Abstract
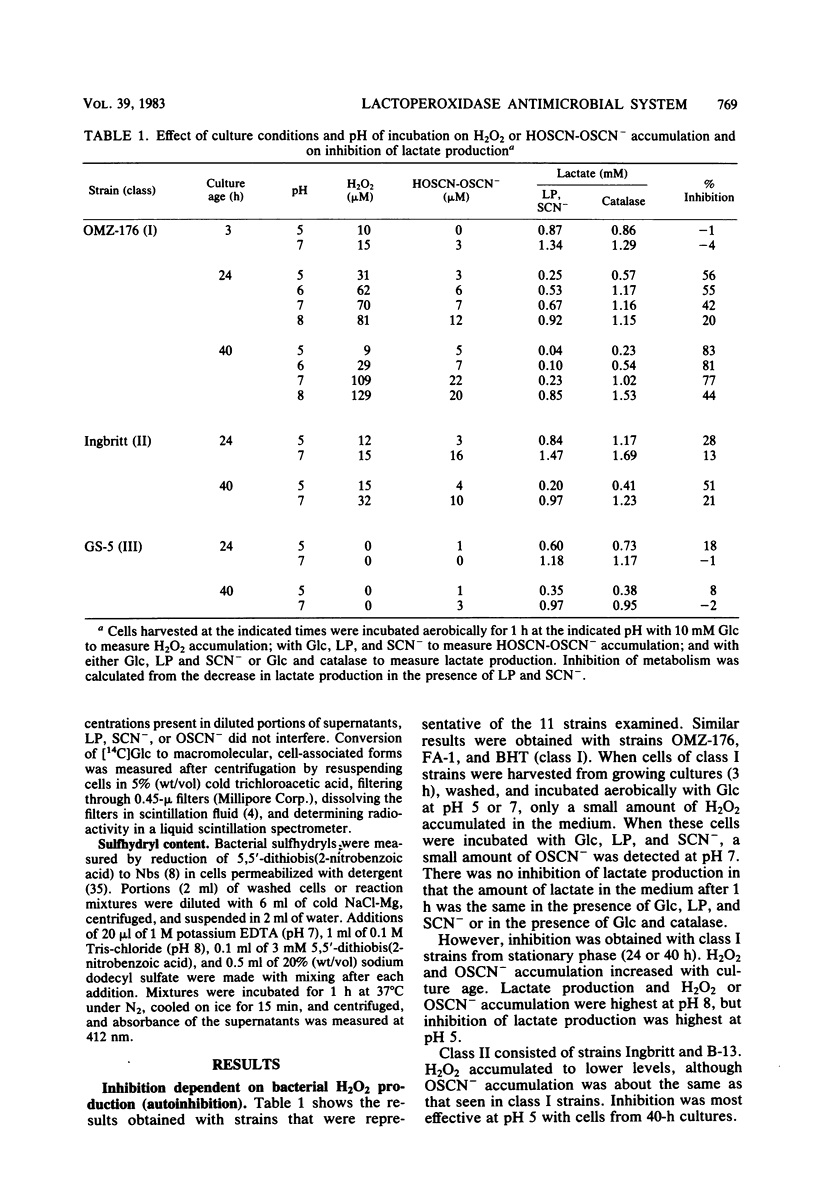
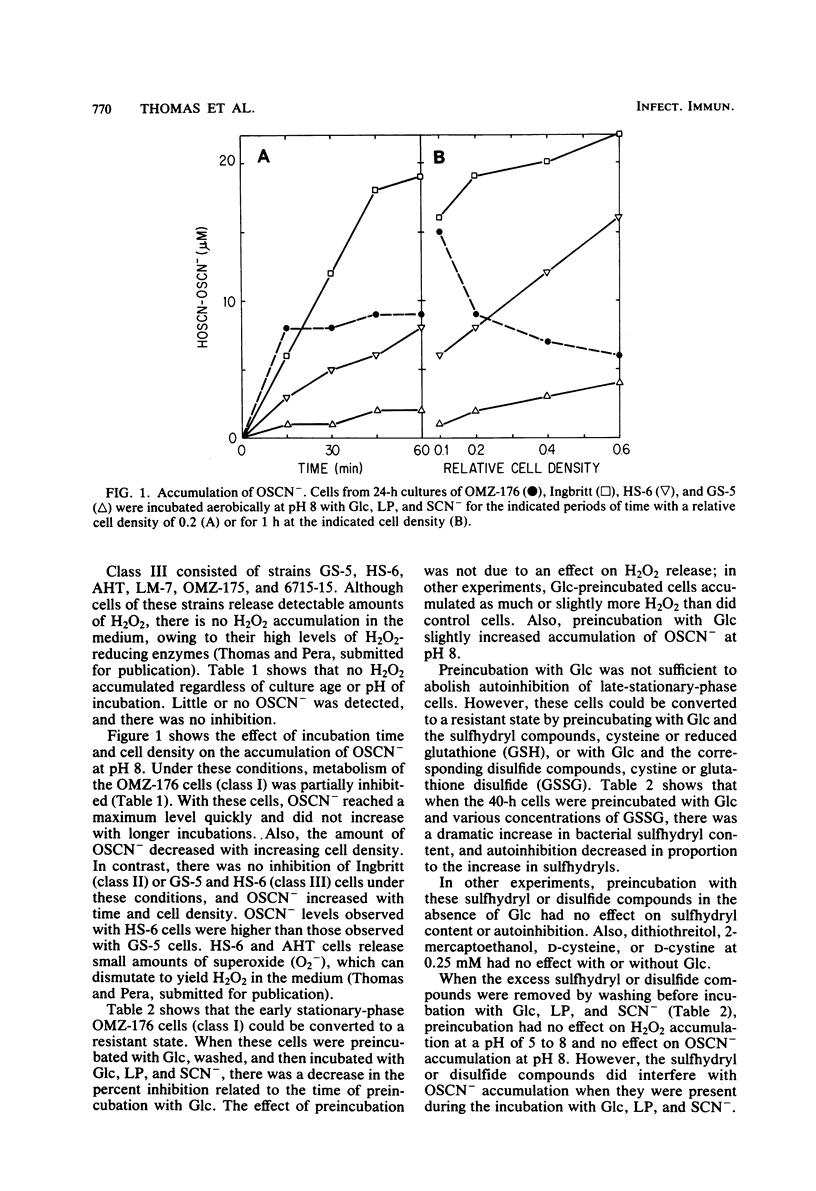
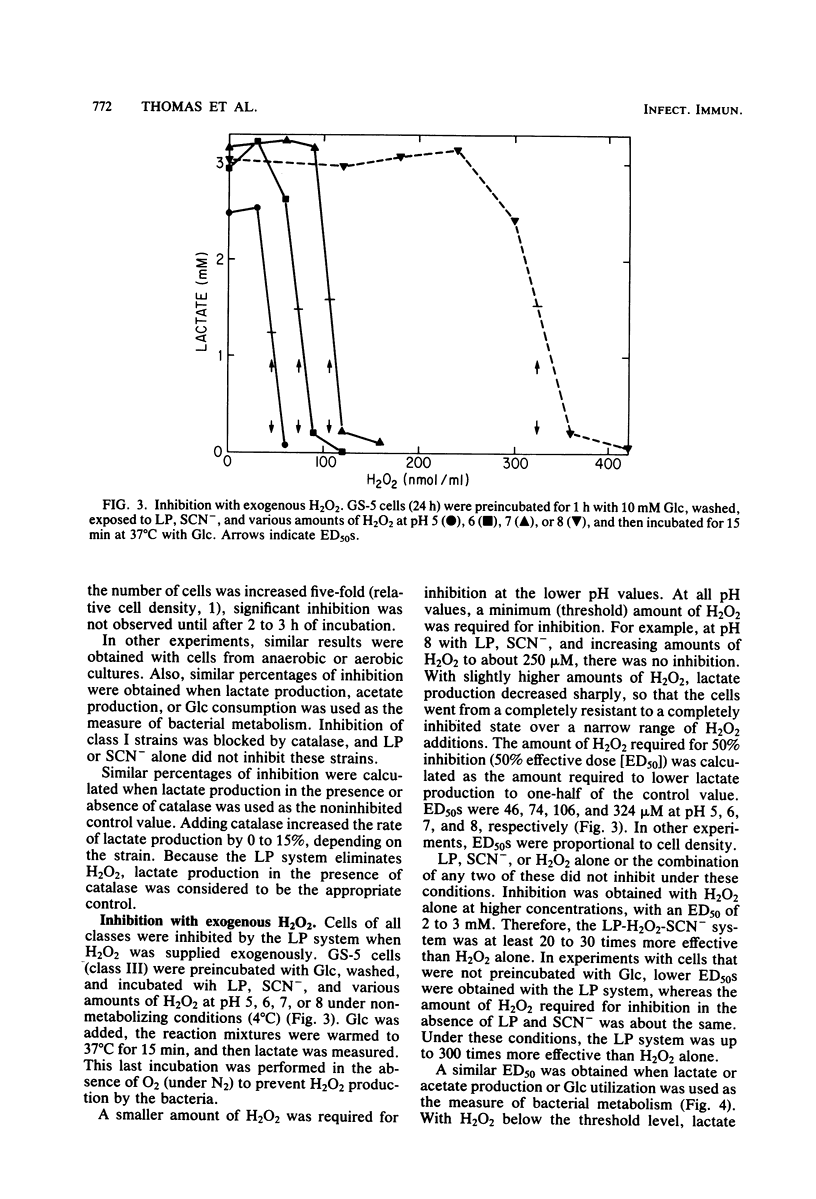
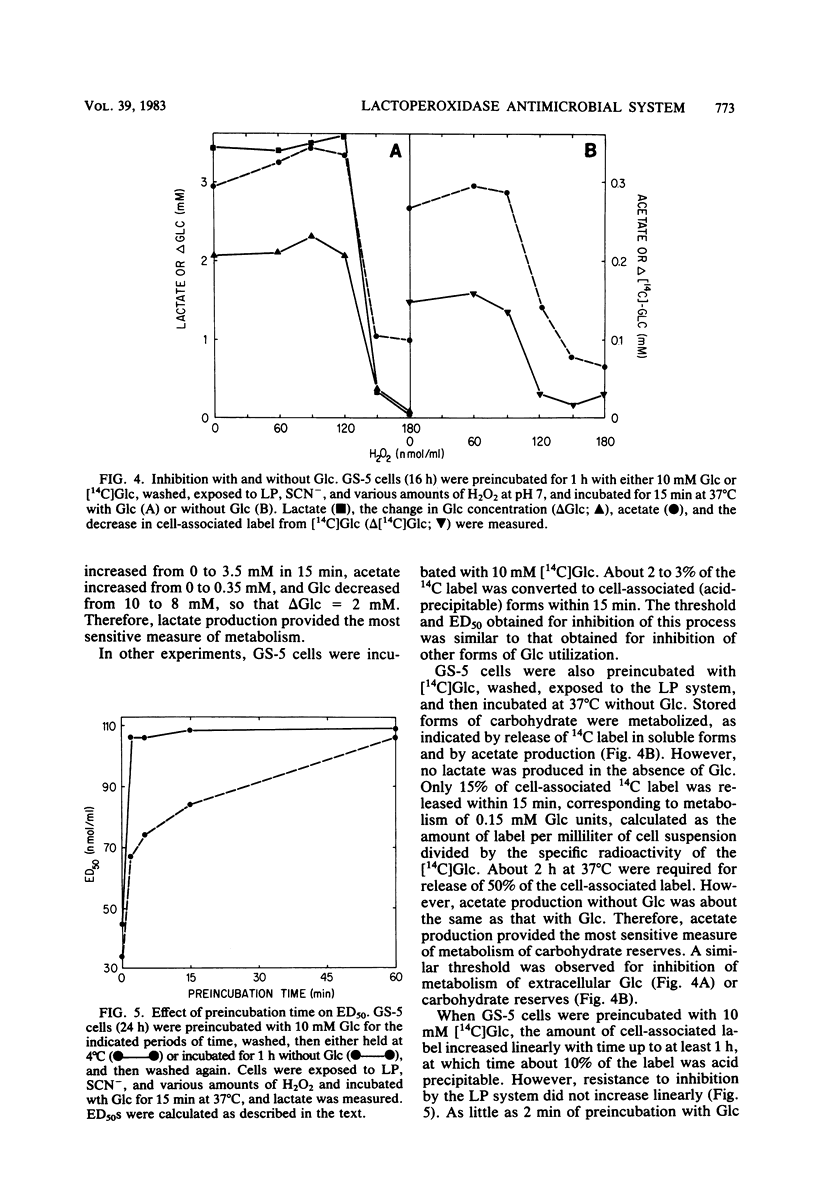
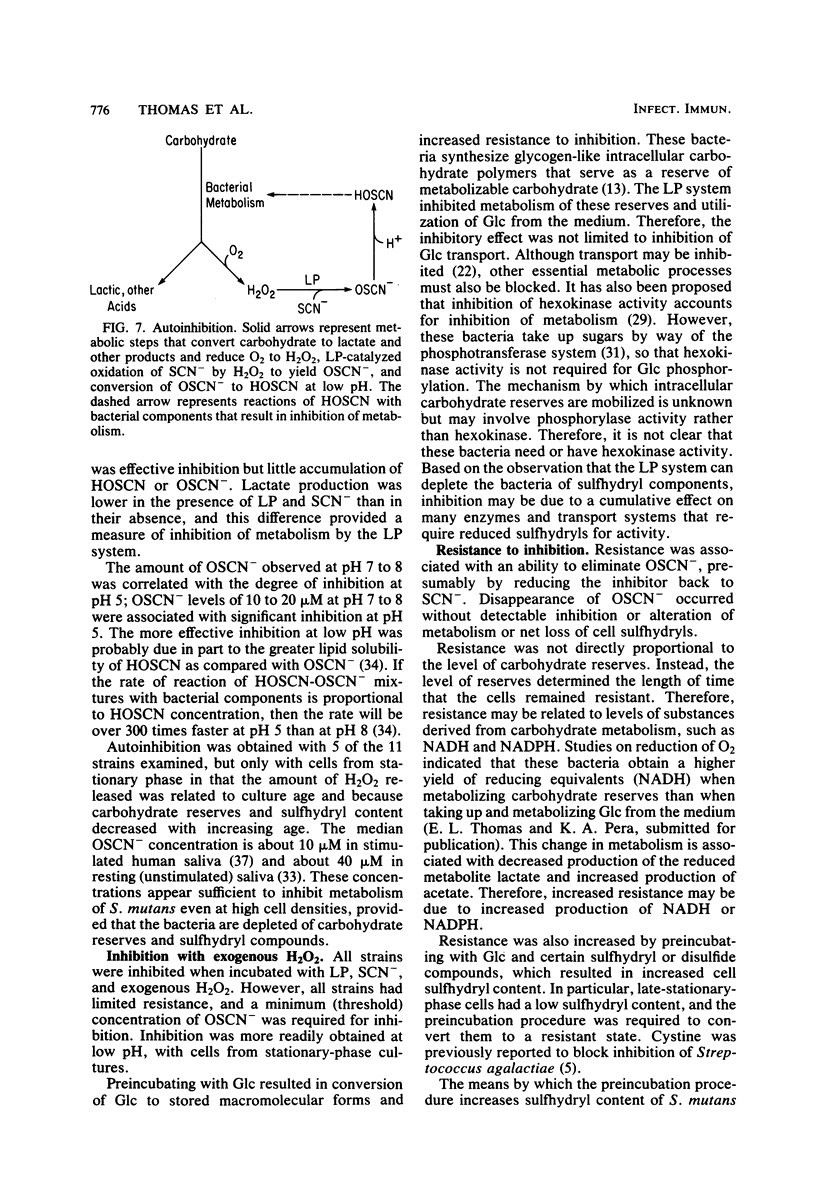
Inhibition of bacterial metabolism by the lactoperoxidase (LP)-hydrogen peroxide (H2O2)-thiocyanate system was studied with representatives of serotypes a through g of Streptococcus mutans. The aims were to determine whether the amount of H2O2 released from these catalase-negative bacteria is sufficient to activate the LP system and whether these oral bacteria are resistant to inhibition by the LP system, which is active in human saliva. When the washed, stationary-phase cells were incubated aerobically with LP, thiocyanate, and glucose (Glc), greater than 90% inhibition of Glc utilization and lactate production was obtained with strains that released large amounts of H2O2 (BHT, FA-1, OMZ-176); 20 to 50% inhibition was obtained with strains that released about half as much H2O2 (B-13, Ingbritt); and no inhibition was obtained with strains that released only small amounts of H2O2 (AHT, HS-6, GS-5, LM-7, OMZ-175, 6715-15). Inhibition was most effective at pH 5, whereas release of H2O2 and accumulation of the inhibitor (hypothiocyanite ion) were highest at pH 8. With H2O2-releasing cells from early stationary phase, preincubation with Glc abolished inhibition, though it did not influence H2O2 release. Cells harvested 24 h later were depleted of sulfhydryl compounds. Inhibition of these cells was abolished by preincubation with Glc and certain sulfhydryl or disulfide compounds (reduced or oxidized glutathione, cysteine or cystine). This preincubation increased cell sulfhydryl content but had no effect on H2O2 release. All strains were inhibited when incubated with LP, thiocyanate, and added (exogenous) H2O2. Smaller amounts of H2O2 were required to inhibit at pH 5, and larger amounts were required to inhibit cells preincubated with Glc or with Glc and the sulfhydryl or disulfide compounds. The results indicate that pH, amount of H2O2, cell sulfhydryl content, and stored-carbohydrate content determine susceptibility to inhibition.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aune T. M., Thomas E. L. Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur J Biochem. 1977 Oct 17;80(1):209–214. doi: 10.1111/j.1432-1033.1977.tb11873.x. [DOI] [PubMed] [Google Scholar]
- Aune T. M., Thomas E. L. Oxidation of protein sulfhydryls by products of peroxidase-catalyzed oxidation of thiocyanate ion. Biochemistry. 1978 Mar 21;17(6):1005–1010. doi: 10.1021/bi00599a010. [DOI] [PubMed] [Google Scholar]
- Brown R. W., Mickelson M. N. Lactoperoxidase, thiocyanate, and free cystine in bovine mammary secretions in early dry period and at the start of lactation and their effect on Streptococcus agalactiae growth. Am J Vet Res. 1979 Feb;40(2):250–255. [PubMed] [Google Scholar]
- Coykendall A. L. Four types of Streptococcus mutans based on their genetic, antigenic and biochemical characteristics. J Gen Microbiol. 1974 Aug;83(2):327–338. doi: 10.1099/00221287-83-2-327. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fahey R. C., Brown W. C., Adams W. B., Worsham M. B. Occurrence of glutathione in bacteria. J Bacteriol. 1978 Mar;133(3):1126–1129. doi: 10.1128/jb.133.3.1126-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Tellefson L. M. Effect of human saliva on glucose uptake by Streptococcus mutans and other oral microorganisms. Infect Immun. 1981 Feb;31(2):598–607. doi: 10.1128/iai.31.2.598-607.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg D. M., Jago G. R. The antibacterial action of lactoperoxidase. The nature of the bacterial inhibitor. Biochem J. 1970 May;117(4):779–790. doi: 10.1042/bj1170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendoorn H., Piessens J. P., Scholtes W., Stoddard L. A. Hypothiocyanite ion; the inhibitor formed by the system lactoperoxidase-thiocyanate-hydrogen peroxide. I. Identification of the inhibiting compound. Caries Res. 1977;11(2):77–84. doi: 10.1159/000260252. [DOI] [PubMed] [Google Scholar]
- KRAUS F. W., NICKERSON J. F., PERRY W. I., WALKER A. P. Peroxide and peroxidogenic bacteria in human saliva. J Bacteriol. 1957 Jun;73(6):727–735. doi: 10.1128/jb.73.6.727-735.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Clem W. H., Luebke R. G. The peroxidase-thiocyanate-hydrogen peroxide antimicrobial system. Biochim Biophys Acta. 1966 Mar 28;117(1):63–72. doi: 10.1016/0304-4165(66)90152-8. [DOI] [PubMed] [Google Scholar]
- MORRISON M., HULTQUIST D. E. LACTOPEROXIDASE. II. ISOLATION. J Biol Chem. 1963 Aug;238:2843–2849. [PubMed] [Google Scholar]
- Mickelson M. N. Antibacterial action of lactoperoxidase-thiocyanate-hydrogen peroxide on Streptococcus agalactiae. Appl Environ Microbiol. 1979 Nov;38(5):821–826. doi: 10.1128/aem.38.5.821-826.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson M. N. Effect of lactoperoxidase and thiocyanate on the growth of Streptococcus pyogenes and Streptococcus agalactiae in a chemically defined culture medium. J Gen Microbiol. 1966 Apr;43(1):31–43. doi: 10.1099/00221287-43-1-31. [DOI] [PubMed] [Google Scholar]
- Mickelson M. N. Glucose transport in Streptococcus agalactiae and its inhibition by lactoperoxidase-thiocyanate-hydrogen peroxide. J Bacteriol. 1977 Nov;132(2):541–548. doi: 10.1128/jb.132.2.541-548.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M., Allen P. Z. Lactoperoxidase: identification and isolation from Harderian and lacrimal glands. Science. 1966 Jun 17;152(3729):1626–1628. doi: 10.1126/science.152.3729.1626. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The effect of the inhibitory system on susceptible and resistant strains of group N streptococci. Biochem J. 1966 Aug;100(2):373–381. doi: 10.1042/bj1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The inhibition of streptococci by lactoperoxidase, thiocyanate and hydrogen peroxide. The oxidation of thiocyanate and the nature of the inhibitory compound. Biochem J. 1966 Aug;100(2):382–388. doi: 10.1042/bj1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter B., Marshall V. M., BjörckL, Rosén C. G. Nonspecific bactericidal activity of the lactoperoxidases-thiocyanate-hydrogen peroxide system of milk against Escherichia coli and some gram-negative pathogens. Infect Immun. 1976 Mar;13(3):800–807. doi: 10.1128/iai.13.3.800-807.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Mayo J. A. Phosphoenolpyruvate-dependent glucose transport in oral streptococci. J Dent Res. 1973 Nov-Dec;52(6):1209–1215. doi: 10.1177/00220345730520060801. [DOI] [PubMed] [Google Scholar]
- Tenovuo J., Mansson-Rahemtulla B., Pruitt K. M., Arnold R. Inhibition of dental plaque acid production by the salivary lactoperoxidase antimicrobial system. Infect Immun. 1981 Oct;34(1):208–214. doi: 10.1128/iai.34.1.208-214.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenovuo J., Pruitt K. M., Thomas E. L. Peroxidase antimicrobial system of human saliva: hypothiocyanite levels in resting and stimulated saliva. J Dent Res. 1982 Aug;61(8):982–985. doi: 10.1177/00220345820610081301. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect Immun. 1978 May;20(2):456–463. doi: 10.1128/iai.20.2.456-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Aune T. M. Oxidation of Escherichia coli sulfhydryl components by the peroxidase-hydrogen peroxide-iodide antimicrobial system. Antimicrob Agents Chemother. 1978 Jun;13(6):1006–1010. doi: 10.1128/aac.13.6.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L., Bates K. P., Jefferson M. M. Hypothiocyanite ion: detection of the antimicrobial agent in human saliva. J Dent Res. 1980 Sep;59(9):1466–1472. doi: 10.1177/00220345800590090201. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Bates K. P., Jefferson M. M. Peroxidase antimicrobial system of human saliva: requirements for accumulation of hypothiocyanite. J Dent Res. 1981 Apr;60(4):785–796. doi: 10.1177/00220345810600040401. [DOI] [PubMed] [Google Scholar]
- Thomas E. L. Lactoperoxidase-catalyzed oxidation of thiocyanate: equilibria between oxidized forms of thiocyanate. Biochemistry. 1981 May 26;20(11):3273–3280. doi: 10.1021/bi00514a045. [DOI] [PubMed] [Google Scholar]



