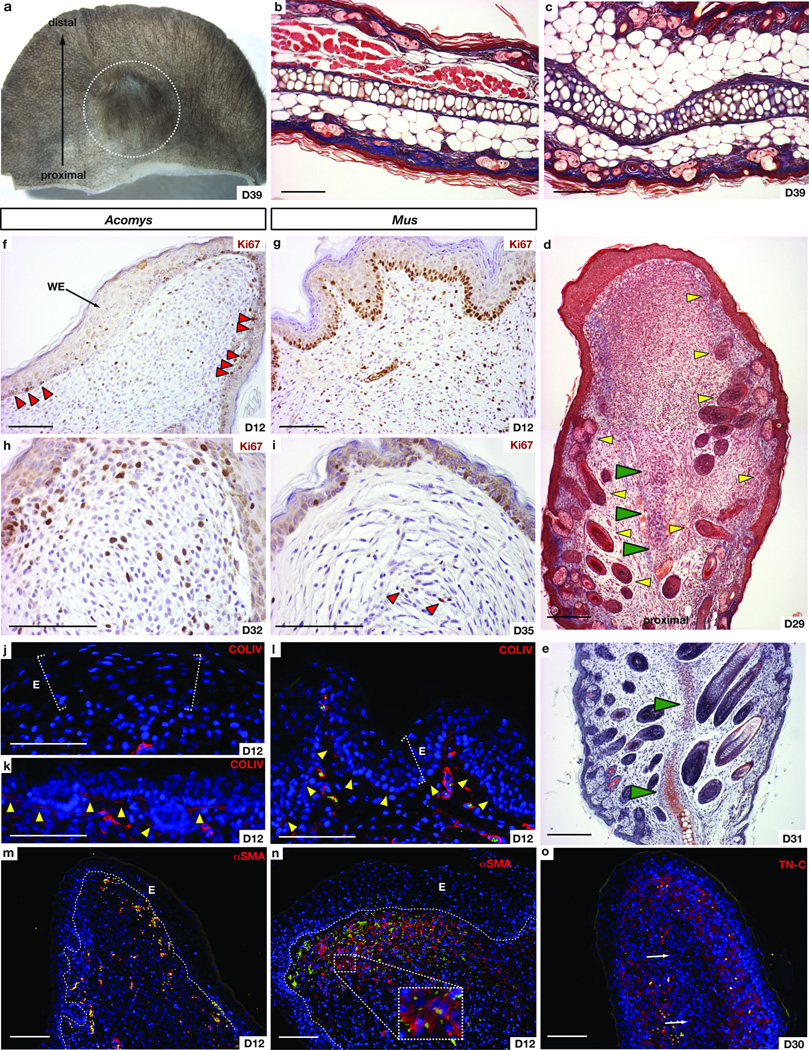
Adult mammal skin is normally unable to regenerate epidermally-derived structures in response to wounding (e.g. glands and hair follicles). An exception to this is the observation of spontaneous folliculargenesis in large excisional wounds in rabbits, and more recently in lab mice (C57BL6/SJ, SJL or mixed strain)16,17,18. Rabbits are also one of the few mammalian species capable of regenerating large ear punch wounds19. We hypothesized that the regenerative capacity observed in Acomys might extend to their ear tissue as well. To test this we made 4mm punches through the ears of both Acomys species and, to our surprise, found they were capable of closing these large punches (Fig. 4a–c and Fig. S7a–c). Uninjured ear tissue contains skin (epidermis and dermis), associated hair follicles, adipose cells, muscle and cartilage; we found that Acomys were capable of completely regenerating all of these tissues with high fidelity except muscle (Fig. 4b–c). Twelve days after injury we observed an accumulation of cells around the circumference of the wound beneath the epidermis and although regeneration of new tissue was centripetal, cells accumulated to a greater degree on the proximal side of the punch. Hair follicle and cartilage regeneration proceeded in a proximal to distal wave (Fig. 4d, e) and similar to the skin, follicular epidermis in the ear activated Wnt-signaling (Fig. S6d, e). In contrast to Acomys, we found Mus were incapable of regenerating 4mm ear punches and instead formed scar tissue (Fig. S8a, b). Interestingly, despite scar formation, Mus ear repair resulted in the de novo formation of cartilage condensations distal to the cut cartilage suggesting Mus might initiate, but not maintain, a regenerative response following ear wounding (Fig, S8b).
Figure 4. Acomys regenerate hair follicles, sebaceous glands, dermis, adipose tissue and cartilage in 4mm ear punches.
(a) Regenerated 4mm ear punch in A. percivali. (b) Unwounded tissue in Acomys ear pinna. (c) Regenerated dermis, hair follicles, cartilage and adipose tissue within biopsy punched area. Days are post injury. White circle = original punch area. (d) Regenerating hair follicles (yellow arrows) and cartilage (green arrows) differentiate proximal to distal. (e) Safranin-O/Fast Green indicates chondrogenesis (green arrows). (f–i) Proliferating cells (Ki67+) in early (f–g) and late (h–i) Acomys and Mus ears. Proliferation is restricted proximal to the wound epidermis (WE) (red arrows) in Acomys (f) and is continuous in basal keratinocytes of Mus (g). Proliferation is maintained in Acomys at D32 (h) with very few proliferating cells persisting in Mus (i) (red arrows). (j–l) Collagen IV stained mature basement membrane is absent beneath the wound epidermis in Acomys (j), but is present near the amputation (k) and distally in Mus (l). Yellow arrows indicate basement membrane; e=epidermis, and white brackets indicate epidermal thickness. (m–n) Almost no αSMA positive fibroblasts are present in Acomys (m) whereas αSMA positive myofibroblasts are present in healing Mus ear (n). Inset shows stress fibers in individual myofibroblasts. (o) TN-C disappears where new cartilage differentiates (white arrows) in Acomys. Yellow/green cells (j–o) are autofluorescing blood cells in GFP channel. Scale bars = 100 µm.
It remains unclear whether mammalian regeneration proceeds through formation of a blastema, or is instead an exaggerated version of hyperplastic growth20,21,22. Blastema formation is considered a hallmark of epimorphic regeneration. One characteristic of a regeneration blastema is that it contains proliferating cells and maintains proliferation during regeneration23. We observed widespread proliferation throughout the ear regenerate in Acomys and surprisingly, throughout healing ear tissue in Mus (Fig. 4f, g). However, we noted a lack of proliferation in the distal epidermis of Acomys, whereas we detected proliferation throughout Mus epidermis extending to the distal tip (Fig. 4f, g). While proliferation was maintained in Acomys ears, we observed almost no proliferating cells in later-staged Mus ears (Fig. 4h, i).
A second characteristic of a blastema is the formation of a specialized epidermal signaling center (the wound epidermis) which is required for proliferating blastemal cells to remain in the cell cycle24 and is characterized by a loss of epidermal stratification, loss of basal keratinocyte polarity, and lack of a mature basal lamina25. Following re-epithelialization in Acomys, we noted a thickening of the distal epidermis, disorganization of basal keratinocytes, and absence of a mature basement membrane (Fig. 4j). Comparatively, the epidermis near the amputation plane exhibited normal stratification and possessed a prominent basement membrane (Fig. 4k). In contrast, Mus appeared to form a wound epidermis only transiently following re-epithelialization, with a proportionately smaller distal area exhibiting these characteristics for a short time (data not shown). By D12 in Mus, collagen type IV staining revealed a mature basement membrane beneath the entire epidermis of the healing ear (Fig. 4l). In addition, the epidermis exhibited normal stratification and proper apical-basal polarity of the basal keratinocytes (Fig. 4g, l).
In addition to sustained proliferation and formation of the wound epidermis, extracellular matrix (ECM) molecules plays a key role in supporting proliferation and directing subsequent differentiation during regeneration26. In contrast, molecules such as laminin and collagen type I, which favor differentiation, are downregulated in the blastema during amphibian limb regeneration and are expressed as differentiation of the musculoskeletal system proceeds26,27. Histological examination of Acomys ears at D12 revealed high levels of fibronectin (FN), some tenascin-C (TN-C) surrounding densely packed cells, but very low levels of collagen type I (Fig. S9a–c). Collagen type III was also more abundant than collagen type I during regeneration (Fig. S9d–d’). TN-C became restricted from areas where new cartilage began differentiating and within these differentiating cells we found activation of the Bmp-signaling pathway in cells giving rise to new auricular cartilage (Fig. 4o and Fig. S10). During hyperplastic growth in Mus ears, the ECM initially displayed high levels of FN and low levels of TN-C as did Acomys ears, but produced relatively higher levels of collagen type I (Fig. S9e–g). Collagen production in Mus was not only faster and more abundant, but also exhibited a higher ratio of collagen type I to III (Fig. S9h, h’). Given the exuberant production of collagen type I in Mus, we asked if resident fibroblasts were differentiating into myofibroblasts, which contribute to scarring in lieu of regeneration (reviewed in28). Using alpha smooth muscle actin (αSMA), we found myofibroblasts in high abundance throughout ear tissue in Mus, while they were almost completely absent in Acomys ears (Fig. 4m, n). These data corroborate the importance of the wound ECM to promote proliferation while antagonizing differentiation and support previous work showing precocious collagen type I formation antagonizes appendage regeneration27.
Our data suggest that reparative ear regeneration in Acomys is a balance between premature reformation of the dermis (scarring) and maintenance of cell proliferation within a pro-regenerative environment. In contrast, Mus fails to form (or maintain) a wound epidermis, which is coincident with precocious formation of the basement membrane and stratification of the epidermis. This leads to loss of cell proliferation, increased collagen type I deposition (in lieu of collagen type III), myofibroblast activation and ultimately, scar formation. While our data suggest ear regeneration shares similar characteristics with blastema formation, understanding the molecular signals required to organize and maintain a wound epidermis and identifying the lineage of regenerating cells is crucial to address how regeneration occurs in these animals. Future work investigating how Acomys are capable of controlling fibrosis will shed light on how regeneration and scarring can be balanced in the face of infection and inflammation in wild mammals and provides an ideal model system in which to examine epimorphic regeneration in mammals.
METHODS SUMMARY
Animals
Specimens of Acomys kempi and Acomys percivali were live-captured in Laikipia, Kenya at Mpala Research Centre. Experimental animals were held in an open-air field laboratory under ambient conditions. For comparative experiments Swiss Webster mice were obtained from a local supplier in Nairobi and in the United States from Charles River.
Wounding
All experiments conducted in Kenya were performed in accordance with approved animal practices. Experiments performed in the USA were approved by the Institutional Review Board on Animal Care at the University of Florida and in Kenya by the University of Nairobi. Spiny mice were halothane-anesthetized and small (4 mm) and large (1.5 cm) full-thickness, excisional wounds (FTE) were made on the dorsum.
Strength Measurements
Strength measures were made using a Hounsfield Tensometer equipped with an automatic motor and stretched at a rate of 20 mm min−1. Full-thickness skin strips (including the panniculus carnosus) measuring ~20 mm × 40 mm were used. Force (N) and displacement (mm) were measured on a xy plotter and these points were subsequently recorded as stress (σ=force per cross-sectional area) and strain (ε=change in length/initial length) and re-plotted in Excel. The work (W) required to achieve the breaking strength was calculated as the area under each stress/strain curve and expressed as the mean work for each genus.
Histology and Immunohistochemistry
For histological analysis, samples were fixed in 10% NBF at 4°C for 16–24hr, washed in PBS, dehydrated in ethanol and infiltrated with paraffin. Samples were cut at 5 µm. For immunohistochemistry, slides were incubated with 1° antibodies (see Methods) and visualized using either DAB or Alexa-Flour 594. Negative controls were run using appropriate Ig isotypes at the same concentration as 1° antibody. For all IHC comparisons a minimum of n=4 per species were used.
METHODS
Animals
Male and female Acomys kempi and A. percivali were live-captured using Sherman Traps in Laikipia, Kenya at Mpala Research Centre, between 2009–2011. Fifty traps were set in the late afternoon at various kopjes (rock outcroppings) and traps were checked the following morning for animals. Captured animals were transported to an open-air field laboratory and held under ambient conditions in species-specific groups with access to water ad libitum. Animals were fed twice daily on peanut butter and oats. Some animals were transported to the University of Nairobi where they were maintained under similar conditions. For comparative experiments Swiss Webster mice were obtained from a local supplier in Nairobi and in the United States from Charles River.
Wounding
Both male and female mice were used, but pregnant females were excluded. Three types of wounds were used for this study; (1) natural wounds, (2) small circular wounds (4 mm diameter), and (3) large circular wounds (1.5 cm diameter). Spiny mice were halothane-anesthetized and shaved at least one day prior to wounding. Natural wounds were not made by the investigators and occurred either in the field or in captivity. Full-thickness, excisional wounds (FTE) were made by pinching the skin with forceps and cutting beneath to form a 4mm circle (small) or cutting with iridectomy scissors a 1.5 cm diameter circle (large). For small wounds, 4 wounds were made on the dorsum posterior to the forelimbs and anterior to the hindlimbs. Wounds were allowed to heal and were not treated during healing. Calipers were used to measure wound width (mm) and wounds were recorded using a Canon S900 (1x–4x Macro zoom).
Strength Measurements
Following sacrifice, the entire dorsal skin including the panniculus carnosus was removed and placed face down in PBS. Two strips of dorsal skin measuring 20 mm × 40 mm were excised from this preparation maintaining the anteroposterior orientation and the second strip was returned to PBS while the first strip was measured. Strips for mechanical testing were placed in metal screw clamps with rubber pieces covering the clamped ends. Clamps were placed in a Hounsfield Tensometer equipped with an automatic motor and stretched at a rate of 20 mm min−1. Tissue measurements for width (mm) and length (mm) between clamps were measured prior to stretching, and PBS was applied prior to loading to keep the samples moist. Force (N) and displacement (mm) were measured on a xy plotter and these points were subsequently recorded as stress (σ=force per cross-sectional area) and strain (ε=change in length/initial length) and re-plotted in Excel.
Load was applied to skin strips parallel to the long body axis and the tensile strength (σm) and failure strain (εf) were recorded (M. musculus n=6, A. kempi n=5, A. percivali n=5). The work (W) required to achieve the breaking strength was calculated as the area under each stress-strain curve and expressed as the mean work for each genus.
Immunohistochemistry
Primary antibodies and antigen retrieval used were: Tenascin-C 1:50 (Abcam; ab3970) with pronase at 1ug/ml for 10 mins at room temperature, Fibronectin 1:500 (Abcam; ab23750) with heat-induced epitope retrieval (HIER) in citrate buffer pH=6.0, pan Cytokeratin 1:1000 (DAKO; Z0622) with proteinases-K (PK) (DAKO) for 2 mins at room temperature, Cytokeratin 17 1:400 (Abcam; ab53707) with PK 2 mins, Ki67 (Abcam; ab15580) 1:2000 with HIER Tris/EDTA pH=9.0, LEF-1 1:4000 (Gift of Prof. Otmar Huber) with HIER citrate pH=6.0 followed by treatment with 0.1% trypsin 10 mins room temperature, β-catenin 1:1000 (Sigma; HPA 029159) with HIER citrate pH=6.0, pSMAD1/5/8 1:200 (Cell Signaling; #9511) with HIER Tris/EDTA pH=9.0, SOX2 1:150 (Abcam; ab97959) with HIER citrate pH=6.0, Collagen type IV 1:500 (Rockland; 600-401-106-0.1) with PK 2 mins, αSMA 1:200 (Abcam; ab32575) with HIER citrate pH=6.0, Collagen type I 1:500, (Abcam; ab34710) with HIER citrate pH=6.0 followed by PK 2 mins. For detection of rabbit antibodies Vector Elite anti-rabbit kits were used and for mouse monoclonal antibodies (Tenascin-C) Vector M.O.M kits were used. Antibodies were visualized with either DAB (Vector) or a streptavidin conjugated Alexa-Flour 594 antibody after incubation with biotinylated-anti secondary.
Estimation of the proportion of the adnexa in the dermis (Vv(a,d))
The volume density of the adnexa in the dermis Vv(a,d) is the ratio of the total volume of follicles and sebaceous glands to the total volume of the dermis. This ratio can be estimated by point counting on plane sections29,30,31. For this purpose, one histological section was randomly sampled from each animal. The corresponding micrographs were projected on a screen, and a transparent test grid bearing a square lattice of points overlaid with random positions on each projected image (Equation 1).
| (1) |
The total number of test points falling on profiles of the adnexa P(a) and on the entire dermis P(d) were counted. An estimator of the volume density of the adnexa was then calculated which is unbiased (i.e., there is no systematic error due to the sampling or counting procedures) provided that the plane section and the test grid are randomly positioned.
Statistical Analysis
To test for significant differences between percent adnexa, the student’s two-tailed t-test function in Excel was used to calculate P values. Alpha was set at 0.05 and means were reported ± standard error.
Descriptive Statistics
Mean ± standard error of the mean (s.e.m.). For strength measure, n=6 for M. musculus and n=5 for A. kempi and A. percivali. Mean failure strain (εf) for Mus (0.61±0.05), A. kempi (0.14±0.05) and A. percivali (0.08±0.02). Mean tensile strength (MPa) Mus (2.3 ±0.19), A. kempi (0.15±0.04), A. percivali (0.08±0.03) and Acomys (both species) (0.11±0.03). Mean toughness (MJ m−3). Mus (0.77 ±0.08), A. kempi (0.012±0.006), A. percivali (0.005±0.003) and Acomys (both species) (0.01±0.004). Percent adnexa, n=6 for each species. A. percivali (55.61% ±4.28) compared to M. musculus (43.65% ±4.62) (t=1.9, P=0.043). Percent wound contraction A. percivali, n=12, (95.5%±0.7).
Supplementary Material
Acknowledgements
We thank John Kahiro for assisting during materials testing and the Department of Mechanical Engineering, University of Nairobi, for use of their equipment. We thank Dr. John Kimani, Stanley Marete and Jackson Mugweru, for help with animal care and materials procurement in Nairobi, Ekiru Ekaran for field assistance, and Bernard Agwanda, Darcy Ogada, and Hillary Young for help with identification and natural history of Acomys. Conversations with Steve Takata and Truman Young drew our attention to this phenomenon.
Footnotes
Supplementary Information
Supplementary information accompanies this paper.
Author Contributions
A.W.S, J.R.G, T.M.P and M.M. formulated the research. A.W.S, with assistance from M.G.S, M.M and S.G.K, performed the research and analyzed the data. A.W.S wrote the manuscript and all authors discussed the results, commented on and edited the manuscript.
The authors declare no competing financial interests.
REFERENCES
- 1.Maginnis TL. The costs of autotomy and regeneration in animals: a review and framework for future research. Behavioral Ecology. 2006;17:857–872. [Google Scholar]
- 2.Shargal E, Rath-Wolfson L, Kronfeld N, Dayan T. Ecological and histological aspects of tail loss in spiny mice (Rodentia : Muridae, Acomys) with a review of its occurrence in rodents. Journal of Zoology. 1999;249:187–193. [Google Scholar]
- 3.Bauer AM, Russell AP, Shadwick RE. Mechanical-Properties and Morphological Correlates of Fragile Skin in Gekkonid Lizards. Journal of Experimental Biology. 1989;145:79–102. [Google Scholar]
- 4.Kragl M, et al. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–65. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- 5.Dubost G, Gasc JP. The process of total tail autotomy in the South-American rodent, Proechimys. Journal of Zoology (London) 1987;212:563–572. [Google Scholar]
- 6.Vogel HG. Correlation between Tensile-Strength and Collagen Content in Rat Skin - Effect of Age and Cortisol Treatment. Connective Tissue Research. 1974;2:177–182. doi: 10.3109/03008207409152242. [DOI] [PubMed] [Google Scholar]
- 7.Seifert AW, Monaghan JR, Voss SR, Maden M. Skin regeneration in adult axolotls: a blueprint for scar-free healing in vertebrates. PLoS One. 2012;7:e32875. doi: 10.1371/journal.pone.0032875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dang CM, et al. Scarless fetal wounds are associated with an increased matrix metalloproteinase-to-tissue-derived inhibitor of metalloproteinase ratio. Plast Reconstr Surg. 2003;111:2273–2285. doi: 10.1097/01.PRS.0000060102.57809.DA. [DOI] [PubMed] [Google Scholar]
- 9.Soo C, et al. Differential expression of matrix metalloproteinases and their tissue-derived inhibitors in cutaneous wound repair. Plast Reconstr Surg. 2000;105:638–647. doi: 10.1097/00006534-200002000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Yannas IV. Tissue and Organ Regeneration in Adults. Springer; 2001. [Google Scholar]
- 11.McGowan KM, Coulombe PA. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. J Cell Biol. 1998;143:469–486. doi: 10.1083/jcb.143.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- 13.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 14.Botchkarev VA, Sharov AA. BMP signaling in the control of skin development and hair follicle growth. Differentiation. 2004;72:512–526. doi: 10.1111/j.1432-0436.2004.07209005.x. [DOI] [PubMed] [Google Scholar]
- 15.Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Billingham RE, Russell PS. Incomplete wound contracture and the phenomenon of hair neogenesis in rabbits' skin. Nature. 1956;177:791–792. doi: 10.1038/177791b0. [DOI] [PubMed] [Google Scholar]
- 17.Breedis C. Regeneration of hair follicles and sebaceous glands from the epithelium of scars in the rabbit. Cancer Res. 1954;14:575–579. [PubMed] [Google Scholar]
- 18.Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 19.Vorontsova MAa, L LD. Asexual propagation and regeneration. Pergamon Press; 1960. [Google Scholar]
- 20.Borgens RB. Mice Regrow the Tips of Their Foretoes. Science. 1982;217:747–750. doi: 10.1126/science.7100922. [DOI] [PubMed] [Google Scholar]
- 21.Muneoka K, Allan CH, Yang X, Lee J, Han M. Mammalian regeneration and regenerative medicine. Birth Defects Research Part C: Embryo Today: Reviews. 2008;84:265–280. doi: 10.1002/bdrc.20137. [DOI] [PubMed] [Google Scholar]
- 22.Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clinical Immunology and Immunopathology. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- 23.Chalkley DT. A quantitative histological analysis of forelimb regeneration in Triturus viridescens. Journal of Morphology. 1954;94:21–70. [Google Scholar]
- 24.Globus M, Vethamany-Globus S, Lee YC. Effect of apical epidermal cap on mitotic cycle and cartilage differentiation in regeneration blastemata in the newt, Notophthalmus viridescens. Dev Biol. 1980;75:358–372. doi: 10.1016/0012-1606(80)90169-4. [DOI] [PubMed] [Google Scholar]
- 25.Neufeld DA, Day FA. Perspective: a suggested role for basement membrane structures during newt limb regeneration. Anat Rec. 1996;246:155–161. doi: 10.1002/(SICI)1097-0185(199610)246:2<155::AID-AR1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Calve S, Odelberg SJ, Simon HG. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Developmental Biology. 2010;344:259–271. doi: 10.1016/j.ydbio.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satoh A, makanae A, Hirata A, Satou Y. Blastema induction in aneurogenic state and Prrx-1 regulation by MMPs and FGFs in Ambystoma mexicanum limb regeneration. Dev Biol. 2011;355:263–274. doi: 10.1016/j.ydbio.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 29.Gundersen HJG. Notes on the estimation of the numerical density of arbitrary profiles: the edge effect. Journal of Microscopy. 1977;111:219–223. [Google Scholar]
- 30.Kiama SG, Maina JN, Bhattacharjee J, Weyrauch KD. Functional morphology of the pecten oculi in the nocturnal spotted eagle owl (Bubo bubo africanus), and the diurnal black kite (Milvus migrans) and domestic fowl (Gallus gallus var. domesticus): a comparative study. Journal of Zoology. 2001;254:521–528. [Google Scholar]
- 31.Weibel ER. Stereological methods. London San Francisco: Academic Press; 1979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.