Abstract
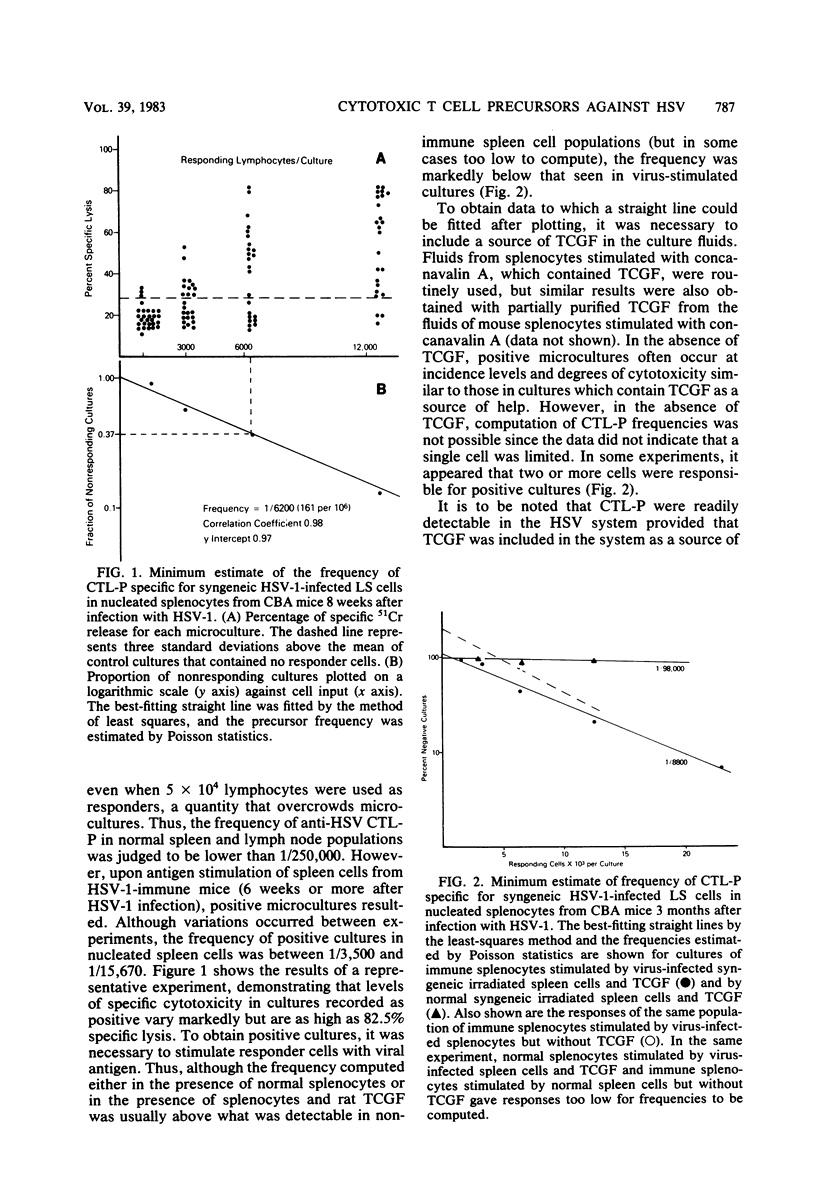
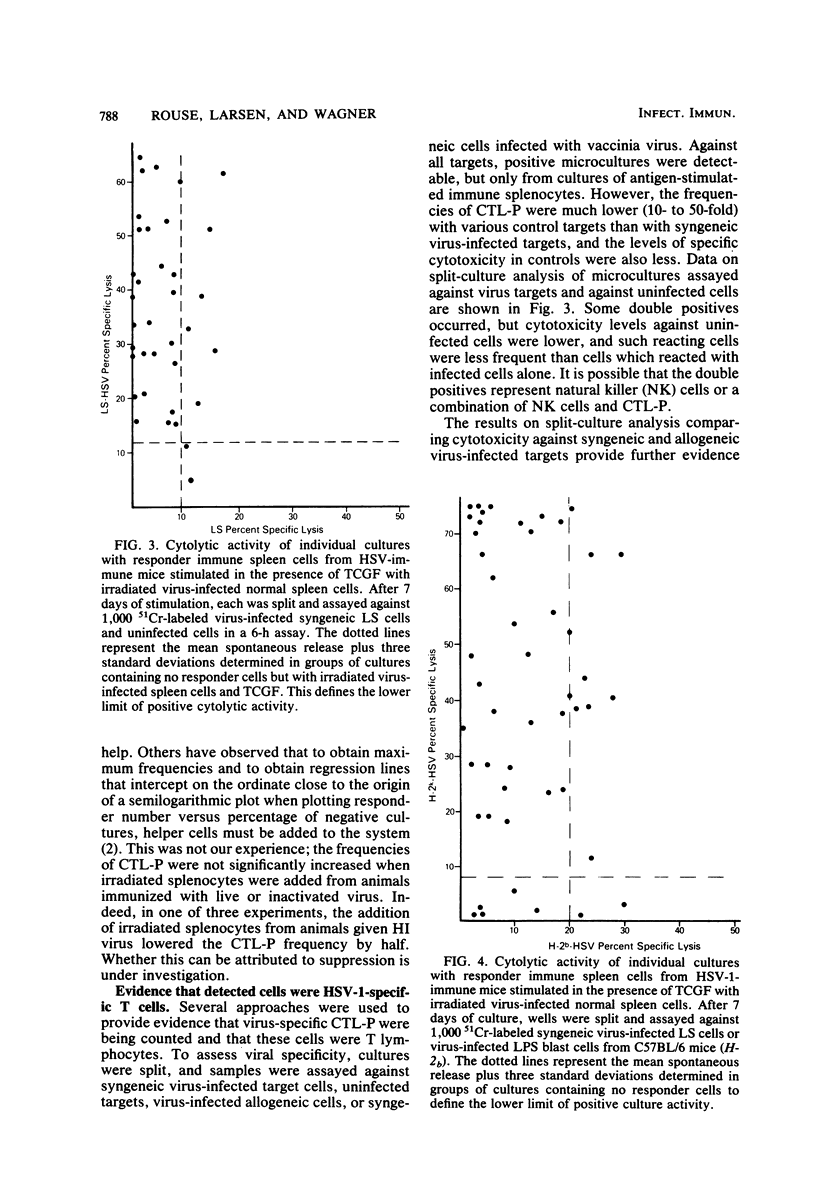
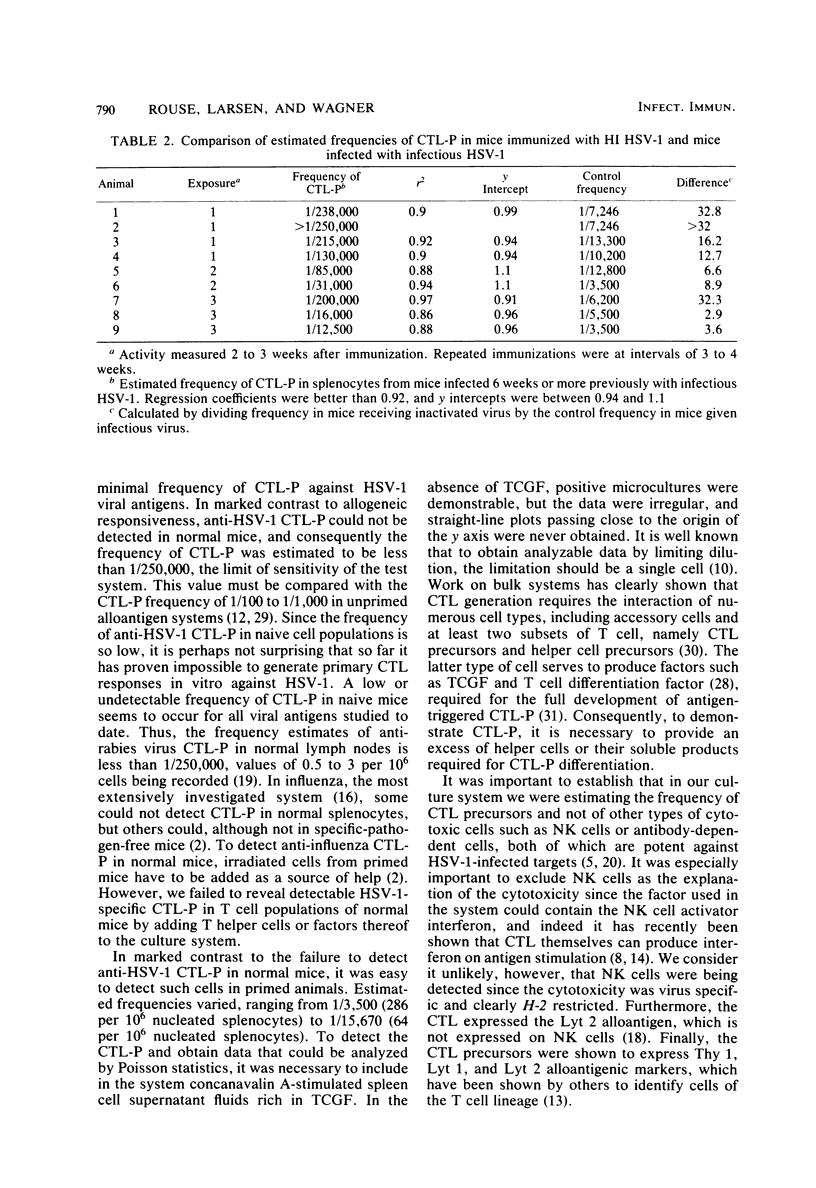
The conditions for establishing a limiting dilution assay to measure cytotoxic T lymphocyte precursors (CTL-P) against herpes simplex virus type 1 (HSV-1) were determined. Analysis by Poisson statistics demonstrated that the estimated frequency of HSV-1-reactive cells in the spleens of normal mice was less than 1/250,000. In contrast, mice immunized previously with infectious HSV-1 demonstrated a CTL-P frequency between 1/3,500 and 1/15,670. The generation of a maximum cytotoxic T lymphocyte response required that mice be primed in vivo with infectious virus. Immunization with inactivated virus either failed to elicit detectable CTL-P frequencies or gave frequencies markedly less than those induced with infectious virus. To obtain positive cultures, the responder cell population had to be exposed to stimulator splenocytes expressing viral antigens. Normal splenocytes without virus or normal splenocytes with T cell growth factor did not result in significant cytotoxicity. Split culture analysis comparing cytotoxicity against syngeneic and allogeneic virus-infected targets provided evidence for specificity, H-2 restriction, and the T cell nature of the CTL-P. It was determined that precursors were eliminated by treatment with anti-Thy 1, Lyt 2.1, or Lyt 1.1, indicating the CTL-P were Lyt 1+2+ cells. Cytotoxicity was reduced after treatment of the responders with anti-Lyt 2 plus complement, which gave further evidence of the T cell nature of the cytotoxic T lymphocytes. These experiments demonstrated the feasibility of using the limiting dilution approach as a highly sensitive and quantitative means to measure the cell-mediated immune response to HSV-1 antigens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Leung K. N., Ertl H. An analysis of effector T cell generation and function in mice exposed to influenza A or Sendai viruses. Immunol Rev. 1981;58:5–24. doi: 10.1111/j.1600-065x.1981.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Askonas B. A., Mullbacher A., Ashman R. B. Cytotoxic T-memory cells in virus infection and the specificity of helper T cells. Immunology. 1982 Jan;45(1):79–84. [PMC free article] [PubMed] [Google Scholar]
- Bone D. R., Courtney R. J. A temperature-sensitive mutant of herpes simplex virus type 1 defective in the synthesis of the major capsid polypeptide. J Gen Virol. 1974 Jul;24(1):17–27. doi: 10.1099/0022-1317-24-1-17. [DOI] [PubMed] [Google Scholar]
- Braciale T. J., Yap K. L. Role of viral infectivity in the induction of influenza virus-specific cytotoxic T cells. J Exp Med. 1978 Apr 1;147(4):1236–1252. doi: 10.1084/jem.147.4.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching C., Lopez C. Natural killing of herpes simplex virus type 1-infected target cells: normal human responses and influence of antiviral antibody. Infect Immun. 1979 Oct;26(1):49–56. doi: 10.1128/iai.26.1.49-56.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Komatsu Y., Nawa Y., Bellamy A. R., Marbrook J. Clones of cytotoxic lymphocytes can recognise uninfected cells in a primary response against influenza virus. Nature. 1978 Aug 24;274(5673):802–804. doi: 10.1038/274802a0. [DOI] [PubMed] [Google Scholar]
- Krammer P. H., Marcucci F., Waller M., Kirchner H. Heterogeneity of soluble T cell products. I. Precursor frequency and correlation analysis of cytotoxic and immune interferon (IFN-gamma)-producing spleen cells in the mouse. Eur J Immunol. 1982 Mar;12(3):200–204. doi: 10.1002/eji.1830120306. [DOI] [PubMed] [Google Scholar]
- Lawman M. J., Rouse B. T., Courtney R. J., Walker R. D. Cell-mediated immunity against herpes simplex induction of cytotoxic T lymphocytes. Infect Immun. 1980 Jan;27(1):133–139. doi: 10.1128/iai.27.1.133-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl K. F., Wilson D. B. Histocompatibility antigen-activated cytotoxic T lymphocytes. II. Estimates of the frequency and specificity of precursors. J Exp Med. 1977 Mar 1;145(3):508–522. doi: 10.1084/jem.145.3.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Cerottini J. C., Ryser J. E., Maryanski J. L., Taswell C., Widmer M. B., Brunner K. T. Quantitation and cloning of cytolytic T lymphocytes and their precursors. Immunol Rev. 1980;51:93–123. doi: 10.1111/j.1600-065x.1980.tb00318.x. [DOI] [PubMed] [Google Scholar]
- McKenzie I. F., Potter T. Murine lymphocyte surface antigens. Adv Immunol. 1979;27:179–338. doi: 10.1016/s0065-2776(08)60263-1. [DOI] [PubMed] [Google Scholar]
- Morris A. G., Lin Y. L., Askonas B. A. Immune interferon release when a cloned cytotoxic T-cell line meets its correct influenza-infected target cell. Nature. 1982 Jan 14;295(5845):150–152. doi: 10.1038/295150a0. [DOI] [PubMed] [Google Scholar]
- Naylor P. T., Larsen H. S., Huang L., Rouse B. T. In vivo induction of anti-herpes simplex virus immune response by type 1 antigens and lipid A incorporated into liposomes. Infect Immun. 1982 Jun;36(3):1209–1216. doi: 10.1128/iai.36.3.1209-1216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J. A., Allouche M., Doherty P. C. Limiting dilution analysis of the specificity of influenza-immune cytotoxic T cells. Cell Immunol. 1982 Feb;67(1):49–59. doi: 10.1016/0008-8749(82)90198-8. [DOI] [PubMed] [Google Scholar]
- Pfizenmaier K., Jung H., Starzinski-Powitz A., Röllinghoff M., Wagner H. The role of T cells in anti-herpes simplex virus immunity. I. Induction of antigen-specific cytotoxic T lymphocytes. J Immunol. 1977 Sep;119(3):939–944. [PubMed] [Google Scholar]
- Pollack S. B., Tam M. R., Nowinski R. C., Emmons S. L. Presence of T cell-associated surface antigens on murine NK cells. J Immunol. 1979 Oct;123(4):1818–1821. [PubMed] [Google Scholar]
- Reddehase M. J., Cox J. H., Koszinowski U. H. Frequency analysis of cytolytic T cell precursors (CTL-P) generated in vivo during lethal rabies infection of mice. I. Distinction of CTL-P with different interleukin 2 sensitivity. Eur J Immunol. 1982 Jun;12(6):519–523. doi: 10.1002/eji.1830120613. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Mechanisms of recovery from Herpesvirus infections -a review. Can J Comp Med. 1978 Oct;42(4):414–427. [PMC free article] [PubMed] [Google Scholar]
- Ryser J. E., MacDonald H. R. Limiting dilution analysis of alloantigen-reactive T lymphocytes. I. Comparison of precursor frequencies for proliferative and cytolytic responses. J Immunol. 1979 May;122(5):1691–1696. [PubMed] [Google Scholar]
- Skinner M. A., Marbrook J. An estimation of the frequency of precursor cells which generate cytotoxic lymphocytes. J Exp Med. 1976 Jun 1;143(6):1562–1567. doi: 10.1084/jem.143.6.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Taswell C., MacDonald H. R., Cerottini J. C. Limiting dilution analysis of alloantigen-reactive T lymphocytes. II. Effect of cortisone and cyclophosphamide on cytolytic T lymphocyte precursor frequencies in the thymus. Thymus. 1979 Sep;1(1-2):119–131. [PubMed] [Google Scholar]
- Teh H. S., Harley E., Phillips R. A., Miller R. G. Quantitative studies on the precursors of cytotoxic lymphocytes. I. Characterization of a clonal assay and determination of the size of clones derived from single precursors. J Immunol. 1977 Mar;118(3):1049–1056. [PubMed] [Google Scholar]
- Wagner H., Hardt C., Rouse B. T., Röllinghoff M., Scheurich P., Pfizenmaier K. Dissection of the proliferative and differentiative signals controlling murine cytotoxic T lymphocyte responses. J Exp Med. 1982 Jun 1;155(6):1876–1881. doi: 10.1084/jem.155.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H., Hardt C., Stockinger H., Pfizenmaier K., Bartlett R., Röllinghoff M. Impact of thymus on the generation of immunocompetence and diversity of antigen-specific MHC-restricted cytotoxic T-lymphocyte precursors. Immunol Rev. 1981;58:95–129. doi: 10.1111/j.1600-065x.1981.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Wagner H., Pfizenmaier K., Röllinghoff M. The role of the major histocompatibility gene complex in murine cytotoxic T cell responses. Adv Cancer Res. 1980;31:77–124. doi: 10.1016/s0065-230x(08)60657-0. [DOI] [PubMed] [Google Scholar]
- Wagner H., Röllinghoff M. T-T-cell interactions during the vitro cytotoxic allograft responses. I. Soluble products from activated Lyl+ T cells trigger autonomously antigen-primed Ly23+ T cells to cell proliferation and cytolytic activity. J Exp Med. 1978 Dec 1;148(6):1523–1538. doi: 10.1084/jem.148.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Hengartner H., Nabholz M., Lernhardt W., Schreier M. H., Haas W. Fine specificity of a continuously growing killer cell clone specific for H-Y antigen. Eur J Immunol. 1979 Aug;9(8):592–597. doi: 10.1002/eji.1830090804. [DOI] [PubMed] [Google Scholar]



