Abstract
Intravascular stents were first introduced in the 1980s as an adjunct to primary angioplasty for management of early complications, including arterial dissection, or treatment of an inadequate technical outcome due to early elastic recoil of the atherosclerotic lesion. Despite the beneficial effects of stenting, persistent high rates of restenosis motivated the design of drug eluting stents for delivery of agents to limit the proliferative and other inflammatory responses within the vascular wall that contribute to the development of a restenotic lesion. These strategies have yielded a significant reduction in the incidence of restenosis, but challenges remain, including incomplete repair of the endothelium at the site of vascular wall injury that may be associated with a late risk of thrombosis. A failure of vessel wall healing has been attributed to primarily to the use of polymeric stent coatings, but the effects of the eluted drug and other material properties or design features of the stent cannot be excluded. Improvements in stent microfabrication, as well as the introduction of alternative materials may help to address those limitations that inhibit stent performance. This review describes the application of novel microfabrication processes and the evolution of new nanotechnologies that hold significant promise in eliminating existing shortcomings of current stent platforms.
1.0 Introduction to the Current Stent Market
Cardiovascular disease, most frequently due to atherosclerosis continues to be the leading cause of death in the western world. Although the application of intravascular stents have revolutionized the treatment of coronary and peripheral arterial disease acknowledged limitations have lead to rapid technological innovation over the past decade. Multiple parallel strategies remain under investigation in an attempt to optimize stent design for deployment in distinct vascular beds with differing biomechanical environments, vessel sizes, as well as unique lesion and patient characteristics.
1.1 Drawbacks and Limitations of Current Stent Platforms
Recurrent stenosis and late stage thrombosis remain significant limitations after stenting of peripheral or coronary atherosclerotic lesions (1, 2). Restenosis arises from the proliferation of smooth muscle cells in the vessel wall in response to acute vessel wall injury induced by angioplasty. The recent advent of drug eluting stents (DES) with the local release of an anti-proliferative agent has lead to a significant reduction in the risk of restenosis. While solving one problem, the use of DES has been associated with a small but increased risk of late thrombosis due to a failure of vessel wall healing (3-5). All patients who have received a DES have an ongoing requirement for dual antiplatelet therapy consisting of aspirin and a thienopyridine.
Prior studies have proven the value of a functional and intact endothelium in the prevention and attenuation of restenosis and thrombosis. The quest for a stent that has a high rate of endothelialization has lead to the investigation of unique surface topographic features and the attachment of various peptides to enhance the binding and proliferation of endothelial cells and circulating endothelial progenitor cells.
1.2 Drug Delivery
Drug eluting stents typically consist of a bare metal stent (BMS), the drug and a polymer drug delivery system (6, 7). This review will focus on the use of new fabrication technologies currently under investigation to overcome polymer based drug delivery systems presumed responsible for the shortcomings of DES. Investigators have been able to circumvent the need for polymer delivery systems by incorporating drug releasing reservoirs into stents, fabricating porous surfaces, and applying novel coating strategies to existing stent platforms.
1.3 Stent Design and Current Material Selection
Efforts persist in minimizing the dimensions of stent struts to facilitate delivery, allow for use in smaller vessels, and to minimize the contact area between the stent and the blood vessel wall. Stent fabrication begins with a stent design, which has traditionally consisted of a cell pattern that has circumferential rings connected by longitudinal struts. While differences exist, the vast majority of stent designs follow this basic principle. Changes that have been implemented include, the incorporation of drug reservoirs within the struts and a slide and lock design to facilitate the use polymeric materials, which do not deform plastically.
Material selection for stent applications has been the subject of numerous reviews (8-11). It requires the balancing of several factors to ensure the adequacy of the material's hoop strength, biocompatibility, thromboresistivity and radiopacity. The first stents and the majority of current stents are produced from 316L stainless steel because of its mechanical attributes and corrosion resistance. Another common material is the shape memory metal, nitinol, which is used for the fabrication of self-expanding stents deployed mainly in the peripheral arteries. The limitations of these materials reside in their minimal radiopacity and release of nickel. In an effort to increase radiopacity, researchers coated 316L stents with gold, though the results were unsatisfactory (12-14). Greater success was achieved however, in the fluoroscopic visualization of nitinol stents by the addition of radiopaque markers riveted onto stent extremes (10). To overcome the problems associated with release of nickel from 316L and nitinol stents(15, 16), researchers have applied surface treatments(17-19) effectively lowering the nickel content of the surface and increasing corrosion resistance. A brief look at the modifications these materials have undergone illustrates the optimization that is required for a stent to be of clinical utility. These materials continue to serve as the standard against which new materials will be compared.
More recently stent platforms have expanded to include cobalt chromium alloys(20), which have increased strength that allows for thinner struts(21). Also a number of bioabsorbable materials have been investigated under the postulation that improved clinical outcomes may be achievable through their use in stents that provide short-term mechanical scaffolding during initial vessel remodeling followed by absorption with complete vessel wall healing. Bioabsorbable stents also offer an opportunity to incorporate drugs throughout the stent, thereby, increasing the amount of drug that can be delivered.
2.0 Stent Fabrication
Fabrication of both bioabsorbable and inert polymeric and metallic stents have been pursued through a variety of schemes including both top-down fabrication approaches, such as laser machining, molding, electroforming, and bottom up strategies, such as 3-D printing and solvent casting. The multitude of fabrication modalities have resulted from a divergent set of materials used to develop stent platforms and advanced design considerations. Table 1 provides a brief overview of the fabrication modalities that have been used to produce stents or stent like devices in both academic and industrial settings. Exploration of both academic and industrial fabrication strategies has given insight into fabrication technologies that will need to be adopted as stent design continues to advance from both a material and design standpoint. The advancement of materials utilized for stent platforms and the complexity of current stent designs require an expanding range of diverse fabrication processes. This is already beginning in academia and industry thus providing a foundation for producing the next round of stents with sophisticated geometries and tight tolerances from cutting edge materials.
Table 1.
Stent Fabrication Strategies
| Fabrication Technique | Material Choiee | Research Arena | ||
|---|---|---|---|---|
| Metals | Polymers | Industry | Academia | |
| Nd:YAG Laser | ✓ | x | ✓ | ✓ |
| Feintosecoiid Laser | ✓ | ✓ | ✓ | x |
| Compression Molding | ✓ | ✓ | x | ✓ |
| Photochemical Etching | ✓ | ✓ | ✓ | ✓ |
| Electroforming | ✓ | x | ✓ | ✓ |
| Sputter Coating | ✓ | x | x | ✓ |
| Micro Electro Discharge | ✓ | x | x | ✓ |
| Lithography | ✓ | ✓ | x | ✓ |
| Fused Deposition Modeling | x | ✓ | x | ✓ |
| Solvent Casting | x | ✓ | x | ✓ |
2.1 Laser machining
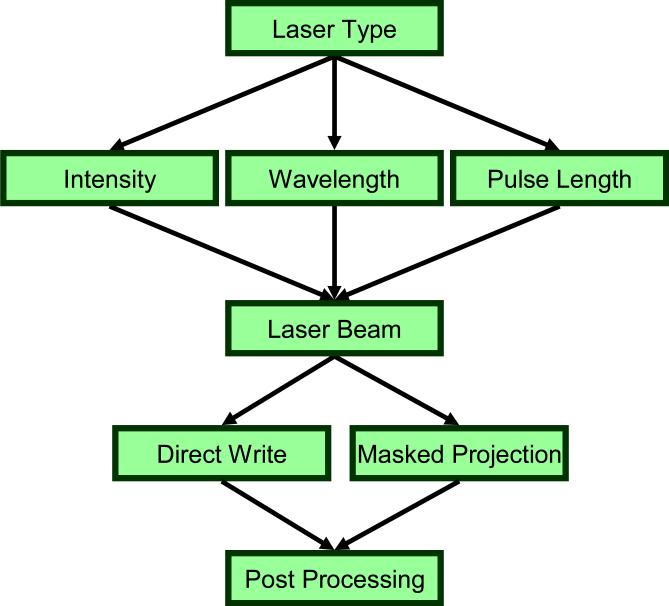
Laser machining of stents from thin walled tubing is the most common form of stent fabrication. Stents can be machined through a direct write method where the laser is focused directly on the substrate or a masked projection method in which the laser passes through a mask before hitting the substrate. Laser micromachining creates stents by removing material to create open cells via sublimation, melting, or oxygen reaction. Sublimation is not commonly used because the cutting speed is slow. However, this technique does minimize the adherence of solid impurities or dross and the extent of the heat affected zone in which adjacent microstructure and mechanical properties are altered by heat. Melting and oxygen reaction remain industry standards because of their high processing speeds, but often lead to the deposition of the removed material on the surface, oxidation at the cuts, and burr formation. Dross and spatter that stick to the backside of the cut can be removed with additional finishing steps, such as microblasting with aluminum oxide powder, pickling, or soft etching. Schemes to manipulate laser type, pulse length, power level, and different mediums that contain the laser have been sought to address these drawbacks (Fig. 1). One example of a novel strategy is the Synova Laser Microjet™ that passes the laser through a continuous stream of water to facilitate accurate cutting with minimal thermal and structural distortion.
Figure 1.
A number of laser specific variables, including intensity, wavelength, and pulse length, as well as material ablation formats are selected with respect to specific material properties for optimal stent fabrication.
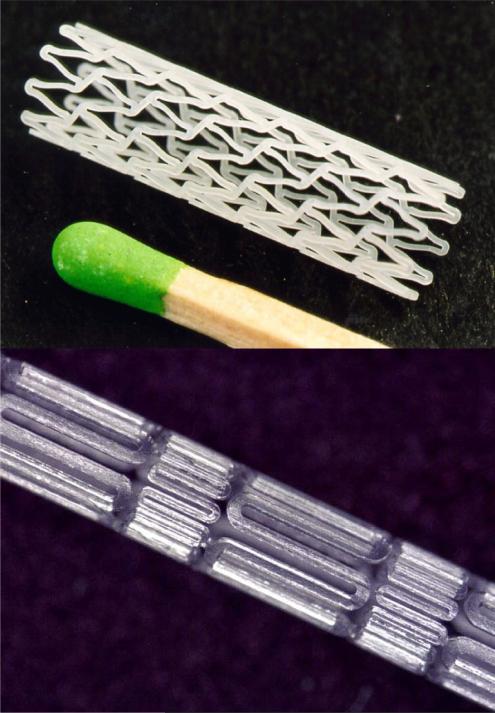
The neodymium:yttrium-aluminum-garnet (Nd:YAG) solid-state laser is the most frequently used laser for stent fabrication because of its short fundamental wavelength of 1064 nm. Nd:YAG lasers have been shown to make slits on the order of 50 - 100 μm with short pulses and high repetitions (22). The main drawback of the Nd:YAG laser is the size of the heat affected zone and dross adherence. Metallic materials exhibit exceptional absorption of infrared (IR) light, which allows for efficient and rapid machining with IR lasers. However, bioabsorbable polymers are optically transparent, therefore different lasers are required. Femtosecond and excimer lasers are capable of machining optically transparent materials, such as bioabsorbable polymers with high fidelity (Fig. 2) (23). Femtosecond lasers are usually based on the titanium:sapphire solid state laser and can machine objects on the nanometer scale with a cut width of 30 μm on nitinol (24, 25). The femtosecond laser has also been used to fabricate biodegradable magnesium alloy stents (26). Their major shortcoming is cost. Excimer lasers do not cause ablation by heat but through disruption of molecular bonds through absorption of laser light in the ultraviolet range. While adverse heat effects do not exist, lateral resolution is about 10 μm with beam quality not as good as other laser systems (27). Polymeric stents have also been machined using CO2 lasers (28, 29). However, CO2 lasers may induce chemical changes in the treated material (30).
Figure 2.
Example of two laser machined bioabsorbable stents. The top stent is courtestsy Laser Zentrum Hannover(© Laser Zentrum Hannover e.V. (LZH)) and the bottom stent is courtesy of Resonetics(©Resonetics)
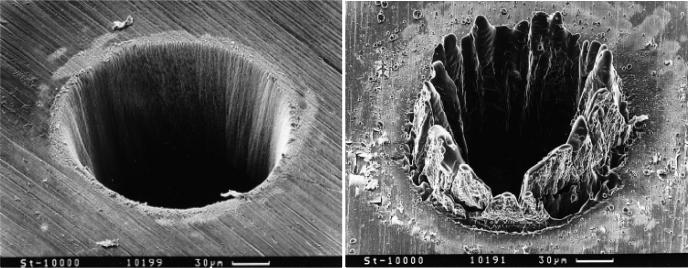
Laser parameters that can be varied are the pulse length and energy level. Pulses longer than 10 ns are associated with heat diffusion and conduction, which can impact the properties of material in the vicinity of the pulse beam (Fig 3.) (31). Shorter pulses, in the femtosecond range, and higher pulse repetition reduce the heat affected zone, which is especially critical for polymeric materials (32).
Figure 3.
The difference between femtosecond laser pulses and nanosecond pulses on thin steel films. The SEM image on the left shows a hole drilled in a 100μm thick steel foil with 200 fs, 120 μJ, F = 0.5J/cm2 laser pulses at 780nm. The SEM image on the right shows the molten material left behind when holes were drilled in a 100μm thick steel foil with 3.3 ns, 1 mJ, F = 4.2J/cm2 laser pulses at 780nm.(©Springer-Verlag 1996 ) (115)
2.2 Photoetching, Electroforming, and Microelectrodischarge Machining for Fabrication of Metallic Stents
Alternative stent manufacturing techniques include photoetching, electroforming, and micro-electrodischarge machining (μEDM), which are capable of producing burr/dross free cuts with minimal roughness. The μEDM process has been used to fabricate stents from planar 50 μm thick stainless steel foil (33) and demonstrate the capacity to incorporate sensor systems to monitor intravascular pressure and flow (34). Photochemical etching uses a photoresist and etchants to chemically remove unmasked material from metallic sheets or tubes in order to produce complex designs with high resolution(35). The stainless steel NIR (Boston Scientific, Inc), the stainless steel LP (Interventional Technologies, Inc) (36) and the nitinol aSpire (Vascular Architects, Inc.) stents were produced via this process. Photochemical etching has also been used in the fabrication of an origami stent fabricated from nitinol foil (37) and in conjunction with 3D lithography to produce patterns on nitinol films formed via magnetron sputtering (38, 39).
Electroforming, as a stent manufacturing tool, has been reported by Electroformed Stents Inc and Moravej et al. (40). In brief, a stent is produced by a process of electrodeposition onto a metallic mandrel, usually aluminum, which has been placed in a plating bath. The electroforming process has also been used to produce molds with micro- and nanoscale features.
Higher costs and slower fabrication times are acknowledged disadvantages of these processes and with the exception of electroforming, these techniques are best suited for fabrication of planar devices. Nonetheless, these approaches may lead to the development of batch compatible processes with high throughput production and reduced costs.
2.3 Fabrication Techniques for Production of Polymeric Stents
Laser machining has been used to produce polymeric stents, such as the REVA™ stent (41), however, concerns related to thermal or chemically induced alterations in polymer properties have motivated the evaluation of injection and compression molding processes. These techniques are particularly suitable for polymeric materials because of their thermoplastic behavior with recent efforts directed at optimizing micro- and nanomolding processes (42-46). Solvent cast films have been fabricated into stents by cutting the films into strips and wrapping them around a mandrel into a coil like structure (47). As a related process, fused deposition molding (FDM), which consists of repeated deposition of thin layers of polymer, has been evaluated for the production of 3D microstructures that display micron level resolution with short processing times (48). Likewise, melt spinning has also been used to form bioabsorbable polymer fibers on the range of 150 μm to 200 μm which can then be woven or knitted into stent-like structures (49, 50). The technique of controlled expansion of saturated polymers allows for drug loading during the fabrication process (51) and can form bioabsorbable stents that posses the proper mechanical integrity (52). To date, photolithography has been used to produce biodegradable scaffolds for tissue engineering applications, but has not been utilized in the fabrication of stents.
2.4 Drug Reservoirs
A number of stents, including the Janus™ and Conor™ (Table 2) stents have now been designed with drug containing reservoirs of various form and size. As previously noted, drug eluting stents based upon the application of a thin polymer film coating have lead to a significant reduction in the incidence of restenosis. Nonetheless, DES have been associated with delayed vascular wall healing and alternative designs sought for local drug delivery. One such approach has been the incorporation of localized reservoirs within the body of the stent (Fig. 4). In principle, reservoirs increase the total drug carrying capacity of the stent, reduce the mass of required polymer carrier, facilitate local delivery of multiple drugs, and provide greater flexibility in tailoring the site and kinetics of drug release (53). Reservoirs are sculpted into the struts of the Janus Carbostent™ and at hinge points in the Conor™ stent (Fig. 5) (53). Clinical outcomes have been mixed for both stent systems; reflecting the need for further studies to optimize polymer-drug formulations (54).
Table 2.
Polymer Free Drug Eluting Stents
| Company_Stent Name | Drug Delivery Type | Surface Fabrication Technique | Drug Application | Pore Size | Clinical Trials |
|---|---|---|---|---|---|
| Sorin_Janus Flex | Surface Reservoir | Sculpted | Adhered onto the Outer Surface | NA | Jupiter I,II |
| Conor/J&J_Conor, Costar, NEVO | Strut Reservoirs | Laser Cut | Automated MicroJet System | NA | PISCES, COSTAR I,II |
| Translumina_Yukon | Microporous Surface | Sand Blasting | Onsite Coating | ~2μm | ISAR-TEST |
| Abbott/Jomed_Nanoporous Stent | Nanoporous Surface | Electrochemical Oxidation of Aluminum Coating | Dipping Coating | 5-15nm | Present I,II |
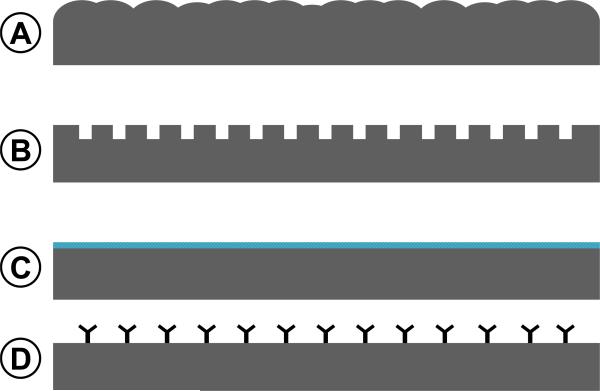
Figure 4.
Micro- and nano- fabrication has facilitated the design stents that harbor reservoirs for local drug delivery. (A) A single strut of a bare metal stent expanded in the vessel pressed against the endothelium with the smooth muscle cells beyond the endothelium. (B) Drug containing polymer coated strut and represents the first generation of drug eluting stents. Newer strategies include the incorporation of (C) microtopographic features that provide increased surface area for drug loading, (D) lumen-oriented reservoirs, and (E) drug reservoirs that extend throughout the strut structure.
Figure 5.
The Cordis NEVO™ stents has reservoirs machined into the stent struts(Courtesy of Cordis Corporation)
3.0 Surface Modifications
The bridge between bulk fabrication and applied coatings resides in the ability to change the surface properties of current stents to achieve different biological responses. There is no single material currently available that possesses both the requisite mechanical properties and elicits an optimal biological response. This has lead efforts to develop non-thrombogenic surface modifications that facilitates vascular wall healing in a manner that leads to the generation of an intact normal functional endothelium. Surface modifications have focused on three major areas - improvement of blood contacting characteristics, enhancement of endothelial cell migration, attachment and proliferation, and development of porous surface films for drug release.
3.1 Chemical and Physical Strategies for Stent Surface Modification
Surface modification strategies are inclusive of the physical or chemical modification of the surface of the pre-existing bulk material and deposition of a new material onto a base platform. Radiofrequency plasma treatment can alter the surface properties of metals and polymers by chemically modifying the molecular structure of the first few atomic layers, grafting different functional groups, or by creating reactive surfaces that can be used for additional surface modifications such as the covalent attachment of biopharmaceuticals. Plasma or chemical based etching and sanding, as well as polishing and microblasting, have been used to modify surface topography and enhance surface roughness. Plasma processing is also capable of forming nanopillar arrays on the surface of a stent (55) providing a surface that could be used for cell proliferation or drug delivery. A combinational approach could also be used in which a stent surface would be micropatterned and subsequently plasma coated allowing for control over the physical and chemical properties (56) of the stent surface.
The deposition of a thin film layer onto the non-uniform geometry that is characteristic of most stents can be accomplished by chemical (CVD) or physical vapor deposition (PVD) and by plasma polymerization. CVD and PVD afford strongly adherent pinhole free films, in addition to the optimal adhesion and integrity upon stent expansion observed with thinner films (~36 nm) (57). Plasma deposition has the potential to create stable films that can enhance corrosion resistance and functionalize stent surfaces with a high density of grafting sites(58) such as amino groups that facilitate covalent attachment of biopharmaceuticals (59, 60) and pharmaceuticals (61). PVD techniques, such as matrix assisted pulsed laser evaporation have also been used for the deposition of both organic and biological materials (62). Recently, PTFE and 316L stainless steel have been co-deposited by radiofrequency magnetron sputtering to produce a gradient surface coating that progresses from a metal base to PTFE at the surface (63). Finally, the use of CVD to apply thin films of diamond-like carbon has shown the potential to allow tailorable drug release from underlying polymers based on CVD film surface area(64).
3.2 Enhancing Surface Blood Contacting Properties
In vitro tests of thrombogenic potential are somewhat limited in predicting in vivo performance. Nonetheless, recent investigations suggest that plasma and chemical based etching can improve the hemocompatibility of stents (65). Fluorinated diamond-like carbon films of 40-50 nm in thickness, prepared using plasma enhanced CVD, showed a significant reduction in platelet adhesion (66, 67), as did a PLLA stent that was subjected to plasma polymerization of diethylene glycol (49). Strategies based upon the attachment of anti-thrombogenic molecules have been reviewed in detail elsewhere(68).
3.3 Enhancing Endothelial Cell Adhesion and Growth: Inductive and Conductive Coatings
Surface modifications that alter surface chemistry or topography can influence protein and cell adhesion leading to enhanced endothelial cell growth and proliferation (Fig. 6) (69, 70). Recent examples include sputtering TiO2 onto 316L stainless steel (71) and binding tropoelastin to plasma treated metal surfaces, which enhanced cell attachment and proliferation (72). Likewise, microblasting and reactive ion etching lead to topologically rough surfaces with improved cell attachment (73) and nanoscale surface features produced by polishing influenced endothelial cell proliferation and protein expression. Patterned Ti with grooves ranging from 750 nm to 200 μm showed enhanced endothelial coverage on nanometer scale patterns compared to micron scale patterns or random nanostructured surfaces (74). Nanometer patterns can also be produced in a manner that leads to endothelial cell alignment, which mimics native endothelium (75). Of note, surfaces with a defined nanopatterned grid demonstrated a greater degree of endothelial cell adhesion, as compared to responses observed on random nanostructured surfaces (76). Nanostructured surfaces, when compared to microstrutured surfaces, appear to afford greater adhesion of endothelial cells than smooth cells (77), lead to higher cell densities (78), and enhanced adhesion and spreading (79). Interestingly, features between 100 nm and 1 μm have yielded greater cell adhesion densities than those less than 100 nm (80). The molecular mechanisms of these effects have not been well defined, but may be related, in part, to changes in protein adsorption profiles.
Figure 6.
Surface Modifications strategies to enhance endothelialization include: (A) surface roughening to increase topography, (B) discreet patterning, (C) chemical modification of the surface, and (D) covalent attachment of biopharmaceuticals.
Layer-by-layer (54) polymer assembly has been employed to deposit a thin film on stents. LbL assembly on NiTi surfaces reduced in vitro platelet adhesion and facilitated the incorporation of a nitric oxide donor (81). DNA loaded into an LbL film has been successfully transferred from a stent into the vascular wall (82). Self-assembled monolayers (SAMs) form highly ordered monolayers a few nanometers thick and facilitate the presentation of a variety of unique chemical groups, including those that chelate radioisotopes (83). SAMs can persist on a stainless steel stent for 14-21 days before oxidation and desorption of the thin film from the stent (84). As a variant of film deposition strategies based upon molecular self-assembly, Stupp and colleagues have described the deposition of self-assembled peptide nanofibers to enhance cell adhesion and proliferation (85). Ink-jet techniques have also shown some promise as a method coat stents with drug-polymer formulations with the flexibility of deposition at spatially discrete sites (86).
3.4 Microporous and Nanoporous Stents for Drug Delivery
Microporous and nanoporous stent surfaces are intriguing because of their capacity to increase drug loading and influence drug release kinetics without the need for a polymer coating (Fig. 7, Table 2). Stents sand blasted to produce a rough finish are at least as safe as non-treated stents, but their potential to have a meaningful clinical impact remains to be demonstrated (87). Porous surfaces have been created either by direct treatment of the stent or after deposition of a nonpolymeric coating.
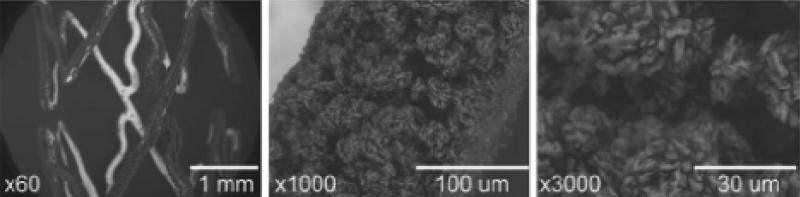
Figure 7.
Scanning Electron Microscope images at different magnifications of the expanded Translumina YUKON®DES Coronary Stent System with PEARL surface coated with leflunomide. The microporous stent surface allows for the incorporation of drugs with slower release kinetics without the need for a polymer coating. The roughness of the stent surface is 1.96±0.21 μm(Reprinted with permission of the Elsevier Ireland Ltd. © 2008) (116)
Microporous stents
Recent examples of stents that contain a microporous coating include the Corel-C™ stent that is fabricated from CoCr with a carbon nanoparticle coating (Relysis Medical, India) (88). Microporous surfaces have also been incorporated into polymer based bioabsorbable stents (89). As noted, sandblasting has been used to create pores between 1 to 100 μm on the surface of stainless steel stents, an example of which is the Yukon™ stent platform (Translumina, Hechingen, Germany) (90). Adsorption of rapamycin along with other drugs has proven feasible and showed a dose-dependent efficacy in prevention of restenosis (91). However, the rapamycin eluting Yukon™ stent was found to be clinically inferior to the Cypher™ stent, as well as a version of the Yukon™ stent coated in a drug containing bioabsorbable polymer (92).
Nanoporous surfaces
At least two examples exist of nanoporous stent surfaces. The Jomed™ coating consists of a thin aluminum base layer, which is then subjected to an acidic solution that converts the aluminum into a thin ceramic nanoporous aluminum oxide (93). A recent report suggests that particle debris may be released from the stent surface (94). MIV Therapeutics, Inc. has developed a stent with a hydroxyapatite coating that is 0.30 to 1 μm in thickness with a porosity of 40-60% in volume (95). This stent has shown promising responses in both animal studies and in an initial clinical study after adsorption of sirolimus (96).
4.0 Biopharmaceuticals
Peptides (97), DNA (98), and heparin (99), as well as anti-platelet agents, such as abciximab (59), growth factors and integrin binding sequences have been incorporated onto stents to reduce thrombosis or increase endothelial migration. Plasmids coding for vascular endothelial growth factor (VEGF) (100) and VEGF itself (101) have been coated onto stents to increase the rate of endothelialization. Cyclic Arg-Gly-Asp (cRGD), as well as anti-CD34 and anti-kinase insert domain antibodies have been applied to increase the recruitment and retention of endothelial progenitor cells (97). However, a recent report have raised efficacy and safety concerns of EPC capture stents (102).
5.0 Microspheres and Nanoparticles
Microspheres and nanoparticles have been used to nano-texture stents, in molding processes to make stents, and for drug delivery from stents. Proteins have been incorporated within 1-2 μm diameter dextran particles for use in polymer based delivery devices (103). Drug loaded microspheres and nanoparticles have been administered systemically and delivered locally via microporous catheters (104). Drug loaded nanoparticles have also been deposited onto stents and exhibited a delivery profile that was superior to that of dip coated stents (105). Microspheres have been dispersed within polymeric stents (106) and embedded within polymer containing channels etched in struts for abluminal drug delivery (107). Both elastase inhibitors (108) and nitric oxide donors (109) have been delivered from nanoparticles in this fashion.
Stents have been used as homing sites for drug containing magnetic microspheres and nanoparticles (Fig. 8). Magnetic targeting would allow an agent to be delivered on demand to an in vivo site with different dosing regimes (110). Magnetic nanoparticles have been used to deliver genes to the lumen of a vessel (111) and the ability to capture particles from 2 μm to 370 nm in diameter has been shown under simulated in vivo flow conditions (112). Cells can also endocytose magnetic microspheres and nanoparticles for targeting to stent surfaces (113, 114).
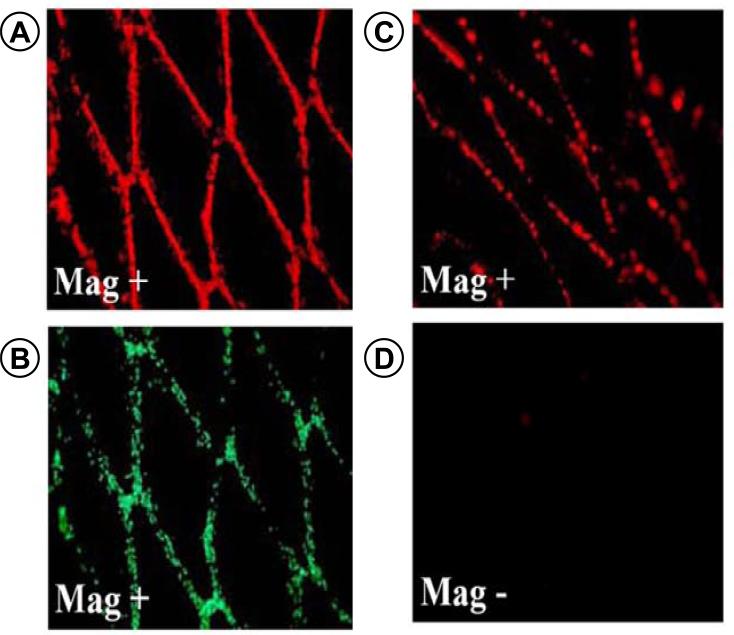
Figure 8.
Illustrates the magnetic targeting of bovine aortic endothelial cells(BAECs) under flow conditions to stainless-steel stents in vitro and in vivo. Magnetically responsive BAECS are shown captured on SS stents based on (A) red fluorescence of the MNPs and (B) Calcein staining of live cells. To determine in vivo capture BAECs were loaded with fluorescent MNPs and injected into the ventricular cavity. (C) Rats were exposed to a magnetic field of 1,000 G for 5 minutes then sacrificed and the stents were removed and imaged. (D) Control rats were not exposed to magnetic field. (© 2008 The National Academy of Sciences of the USA) (113)
6.0 Conclusions
Stent design continues to mature in a manner that has lead to the adoption of novel strategies derived from the fields of material science, microfabrication, and nanotechnology. The next generation of stent designs is leveraging advancements in material science to create stent platforms from diverse materials ranging from new alloys to bioabsorbable polymers. New alloys have allowed for more sophisticated stent designs, while bioabsorbable stents offer the potential of treating a vessel for a transient period of time before absorbing into the vessel resulting in a healthy vessel with no permanent foreign body. Platforms are leaning towards a future in which they have minimal radial profiles and tissue contact while maintaining adequate mechanical strength, and deliverability. Stent platforms are utilizing alterations in structure to improve drug delivery and targeting. Drug delivery reservoirs have been incorporated into stents in an attempt to improve spatially targeted drug delivery while minimizing the presence of the polymer carrier. Current research has focused upon improved stent coatings that will allow stents to serve as vehicles for local drug delivery and act as inductive scaffold for endothelial repair. The synergy of micro- and nanoparticle technology and stent design will continue to be employed to enhance the flexibility of local drug release and demonstrate the potential to target treatment to the site of the deployed stent. Stent surfaces have been modified to yield improvements in blood contacting properties, drug delivery to reduce restenosis, and accelerate endothelialization through targeting local healing events in the vascular wall and the capture of circulating progenitor cells. Stents have evolved into multifaceted devices that have benefited from advances in a variety of fields and will continue to incorporate new advances from these fields to overcome current limitations and create the next generation of stents.
REFERENCES
- 1.Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. American Heart Journal. 2006;151(6):1260–4. doi: 10.1016/j.ahj.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Doyle B, Rihal CS, O'Sullivan CJ, Lennon RJ, Wiste HJ, Bell M, et al. Outcomes of stent thrombosis and restenosis during extended follow-up of patients treated with bare-metal coronary stents. Circulation. 2007 Nov 20;116(21):2391–8. doi: 10.1161/CIRCULATIONAHA.107.707331. [DOI] [PubMed] [Google Scholar]
- 3.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006 Jul 4;48(1):193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 4.Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007 Feb 27;115(8):1051–8. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 5.Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007 Mar 8;356(10):998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 6.Kamath KR, Barry JJ, Miller KM. The Taxus drug-eluting stent: a new paradigm in controlled drug delivery. Adv Drug Deliv Rev. 2006 Jun 3;58(3):412–36. doi: 10.1016/j.addr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Puskas JE, Munoz-Robledo LG, Hoerr RA, Foley J, Schmidt SP, Evancho-Chapman M, et al. Drug-eluting stent coatings. Wires Nanomed Nanobi. 2009 Jul-Aug;1(4):451–62. doi: 10.1002/wnan.38. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien B, Carroll W. The evolution of cardiovascular stent materials and surfaces in response to clinical drivers: A review. Acta Biomaterialia. 2009 May;5(4):945–58. doi: 10.1016/j.actbio.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Mani G, Feldman MD, Patel D, Agrawal CM. Coronary stents: a materials perspective. Biomaterials. 2007 Mar;28(9):1689–710. doi: 10.1016/j.biomaterials.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Stoeckel D, Pelton A, Duerig T. Self-expanding nitinol stents: material and design considerations. Eur Radiol. 2004 Feb;14(2):292–301. doi: 10.1007/s00330-003-2022-5. [DOI] [PubMed] [Google Scholar]
- 11.Waksman R. Update on bioabsorbable stents: from bench to clinical. J Interv Cardiol. 2006 Oct;19(5):414–21. doi: 10.1111/j.1540-8183.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- 12.Reifart N, Morice MC, Silber S, Benit E, Hauptmann KE, de Sousa E, et al. The NUGGET study: NIR ultra gold-gilded equivalency trial. Catheterization and Cardiovascular Interventions. 2004 May;62(1):18–25. doi: 10.1002/ccd.20026. [DOI] [PubMed] [Google Scholar]
- 13.Kastrati A, Schomig A, Dirschinger J, Mehilli J, von Welser N, Pache J, et al. Increased Risk of Restenosis After Placement of Gold-Coated Stents : Results of a Randomized Trial Comparing Gold-Coated With Uncoated Steel Stents in Patients With Coronary Artery Disease. Circulation. 2000 May 30;101(21):2478–83. doi: 10.1161/01.cir.101.21.2478. 2000. [DOI] [PubMed] [Google Scholar]
- 14.vom Dahl J, Haager PK, Grube E, Gross M, Beythien C, Kromer EP, et al. Effects of gold coating of coronary stents on neointimal proliferation following stent implantation. Am J Cardiol. 2002 Apr 1;89(7):801–5. doi: 10.1016/s0002-9149(02)02188-4. [DOI] [PubMed] [Google Scholar]
- 15.Koster R, Vieluf D, Kiehn M, Sommerauer M, Kahler J, Baldus S, et al. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet. 2000 Dec 2;356(9245):1895–7. doi: 10.1016/S0140-6736(00)03262-1. [DOI] [PubMed] [Google Scholar]
- 16.Heintz C, Riepe G, Birken L, Kaiser E, Chakfe N, Morlock M, et al. Corroded nitinol wires in explanted aortic endografts: an important mechanism of failure? J Endovasc Ther. 2001 Jun;8(3):248–53. doi: 10.1177/152660280100800303. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien B, Carroll WM, Kelly MJ. Passivation of nitinol wire for vascular implants - a demonstration of the benefits. Biomaterials. 2002 Apr;23(8):1739–48. doi: 10.1016/s0142-9612(01)00299-x. [DOI] [PubMed] [Google Scholar]
- 18.Chu CL, Wang RM, Yin LH, Pu YP, Dong YS, Guo C, et al. Surface treatment of NiTi shape memory alloy by modified advanced oxidation process. T Nonferr Metal Soc. 2009 Jun;19(3):575–80. [Google Scholar]
- 19.Firstov GS, Vitchev RG, Kumar H, Blanpain B, Van Humbeeck J. Surface oxidation of NiTi shape memory alloy. Biomaterials. 2002 Dec;23(24):4863–71. doi: 10.1016/s0142-9612(02)00244-2. [DOI] [PubMed] [Google Scholar]
- 20.Kereiakes DJ, Cox DA, Hermiller JB, Midei MG, Bachinsky WB, Nukta ED, et al. Usefulness of a cobalt chromium coronary stent alloy. Am J Cardiol. 2003 Aug 15;92(4):463–6. doi: 10.1016/s0002-9149(03)00669-6. [DOI] [PubMed] [Google Scholar]
- 21.Sketch MH, Ball M, Rutherford B, Popma JJ, Russell C, Kereiakes DJ, et al. Evaluation of the medtronic (Driver) cobalt-chromium alloy coronary stent system. American Journal of Cardiology. 2005 Jan 1;95(1):8–12. doi: 10.1016/j.amjcard.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 22.Kathuria YP. Biocompatible metallic stent for medical therapy. Window on the Laser Medicine World. 2003;5287:52–61. 180. [Google Scholar]
- 23.Aguilar CA, Lu Y, Mao S, Chen SC. Direct micro-patterning of biodegradable polymers using ultraviolet and femtosecond lasers. Biomaterials. 2005 Dec;26(36):7642–9. doi: 10.1016/j.biomaterials.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Ishizuka M, Liu XB, Sugimoto Y, Ikeda N, Asakawa K. Nanostructuring in submicron-level waveguides with femtosecond laser pulses. Optics Communications. 2002 Oct 15;212(1-3):159–63. [Google Scholar]
- 25.Li CD, Nikumb S, Wong F. An optimal process of femtosecond laser cutting of NiTi shape memory alloy for fabrication of miniature devices. Optics and Lasers in Engineering. 2006 Oct;44(10):1078–87. [Google Scholar]
- 26.Heublein B, Rohde R, Kaese V, Niemeyer M, Hartung W, Haverich A. Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology? Heart. 2003 Jun;89(6):651–6. doi: 10.1136/heart.89.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia M, Tu YL. An investigation of femtosecond laser micromachining. 2005 International Conference on MEMS, NANO and Smart Systems, Proceedings. 2005:296–300. [Google Scholar]
- 28.Grabow N, Bunger CM, Schultze C, Schmohl K, Martin DP, Williams SF, et al. A biodegradable slotted tube stent based on poly(L-lactide) and poly(4-hydroxybutyrate) for rapid balloon-expansion. Ann Biomed Eng. 2007 Dec;35(12):2031–8. doi: 10.1007/s10439-007-9376-9. [DOI] [PubMed] [Google Scholar]
- 29.Grabow N, Schlun M, Sternberg K, Hakansson N, Kramer S, Schmitz KP. Mechanical properties of laser cut poly(L-lactide) micro-specimens: implications for stent design, manufacture, and sterilization. J Biomech Eng. 2005 Feb;127(1):25–31. doi: 10.1115/1.1835349. [DOI] [PubMed] [Google Scholar]
- 30.Lootz D, Behrend D, Kramer S, Freier T, Haubold A, Benkiesser G, et al. Laser cutting: influence on morphological and physicochemical properties of polyhydroxybutyrate. Biomaterials. 2001 Sep;22(18):2447–52. doi: 10.1016/s0142-9612(00)00245-3. [DOI] [PubMed] [Google Scholar]
- 31.Tonshoff HK, Ostendorf A, Nolte S, Korte F, Bauer T. Micro-machining using femtosecond lasers. 1St International Symposium on Laser Precision Microfabrication. 2000;4088:136–9. 410. [Google Scholar]
- 32.Kathuria YP. Laser microprocessing of metallic stent for medical therapy. Journal of Materials Processing Technology. 2005 Dec 30;170(3):545–50. [Google Scholar]
- 33.Takahata K, Gianchandani YB. A planar approach for manufacturing cardiac stents: Design, fabrication, and mechanical evaluation. Journal of Microelectromechanical Systems. [Article] 2004 Dec;13(6):933–9. [Google Scholar]
- 34.Takahata K, Gianchandani YB, Wise KD. Micromachined antenna stents and cuffs for monitoring intraluminal pressure and flow. Journal of Microelectromechanical Systems. [Article] 2006 Oct;15(5):1289–98. [Google Scholar]
- 35.Shape Memory and Superelastic Technologies. SMST, The International Organization on Shape Memory and superelastic Technology; Pacific Grove, California, USA: 2000. SMST-2000: proceedings of the International Conference on Shape Memory and Superelastic Technologies. [Google Scholar]
- 36.Craig CH, Radisch HR, Trozera TA, Turner PC, Govier RD, Vesely EJ, et al. Development of a platinum-enhanced radiopaque stainless steel (PERSS (R))(7). Am Soc Test Mater. 2003;1438:28–38. [Google Scholar]
- 37.Kuribayashi K, Tsuchiya K, You Z, Tomus D, Umemoto M, Ito T, et al. Self-deployable origami stent grafts as a biomedical application of Ni-rich TiNi shape memory alloy foil. Materials Science and Engineering a-Structural Materials Properties Microstructure and Processing. 2006 Mar 15;419(1-2):131–7. [Google Scholar]
- 38.de Miranda RL, Zamponi C, Quandt E. Fabrication of TiNi thin film stents. Smart Mater Struct. 2009 Oct;18(10) [Google Scholar]
- 39.Rumpf H, Wipperfurth V, Zamponi C, Quandt E. Near net-shape fabrication of superelastic NiTi devices by sputtering and photoetching. Mater Trans. 2006 Mar;47(3):523–6. [Google Scholar]
- 40.Moravej M, Prima F, Fiset M, Mantovani D. Electroformed iron as new biomaterial for degradable stents: Development process and structure-properties relationship. Acta Biomaterialia. 2010;6(5):1726–35. doi: 10.1016/j.actbio.2010.01.010. [doi: DOI: 10.1016/j.actbio.2010.01.010] [DOI] [PubMed] [Google Scholar]
- 41.Tolken S. Firms Investing in angio-plastics solution. Plastics News. 2005;16(46) 1/24/2005. [Google Scholar]
- 42.Park JH, Davis S, Yoon YK, Prausnitz MR, Allen MG. Micromachined biodegradable microstructures. Mems-03: Ieee the Sixteenth Annual International Conference on Micro Electro Mechanical Systems. 2003:371–4. [Google Scholar]
- 43.Armani DK, Liu C. Mircofabrication technology for polycaprolactone, a biodegradable polymer. J Micromech Microeng. [Article] 2000 Mar;10(1):80–4. [Google Scholar]
- 44.Lafont A, Li SM, Garreau H, Cornhill F, Vert M. PLA stereocopolymers as sources of bioresorbable stents: Preliminary investigation in rabbit. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2006 May;77B(2):349–56. doi: 10.1002/jbm.b.30391. [DOI] [PubMed] [Google Scholar]
- 45.Grayson ACR, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, et al. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat Mater. [Article] 2003 Nov;2(11):767–72. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 46.Ryu W, Fasching RJ, Vyakarnam M, Greco RS, Prinz FB. Microfabrication technology of biodegradable polymers for interconnecting microstructures. Journal of Microelectromechanical Systems. 2006 Dec;15(6):1457–65. [Google Scholar]
- 47.Chen MC, Liu CT, Tsai HW, Lai WY, Chang Y, Sung HW. Mechanical properties, drug eluting characteristics and in vivo performance of a genipin-crosslinked chitosan polymeric stent. Biomaterials. 2009 Oct;30(29):5560–71. doi: 10.1016/j.biomaterials.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 48.Yamada A, Niikura F, Ikuta K. A three-dimensional microfabrication system for biodegradable polymers with high resolution and biocompatibility. J Micromech Microeng. 2008 Feb;18(2) doi: 10.1109/IEMBS.2004.1403769. [DOI] [PubMed] [Google Scholar]
- 49.Su SH, Chao RYN, Landau CL, Nelson KD, Timmons RB, Meidell RS, et al. Expandable bioresorbable endovascular stent. I. Fabrication and properties. Annals of Biomedical Engineering. [Article] 2003 Jun;31(6):667–77. doi: 10.1114/1.1575756. [DOI] [PubMed] [Google Scholar]
- 50.Han DW, Lee JJ, Jung DY, Park JC, Hyon SH. Development of epigallocatechin gallate-eluting polymeric stent and its physicochemical, biomechanical and biological evaluations. Biomed Mater. [Article] 2009 Aug;4(4) doi: 10.1088/1748-6041/4/4/044104. [DOI] [PubMed] [Google Scholar]
- 51.Vogt F, Stein A, Rettemeier G, Krott N, Hoffmann R, vom Dahl J, et al. Long-term assessment of a novel biodegradable paclitaxel-eluting coronary polylactide stent. European Heart Journal. 2004 Aug;25(15):1330–40. doi: 10.1016/j.ehj.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 52.Blindt R, Hoffmeister KM, Bienert H, Pfannschmitt O, Bartsch G, Thissen H, et al. Development of a new biodegradable intravascular polymer stent with simultaneous incorporation of bioactive substances. International Journal of Artificial Organs. 1999 Dec;22(12):843–53. [PubMed] [Google Scholar]
- 53.Finkelstein A, McClean D, Kar S, Takizawa K, Varghese K, Baek N, et al. Local drug delivery via a coronary stent with programmable release pharmacokinetics. Circulation. 2003 Feb 11;107(5):777–84. doi: 10.1161/01.cir.0000050367.65079.71. [DOI] [PubMed] [Google Scholar]
- 54.Krucoff MW, Kereiakes DJ, Petersen JL, Mehran R, Hasselblad V, Lansky AJ, et al. A novel bioresorbable polymer paclitaxel-eluting stent for the treatment of single and multivessel coronary disease. Journal of the American College of Cardiology. [Article] 2008 Apr;51(16):1543–52. doi: 10.1016/j.jacc.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 55.Loya MC, Park E, Chen LH, Brammer KS, Jin S. Radially arrayed nanopillar formation on metallic stent wire surface via radio-frequency plasma. Acta Biomaterialia. 2010;6(4):1671–7. doi: 10.1016/j.actbio.2009.11.015. [doi: DOI: 10.1016/j.actbio.2009.11.015] [DOI] [PubMed] [Google Scholar]
- 56.Shen Y, Wang GX, Chen L, Li H, Yu P, Bai MJ, et al. Investigation of surface endothelialization on biomedical nitinol (NiTi) alloy: Effects of surface micropatterning combined with plasma nanocoatings. Acta Biomaterialia. 2009 Nov;5(9):3593–604. doi: 10.1016/j.actbio.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 57.Lewis F, Horny P, Hale P, Turgeon S, Tatoulian M, Mantovani D. Study of the adhesion of thin plasma fluorocarbon coatings resisting plastic deformation for stent applications. J Phys D, Appl Phys. 2008 Feb 21;41(4):045310. (7 pp.)- (7 pp.) [Google Scholar]
- 58.Chen CH, Yang MR, Wu SK. Polymerized hexamethyldisilazane coated on equiatomic TiNi shape memory alloy using DC-pulsed plasma assisted chemical vapor deposition. Surf Coat Technol. 2008 Mar 15;202(12):2709–14. [Google Scholar]
- 59.Hong YJ, Jeong MH, Kim W, Lim SY, Lee SH, Hong SN, et al. Effect of abciximab-coated stent on in-stent intimal hyperplasia in human coronary arteries. American Journal of Cardiology. 2004 Oct 15;94(8):1050–4. doi: 10.1016/j.amjcard.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 60.Yang ZL, Wang J, Luo RF, Maitz MF, Jing FJ, Sun H, et al. The covalent immobilization of heparin to pulsed-plasma polymeric allylamine films on 316L stainless steel and the resulting effects on hemocompatibility. Biomaterials. 2010 Mar;31(8):2072–83. doi: 10.1016/j.biomaterials.2009.11.091. [DOI] [PubMed] [Google Scholar]
- 61.Song SJ, Kim KS, Kim KH, Li HJ, Kim JH, Jeong MH, et al. Preparation of a biocompatible stent surface by plasma polymerization followed by chemical grafting of drug compounds. J Mater Chem. 2009;19(20):3248–52. [Google Scholar]
- 62.Patz TM, Doraiswamy A, Narayan RJ, Menegazzo N, Kranz C, Mizaikoff B, et al. Matrix assisted pulsed laser evaporation of biomaterial thin films. Materials Science & Engineering C-Biomimetic and Supramolecular Systems. 2007 Apr;27(3):514–22. [Google Scholar]
- 63.Piedade AP, Nunes J, Vieira MT. Thin films with chemically graded functionality based on fluorine polymers and stainless steel. Acta Biomaterialia. [Article] 2008 Jul;4(4):1073–80. doi: 10.1016/j.actbio.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 64.Enomoto K, Hasebe T, Asakawa R, Kamijo A, Yoshimoto Y, Suzuki T, et al. Controlling the drug release rate from biocompatible polymers with micro-patterned diamond-like carbon (DLC) coating. Diam Relat Mat. [doi: DOI: 10.1016/j.diamond.2010.01.053] In Press, Corrected Proof. [Google Scholar]
- 65.Lahann J, Klee D, Thelen H, Bienert H, Vorwerk D, Hocker H. Improvement of haemocompatibility of metallic stents by polymer coating. Journal of Materials Science-Materials in Medicine. 1999 Jul;10(7):443–8. doi: 10.1023/a:1008939400812. [DOI] [PubMed] [Google Scholar]
- 66.Hasebe T, Ishimaru T, Kamijo A, Yoshimoto Y, Yoshimura T, Yohena S, et al. Effects of surface roughness on anti-thrombogenicity of diamond-like carbon films. Diam Relat Mat. [Proceedings Paper] 2007 Apr-Jul;16(4-7):1343–8. [Google Scholar]
- 67.Hasebe T, Shimada A, Suzuki T, Matsuoka Y, Saito T, Yohena S, et al. Fluorinated diamond-like carbon as antithrombogenic coating for blood-contacting devices. Journal of Biomedical Materials Research Part A. 2006 Jan;76A(1):86–94. doi: 10.1002/jbm.a.30512. [DOI] [PubMed] [Google Scholar]
- 68.Jordan SW, Chaikof EL. Novel thromboresistant materials. Journal of Vascular Surgery. 2007 Jun;45:104A–15A. doi: 10.1016/j.jvs.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 69.De S, Sharma R, Ali N, Mazumder MK. Enhancement of blood compatibility of implants by helium plasma treatment. Conference Record of the 2004 Ieee Industry Applications Conference, Vols 1-4 - Covering Theory to Practice. [Proceedings Paper] 2004:932–6. [Google Scholar]
- 70.Parker ER, Thibeault BJ, Aimi MF, Rao MP, MacDonald NC. Inductively coupled plasma etching of bulk titanium for MEMS applications. Journal of the Electrochemical Society. 2005;152(10):C675–C83. [Google Scholar]
- 71.Yeh HI, Lu SK, Tian TY, Hong RC, Lee WH, Tsai CH. Comparison of endothelial cells grown on different stent materials. Journal of Biomedical Materials Research Part A. 2006 Mar 15;76A(4):835–41. doi: 10.1002/jbm.a.30595. [DOI] [PubMed] [Google Scholar]
- 72.Yin YB, Wise SG, Nosworthy NJ, Waterhouse A, Bax DV, Youssef H, et al. Covalent immobilisation of tropoelastin on a plasma deposited interface for enhancement of endothelialisation on metal surfaces. Biomaterials. [Article] 2009 Mar;30(9):1675–81. doi: 10.1016/j.biomaterials.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 73.Pypen CMJM, Plenk H, Ebel MF, Svagera R, Wernisch J. Characterization of microblasted and reactive ion etched surfaces on the commercially pure metals niobium, tantalum and titanium. Journal of Materials Science-Materials in Medicine. 1997 Dec;8(12):781–4. doi: 10.1023/a:1018568830442. [DOI] [PubMed] [Google Scholar]
- 74.Palmaz JC, Benson A, Sprague EA. Influence of surface topography on endothelialization of intravascular metallic material. Journal of Vascular and Interventional Radiology. 1999 Apr;10(4):439–44. doi: 10.1016/s1051-0443(99)70063-1. [DOI] [PubMed] [Google Scholar]
- 75.Lu J, Rao MP, MacDonald NC, Khang D, Webster TJ. Improved endothelial cell adhesion and proliferation on patterned titanium surfaces with rationally designed, micrometer to nanometer features. Acta Biomaterialia. 2008 Jan;4(1):192–201. doi: 10.1016/j.actbio.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 76.Jing Lu aTJW., editor. Endothelial Cell Adhesion on highly Controllable Compared to Random Nanostuctured Titanium Surface Features. Materials Research Society Symposium. 2007 [Google Scholar]
- 77.Choudhary S, Haberstroh KM, Webster TJ. Enhanced functions of vascular cells on nanostructured Ti for improved stent applications. Tissue Engineering. 2007 Jul;13(7):1421–30. doi: 10.1089/ten.2006.0376. [DOI] [PubMed] [Google Scholar]
- 78.Samaroo HD, Lu J, Webster TJ. Enhanced endothelial cell density on NiTi surfaces with sub-macron to nanometer roughness. International Journal of Nanomedicine. 2008;3(1):75–82. doi: 10.2147/ijn.s2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choudhary S, Berhe M, Haberstroh KM, Webster TJ. Increased endothelial and vascular smooth muscle cell adhesion on nanostructured titanium and CoCrMo. International Journal of Nanomedicine. 2006;1(1):41–9. doi: 10.2147/nano.2006.1.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khang D, Lu J, Yao C, Haberstroh KM, Webster TJ. The role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titanium. Biomaterials. 2008 Mar;29(8):970–83. doi: 10.1016/j.biomaterials.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 81.Thierry B, Winnik FM, Merhi Y, Silver J, Tabrizian M. Bioactive coatings of endovascular stents based on polyelectrolyte multilayers. Biomacromolecules. 2003 Nov-Dec;4(6):1564–71. doi: 10.1021/bm0341834. [DOI] [PubMed] [Google Scholar]
- 82.Yamauchi F, Koyamatsu Y, Kato K, Iwata H. Layer-by-layer assembly of cationic lipid and plasmid DNA onto gold surface for stent-assisted gene transfer. Biomaterials. 2006 Jun;27(18):3497–504. doi: 10.1016/j.biomaterials.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 83.van Bommel KJC, Friggeri A, Mateman D, Geurts FAJ, van Leerdam KGC, Verboom W, et al. Self-assembled monolayers on gold for the fabrication of radioactive stents. Advanced Functional Materials. 2001 Apr;11(2):140–6. [Google Scholar]
- 84.Mahapatro A, Johnson DM, Patel DN, Feldman MD, Ayon AA, Agrawal CM. The use of alkanethiol self-assembled monolayers on 316L stainless steel for coronary artery stent nanomedicine applications: an oxidative and in vitro stability study. Nanomedicine. 2006 Sep;2(3):182–90. doi: 10.1016/j.nano.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 85.Sargeant TD, Rao MS, Koh CY, Stupp SI. Covalent functionalization of NiTi surfaces with bioactive peptide amphiphile nanofibers. Biomaterials. 2008 Mar;29(8):1085–98. doi: 10.1016/j.biomaterials.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tarcha PJ, Verlee D, Hui HW, Setesak J, Antohe B, Radulescu D, et al. The application of ink-jet technology for the coating and loading of drug-eluting stents. Annals of Biomedical Engineering. 2007 Oct;35(10):1791–9. doi: 10.1007/s10439-007-9354-2. [DOI] [PubMed] [Google Scholar]
- 87.Dibra A, Kastrati A, Mehilli J, Pache J, von Oepen R, Dirschinger J, et al. Influence of stent surface topography on the outcomes of patients undergoing coronary stenting: A randomized double-blind controlled trial. Catheterization and Cardiovascular Interventions. 2005 Jul;65(3):374–80. doi: 10.1002/ccd.20400. [DOI] [PubMed] [Google Scholar]
- 88.Bhargava B, Reddy NK, Karthikeyan G, Raju R, Mishra S, Singh S, et al. A novel paclitaxel-eluting porous carbon-carbon nanoparticle coated, nonpolymeric cobalt-chromium stent: Evaluation in a porcine model. Catheterization and Cardiovascular Interventions. 2006 May;67(5):698–702. doi: 10.1002/ccd.20698. [DOI] [PubMed] [Google Scholar]
- 89.Ye YW, Landau C, Willard JE, Rajasubramanian G, Moskowitz A, Aziz S, et al. Bioresorbable microporous stents deliver recombinant adenovirus gene transfer vectors to the arterial wall. Ann Biomed Eng. 1998 May-Jun;26(3):398–408. doi: 10.1114/1.62. [DOI] [PubMed] [Google Scholar]
- 90.Wessely R, Hausleiter J, Michaelis C, Jaschke B, Vogeser M, Milz S, et al. Inhibition of neointima formation by a novel drug-eluting stent system that allows for dose-adjustable, multiple, and on-site stent coating. Arterioscler Thromb Vasc Biol. 2005 Apr;25(4):748–53. doi: 10.1161/01.ATV.0000157579.52566.ee. [DOI] [PubMed] [Google Scholar]
- 91.Hausleiter J, Kastrati A, Wessely R, Dibra A, Mehilli J, Schratzenstaller T, et al. Prevention of restenosis by a novel drug-eluting stent system with a dose-adjustable, polymer-free, on-site stent coating. Eur Heart J. 2005 Aug;26(15):1475–81. doi: 10.1093/eurheartj/ehi405. [DOI] [PubMed] [Google Scholar]
- 92.Mehilli J, Byrne RA, Wieczorek A, Iijima R, Schulz S, Bruskina O, et al. Randomized trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis. Eur Heart J. 2008 Aug;29(16):1975–82. doi: 10.1093/eurheartj/ehn253. [DOI] [PubMed] [Google Scholar]
- 93.Wieneke H, Dirsch O, Sawitowski T, Gu YL, Brauer H, Dahmen U, et al. Synergistic effects of a novel nanoporous stent coating and tacrolimus on intima proliferation in rabbits. Catheterization and Cardiovascular Interventions. 2003 Nov;60(3):399–407. doi: 10.1002/ccd.10664. [DOI] [PubMed] [Google Scholar]
- 94.Kollum M, Farb A, Schreiber R, Terfera K, Arab A, Geist A, et al. Particle debris from a nanoporous stent coating obscures potential antiproliferative effects of tacrolimus-eluting stents in a porcine model of restenosis. Catheter Cardiovasc Interv. 2005 Jan;64(1):85–90. doi: 10.1002/ccd.20213. [DOI] [PubMed] [Google Scholar]
- 95.Rajtar A, G.L. K. Hydroxyapatite-coated cardiovascular stents. EuroIntervention. 2006;2:113–5. [PubMed] [Google Scholar]
- 96.Costa JR, Jr., Abizaid A, Costa R, Feres F, Tanajura LF, Abizaid A, et al. 1-year results of the hydroxyapatite polymer-free sirolimus-eluting stent for the treatment of single de novo coronary lesions: the VESTASYNC I trial. JACC Cardiovasc Interv. 2009 May;2(5):422–7. doi: 10.1016/j.jcin.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 97.Blindt R, Vogt F, Astafieva I, Fach C, Hristov M, Krott N, et al. A novel drug-eluting stent coated with an integrin-binding cyclic Arg-Gly-Asp peptide inhibits neointimal hyperplasia by recruiting endothelial progenitor cells. Journal of the American College of Cardiology. 2006 May 2;47(9):1786–95. doi: 10.1016/j.jacc.2005.11.081. [DOI] [PubMed] [Google Scholar]
- 98.Klugherz BD, Jones PL, Cui XM, Chen WL, Meneveau NF, DeFelice S, et al. Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nature Biotechnology. 2000 Nov;18(11):1181–4. doi: 10.1038/81176. [DOI] [PubMed] [Google Scholar]
- 99.Lin PH, Chronos NA, Marijianowski MM, Chen C, Bush RL, Conklin B, et al. Heparin-coated balloon-expandable stent reduces intimal hyperplasia in the iliac artery in baboons. J Vasc Interv Radiol. 2003 May;14(5):603–11. doi: 10.1097/01.rvi.0000071088.76348.23. [DOI] [PubMed] [Google Scholar]
- 100.Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirkwood L, Stratford PW, et al. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Circulation. 2004 Jul 6;110(1):36–45. doi: 10.1161/01.CIR.0000133324.38115.0A. [DOI] [PubMed] [Google Scholar]
- 101.Swanson N, Hogrefe K, Javed Q, Gershlick AH. In vitro evaluation of vascular endothelial growth factor (VEGF)-eluting stents. International Journal of Cardiology. 2003 Dec;92(2-3):247–51. doi: 10.1016/s0167-5273(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 102.Rossi ML, Zavalloni D, Gasparini GL, Mango R, Belli G, Presbitero P. The first report of late stent thrombosis leading to acute myocardial infarction in patient receiving the new endothelial progenitor cell capture stent. Int J Cardiol. 2009 Jan 8; doi: 10.1016/j.ijcard.2008.11.134. [DOI] [PubMed] [Google Scholar]
- 103.Jin T, Zhu HH, Wu F, Yuan WE, Geng LL, Zhu H. Preparing polymer-based sustained-release systems without exposing proteins to water-oil or water-air interfaces and cross-linking reagents. Journal of Controlled Release. 2008 May 22;128(1):50–9. doi: 10.1016/j.jconrel.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 104.Brito L, Amiji M. Nanoparticulate carriers for the treatment of coronary restenosis. International Journal of Nanomedicine. 2007;2(2):143–61. [PMC free article] [PubMed] [Google Scholar]
- 105.Nakano K, Egashira K, Masuda S, Funakoshi K, Zhao G, Kimura S, et al. Formulation of nanoparticle-eluting stents by a cationic electrodeposition coating technology: efficient nano-drug delivery via bioabsorbable polymeric nanoparticle-eluting stents in porcine coronary arteries. JACC Cardiovasc Interv. 2009 Apr;2(4):277–83. doi: 10.1016/j.jcin.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 106.Sarisozen C, Arica B, Atilla Hincal A, Calis S. Development of biodegradable drug releasing polymeric cardiovascular stents and in vitro evaluation. J Microencapsul. 2008 Dec 3;:1–11. doi: 10.1080/02652040802465792. [DOI] [PubMed] [Google Scholar]
- 107.Elkins CJ, Waugh JM, Amabile PG, Minamiguchi H, Sugimoto K, Ganaha F, et al. Development of a platform to evaluate and limit in-stent restenosis. Tissue Engineering. 2002 Jun;8(3):395–407. doi: 10.1089/107632702760184664. [DOI] [PubMed] [Google Scholar]
- 108.Ganaha F, Ohashi K, Do YS, Lee J, Sugimoto K, Minamiguchi H, et al. Efficient inhibition of in-stent restenosis by controlled stent-based inhibition of elastase: A pilot study. Journal of Vascular and Interventional Radiology. 2004 Nov;15(11):1287–93. doi: 10.1097/01.RVI.0000141340.67588.4F. [DOI] [PubMed] [Google Scholar]
- 109.Do YS, Kao EY, Ganaha F, Minamiguchi H, Sugimoto K, Lee J, et al. In-stent restenosis limitation with stent-based controlled-release nitric oxide: Initial results in rabbits. Radiology. 2004 Feb;230(2):377–82. doi: 10.1148/radiol.2302020417. [DOI] [PubMed] [Google Scholar]
- 110.Rosengart AJ, Kaminski MD, Chen HT, Caviness PL, Ebner AD, Ritter JA. Magnetizable implants and functionalized magnetic carriers: A novel approach for noninvasive yet targeted drug delivery. Journal of Magnetism and Magnetic Materials. 2005 May 1;293(1):633–8. [Google Scholar]
- 111.Chorny M, Fishbein I, Alferiev I, Levy RJ. Magnetically Responsive Biodegradable Nanoparticles Enhance Adenoviral Gene Transfer in Cultured Smooth Muscle and Endothelial Cells. Mol Pharm. 2009 Jun 4; doi: 10.1021/mp900017m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yellen BB, Forbes ZG, Halverson DS, Fridman G, Barbee KA, Chorny M, et al. Targeted drug delivery to magnetic implants for therapeutic applications. Journal of Magnetism and Magnetic Materials. 2005 May 1;293(1):647–54. [Google Scholar]
- 113.Polyak B, Fishbein I, Chorny M, Alferiev I, Williams D, Yellen B, et al. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steed stents. Proceedings of the National Academy of Sciences of the United States of America. 2008 Jan 15;105(2):698–703. doi: 10.1073/pnas.0708338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pislaru SV, Harbuzariu A, Gulati R, Witt T, Sandhu NP, Simari RD, et al. Magnetically targeted endothelial cell localization in stented vessels. Journal of the American College of Cardiology. 2006 Nov 7;48(9):1839–45. doi: 10.1016/j.jacc.2006.06.069. [DOI] [PubMed] [Google Scholar]
- 115.Chichkov BN, Momma C, Nolte S, vonAlvensleben F, Tunnermann A. Femtosecond, picosecond and nanosecond laser ablation of solids. Applied Physics a-Materials Science & Processing. 1996 Aug;63(2):109–15. [Google Scholar]
- 116.Deuse T, Erben RG, Ikeno F, Behnisch B, Boeger R, Connolly AJ, et al. Introducing the first polymer-free leflunomide eluting stent. Atherosclerosis. 2008 Sep;200(1):126–34. doi: 10.1016/j.atherosclerosis.2007.12.055. [DOI] [PubMed] [Google Scholar]