Abstract
From its origins in how the brain controls the endocrine system via the hypothalamus and pituitary gland, neuroendocrinology has evolved into a science that now includes hormone action on many aspects of brain function. These actions involve the whole central nervous system and not just the hypothalamus. Advances in our understanding of cellular and molecular actions of steroid hormones have gone beyond the important cell nuclear actions of steroid hormone receptors to include signaling pathways that intersect with other mediators such as neurotransmitters and neuromodulators. This has, in turn, broadened the search for and identification of steroid receptors to include non-nuclear sites in synapses, dendrites, mitochondria and glial cells, as well as cell nuclei. The study of estrogen receptors and estrogen actions on processes related to cognition, mood, autonomic regulation, pain and neuroprotection, among other functions, has led the way in this new view of hormone actions on the brain. In this review we summarize past and current work in our laboratory on this topic. This exciting and growing field involving many laboratories continues to reshape our ideas and approaches to neuroendocrinology both at the bench and the bedside.
Keywords: estrogens, progesterone, rapid non-genomic actions, hippocampus, cognition, mood, autonomic regulation
Introduction
Circulating hormones have turned out to be a very effective way to probe brain structure and function. They have helped to demonstrate the plasticity of the adult and developing brain, not only in relation to the important events in the hypothalamus that regulate reproductive functions and homeostasis, but also higher cognitive processes as well as emotional regulation and decision-making. This review will highlight our laboratories’ role in this story. We will do so in the context of a brief overview of the history of neuroendocrinology dating back to the work of Geoffrey Harris and subsequent pioneers on the connections between the brain and the endocrine system via the hypothalamus and the portal blood vessels, which carry releasing factors from the hypothalamus to the pituitary gland (Harris 1948; Meites 1992). Then we shall move to the present and discuss the remarkable changes in our understanding of how steroid hormones affect cell functions, beginning with the nuclear receptors and expanding to non-nuclear receptors and indirect genomic as well as non-genomic actions. This new view has broadened the sites of steroid hormone action, especially for estrogens, to include the entire central nervous system (CNS).
A brief history
After the portal blood supply was shown to carry blood from the hypothalamus to the anterior pituitary (Harris 1948), heroic efforts using hypothalami from slaughterhouse animals led to the isolation and structural identification of peptide releasing factors (Guillemin 1978; Schally et al 1973). The feedback regulation of hypothalamic and pituitary hormones implied the existence of receptor mechanisms for gonadal, adrenal and thyroid hormones. Then the identification of cell nuclear hormone receptors in peripheral tissues (Jensen et al 1981) using tritiated steroid and iodinated thyroid hormones led to the demonstration of similar receptor mechanisms in the hypothalamus and pituitary gland (Pfaff & Keiner 1973; Stumpf & Sar 1976). For this, it was necessary to use autoradiographic methods because of the discrete nature of these receptor-containing cells, although more conventional cell fractionation methods also were used along with sucrose density gradient centrifugation to demonstrate receptors with molecular sizes similar to those in the peripheral tissues (Gerlach & McEwen 1972; McEwen & Plapinger 1970; Pfaff & Keiner 1973; Zigmond & McEwen 1970).
What about behavior?
Before the demonstration of nuclear estrogen and androgen receptors in hypothalamus, some suggested that sex hormones acted indirectly to activate sex behavior (Young 1961). Yet, the demonstration of binding sites and receptors for estrogens in hypothalamus led to studies using hormone implants as well as sophisticated neuroanatomical and neurophysiologic methods that demonstrated that sex hormones facilitate sex behavior via receptors in the hypothalamus (Davis et al 1979; Pfaff 1980).
But there are other brain functions and behaviors besides sex behavior that are influenced by estrogens (McEwen et al 1998; McEwen & Alves 1999). These include fine motor control, motor coordination, pain, mood regulation, cognitive function, cardiovascular regulation, neuroprotection and many others. These behaviors and brain functions involve brain areas beyond the hypothalamus, including the spinal cord, cerebellum, nigrostriatal and mesolimbic system, amygdala, hippocampus, cerebral cortex and brainstem, as well as a large array of neurotransmitter and neuromodulator systems, including cholinergic, noradrenergic, serotonergic, dopaminergic, glutamatergic, neuropeptide Y and opioidergic systems.
How do these effects come about? First, tritiated steroid hormone cell nuclear uptake and retention was not all confined to the hypothalamus, although, in the case of sex hormones, the major concentration of such receptors is in the hypothalamus and the amygdala (Pfaff & Keiner 1973). The big surprise was the discovery of cell nuclear receptor sites for glucocorticoids in the hippocampus, not only of rodents but also monkeys with extension to other species (Gerlach & McEwen 1972; Gerlach et al 1976; McEwen et al 1968). This unexpected finding directed us to brain functions above the hypothalamus and, in particular, to the function of the hippocampus, a brain region important for memory and other aspects of behavioral regulation (Eichenbaum & Otto 1992).
Moreover, as it turned out, a further serendipitous finding of estrogen receptors in the hippocampus (Loy et al 1988) also represented a turning point in our realization that not all steroid hormone actions occur via cell nuclear receptors but rather operate via receptors in other parts of the cell using a variety of signaling pathways (McEwen & Milner 2007; Milner et al 2001). This is now recognized to be the case for all classes of steroid hormones (Edwards 2005), including Vitamin D (Huhtakangas et al 2004), aldosterone (Wehling et al 1992), androgens (Tabori et al 2005), glucocorticoids (Johnson et al 2005), as well as estrogens and progestins, as will be discussed in this article.
Non-genomic estrogen receptors and estrogen-regulated synapse formation
The discovery of non-nuclear estrogen receptors
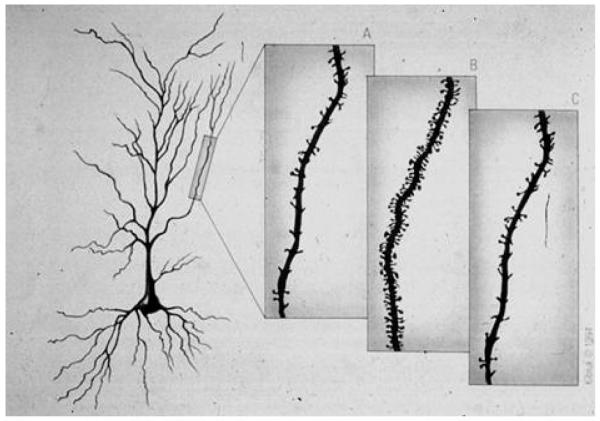
Because of the paucity of cell nuclear estrogen receptors in the hippocampus (Loy et al 1988; Pfaff & Keiner 1973), it was a surprise to find that hippocampal CA1 pyramidal neurons demonstrate reversible synaptogenesis (Figure 1) in response to ovarian steroids, a process that is regulated by excitatory amino acids via NMDA receptors in female rats (McEwen et al 1995; Woolley 1999). CA1 synaptic remodeling is a relatively rapid event, occurring during the female rats’ 5 day estrous cycle: the synapses take several days to be induced under the influence of estrogens and activated NMDA receptors and then disappear within 12 hours under the influence of the proestrus surge of progesterone (McEwen et al 1995). Treatment of ovariectomized females with estradiol benzoate for 72 hours, confirmed estrogen’s short-term effects on spine and synapses (Woolley & McEwen 1992). Interestingly, reports of an even more rapid induction of spine-like profiles within 40-60 minutes in which the normally inactive estradiol 17α is effective along with estradiol 17 β (MacLusky et al 2005) as is an estrogen receptor alpha (ERα) agonist but not an estrogen receptor beta (ERβ) agonist (Phan et al 2011), suggest that the relative importance of each estrogen receptor changes during different stages of spine development, although there is no indication how mature and functional these spines may be within a few hours of estrogen treatment.
Figure 1.
Representation of CA1 pyramidal neurons in the female rat hippocampus during the 4-5 day estrous cycle: (A) diestrus, when estradiol levels begin to gradually increase; (B) proestrus, before ovulation; (C) estrus, after ovulation. From McEwen and Schmeck The Hostage Brain Rockefeller University Press, 1994. Drawing by Lidia Kibiuk.
Estrogen induction of spine synapses was first presaged with the finding of cyclic variations in the threshold of the dorsal hippocampus to elicitation of seizures, with the greatest sensitivity occurring during proestrus (Terasawa & Timiras 1968). We subsequently found that estradiol regulates expression of pre- and post-synaptic proteins in the female rat hippocampus (Waters et al 2009) and female mouse hippocampus (Li et al 2004; Spencer et al 2008). In rats, the ability of estradiol to increase spines and thus new synaptic connections between hippocampal cells through multisynaptic boutons connecting different neurons (Yankova et al 2001) suggests a two-step process that first produces new filopodia and then induces their maturation into mature spine synapses. However, mice differ from rats in that estradiol did not increase the density of dendritic spines in mouse CA1 but rather increased the number of mushroom-shaped spines; these are presumed to be mature, functional spine synapses (Li et al 2004).
Spine maturation is also regulated by ovarian hormones in the rat hippocampus (Gonzalez-Burgos et al 2005). Estrogen receptors in the hippocampus are largely non-nuclear with the exception of nuclear receptors in scattered interneurons (Loy et al 1988; McEwen & Milner 2007). Electron microscopic autoradiography studies with 125I estradiol show these non-nuclear ER’s appear capable of binding estradiol and thus may be functional receptors (Milner et al 2008b). Estrogen receptors are found in dendrites, dendritic spines and certain types of presynaptic terminals as well as glia (Hart et al 2007; Herrick et al 2006; Ledoux et al 2009; Milner et al 2005; Milner et al 2001; Towart et al 2003). Labeling for ERα and ERβ is also associated with membranes. In terminals, ERα-immunoreactivity (-ir) is often associated with synaptic vesicles (Hart et al 2007). Moreover, ERβ–ir is often associated with mitochondria in both pre- and post-synaptic profiles (Milner et al 2005).
The expression of ERs in the hippocampus is dynamic, suggesting a possible role in hippocampal excitability/activity or function as estrogen levels change with ovarian cycling and aging. The numbers of ERα– and ERβ–containing profiles fluctuate across the estrous cycle in mice (Mitterling et al 2010). Post-synaptic localization of ERα is regulated by estradiol in young but not aged female rats; however, ERβ is estrogen sensitive in both young and aged females (Adams et al 2002; Waters et al 2011). In particular, more ERα- and ERβ-labeled dendritic spines are observed at diestrus, the phase when estrogens are the lowest (Mitterling et al 2010) (Milner et al 2008b). Both receptors contribute to estrogen regulation of synaptic proteins; estradiol increases the expression of PSD-95 as does agonists for ERα and ERβ, however, only the ERβ agonist regulated the expression of AMPA receptor subunits GluR2 and 3 (Waters et al 2009).
Non-classical estrogen receptors also may contribute to membrane-initiated signaling in the brain. This category of receptors would include ER-X, although its biochemical structure has yet to be fully characterized (Toran-Allerand et al 2002) and the seven-transmembrane G-protein coupled estrogen receptor 1 (GPER1; formerly GPR30) (Prossnitz et al 2008). GPER1 is a novel estrogen receptor first identified in breast cancer cells that is also expressed in the hippocampus (Brailoiu et al 2007). Studies have demonstrated that endogenous GPER1 protein is localized at the plasma membrane (Filardo et al 2007; Funakoshi et al 2006) and in endoplasmic reticulum (Langer et al 2010), and we have identified GPER1 with immunoelectron microscopy in pre- and post-synaptic compartments in the hippocampus, similar to ERα and ERβ (T. Milner, E. Waters, K. Akama unpublished). Treatment with a GPER1 agonist, G1 (Bologa et al 2006), increases PSD-95 expression in the female mouse hippocampus 48 hours after treatment (E. Waters, T. Milner unpublished data). A recent study also used the GPER1 agonist, G1 to address the role of GPER1 on basal forebrain cholinergic neurons and to demonstrate that GPER1 can mediate estrogen effects on cognitive performance (Hammond & Gibbs 2011). Altogether these reports along with overlapping expression profiles suggest a complex interplay between the various estrogen receptors to coordinate rapid and sub-chronic attributes of estrogen signaling and synaptic plasticity.
Progestin receptors and actions
The hippocampus also expresses estrogen-inducible progestin receptors (PRs), albeit at much lower levels than in the hypothalamus (Parsons et al 1982). PR-ir in hippocampus is present in axons, dendrites and synaptic terminals, as well as glial cell processes in both rats and mice (Waters et al 2008)The critical involvement of progesterone in ovarian steroid regulation of dendritic spine density was demonstrated in rats, where progesterone administration rapidly potentiated estrogen-induced spine formation, but then triggered the down-regulation of spines on CA1 neurons; moreover, the natural down-regulation of dendritic spines between the proestrus peak and the trough on the day of estrus was blocked by the progesterone antagonist, RU38486 (Woolley & McEwen 1993). Progesterone administration also decreases estrogen mediated increased expression of PSD-95 and synaptophysin in the rat CA1 stratum radiatum and in hippocampal primary cultures (E. Waters unpublished). Progesterone’s effects on dendritic spines is likely dependent on the classical PR; however, a number of novel membrane progesterone receptors (Thomas 2008) that are present in the hippocampus (E. Waters unpublished) may also contribute to progesterone’s actions, particularly neuroprotection.
Androgen receptors and actions
Dendritic spines are up-regulated by testosterone in gonadectomized female and male rats (Leranth et al 2004). This could occur via aromatization of androgens to estrogen, but estradiol benzoate fails to increase spines in male rats, suggesting a direct effect of testosterone (Leranth et al 2003; Lewis et al 1995). In rat hippocampus, androgen receptor (AR) immunoreactivity is prominent in CA1 pyramidal cell nuclei (Tabori et al 2005). Like ERα, ERβ and PR, ARs are located at extranuclear sites. AR-ir is found in dendritic spines, many arising from pyramidal and granule cell dendrites. AR-ir also is associated with clusters of small, synaptic vesicles in axon terminals and axons, particularly those arising from granule cells (i.e., mossy fibers) (Tabori et al 2005). In addition, AR labeling is prominent in astrocytic profiles (Tabori et al 2005). Dendritic spines are upregulated by testosterone in gonadectomized female and male rats (Leranth et al 2004). This could occur via aromatization of androgens to estrogen, but estradiol benzoate fails to increase spines in male rats (Leranth et al 2003; Lewis et al 1995). Interestingly, in Tfm rats, lacking ARs, testosterone and dihydrotesterone are still able to modulate spine number suggesting that novel ARs remain to be discovered (MacLusky et al 2006).
Role of NMDA receptors and excitatory amino acids
Besides progesterone, the single most novel feature of estrogen-induced spinogenesis on CA1 pyramidal neurons is that it is blocked by concurrent administration of NMDA receptor antagonists but not by AMPA-kainate receptor antagonists (Woolley & McEwen 1994). Spine synapses are excitatory and likely express NMDA receptors; one of the long-term effects of estradiol is to induce NMDA receptor binding sites in the CA1 region of the hippocampus (Weiland 1992). However, while increases in NMDAR1 subunit immunoreactivity in both the cell bodies and dendrites of CA1 pyramidal neurons have been reported (Gazzaley et al 1996); other reports have found no changes in NMDAR1 or NMDAR2 A and B (Snyder et al 2011). Furthermore, the actions of estradiol on NMDA receptor binding are mimicked by enhancing cholinergic function, just as the actions of estradiol are blocked by antagonizing cholinergic activity (Daniel & Dohanich 2001). Estrogen receptor activation may influence the cholinergic system directly by influencing cholinergic afferents (Towart et al 2003) or indirectly by influencing inhibitory GABAergic interneurons (Rudick et al 2003; Rudick & Woolley 2001). Activation of NMDA receptors themselves could lead to induction of new synapses, in which case estrogen/cholinergic induction of NMDA receptors would be a primary event leading to synapse formation.
Second messenger pathways
Non-nuclear estrogen receptors are able to interact with second messenger pathways, as shown in a seminal study (Razandi et al 1999) reporting that transfection of ERα and ERβ into Chinese hamster ovarian cells led to coupling of the estrogen receptors with second messenger systems that are stimulated by estrogens and blocked, at least partially, by non-steroidal estrogen antagonists. The association between the ERα-ir and ERβ-ir and dendrites in hippocampus (McEwen & Milner 2007) supports a possible local, non-genomic role for these estrogen receptors in regulation of dendritic spine density via second messenger systems.
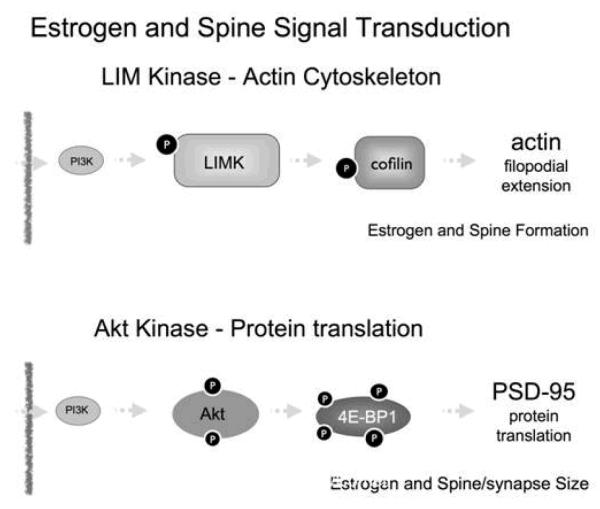
In addition to their direct and rapid effects on synaptic function and morphology, non-nuclear estrogen receptors can also initiate signaling pathways that regulate nuclear transcriptional events. Studies performed both in vivo and in vitro examining one second messenger pathway, the phosphorylation of CREB (pCREB), have indicated that estrogen acts within as little as 15 minutes to increase pCREB-ir in cell nuclei of hippocampal pyramidal neurons (Lee et al 2004). Another estrogen-sensitive pathway involves phosphoinositol-3 kinase (PI3K), and the phosphorylation of Akt, (Akama & McEwen 2003; Datta et al 1999; Spencer et al 2008; Znamensky et al 2003) and another, the phosphorylation of LIM kinase (Spencer et al 2008; Yildirim et al 2008; Yuen et al 2010). In vitro cell models followed by in vivo studies using light and electron microscopic immunocytochemistry have consistently demonstrated the involvement of these pathways. As noted in Figure 2, the LIMK pathway is associated with actin polymerization via cofilin phosphorylation, whereas the pAkt pathway leads to PSD-95 de novo translation and spine maturation. Investigations in the mouse have shown the fluctuation of the pAkt and pLIMK pathways in hippocampus during the estrous cycle, suggesting that this phenomenon is physiologically relevant. (Figures 3 and 4).
Figure 2.
Schematic summaries of the postulated role of estrogen stimulation of two pathways that can lead to spine synapse formation and maturation. First, estradiol signaling activates PI3 kinase and leads to phosphorylation of Lim Kinase 1 (LIMK1) leading, in turn, to phosphorylation of cofilin. This phosphorylation disinhibits actin polymerizatin and leads to filopodia formation that leads, in turn, to contacts with presynaptic elements that can lead to new, stable synaptic contact. In addition, PI3K increased phosphorylation of AKT and subsequent phosphorylation of 4E-BP1 leading to increased PSD95 translation. This effect promotes spine and synapse maturation.
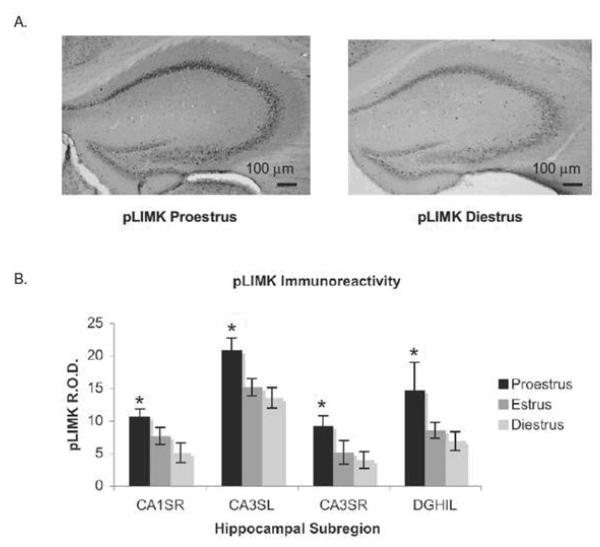
Fig. 3.
LIMK is activated during proestrus. (A) Images of peroxidase labeling of phosphorylated LIMK in the dorsal hippocampal formation of representative sections from one proestrus and one diestrus mouse. pLIMK-ir is darker throughout the hippocampus in proestrus than in diestrus. (B) Quantification of pLIMK-ir in four hippocampal subregions across the estrous cycle. pLIMK-ir was significantly higher in proestrus than in estrus (P<0.01) or diestrus. From (Spencer et al 2008). (P<0.0001) by permission.
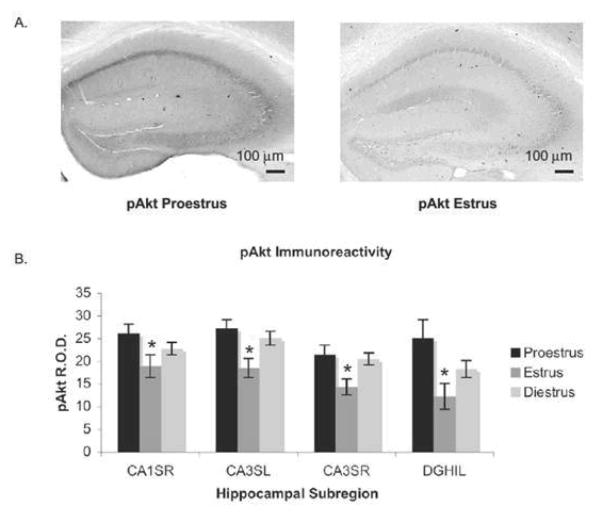
Fig. 4.
Akt is activated during proestrus and inactivated during estrus. (A) Images of peroxidase labeling of phosphorylated Akt in the dorsal hippocampal formation of representative sections from one proestrus and one estrus mouse. pAkt-ir is darker throughout the hippocampus inproestrus than in estrus. (B) Quantification of pAkt-ir in four hippocampal subregions across the estrus cycle. pAkt-ir was significantly higher in proestrus compared with estrus (P<0.0001), and in diestrus compared with estrus (P<0.0001). From (Spencer et al 2008) by permission.
How estradiol might feed into these signal transduction pathways is under current investigation. Although estrogen receptors do not have intrinsic kinase activity, membrane-initiated estrogen signaling in neurons likely stems from interactions of membrane-associated estrogen receptors with signaling molecules and scaffolding proteins to form multimolecular complexes, as well as actions of novel, non-classical estrogen receptors like GPER1. Tissue- and cell-type specific estrogen receptor complexes would allow for the wide range of estrogen mediated outcomes reported to date, particularly in regards to cross-talk between estrogen signaling and other ligand-activated pathways. Since a direct interaction between ERα and PI3K was discovered (Simoncini et al 2000), numerous kinase and phosphatases have been implicated in ERα and ERβ actions (Levin 1999), likely mediated through an estrogen receptor signaling complex. In addition, the novel estrogen receptor GPER1 has been shown to initiate rapid Ca2+, cAMP, and ERK-dependent signaling in vitro (Filardo et al 2007; Kuo et al 2010; Quinn et al 2009).
Scaffolding proteins, such as the recently identified MNAR/PELP1 protein (Brann et al 2008), that may form an ER signaling complex were first identified and well studied in breast cancer cell lines (Boonyaratanakornkit 2011). PELP1 is capable of coupling estrogen receptors together with signaling intermediates such as Src kinase and PI3K (Cheskis et al 2008), and in breast cancer cells, PELP1 knockdown demonstrates its essential role for mediating estrogen-stimulated Akt activation (Dimple et al 2008). PELP1 protein expression is identified in CNS structures, including the hippocampus, where it is observed to co-localize with ERα (Khan et al 2005). Ultrastructurally, PELP1-ir is found in dendritic spines (T. Milner, K. Akama unpublished). The co-localization with ERα has led to preliminary studies that suggest that PELP1 might play a similarly significant role in non-genomic estrogen action on kinase activity in the brain (Raz et al 2008). PELP1 scaffolding protein may be involved in recruiting estrogen receptors to non-nuclear locations in hippocampal neurons and provide a means by which estrogen receptors can activate membrane-initiated, non-genomic signaling in the brain.
Involvement of brain-derived neurotrophic factor
Both mouse and rat show ovarian hormone-dependent variation in brain derived neurotrophic factor (BDNF) immunoreactivity and phosphorylation of the BDNF receptor TrkB in hippocampus (Figure 5) (Scharfman & MacLusky 2006; Spencer et al 2008; Spencer-Segal et al 2011). The observation that this estradiol effect is not apparent 6 hours but rather 48 hours after treatment provides evidence that rapid and short term effects of estradiol work synergistically to both prime and activate signaling systems (Spencer-Segal et al., unpublished). Estradiol induction of the BDNF system had been linked to its epileptogenic potential, as well as to its role in neuroprotection. We explored the location and estrogen-sensitivity of the activated TrkB receptor in mouse hippocampus, where pre-synaptic pTrkB appears to be a key target (Spencer-Segal et al 2011). In our further studies using the transgenic BDNF Val66Met mouse we found that BDNF genotype predicts estrous cycle variations in mood and cognition and hippocampal signaling pathways (Spencer et al 2010). Thus, BDNF is likely an important target for estradiol actions on hippocampal-dependent behaviors.
Figure 5.
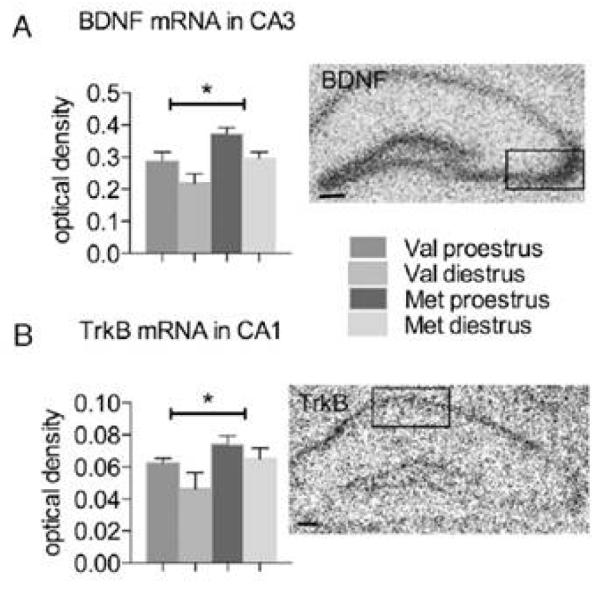
BDNF Val66Met mice have increased hippocampal BDNF and TrkB expression. (A) Optical density of BDNF mRNA in the CA3 pyramidal cell layer (box) from in situ hybridization films. (B) Optical density of TrkB mRNA in the CA1 pyramidal cell layer (box) from in situ hybridization films. *, P < 0.05 for genotype. n = 6 for Val proestrus, 4 for Val diestrus, 5 for Met proestrus, and 4 for Met diestrus. Error bars represent SEM. (Scale bars: 100 μm.) From (Spencer et al 2010) by permission.
It has been suggested that one action of BDNF that converges with estrogen’s effects is the induction of NPY synthesis. This represents one of the ways by which both estradiol and BDNF produce effects on the brain, such as the ability to attenuate excitability (Scharfman & MacLusky 2006). In addition, estradiol facilitation of the release of NPY in which results in the attenuation of seizures is an example of a non-genomic presynaptic action of estradiol (Ledoux et al 2009). This effect is not rapid per se but rather short-term, requiring treatment 24 hours prior to testing. During a similar 24 hour period, estradiol treatment also upregulates expression of NPY mRNA in CA1, possibly through BDNF (Scharfman & MacLusky 2006). Treatment with CI628, a selective estrogen response modulator that acts as an antagonist for nuclear estrogen receptor, blocked estrogen-induced increase of NPY mRNA levels but did not alter the number of parvalbumin, calretinin, and cholecystokinin immunoreactive cells, nor mRNA levels for parvalbumin and cholecystokinin (Nakamura & McEwen 2005). Interestingly, there was no estrogen effect on the number of neurons with NPY mRNA or immunoreactivity in the dentate gyrus or the CA3 region (T. Milner unpublished). Given the ability of estrogens to non-genomically regulate NPY release (Ledoux et al 2009) and increase seizure suspectibility, the ability of estrogen-induced BDNF to also upregulate NPY after seizure as well as independent of seizure (Poulsen et al 2002) maybe contribute to recovery. These links between estrogen, BDNF and NPY expression and release (Scharfman & MacLusky 2006), contribute to the regulation of glutamate-dependent neuronal activity in the adult rat hippocampus.
Aging in hippocampus and prefrontal cortex (PFC)
The female rat hippocampus loses the ability to show estrogen-induced spine synapse formation by 22 months (Adams et al PNAS 2001). This is accompanied by loss of ERα in dendrites of CA1 pyramidal cells (Adams et al 2002) as well as reduction in the ability of estrogen treatment to increase pLIMK-ir in the CA1 region of the aging brain (Yildirim et al 2008), both of which appear to be part of the mechanism for spine synapse formation (Yuen et al 2010). Another glimpse into age related loss of plasticity in the rhesus monkey brain is the loss of thin spine synapses that accompanies impaired PFC function (Dumitriu et al 2010a; Dumitriu et al 2010b). Estrogen treatment is able to reduce this loss of function by inducing spine formation in the aging rhesus monkey brain (Dumitriu et al 2010b), and it should be noted that the aging rhesus brains studied so far are not as old relative to lifespan as is the case in the 22 month old rat. Therefore, it cannot be ruled out that the rhesus brain will also lose the ability to respond to estradiol at even older ages. How can this be prevented? There are indications from investigations of the aging rodent brain that estrogen treatment during the aging processes prevents age-related loss of memory capacity (Gibbs 2000), and there is evidence that this occurs in humans as well (Sherwin 2009).
Sex differences
There are sex differences in spine synapse formation. Male rats castrated as adults do not exhibit hippocampal synaptogenesis in response to estrogens (Leranth et al 2003). However, if the process of sexual differentiation has been blocked by aromatase inhibitors immediately after birth, adult males show an estrogen-induced increase in dendritic spines in (Lewis et al 1995). Yet castrated male rats show a decrease in spine synaptic density in hippocampus that is restored by treatment with testosterone or dihydrotestosterone (Kovacs et al 2003). Along with evidence that spatial memory processes show sex differences programmed by the aromatization of estrogens early in neonatal life (Williams & Meck 1991), these results indicate that the hippocampus undergoes sexual differentiation.
Estrogen actions in autonomic circuitry
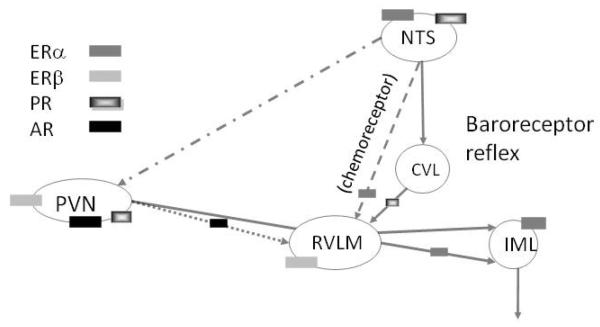
Estrogens act in other extra-hypothalamic brain regions, including the nigrostriatal system, cerebellum, brain stem and spinal cord by both direct and indirect genomic and non-genomic mechanisms (McEwen & Milner 2007; Milner et al 2003; Monks et al 2001; Smith et al 1988; Zsarnovszky et al 2005). One of the best-studied extra-hypothalamic systems outside of the hippocampus is the central autonomic system of the brainstem. Ovarian hormones, particularly estrogens, can modulate central autonomic networks through both genomic (e.g., nuclear receptors) and non-genomic (e.g., extranuclear receptors) mechanisms. Light and electron microscopic immunocytochemical studies in rodents have revealed that the distributions of nuclear and extranuclear estrogen receptor’s, PR’s and AR’s are complementary and overlapping in three major autonomic regions: the rostral ventrolateral medulla (RVLM), nucleus of the solitary tract (NTS) and paraventricular nucleus of the hypothalamus (PVN) (Figure 6). Moreover, electron microscopic autoradiography studies using 125I estradiol indicate that non-nuclear estrogen receptors in autonomic medullary regions detected by immunocytochemistry are capable of binding, and presumably responding to, estradiol (Milner et al 2008b).
Figure 6.
Schematic diagram of distribution of steroid receptors in autonomic circuits. The baroreceptor reflex is formed by projections from the NTS to CVL to RVLM to the interomedial lateral cell column (IML) of the spinal cord. The NTS projects directly to the RVLM (chemoreceptor pathway) and PVN. The PVN projects directly to the RVLM and IML. ERα and PR predominate in neurons in the autonomic regions of the NTS, ERβ predominates in the PVN and RVLM neurons, and AR predominates in the PVN.
Our anatomical and physiological studies have shown that sex and ovarian hormones can alter the expression and subcellular distribution of components of the Angiotensin-II (AngII) signaling pathway in the C1 catecholaminergic RVLM neurons. AngII plays an important role in the central regulation of hypertension (Peterson et al 2006). In particular, AngII injected into the RVLM increases sympathetic nerve activity and blood pressure (Averill et al 1994; Hirooka et al 1997). Catecholaminergic dendrites from young (4 month old) and middle aged (12 month old) female rats had more angiotensin type 1 (AT1) receptor labeling and less labeling for the downstream effector of the AT1 receptor p47, an NADPH oxidase signaling molecule, than males (Pierce et al 2009; Wang et al 2008). Moreover, young proestrus females had more AT1 receptors on the plasma membrane of RVLM catecholaminergic dendrites compared to diestrus females (Pierce et al 2009). In contrast, p47 did not differ across the estrous cycle. In dissociated RVLM neurons from young males and females, AngII induced a comparable production of reactive oxygen species (ROS), an effect mediated by AT1 receptors and NADPH oxidase but larger L-type Ca2+ currents in females (Wang et al 2008). Increased L-type Ca2+ currents may reflect greater plasticity in the central autonomic circuitry in females. All this suggests that estrogen are involved in the relative protection of females against hypertension.
Steroid receptor changes may underlie the adaptive responses that protect young females from the deleterious effects of hypertension (Pamidimukkala et al 2005). In non-hypertensive animals, ERα-labeled nuclei are more abundant than those with PR-ir in all three autonomic regions (Milner et al 2008c). In contrast, ERβ and AR-immunoreactivities are almost exclusively found at extranuclear sites (Milner et al 2007; Milner et al 2010; Wang et al 2006); unpublished observations). At high estrogen levels, the levels of nuclear ERα’s and PR’s fluctuate in the NTS and PVN. Specifically, in proestrus rats or in OVX rats supplemented with estrogen, fewer ERα-labeled nuclei are found in the commissural NTS (cNTS) and PVN (Milner et al 2008a); conversely, the number of PR-immunoreactive nuclei are increased in the cNTS in proestrus or OVX + E supplemented rats (Haywood et al 1999; Milner et al 2008a). In the rat commissural and medial NTS, about half of the neurons containing ERα-labeled nuclei are catecholaminergic whereas fewer ERα-containing nuclei are found in catecholaminergic neurons in the dorsomedial NTS and RVLM (Haywood et al 1999; Lee et al 2000; Milner et al 2008a). Following chronic infusion of AngII, a rat model of hypertension, the levels of nuclear ERα increases whereas nuclear PR decreases in the commissural NTS. Moreover, in the commissural NTS, chronic AngII infusion increases the cytoplasmic-to-nuclear ratio selectively in catecholaminergic neurons (Milner et al 2008a).
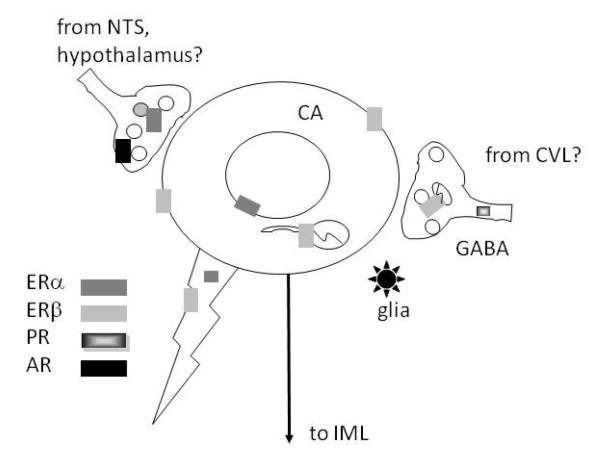
The extranuclear distribution of ER’s, PR’s and AR’s has been investigated most extensively in the RVLM (Figure 7). In this brain region, extranuclear ERβ is found mostly on the somata and dendrites of catecholaminergic neurons (Wang et al 2006). In RVLM as well as the PVN, extranuclear ERβ is found on spinally-projecting neurons (Milner et al 2010; Wang et al 2006) that are importantly involved in the tonic regulation of arterial pressure (Aicher et al 2000; Benarroch 2005). In contrast, extranuclear ERα–, PR- and AR-ir’s are found almost exclusively in axons and terminals (Milner et al 2007; Milner et al 2008c; Wang et al 2006). ERα and AR containing terminals have numerous dense core vesicles, resembling the morphology of NTS chemosensory afferents (Aicher et al 2000) and/or PVN inputs. PR- and ERβ-containing axons and terminals are smaller; PR-labeled presynaptic profiles can contain GABA, possibly originating from the caudal ventrolateral medulla (CVL) (Milner et al 2008c). Some terminals containing ER’s, PR’s and AR’s synapse on catecholamine containing dendrites. Unlike the hippocampus, few glial profiles contain ERα-, ERβ- and PR-immunoreactivities. However, similar to the hippocampus, numerous glial profiles contain AR-labeling (Milner et al 2007). Our electrophysiological data indicates that 17β-estradiol reduced L-type Ca2+ currents in RVLM catecholaminergic bulbospinal neurons either directly through ERβ or indirectly through ERα in selected afferents (Wang et al 2006). The modulation of Ca2+ currents may underlie the decrease in sympathetic tone evoked by local 17 β-estradiol application (Saleh et al 2000). Altogether, these findings provide a potential structural and functional basis through which ovarian hormones could contribute to not only to sex differences in the risk of cardiovascular disease but also to similarities between males and females where estrogens may play a analogous role.
Figure 7.
Schematic diagram of the subcellular distributions of steroid receptors in the RVLM. ERa is found in the nuclei of catecholaminergic (CA) RVLM neurons and in afferent terminals, possibly arising from the NTS or hypothalamus. ERβ is mostly found on the plasma membranes or affiliated with mitochondria of CA neurons and sometimes in terminals. PR is almost exclusively in axons, some colocalized with GABA which possibly arise from the CVL. ARs are in large afferent terminals resembling NTS and hypothalamic afferents and in glia.
Other Directions
What are important next steps? In all of the examples cited in this review, the relative levels and locations of estrogen receptors provides a fruitful ground for understanding the effects of estrogens on neuronal plasticity. ERα and ERβ have been shown to work in a complex, both complementary and sometimes antagonistic manner (Lindberg et al 2003; Monroe et al 2003) and have functionally distinct roles in regulating synaptic connectivity. For example, treatment with ERα agonists rapidly increased dendritic spines in hippocampal CA1 stratum radiatum of ovariectomized females while an ERβ agonist did not (Phan et al 2011). Moreover, ERβ expression in hippocampal neurons attenuates estradiol induced spine formation (Szymczak et al 2006). Both the levels and spatial relationship of estrogen receptors undoubtedly contribute to their rapid effects on synaptic transmission. Estrogen-induced elevations of ERα-ir in the pre-synaptic cytoplasmic pool (Adams et al 2002), where synaptic vesicles reside, may promote neurotransmitter release (Becker 1990; Ledoux et al 2009) and reuptake (O’Malley et al 1987). Moreover, it is possible that ERα enhances fiilopodia formation. In contrast, activation of postsynaptic ERβ by a specific agonist, leads to enhanced fast EPSPs (Kramar et al 2009) and in this way may promote synaptic potentiation and spine maturation. ERα and ERβ are not only present in neuronal compartments at different levels, but they also change differentially as a result of aging and estrogen treatment. Novel estrogen receptors like GPR30/GPER1 also come into play but much remains to be discovered about their regulation and actions in the brain. Together, these findings imply that a changing balance of estrogen receptor expression, possibly in specific cell compartments, underlies differences found in the aging compared to young brains and in male compared to female responses to experience, blood pressure regulation, and inflammation. Further research is needed to understand the interactions between estrogen and estrogen receptors and the mechanisms underlying estrogen actions in both homeostasis and plasticity in the brain.
In addition to the largely neuronal effects of estrogens and the role of the several receptor types, estrogens affect other cell types both genomically and non-genomically. Estradiol, progesterone and testosterone have also been implicated in sex differences in innate, as well as acquired and auto-immunity (Marriott & Huet-Hudson 2006; Whitacre et al 1999). Not surprisingly, this is a complicated relationship, because although estrogens enhance normal immunity and autoimmunity (Cunningham & Gilkeson 2011), females also experience higher rates of some autoimmune disorders such as lupus, rheumatoid arthritis and multiple sclerosis (McCombe et al 2009; Whitacre 2001). In contrast, androgens are generally shown to suppress immune system responses, although males do experience higher rates of psoraisis and Guillain-Barré Syndrome than females (McCombe et al 2009). Numerous reports suggest both pro- and anti-inflammatory actions of estrogen, while expression of ERα and ERβ within the various peripheral immune cells may vary with immune state (Straub 2007). In the central nervous system ERα and ERβ are found in astrocytes (Milner et al 2005; Milner et al 2001; Spence et al 2011) but very little ERα and no ERβ in microglia (Sierra et al 2008). A relatively greater influence of ERα on innate and adaptive immunity is suggested by the role estrogen plays in the differentiation of dendritic cells: utilization of estrogen receptor agonists suggest that ERα and not ERβ enhances dendritic cell function (Douin-Echinard et al 2008). Although reports offer conflicting results about the actions of estrogens during immune disease states, higher estrogen levels in females likely contributes to sex differences in immunity noted during normal and disease states. The mechanism by which estrogens can exert anti-inflammatory effects that are evident in neurological diseases and in the aging process demands further investigation.
Conclusions
The investigation of cellular and molecular actions of estrogens on the brain has contributed to at least two important areas of knowledge. First, the mechanism of estrogen action has expanded to include indirect genomic and non-genomic actions of these hormones along with their traditional genomic effects. This has led to the finding of estrogen receptors in many parts of the brain in neuron cell bodies, dendrites, pre-synaptic terminals, mitochondria and glial cell processes using high-resolution immunocytochemical techniques involving electron microscopy. Second, these findings have revitalized studies of estrogen actions on diverse functions in many parts of the brain, such as the action of estradiol on hippocampus and prefrontal cortex in both rodent and monkey models and the effects of aging on estrogen actions and on autonomic centers in the brain stem. These potentially affect cognition, mood, and blood pressure regulation, and potentially alter inflammatory processes and innate and acquired immune function not only in the brain but throughout the body.
Acknowledgments
Grant support: NS07080 and PO1-AG16765 (BSM), HL098351, DA08259, HL096571 (TAM), AG039850 (EMW, TAM)
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
Reference List
- Adams MM, Fink SE, Shah RA, Janssen WGM, Hayashi S, et al. Estrogen and aging affect the subcellular distribution of estrogen receptor-a in the hippocampus of female rats. J. Neurosci. 2002;22:3608–14. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher SA, Milner TA, Pickel VM, Reis DJ. Anatomical substrates for baroreflex sympathoinhibition in the rat. Brain Res Bull. 2000;51:10–10. doi: 10.1016/s0361-9230(99)00233-6. [DOI] [PubMed] [Google Scholar]
- Akama KT, McEwen BS. Estrogen stimulates postsynaptic densit-95 rapid protein synthesis via the Akt/protein kinase B pathway. J. Neurosci. 2003;23:2333–9. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill DB, Tsuchihashi T, Khosla MC, Ferrario CM. Losartan, nonpeptide angiotensin II-type 1 (AT1) receptor antagonist, attenuates pressor and sympathoexcitatory responses evoked by angiotensin II and L-glutamate in rostral ventrolateral medulla. Brain Res. 1994;665:245–52. doi: 10.1016/0006-8993(94)91344-7. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17b-estradiol on striatum: Sex differences in dopamine release. Synapse. 1990;5:157–64. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Paraventricular nucleus, stress response, and cardiovascular disease. Clin Auton Res. 2005;15:254–63. doi: 10.1007/s10286-005-0290-7. [DOI] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006;2:207–12. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit V. Scaffolding proteins mediating membrane-initiated extra-nuclear actions of estrogen receptor. Steroids. 2011 doi: 10.1016/j.steroids.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J. Endocrin. 2007;193:311–21. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Brann DW, Zhang QG, Wang RM, Mahesh VB, Vadlamudi RK. PELP1--a novel estrogen receptor-interacting protein. Mol Cell Endocrinol. 2008;290:2–7. doi: 10.1016/j.mce.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheskis BJ, Greger J, Cooch N, McNally C, McLarney S, et al. MNAR plays an important role in ERa activation of Src/MAPK and PI3K/Akt signaling pathways. Steroids. 2008;73:901–5. doi: 10.1016/j.steroids.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. 2011;40:66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J. Neurosci. 2001;21:6949–56. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes & Devel. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Davis P, McEwen BS, Pfaff DW. Localized behavioral effects of tritiated estradiol implants in the ventromedial hypothalamus of female rats. Endocrinology. 1979;104:89–903. doi: 10.1210/endo-104-4-898. [DOI] [PubMed] [Google Scholar]
- Dimple C, Nair SS, Rajhans R, Pitcheswara PR, Liu J, et al. Role of PELP1/MNAR signaling in ovarian tumorigenesis. Cancer Res. 2008;68:4902–9. doi: 10.1158/0008-5472.CAN-07-5698. [DOI] [PubMed] [Google Scholar]
- Douin-Echinard V, Laffont S, Seillet C, Delpy L, Krust A, et al. Estrogen receptor alpha, but not beta, is required for optimal dendritic cell differentiation and [corrected] CD40-induced cytokine production. J Immunol. 2008;180:3661–9. doi: 10.4049/jimmunol.180.6.3661. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010a;30:7507–15. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH. Estrogen and the aging brain: an elixir for the weary cortical network. Ann N Y Acad Sci. 2010b;1204:104–12. doi: 10.1111/j.1749-6632.2010.05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–76. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T. The hippocampus - what does it do? Behav.Neural.Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, et al. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–45. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun. 2006;346:904–10. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential Regulation of NMDAR1 mRNA and Protein by Estradiol in the RAt Hippocampus. J.Neurosci. 1996;16:6830–8. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach J, McEwen BS. Rat brain binds adrenal steroid hormone: radioautography of hippocampus with corticosterone. Science. 1972;175:1133–6. doi: 10.1126/science.175.4026.1133. [DOI] [PubMed] [Google Scholar]
- Gerlach J, McEwen BS, Pfaff DW, Moskovitz S, Ferin M, et al. Cells in regions of rhesus monkey brain and pituitary retain radioactive estradiol, corticosterone and cortisol differently. Brain Res. 1976;103:603–12. doi: 10.1016/0006-8993(76)90463-7. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol. of Aging. 2000;21:107–16. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos I, Alejandre-Gomez M, Cervantes M. Spine-type densities of hippocampal CA1 neurons vary in proestrus and estrus rats. Neurosci. Lett. 2005;379:52–4. doi: 10.1016/j.neulet.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Guillemin R. Peptides in the brain: the new endocrinology of the neuron. Science. 1978;202:390–402. doi: 10.1126/science.212832. [DOI] [PubMed] [Google Scholar]
- Hammond R, Gibbs RB. GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Res. 2011;1379:53–60. doi: 10.1016/j.brainres.2010.11.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GW. Electrical stimulation of the hypothalamus and the mechanism of neural control of the adenohypophysis. J. Physiol. 1948;107:418–29. doi: 10.1113/jphysiol.1948.sp004286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-a-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J. Neurosci. 2007;27:2102–11. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood SA, Simonian SX, van der Beek EM, Bicknell RJ, Herbison AE. Fluctuating estrogen and progesterone receptor expression in brainstem norepinephrine neurons through the rat estrous cycle. Endocrinology. 1999;140:3255–63. doi: 10.1210/endo.140.7.6869. [DOI] [PubMed] [Google Scholar]
- Herrick SP, Waters EM, Drake CT, McEwen BS, Milner TA. Extranuclear estrogen receptor beta immunoreactivity is on doublecortin-containing cells in the adult and neonatal rat dentate gyrus. Brain Res. 2006 doi: 10.1016/j.brainres.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Hirooka Y, Potts PD, Dampney RA. Role of angiotensin II receptor subtypes in mediating the sympathoexcitatory effects of exogenous and endogenous angiotensin peptides in the rostral ventrolateral medulla of the rabbit. Brain Res. 1997;772:107–14. doi: 10.1016/s0006-8993(97)00861-5. [DOI] [PubMed] [Google Scholar]
- Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1a,25(OH)2-vitamin D3 in vivo and in vitro. Mol. Endocrin. 2004;18:2660–71. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- Jensen E, Geoffrey L, Greene L, Closs L, E D, Nadji M. Receptors reconsidered a 20-year perspective. In: Greep R, editor. Recent Progress in Hormone Research. Academic Press; New York: 1981. pp. 1–40. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience. 2005;136:289–99. doi: 10.1016/j.neuroscience.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Khan MM, Hadman M, Wakade C, De Sevilla LM, Dhandapani KM, et al. Cloning, expression, and localization of MNAR/PELP1 in rodent brain: Colocalization in estrogen receptor-a-but not in gonadotropin-releasing hormone-positive neurons. Endocrinology. 2005;146:5215–27. doi: 10.1210/en.2005-0276. [DOI] [PubMed] [Google Scholar]
- Kovacs EG, MacLusky NJ, Leranth C. Effects of testosterone on hippocampal CA1 spine synaptic density in the male are inhibited by fimbria/fornix transection. Neuroscience. 2003;122:807–10. doi: 10.1016/j.neuroscience.2003.08.046. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Rex CS, Gall CM, Lynch G. Estrogen’s Place in the Family of Synaptic Modulators. Mol Cell Pharmacol. 2009;1:258–62. [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci. 2010;30:12950–7. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G, Bader B, Meoli L, Isensee J, Delbeck M, et al. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75:603–10. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Ledoux VA, Smejkalova T, May RM, Cooke BM, Woolley CS. Estradiol facilitates the release of neuropeptide Y to suppress hippocampus-dependent seizures. J. Neurosci. 2009;29:1457–68. doi: 10.1523/JNEUROSCI.4688-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E-J, Moore CT, Hosny S, Centers A, Jennes L. Expression of estrogen receptor-a and c-Fos in adrenergic neurons of the female rat during the steroid-induced LH surge. Brain Res. 2000;875:56–65. doi: 10.1016/s0006-8993(00)02622-6. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Campomanes CR, Sikat PT, Greenfield AT, Allen PB, McEwen BS. Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience. 2004;124:549–60. doi: 10.1016/j.neuroscience.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J. Neurosci. 2004;24:495–9. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J. Neurosci. 2003;23:1588–92. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Cellular functions of the plasma membrane estrogen receptor. TEM. 1999;10:374–7. doi: 10.1016/s1043-2760(99)00192-7. [DOI] [PubMed] [Google Scholar]
- Lewis C, McEwen BS, Frankfurt M. Estrogen-induction of dendritic spines in ventromedial hypothalamus and hippocampus: effects of neonatal aromatase blockade and adult castration. Devel.Brain Res. 1995;87:91–5. doi: 10.1016/0165-3806(95)00052-f. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RC, Dunlop JC, Gordon M, et al. Estrogen treatment alters hippocampal dendritic spine shape, enhances synaptic protein immunoreactivity and performance in a spatial working memory task in female C57Bl/6J mice. Proc. Natl. Acad. Sci. USA. 2004;101:2185–90. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, et al. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 2003;17:203–8. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- Loy R, Gerlach J, McEwen BS. Autoradiographic localization of estradiol-binding neurons in rat hippocampal formation and entorhinal cortex. Dev.Brain Res. 1988;39:245–51. doi: 10.1016/0165-3806(88)90028-4. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Johansen JA, Jordan CL, Leranth C. Androgen effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology. 2006;147:2392–8. doi: 10.1210/en.2005-0673. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17a and 17b isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–93. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Marriott I, Huet-Hudson YM. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res. 2006;34:177–92. doi: 10.1385/IR:34:3:177. [DOI] [PubMed] [Google Scholar]
- McCombe PA, Greer JM, Mackay IR. Sexual dimorphism in autoimmune disease. Curr Mol Med. 2009;9:1058–79. doi: 10.2174/156652409789839116. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE, Bulloch K, Weiland NG. Clinically relevant basic science studies of gender differences and sex hormone effects. Psychopharmacology Bulletin. 1998;34:251–9. [PubMed] [Google Scholar]
- McEwen BS, Alves SH. Estrogen Actions in the Central Nervous System. Endocrine Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gould E, Orchinik M, Weiland NG, Woolley CS. Oestrogens and the structural and functional plasticity of neurons: implications for memory, ageing and neurodegenerative processes. In: Goode J, editor. Ciba Foundation Symposium #191 The Non-reproductive Actions of Sex Steroids. CIBA Foundation; London: 1995. pp. 52–73. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. Hippocampal formation: Shedding light on the influence of sex and stress on the brain. Brain Res. Rev. 2007;55:343–55. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Plapinger L. Association of corticosterone-1,2 3H with macromolecules extracted from brain cell nuclei. Nature. 1970;226:263–4. doi: 10.1038/226263a0. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss J, Schwartz L. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–2. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- Meites J. Short history of neuroendocrinology and the International Society of Neuroendocrinology. Neuroendocrinology. 1992;56:1–10. doi: 10.1159/000126201. [DOI] [PubMed] [Google Scholar]
- Milner TA, Alves SE, Hayashi S, McEwen BS. The Identities of Membrane Steroid Receptors. Kluwer Academic Publishers; Boston, MA: 2003. An expanded view of estrogen receptor localization in neurons; pp. 21–5. [Google Scholar]
- Milner TA, Ayoola K, Drake K, Herrick SP, Tabori NE, et al. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 2005 doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, Drake CT, Lessard A, Waters EM, Torres-Reveron A, et al. Angiotensin II-induced hypertension differentially affects estrogen and progestin receptors in central autonomic regulatory areas of female rats. Exp Neurol. 2008a;212:393–406. doi: 10.1016/j.expneurol.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Hernandez FJ, Herrick SP, Pierce JP, Iadecola C, Drake CT. Cellular and subcellular localization of androgen receptor immunoreactivity relative to C1 adrenergic neurons in the rostral ventrolateral medulla of male and female rats. Synapse. 2007;61:268–78. doi: 10.1002/syn.20370. [DOI] [PubMed] [Google Scholar]
- Milner TA, Lubbers LS, Alves SE, McEwen BS. Nuclear and extranuclear estrogen binding sites in the rat forebrain and autonomic medullary areas. Endocrinology. 2008b;149:3306–12. doi: 10.1210/en.2008-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagen L, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J. Comp. Neurol. 2001;429:355–71. [PubMed] [Google Scholar]
- Milner TA, Mitterling KL, Iadecola C, Waters EM. Ultrastructural localization of extranuclear progestin receptors relative to C1 neurons in the rostral ventrolateral medulla. Neurosci Lett. 2008c;431:167–72. doi: 10.1016/j.neulet.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Thompson LI, Wang G, Kievits JA, Martin E, et al. Distribution of estrogen receptor beta containing cells in the brains of bacterial artificial chromosome transgenic mice. Brain Res. 2010;1351:74–96. doi: 10.1016/j.brainres.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, et al. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518:2729–43. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks DA, Arciszewska G, Watson NV. Estrogen-inducible progesterone receptors in the rat lumbar spinal cord: Regulation by ovarian steroids and fluctuation across the estrous cycle. Horm. & Behav. 2001;40:490–6. doi: 10.1006/hbeh.2001.1717. [DOI] [PubMed] [Google Scholar]
- Monroe DG, Johnsen SA, Subramaniam M, Getz BJ, Khosla S, et al. Mutual antagonism of estrogen receptors alpha and beta and their preferred interactions with steroid receptor coactivators in human osteoblastic cell lines. J Endocrinol. 2003;176:349–57. doi: 10.1677/joe.0.1760349. [DOI] [PubMed] [Google Scholar]
- Nakamura NH, McEwen BS. Changes in interneuronal phenotypes regulated by estradiol in the adult rat hippocampus: A potential role for neuropeptide Y. Neuroscience. 2005;136:357–69. doi: 10.1016/j.neuroscience.2005.07.056. [DOI] [PubMed] [Google Scholar]
- O’Malley CA, Hautamaki M, Kelley M, Meyer EM. Effects of ovariectomy and estradiol benzoate on high affinity choline uptake, ACh synthesis, and release from rat cerebral cortical synaptosomes. Brain Res. 1987;403:389–92. doi: 10.1016/0006-8993(87)90082-5. [DOI] [PubMed] [Google Scholar]
- Pamidimukkala J, Xue B, Newton LG, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen facilitation of baroreflex heart rate responses in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H1063–70. doi: 10.1152/ajpheart.01163.2003. [DOI] [PubMed] [Google Scholar]
- Parsons B, Rainbow TC, MacLusky N, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J.Neurosci. 1982;2:1446–52. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JR, Sharma RV, Davisson RL. Reactive oxygen species in the neuropathogenesis of hypertension. Curr Hypertens Rep. 2006;8:232–41. doi: 10.1007/s11906-006-0056-1. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Estrogens and brain function. Springer-Verlag; 1980. [Google Scholar]
- Pfaff DW, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J.Comp.Neurol. 1973;151:121–58. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor alpha and beta selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Kievits J, Graustein B, Speth RC, Iadecola C, Milner TA. Sex differences in the subcellular distribution of angiotensin type 1 receptors and NADPH oxidase subunits in the dendrites of C1 neurons in the rat rostral ventrolateral medulla. Neuroscience. 2009;163:329–38. doi: 10.1016/j.neuroscience.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen FR, Jahnsen H, Blaabjerg M, Zimmer J. Pilocarpine-induced seizure-like activity with increased BNDF and neuropeptide Y expression in organotypic hippocampal slice cultures. Brain Res. 2002;950:103–18. doi: 10.1016/s0006-8993(02)03009-3. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu. Rev. Physiol. 2008;70:165–90. doi: 10.1146/annurev.physiol.70.113006.100518. [DOI] [PubMed] [Google Scholar]
- Quinn JA, Graeber CT, Frackelton AR, Jr., Kim M, Schwarzbauer JE, Filardo EJ. Coordinate regulation of estrogen-mediated fibronectin matrix assembly and epidermal growth factor receptor transactivation by the G protein-coupled receptor, GPR30. Mol Endocrinol. 2009;23:1052–64. doi: 10.1210/me.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neurosignals. 2008;16:140–53. doi: 10.1159/000111559. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: Studies of ERa and ERb expressed in Chinese hamster ovary cells. Mol.Endocrinol. 1999;13:307–19. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J. Neurosci. 2003;23:4479–90. doi: 10.1523/JNEUROSCI.23-11-04479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J. Neurosci. 2001;21:6532–43. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MC, Connell BJ, Saleh TM. Autonomic and cardiovascular reflex responses to central estrogen injection in ovariectomized female rats. Brain Res. 2000;879:105–14. doi: 10.1016/s0006-8993(00)02757-8. [DOI] [PubMed] [Google Scholar]
- Schally AV, Arimura A, Kastin AJ. Hypothalamic regulatory hormones. Science. 1973;179:341–50. doi: 10.1126/science.179.4071.341. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: Complexity of steroid hormone-growth factor interactions in the adult CNS. Front. Neuroendocrin. 2006;27:415–35. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol. 2009;5:620–7. doi: 10.1038/nrendo.2009.193. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56:659–74. doi: 10.1002/glia.20644. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–41. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Locally applied estrogens potentiate glutamate-evoked excitation of cerebellar Purkinje cells. Brain Res. 1988;475:272–82. doi: 10.1016/0006-8993(88)90615-4. [DOI] [PubMed] [Google Scholar]
- Snyder MA, Cooke BM, Woolley CS. Estradiol potentiation of NR2B-dependent EPSCs is not due to changes in NR2B protein expression or phosphorylation. Hippocampus. 2011;21:398–408. doi: 10.1002/hipo.20756. [DOI] [PubMed] [Google Scholar]
- Spence RD, Hamby ME, Umeda E, Itoh N, Du S, et al. Neuroprotection mediated through estrogen receptor-{alpha} in astrocytes. Proc Natl Acad Sci U S A. 2011;108:8867–72. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, Lee FS, McEwen BS. BDNF variant Val66Met interacts with estrous cycle in the control of hippocampal function. Proc. Natl. Acad. Sci. USA. 2010 doi: 10.1073/pnas.0915105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, McEwen BS. Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice. Neuroscience. 2008;155:1106–19. doi: 10.1016/j.neuroscience.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Segal JL, Waters EM, Bath KG, Chao MV, McEwen BS, Milner TA. Distribution of Phosphorylated TrkB Receptor in the Mouse Hippocampal Formation Depends on Sex and Estrous Cycle Stage. J Neurosci. 2011;31:6780–90. doi: 10.1523/JNEUROSCI.0910-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–74. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Sar M. Steroid hormone target sites in the brain: The differential distribution of estrogen, progestin, androgen and glucocorticosteroid. J.Steroid Biochem. 1976;7:1163–70. doi: 10.1016/0022-4731(76)90050-9. [DOI] [PubMed] [Google Scholar]
- Szymczak S, Kalita K, Jaworski J, Mioduszewska B, Savonenko A, et al. Increased estrogen receptor beta expression correlates with decreased spine formation in the rat hippocampus. Hippocampus. 2006;16:453–63. doi: 10.1002/hipo.20172. [DOI] [PubMed] [Google Scholar]
- Tabori NE, Stewart LS, Znamensky V, Romeo RD, Alves SE, et al. Ultrastructural evidence that androgen receptors are located at extranuclear sites in the rat hippocampal formation. Neuroscience. 2005;130:151–63. doi: 10.1016/j.neuroscience.2004.08.048. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Timiras P. Electrical activity during the estrous cycle of the rat: cyclic changes in limbic structures. Endocrinology. 1968;83:207–16. doi: 10.1210/endo-83-2-207. [DOI] [PubMed] [Google Scholar]
- Thomas P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, et al. ER-X: A novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J. Neurosci. 2002;22:8391–401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towart LA, Alves SE, Znamensky V, Hayashi S, McEwen BS, Milner TA. Subcellular relationships between cholinergic terminals and estrogen receptor-a in the dorsal hippocampus. J. Comp. Neurol. 2003;463:390–401. doi: 10.1002/cne.10753. [DOI] [PubMed] [Google Scholar]
- Wang G, Drake CT, Rozenblit M, Zhou P, Alves SE, et al. Evidence that estrogen directly and indirectly modulates C1 adrenergic bulbospinal neurons in the rostral ventrolateral medulla. Brain Res. 2006;1094:163–78. doi: 10.1016/j.brainres.2006.03.089. [DOI] [PubMed] [Google Scholar]
- Wang G, Milner TA, Speth RC, Gore AC, Wu D, et al. Sex differences in angiotensin signaling in bulbospinal neurons in the rat rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1149–57. doi: 10.1152/ajpregu.90485.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Mitterling K, Spencer JL, Mazid S, McEwen BS, Milner TA. Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 2009;1290:1–11. doi: 10.1016/j.brainres.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Torres-Revon AT, McEwen BS, Milner TA. Extranuclear progestin receptor immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 2008;511:34–46. doi: 10.1002/cne.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, et al. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011;1379:86–97. doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling M, Christ M, Thiesen K. Membrane receptors for aldosterone: a novel pathway for mineralocorticoid action. Am.J.Physiol. 1992;263:E974. doi: 10.1152/ajpendo.1992.263.5.E974. [DOI] [PubMed] [Google Scholar]
- Weiland NG. Estradiol selectively regulates agonist binding sites on the N-methyl-D- aspartate receptor complex in the CA1 region of the hippocampus. Endocrinology. 1992;131:662–8. doi: 10.1210/endo.131.2.1353442. [DOI] [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–80. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–8. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology. 1991;16:155–76. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- Woolley C, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. Journal Neuroscience. 1992;12:2549–54. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley C, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J.Comp.Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley C, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor dependent mechanism. Journal Neuroscience. 1994;14:7680–7. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Effects of estrogen in the CNS. Current Opinion Neurobiol. 1999;9:349–54. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]
- Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: A serial electron-microscopic study. Proc. Natl. Acad. Sci. USA. 2001;98:3525–30. doi: 10.1073/pnas.051624598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim M, Janssen WG, Tabori NE, Adams MM, Yuen GS, et al. Estrogen and aging affect synaptic distribution of phosphorylated LIM kinase (pLIMK) in CA1 region of female rat hippocampus. Neuroscience. 2008;152:360–70. doi: 10.1016/j.neuroscience.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young W. The Hormones and Mating Behavior. In: Young W, editor. Sex and Internal Secretions. Williams and Wilkins; Baltimore: 1961. pp. 1173–239. [Google Scholar]
- Yuen GS, McEwen BS, Akama KT. LIM kinase mediates estrogen action on the actin depolymerization factor Cofilin. Brain Res. 2010 doi: 10.1016/j.brainres.2010.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond R, McEwen BS. Selective retention of oestradiol by cell nuclei in specific brain regions of the ovariectomized rats. J.Neurochem. 1970;17:889–99. doi: 10.1111/j.1471-4159.1970.tb02242.x. [DOI] [PubMed] [Google Scholar]
- Znamensky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal Ca1 dendrites. J. Neurosci. 2003;23:2340–7. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsarnovszky A, Le HH, Wang H-S, Belcher SM. Ontogeny of rapid estrogen-mediated extracellular signal-regulated kinase signaling in the rat cerebellar cortex: Potent nongenomic agonist and endocrine disrupting activity of the Xenoestrogen bisphenol A. Endocrinology. 2005;146:5388–96. doi: 10.1210/en.2005-0565. [DOI] [PubMed] [Google Scholar]