Abstract
A hypercoagulable state is a potential mechanism linking elevated blood pressure (BP), adiposity and a sedentary lifestyle to development of coronary heart disease (CHD). We examined relationships among aerobic fitness and adiposity in 76 sedentary subjects with elevated BP. Blood levels of plasminogen activator inhibitor-1 (PAI-1), D-dimer, von Willebrand factor (vWF) and thrombomodulin were assessed as biomarkers of coagulation. In individuals with elevated BP, percent body fat and fitness were associated with biomarkers indicative of a hypercoagulable state, even after demographic and metabolic factors were considered. D-dimer was positively associated with percent body fat (beta=0.37, p=0.003). PAI-1 was higher in men than in women (beta=−0.31, p=0.015) and associated with lower VO2peak (beta=−0.35, p=0.024). Thrombomodulin was positively associated with VO2peak (beta=0.56, p< 0.01). vWF was not significantly associated with fitness or adiposity. Our results emphasise that both percent body fat and physical fitness are important in the maintenance of haemostatic balance.
Keywords: fitness, adiposity, coagulation, hypertension, blood pressure, D-dimer. PAI-1, von Willebrand factor, thrombomodulin
BACKGROUND
The continuing obesity epidemic poses high disease risks, especially given the strong association with coronary heart disease (CHD) and with hypertension - a well-established risk factor for CHD (1). The contribution of obesity and a sedentary lifestyle to CHD risk is also linked by the converse effects of increased aerobic fitness, which confers a protective benefit against the development of CHD (2) and the development of hypertension(3).
Adipose tissue is a complex endocrine organ that releases biologically active substances, including many biological factors that contribute to coagulation and the development of atherosclerosis, a precursor to clinically significant CHD. It has been suggested that increasing coagulation levels may not only be a marker of thrombosis but also a cause of atherogenesis (4). Increasing adiposity has been associated with increasing levels of the coagulation markers D-dimer, thrombomodulin, PAI-1 and vWF (5)(6)(7)(8). Each coagulation marker exhibits different actions, but the cumulative function is to maintain haemostatic balance between thrombosis and fibrinolysis.
D-dimer is a protein fragment found in the blood following degradation of a fibrin clot, hence a direct indicator of fibrinolysis and indirect marker of coagulation (9). PAI-1 is a glycoprotien synthesised by numerous cell types including vascular endothelial cells, vascular smooth muscle cells, and adipocytes. PAI-1 has antifibrinolytic properties, thereby blocking the destruction of fibrin clots (10). vWF is a glycoprotein that is involved in platelet adhesion and aggregation to sites of vascular injury (11).
Elevated levels of D-dimer, PAI-1 and vWF are therefore indicators of a hypercoagulable state and have consistently been found to be independent risk factors of cardiovascular events(12). Endothelial cell membrane-bound thrombomodulin is part of the protein C anticoagulant system which provides an inhibitory mechanism against thrombosis (13)(14). Elevated levels of soluble thrombomodulin have been linked to a reduced risk of suffering a cardiovascular event but are also a marker of endothelial injury (15) and correlate positively with plasma procoagulant activity in patients with type II diabetes (16).
Given the adipose tissue production of these coagulant factors, it has been proposed that adiposity-related alterations in coagulation are a potential mechanism linking obesity to CHD. Indeed, support is provided in a recent review article concluded that both modest and substantial weight loss can significantly reduce PAI-1 levels (17).
Exercise is an important lifestyle intervention that can influence both adiposity and, importantly for CHD risk, blood pressure (BP) and potentially might decrease coagulation even independent of weight loss (14). Prospective studies have found that higher aerobic fitness is associated with lower risk of future CHD related and all-cause mortality(2)(18). Some of the reduction in CHD risk experienced by more fit populations may be due to improvement of the haemostatic system, with fitness promoting a shift from coagulation toward anticoagulation(19)(20). For example, studies from our group have found that higher VO2peak, a measure of aerobic fitness, is independently associated with lower PAI-1(21)(22). Similarly, in women followed for approximately 4 years following a clinically indicated angiography, higher self-reported fitness scores were associated with lower risk for adverse cardiovascular events of which obesity was not independently associated (23).
Given gender differences in adiposity, it may not be surprising that there appear to be gender differences in levels of the coagulation markers; however, these associations are not straightforward. D-dimer and PAI-1 levels have been found to be lower in women than men (24), yet increasing body fat has been related to increasing PAI-1 levels in men but not in women (25). There is some indication that fitness may also be differentially related to coagulation factors across genders. Six months of exercise training produced greater decreases in PAI-1 in men than in women (26). These gender differences may be important clinically. In a study that investigated gender differences in the prognostic value of coagulability markers in the prediction of cardiac events, D-dimer had prognostic value for men but not women (27).
The relative contribution of fitness versus adiposity and potential differences in the relationship between gender and coagulation markers is not well understood, and it has not been well studied in the setting of elevated BP, an important risk factor for development of CHD (27). The purpose of this study was to examine relationships among adiposity, fitness and gender with coagulation in unmedicated individuals with elevated BP. We hypothesised that higher coagulability biomarkers would be directly related to increasing percent body fat, and that increasing aerobic fitness would be associated with decreased coagulability. It was further hypothesised that men D-dimer and PAI-1 levels would be higher in men than in women.
METHODS
Participants
Study participants included 76 otherwise healthy unmedicated men and women with pre-hypertension (>120/80 but <140/90mm/ Hg, N = 34) and stage 1 and stage 2 hypertension (>140/90 but <179/110mmHg; N = 42). Participants had a broad body mass index (BMI) range (23-38 kg/m2) (30.49[3.77] mean[SD]) and were between 25 and 60 years of age (45.40[9.74]).
Women had not been treated with hormone replacement therapy. Participant’s level of habitual physical activity was assessed using the Leisure Time Exercise Questionnaire (LTEQ) and individuals with scores above 40 were excluded. Participants were recruited from the local community through flyer postings, referrals from local doctor’s offices and e-mail list serve distribution. Criteria for exclusion included having an abnormal ECG, history of or current heart disease, recent stroke or neurological impairment, current psychiatric disease or history of major depression, diabetes, anti-inflammatory medication use, current use of prescription medication, current drug or alcohol abuse, diabetes, congestive heart failure and history of myocardial infarction. The study was approved by the University of California San Diego (UCSD) Institutional Review Board. Prior to participation all participants provided written informed consent.
Procedure
The procedures were performed at the UCSD Medical Center General Clinical Research Center. Anthropometric data were obtained through standard procedures by a trained technician or registered nurse and included measurement of height, weight and waist and hip circumference. Six measurements of BP were taken following a 15-min seated rest. Waist circumference was measured midway between the lower rib and the iliac crest while the participant was exhaling. Mean arterial pressure (MAP) was estimated by using the formula: 2/3 diastolic pressure plus 1/3 systolic pressure equals MAP. Hip circumference was obtained at the maximal gluteal protrusion. BMI was calculated as weight in kilograms divided by height in meters squared. Waist circumference was divided by hip circumference to obtain waist to hip ratio.
Adiposity including total percent body fat and percent trunk fat were determined using whole body dual energy x-ray absorptiometry (DEXA) using a Delphi W scanner (Hologic Inc., Bedford, MA) and analysed using Hologic Discovery software (version 12.0). A whole body scan was used to determine total percent body fat and percent trunk fat according to manufacturer’s guidelines in which the truncal region was defined as the region below the chin with bilateral boarders at the glenoid fossae and inferior boarder being the femoral necks converging below the pubic symphysis. Resting BP was measured using a Dinamap Compact BP monitor (Critikon, Tampa, FL) and defined as the average of six seated measures taken after having rested for 15 minutes.
Fitness testing
Cardiorespiratory fitness was determined by a VO2peak treadmill exercise test using the standard Bruce protocol in which treadmill speed and grade were increased gradually from 1.7 mph and 10% grade every 3 minutes (28). Expired gas was analysed by Sensormedics metabolic cart (equipped with Vmax software (version 6-2A). Participants were instructed not to use caffeine or alcohol, exercise vigorously or smoke for 24 h prior to fitness testing.
Biological assays
Participants returned within 7 to 14 days of the fitness test, blood samples were collected at rest between the hours of 10:00am and 12:00 p.m. Samples were kept on ice until being centrifuged and stored at −80oC. Blood collected in tubes containing CTAD (buffered citrate solution, theophylline, adenosine and dipyridamole) was used to determine PAI-1, D-dimer, and vWF levels; blood collected in tubes containing heparin was used to determine thrombomodulin levels. All analyte levels were determined by commercial enzyme-linked immunosorbent assay (ELISA) according to manufacturer’s recommendations (Asserachrom Stago, Asnières, France). Inter and Intra-assay coefficients were <10%.
Data analysis
Variables were examined for normal distribution, and extreme outliers (> 4 standard deviations) were excluded from the analyses. Independent samples t-tests were used to assess group differences between men and women on continuous outcome variables. Pearson correlations were used to assess associations between predictor and outcome variables. Separate linear regression models were developed for each coagulation marker as a dependent variable.
The following predictor variables were entered into each regression model: age, BMI, gender, mean BP (MAP), percent body fat, waist to hip ratio, VO2peak, gender by VO2 peak interaction term, and percent fat by gender interaction term. A p value < 0.05 was chosen to represent statistical significance. All data were analysed using SPSS statistical analysis software version 17.0 for Windows (SPSS, Chicago, IL).
RESULTS
Demographic and coagulation biomarkers characteristics of the participants are presented in Table 1. The 76 participants included 34 women and 42 men (mean age 45.4 [9.74] 9.74). BMI was not significantly different between men and women; yet women had significantly higher percent body fat than men (women averaging 40.7%, men 28.0%; p<.001). Men had significantly higher (weight-adjusted) VO2peak (p<.001) and triglyceride levels (p<.05) and women had higher D-Dimer levels (p=.05). There were no other significant gender differences between the coagulation markers.
Table 1.
Sample Characteristics
| Variables | Men (N=42) | Women (N=34) | P value |
|---|---|---|---|
| Age (years) | 44.74 (9.31) | 47.71 (9.88) | .183 |
| SBP (mmHg) | 142.75 (10.75 | 141.90 (13.13.47) | .761 |
| DBP (mmHg) | 86.42 (902) | 84.85 (8.38) | .371 |
| MAP(mmHg) | 105.01 (8.75) | 103.50 (9.05) | .469 |
| HDL (mg/dl) | 38.59 (6.80) | 54.39 (13.80) | <.001 |
| LDL (mg/dl) | 121.24 (29.31) | 128.97 (31.66) | .297 |
| Triglycerides (mg/dl) | 214.05 (146.55) | 138.21 (86.49) | .007 |
| D-dimer (ng/dl) | 252.12 (154.30) | 344.84 (214.13) | .046 |
| PAI-1(ng/dl) | 76.46 (45.36) | 48.92 (36.25) | .010 |
| Thrombomodulin (ng/dl) | 2.33 (.79) | 2.17 (.64) | .377 |
| vWF % | 178.61 (102.45) | 194.67 (90.04) | .512 |
| Body mass index (kg/m2) | 30.89 (3.87) | 30.05 (3.68) | .351 |
| Percent body fat (DEXA) | 28.02 (5.45) | 40.70 (4.97) | <.001 |
| Waist to hip ratio | 0.99 (0.07) | 0.88 (0.09) | <.001 |
| VO2peak (ml/kg/min) | 30.97 (7.41) | 23.02 (3.99) | <.001 |
Coagulation marker associations with fitness and adiposity
Pearson correlation coefficients among coagulation biomarkers and variables are presented in Table 2. D-dimer was positively associated with percent body fat (p<0.05) and negatively associated with VO2peak (p<0.05). PAI-1 was negatively associated with gender (p<0.05), such that men had higher levels of PAI-1 than women. Thrombomodulin was positively correlated with V02peak (p<0.05). When associations among biomarkers were analysed, PAI-1 was negatively associated with D-dimer (p < 0.05) indicating greater antifibrinolytic activity being related to less fibrin degradation.
Table 2.
Correlation Coefficients of Coagulation Biomarkers and Age, Gender, Blood Pressure, Adiposity and Fitness
| D-Dimer | PAI-1 | Thrombomodulin | vWF | |
|---|---|---|---|---|
| Gender | 0.246* | −0.320** | −0.111 | 0.083 |
| Age | 0.200 | −0.054 | 0.035 | 0.043 |
| MAP | 0.092 | 0.154 | −0.044 | 0.216 |
| Body mass index | 0.211 | −0.043 | 0.067 | 0.002 |
| Percent body fat | 0.367** | −0.211 | −0.097 | 0.033 |
| Waist/hip ratio | −0.066 | 0.080 | −0.106 | 0.030 |
| V02peak | −0.294* | −0.019 | 0.314* | −0.039 |
P < 0.05 level;
P < 0.01 level.
Regression outcomes
For each biomarker, a series of hierarchical liner regressions were performed to determine the measures of fitness and adiposity that contributed to the prediction of coagulation biomarkers (Table 3.).
Table 3.
Regression Outcomes*
| Dependent Coagulation Variables |
Predictor Variables | Model Adjusted R2 | Standardized β | P value |
|---|---|---|---|---|
| D-Dimer | Percent body fat | .121 | .367 | .003 |
| PAI-1 | Gender | .082 | −.312 | .015 |
| VO2peak | .145 | −.351 | .024 | |
| Thrombomodulin | VO2peak | .074 | .558 | <.000 |
| Gender by percent fat | .190 | .442 | .003 | |
| vWf | --- | -- | -- | -- |
Regression analysis consisted of independent variables age, body mass index, gender, mean arterial pressure, percent body fat (DEXA), waist to hip ratio, VO2peak (ml/kg/min), and interaction terms gender by fitness and gender by percent body fat.
D-Dimer
After controlling for the covariates age, BMI, gender and MAP, increasing D-dimer was significantly associated with increasing percent body fat, such that for every one unit increase in percent body fat, D-dimer increased by 9.522 ng/dl (Beta=.367, p=.003). We did not observe significant associations between VO2 max and D-dimer. The final model was: model R2=0.135, adjusted R2=0.121, F (1, 62) = 9.522, p≤0.003 (Table 3.).
PAI-1
After controlling for covariates as described above, PAI-1 levels were significantly associated with gender (Beta= −.312, p=.015) and VO-2peak (Beta=−351, p=.024). The final model was: model R2=0.174, adjusted R2=0.145, F (2, 60) = 6.096, p≤0.004 (Table 3).
Thrombomodulin
After controlling for covariates as described above, thrombomodulin levels were significantly associated with VO2peak (Beta=.558, p<.001). We also found a significant interaction between percent fat and gender for this outcome. Thrombomodulin levels were associated with percent fat (Beta=.442, p=.003) in the opposite direction for men and women with women having higher thrombomodulin as percent body fat increased and men having lower thrombomodulin levels as percent body fat increased. The final model was: model R2=0.216, adjusted R2 =0. 190, F (2, 61)=8.15, p ≤ 0.001 (Figure 1).
Figure 1.
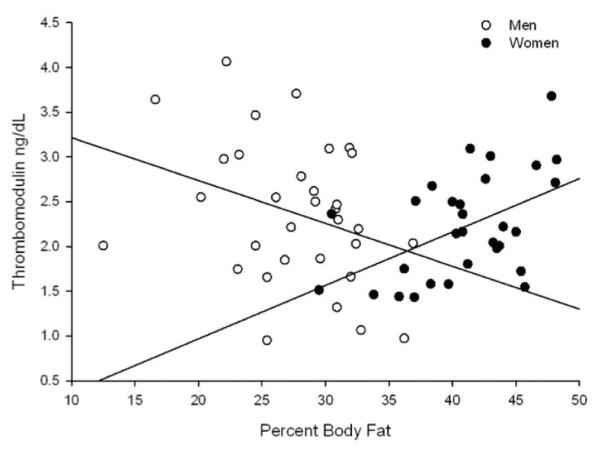
Interaction Among Percent Body Fat, Thrombomodulin and Gender.
Increased percent fat predicted higher thrombomodulin levels in women and lower thrombomodulin levels in men (beta=.442, p=.003).
vWF
There were no significant associations between vWF and variables entered into the model.
DISCUSSION
The aim of this study was to evaluate associations among aerobic fitness and adiposity and markers of coagulation in the setting of elevated BP. Results showed that women had higher D-dimer, lower PAI-1, percentage body fat and VO2peak than men. Regression analyses showed that D-dimer was predicted by percentage fat, and that PAI-1 and thrombomodulin were predicted by VO2peak. Importantly, BP was not significantly related to any of the coagulation markers examined, suggesting that risk for vascular coagulation and fibrinolysis conferred by adiposity and protection via aerobic fitness may be more significant than effects of BP, especially among individuals with mildly to moderately elevated BP.
Our findings are in line with previous reports that increasing D-dimer is related to increasing adiposity in children (29) and older adults (30), extending them to our population of otherwise healthy middle aged individuals with elevated BP. Similarly, our finding that lower PAI-1 is associated with higher aerobic fitness is also in agreement with previous findings (21), however, we did not replicate previous reports that body mass was positively correlated with PAI-1 (31), possibly because the current study has a more narrow range of BMI’s than the previously referenced study. In the current study we found no association between adiposity or fitness and vWF, which have been reported (31).
In the current study, greater aerobic fitness was associated with elevated thrombomodulin levels. Previous studies have proposed that thrombomodulin found in the plasma of healthy individuals may represent a (protective) up-regulation of thrombomodulin by endothelial cells rather than a pathological damage induced cleavage of thrombomodulin (15). Providing support for this idea is a recent study in which thrombomodulin levels were significantly higher in healthy individuals compared to congenital circulation defect patients who are known to be at increased risk for thromboembolism (32).
Plasma levels of thrombomodulin are elevated in women with polycystic ovary syndrome (PCOS) which is an important hormonal disorder in women. PCOS is characterised by increased testosterone production and obesity (33). Adipose tissue possesses aromatase, an enzyme that converts androstenedione to estrone and testosterone to estradiol.
The excess of adipose tissue in obese participants creates the paradox of having both excess androgens (which are responsible for hirsutism and virilisation) and estrogens (which inhibits FSH via negative feedback).Speculation: In women, increased body fat might increase testosterone production. Testosterone increases the expression of thrombomodulin in cultured endothelial cells (33).
Moreover, in women with PCOS who show excess production of testosterone and adiposity, thrombomodulin is elevated (34) Therefore, effects of testosterone on thrombomodulin production in women might become more apparent as the amount of body fat increases. In contrast, estrogen increases in men along with an increase in body fat and such estrogen excess can reduce testosterone levels, thereby perhaps explaining why men showed lower thrombomodulin than women with increasing percent of body fat. Although hypertension is found to be associated with reduced plasma thrombomodulin levels in animal studies (35), human studies examining a broad BP range from normotension to hypertension have not found BP to be associated with plasma thrombomodulin levels (13)(36)(37). Prior studies have found associations between increased D-dimer, PAI-1 and vWF and elevated BP (38)(39)(40), although our results showed no such relationship. One possible explanation for our findings is the relatively restricted range of BP did not provide sufficient variance to detect associations between BP and coagulation, focusing only on individuals with elevated BP. Another possible explanation is that the coagulation markers examined in the current study are influenced to a greater degree by VO2 peak and adiposity than BP.
A recent Chochrane review attempting to quantify the effects of antihypertensive drug treatment on morbidity, stroke, CHD and total cardiovascular events (CVS) in 8,912 adults with mild hypertension (systolic blood pressure (BP) 140-159 mmHg and/or diastolic BP 90-99 mmHg) found that treatment for 4 to 5 years with antihypertensive drugs compared to placebo did not reduce any of the above mentioned events (41). It has also been found that higher cardiorespiratory fitness protects against increases in BP with weight gain (42). When population attributable fractions (PAFs) associated with physical inactivity were calculated for 2008, it is estimated that 6% of the burden of disease from CHD worldwide could be eliminated by the removal of inactivity (43). In light of this evidence, efforts to increase physical activity emerge as a good choice in the effort to reduce BP.
CONCLUSIONS
These findings expand on previous research indicating that physical fitness and lower body fat protect against hypercoagulability, and remain an important risk or protective factor regardless of elevated BP. D-dimer levels are a direct marker of fibrinolysis and an indirect marker of coagulation, and are associated with increased adiposity. This, along with lower PAI-1 levels and higher thrombomodulin levels in more physically fit individuals, provides support that coagulation factors may be a mechanistic pathway through which higher aerobic fitness and lower body mass protect against CHD risk. Our results highlight the importance of lower adiposity and increased cardiorespiratory fitness on coagulation markers in individuals with prehypertension as well as established hypertension. Improvement in the haemostatic system may be just one of the many positive effects of exercise in this population, even without a significant role of BP in coagulation balance.
Acknowledgments
SUPPORT This work was supported by grants HL57265 and HL073355 from the National Institutes of Health and the UCSD General Clinical Research Center (MO1RR-00827)
Footnotes
CONFLICT OF INTEREST The authors have no conflicts of interest to report.
REFERENCES
- 1.Tsigos C, Hainer V, Basdevant A, Finer N, Fried M, Mathus-Vliegen E, et al. Management of obesity in adults: European clinical practice guidelines. Obes Facts. 2008;1:106. doi: 10.1159/000126822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blair SN, Kohl H, Paffenbarger R, Clark DG, Cooper H, Gibbons L. Physical fitness and all-cause mortality. Month. 1989:2395. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 3.Blair SN, Goodyear NN, Gibbons LW, Cooper KH. Physical fitness and incidence of hypertension in healthy normotensive men and women. JAMA: the journal of the American Medical Association. 1984;252(4):487. [PubMed] [Google Scholar]
- 4.Juhan-Vague I, Collen D. On the role of coagulation and fibrinolysis in atherosclerosis. Ann Epidemiol. 1992;2(4):427. doi: 10.1016/1047-2797(92)90092-5. [DOI] [PubMed] [Google Scholar]
- 5.Word I. Study of Soluble Thrombomodulin as an Early Marker of Endothelial Cell Injury in Obese Children. Annals of Pediatric Surgery. 2009;5(2):126. [Google Scholar]
- 6.Mills PJ, Shapiro D, Goldstein IB, Ottaviani C, Pung MA, Khandrika S, et al. Metabolic predictors of inflammation, adhesion, and coagulability in healthy younger-aged adultsObesity (Silver Spring) Obesity. 2008;16(12):2702. doi: 10.1038/oby.2008.420. [DOI] [PubMed] [Google Scholar]
- 7.Porreca E, Di Febbo C, Fusco L, Moretta V, Di Nisio M, Cuccurullo F. Soluble thrombomodulin and vascular adhesion molecule-1 are associated to leptin plasma levels in obese women. Atherosclerosis. 2004;172(1):175. doi: 10.1016/j.atherosclerosis.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Blann AD, McCollum CN. von Willebrand factor, endothelial cell damage and atherosclerosis. Eur J Vasc Surg. 1994;8(1):10. doi: 10.1016/s0950-821x(05)80112-4. [DOI] [PubMed] [Google Scholar]
- 9.Lippi G, Franchini M, Targher G, Favaloro EJ. Help me, Doctor My D-dimer is raised. Ann Med. 2008;40(8):594. doi: 10.1080/07853890802161015. [DOI] [PubMed] [Google Scholar]
- 10.Ha H, Oh EY, Lee HB. The role of plasminogen activator inhibitor 1 in renal and cardiovascular diseases. Nature Reviews Nephrology. 2009;5(4):203. doi: 10.1038/nrneph.2009.15. [DOI] [PubMed] [Google Scholar]
- 11.Smith A, Patterson C, Yarnell J, Rumley A, Ben-Shlomo Y, Lowe G. Which hemostatic markers add to the predictive value of conventional risk factors for coronary heart disease and ischemic stroke?: The Caerphilly Study. Circulation. 2005;112(20):3080. doi: 10.1161/CIRCULATIONAHA.105.557132. [DOI] [PubMed] [Google Scholar]
- 12.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355(25):2631. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 13.Salomaa V, Matei C, Aleksic N, Sansores-Garcia L, Folsom AR, Juneja H, et al. Soluble thrombomodulin as a predictor of incident coronary heart disease and symptomless carotid artery atheroscierosis in the Atherosclerosis Risk in Communities (ARIC) Study: a case-cohort study. The Lancet. 1999;353(9166):1729. doi: 10.1016/s0140-6736(98)09057-6. [DOI] [PubMed] [Google Scholar]
- 14.Edwards KM, Ziegler MG, Mills PJ. The potential anti-inflammatory benefits of improving physical fitness in hypertension. J Hypertens. 2007;25(8):1533. doi: 10.1097/HJH.0b013e328165ca67. [DOI] [PubMed] [Google Scholar]
- 15.Wu KK. Soluble thrombomodulin and coronary heart disease. Curr Opin Lipidol. 2003;14(4):373. doi: 10.1097/00041433-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Aso Y, Fujiwara Y, Tayama K, Inukau T, Takemura Y. Relationship between soluble thrombomodulin in plasma and coagulation of fibrinolysis in type 2 diabetes. Clin Chim Acta. 2000 Nov;301(1-2):135–136. doi: 10.1016/s0009-8981(00)00335-1. 2000. [DOI] [PubMed] [Google Scholar]
- 17.Mertens I, Van Gaal L. Obesity, haemostasis and the fibrinolytic system. Obesity reviews. 2002;3(2):85. doi: 10.1046/j.1467-789x.2002.00056.x. [DOI] [PubMed] [Google Scholar]
- 18.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JAE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351(26):2694. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson ET, Davy KP, Seals DR. Hemostatic, metabolic, and androgenic risk factors for coronary heart disease in physically active and less active postmenopausal women. Arterioscler Thromb Vasc Biol. 1995;15(5):669. doi: 10.1161/01.atv.15.5.669. [DOI] [PubMed] [Google Scholar]
- 20.Bassuk SS, Manson JAE. Physical activity and the prevention of cardiovascular disease. Curr Atheroscler Rep. 2003;5(4):299. doi: 10.1007/s11883-003-0053-7. [DOI] [PubMed] [Google Scholar]
- 21.Von Känel R, Hong S, Pung MA, Mills PJ. Association of blood pressure and fitness with levels of atherosclerotic risk markers pre-exercise and post-exercise. American journal of hypertension. 2007;20(6):670. doi: 10.1016/j.amjhyper.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Hong S, Adler KA, Von Känel R, Nordberg J, Ziegler MG, Mills PJ. Prolonged platelet activation in individuals with elevated blood pressure in response to a moderate exercise challenge. Psychophysiology Psychophysiology. 2009;46(2):276. doi: 10.1111/j.1469-8986.2008.00779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wessel TR, Arant CB, Olson MB, Johnson BD, Reis SE, Sharaf BL, et al. Relationship of physical fitness vs body mass index with coronary artery disease and cardiovascular events in women. JAMA. 2004;292(10):1179. doi: 10.1001/jama.292.10.1179. [DOI] [PubMed] [Google Scholar]
- 24.Rossouw JE. Hormones, genetic factors, and gender differences in cardiovascular disease. Cardiovasc Res. 2002;53(3):550. doi: 10.1016/s0008-6363(01)00478-3. [DOI] [PubMed] [Google Scholar]
- 25.Kockx M, Leenen R, Seidell J, Princen H, Kooistra T. Relationship between visceral fat and PAI-1 in overweight men and women before and after weight loss. Thromb Haemost. 1999;82(5):1490. [PubMed] [Google Scholar]
- 26.Kulaputana O, Macko RF, Ghiu I, Phares DA, Goldberg AP, Hagberg JM. Human gender differences in fibrinolytic responses to exercise training and their determinants. Exp Physiol. 2005;90(6):881. doi: 10.1113/expphysiol.2005.030718. [DOI] [PubMed] [Google Scholar]
- 27.Kalaria VG, Zareba W, Moss AJ, Pancio G, Marder VJ, Morrissey JH, et al. Gender-related differences in thrombogenic factors predicting recurrent cardiac events in patients after acute myocardial infarction* 1. Am J Cardiol. 2000;85(12):1401. doi: 10.1016/s0002-9149(00)00785-2. [DOI] [PubMed] [Google Scholar]
- 28.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92. [PubMed] [Google Scholar]
- 29.Ferguson MA, Gutin B, Owens S, Litaker M, Tracy RP, Allison J. Fat distribution and hemostatic measures in obese children. Am J Clin Nutr. 1998;67(6):1136. doi: 10.1093/ajcn/67.6.1136. [DOI] [PubMed] [Google Scholar]
- 30.Tita-Nwa F, Bos A, Adjei A, Ershler WB, Longo DL, Ferrucci L. Correlates of D-dimer in older persons. Aging Clin Exp Res. 2010 Feb;22(1):20. doi: 10.1007/bf03324810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosito GA, D’Agostino RB, Massaro J, Lipinska I, Mittleman MA, Sutherland P, et al. Association between obesity and a prothrombotic state: the Framingham Offspring Study. Thromb Haemost. 2004 Apr;91(4):683. doi: 10.1160/th03-01-0014. [DOI] [PubMed] [Google Scholar]
- 32.Kajimoto H, Nakazawa M, Murasaki K, Hagiwara N, Nakanishi T. Increased P-selectin expression on platelets and decreased plasma thrombomodulin in Fontan patients. Circ J. 2009 Sep;73(9):1705. doi: 10.1253/circj.cj-08-1087. [DOI] [PubMed] [Google Scholar]
- 33.XU X, LI X, ZHENG Y. Effect of testosterone on the synthesis of thrombomodulin in cultured vascular endothelial cells [J] Editorial Department of Chinese Journal of Geriatric Cardiovascular and Cerebrovascular Disease. 2004;5 [Google Scholar]
- 34.Oral B, Mermi B, Dilek M, Alanoglu G, Sutcu R. Thrombin activatable fibrinolysis inhibitor and other hemostatic parameters in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2009;25(2):110. doi: 10.1080/09513590802549874. [DOI] [PubMed] [Google Scholar]
- 35.Sawada K, Naiki M, Yago H, Matsushita K, Ohtsuki T, Kitagawa K, et al. Hypertension associated with reduced plasma thrombomodulin levels and a hypercoagulable state in rats. Clin Exp Hypertens. 2003;25(2):73. doi: 10.1081/ceh-120017928. [DOI] [PubMed] [Google Scholar]
- 36.Naruse M, Kawana M, Hifumi S, Naruse K, Yoshihara I, Oka T, et al. Plasma immunoreactive endothelin, but not thrombomodulin, is increased in patients with essential hypertension and ischemic heart disease. J Cardiovasc Pharmacol. 1991;17:S471. doi: 10.1097/00005344-199100177-00135. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson T, Hellsten G, Amiral J. Plasma thrombomodulin concentrations in relation to cardiovascular risk factors in a population sample. Blood Coagulation Fibrinol. 1993;4(3):455. doi: 10.1097/00001721-199306000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Landin K, Tengborn L, Smith U. Elevated fibrinogen and plasminogen activator inhibitor (PAI-1) in hypertension are related to metabolic risk factors for cardiovascular disease. J Intern Med. 1990;227(4):273. doi: 10.1111/j.1365-2796.1990.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 39.von Känel R, Le DT, Nelesen RA, Mills PJ, Ancoli-Israel S, Dimsdale JE. The hypercoagulable state in sleep apnea is related to comorbid hypertension. J Hypertens. 2001;19(8):1445. doi: 10.1097/00004872-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Pieper CF, Rao KMK, Currie MS, Harris TB, Cohen HJ. Age, functional status, and racial differences in plasma D-dimer levels in community-dwelling elderly persons. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2000;55(11):M649. doi: 10.1093/gerona/55.11.m649. [DOI] [PubMed] [Google Scholar]
- 41.Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. status and date: New, published in 2012(8) [DOI] [PMC free article] [PubMed]
- 42.Gentile CL, Orr JS, Davy BM, Davy KP. Cardiorespiratory fitness influences the blood pressure response to experimental weight gain. Obesity. 2012;15(12):3005. doi: 10.1038/oby.2007.358. [DOI] [PubMed] [Google Scholar]
- 43.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. The Lancet. 2012 doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


