Abstract
Antiproliferative bioassay-guided fractionation of the ethanol extract of the endemic Malagasy Rubiaceous plant Tarenna grevei led to the isolation of two new antiproliferative oxygenated oleanane triterpene saponins. The structures of the two active compounds were elucidated as 23-hydroxylongispinogenin 3-O-βD-glucopyranoside (1) and longispinogenin 3-O-β-D-glucopyranosyl (1→2)-β-D-glucopyranoside (3) by analyses of their spectral data including 1D- and 2D-NMR spectroscopy and chemical evidence. Compounds 1 and 3 displayed modest antiproliferative activity against the A2780 ovarian cancer cell line with IC50 values of 7.6 and 4 μM, respectively.
Keywords: Tarenna, Rubiaceae, Oxygenated oleanane saponin, Antiproliferative, A2780
As part of our ongoing search for potential anticancer drugs from Madagascan plants in the framework of the International Cooperative Biodiversity Group (ICBG) program, an ethanol extract of leaves and fruit of Tarenna grevei (Drake) Homolle (Rubiaceae) exhibited weak antiproliferative activity (IC50 21 μg/mL) against the A2780 ovarian cancer cell line, and this extract was selected for investigation. The genus Tarenna Gaertn. contains about 180 species distributed in the old world tropics [2]. The presence of iridoid glycosides, lignans, chalcones, sesquiterpenes, cycloartane glycosides, indole alkaloids and other phenolic compounds have been reported to be present in some species of this genus [3 11]. In Madagascar, the powdered bark and the heartwood of Tarenna madagascariensis (Baill.) Homolle, locally called “masonjoany”, has been used as a sunscreen.
Liquid-liquid partition of the ethanol extract of Tarenna grevei using hexanes, ethyl acetate and water gave an active ethyl acetate fraction (IC50 7.9 μg/mL). Successive Sephadex LH-20, octadecylsilyl silica gel and silica-gel column chromatographies afforded two bioactive oxygenated oleanane saponins (1 and 3). The isolation, the structure elucidation and the antiproliferative properties of 1 and 3 are reported herein.
Compound 1 had the molecular formula C36H60O9 as determined by high resolution ESIMS (m/z 659.4098 [M+Na+], calculated for C36H60O9Na, 659.4135). The 1H-NMR spectroscopic data of 1 (Table 1) displayed resonances of the β-anomeric proton of a hexopyranosyl ring at δ 4.39 ppm (d, J = 7.9 Hz), signals for six quaternary methyl groups at δ 0.72, 0.90, 0.93, 1.01, 1.03 and 1.25 (each a singlet), two oxymethylene groups (δ 3.30, J = 11.7 Hz, 1H; δ 3.65, J = 11.7 Hz, 1H; and δ 3.82, J = 12 Hz, 2H) and an olefinic proton at δ 5.24 (brt, J = 2.9 Hz). Inspection of the 13C-NMR revealed the presence of a set of signals ascribable to a β-glucopyranosyl unit (δ 105.8, 75.6, 78.3, 71.5, 77.7 and 62.7) [12], together with 30 carbon resonances (six methyls, eleven methylenes, two of which are oxygenated; six methines, one of which is olefinic, and seven quaternary carbons as observed in the DEPT 135 spectrum, Table 2).
Table 1.
1H-NMR data for compounds 1 and 3 (600 MHz, MeOH)
| Position | 1 | 3 |
|---|---|---|
| 1 | 1.63 m, 0.99 m | 1.63 m, 1.03 m |
| 2 | 1.75 m | 1.44 m, 2.13 m |
| 3 | 3.64 dd (11.7, 4.9) | 3.22 dd (11.5, 5) |
| 5 | 1.62 m | 0.81 brd (12.6) |
| 6 | 1.43 m, 1.54 m | 1.62 m |
| 7 | 1.43 m | 1.43 m |
| 9 | 1.26 overlapped | 1.59 overlapped |
| 11 | 1.92 m | 1.92 m |
| 12 | 5.24 br t (2.9) | 5.26 t (3.4) |
| 15 | 1.33 overlapped, 1.82 t (12.0) | 1.38 overlapped, 1.82 t (12.0) |
| 16 | 4.26 dd (12.0, 4.7) | 4.28 dd (12.0, 4.7) |
| 18 | 2.21 dd (13.8, 4.4) | 2.23 dd (13.8, 4.4) |
| 19 | 1.71 t (13.8), 1.06 m | 1.73 t (13.4), 1.08 m |
| 21 | 1.35 m, 1.73 m | 1.38 m, 1.62 m |
| 22 | 1.43 m, 2.13 m | 1.44 m, 2.13 m |
| 23 | 3.30 d (11.7), 3.65 d (11.7) | 1.11 s |
| 24 | 0.72 s | 0.89 s |
| 25 | 1.01 s | 1.00 s |
| 26 | 1.03 s | 1.06 s |
| 27 | 1.25 s | 1.26 s |
| 28 | 3.82 (12) | 3.86 (11.8) |
| 29 | 0.90 s | 0.90 s |
| 30 | 0.93 s | 0.92 s |
| 3-O-Glc | ||
| 1′ | 4.39 d (7.9) | 4.46 d (7.6) |
| 2′ | 3.17 t (7.9) | 3.58 t (7.6) |
| 3′ | 3.34 m | 3.38 t (7.6) |
| 4′ | 3.28 overlapped | 3.24 t (7.6) |
| 5′ | 3.27 overlapped | 3.29 overlapped |
| 6′ | 3.84 dd (12, 2.9) | 3.86 dd (12, 3.0) |
| 3.66 dd (12, 5.0) | 3.65 dd (12, 5.0) | |
| 2′-O-Glc | ||
| 1″ | 4.69 d (7.7) | |
| 2″ | 3.24 t (7.7) | |
| 3″ | 3.57 t (7.7) | |
| 4″ | 3.29 overlapped | |
| 5″ | 3.29 overlapped | |
| 6″ | 3.84 dd (12, 2.9) | |
| 3.66 dd (12, 5.0) |
Table 2.
13C-NMR data for compounds 1–4 (150 MHz)
| Position | 1a | 2b | 3a | 4b | DEPT |
|---|---|---|---|---|---|
| 1 | 39.6 | 38.6 | 40.4 | 39.5 | CH2 |
| 2 | 26.2 | 25.5 | 26.4 | 26.3 | CH2 |
| 3 | 83.3 | 82.3 | 91.4 | 89.0 | CH |
| 4 | 43.8 | 43.0 | 39.9 | 38.9 | C |
| 5 | 48.0 | 47.4 | 56.9 | 55.9 | CH |
| 6 | 18.7 | 17.9 | 19.3 | 18.4 | CH2 |
| 7 | 34.7 | 32.4 | 34.7 | 33.3 | CH2 |
| 8 | 41.3 | 40.0 | 41.1 | 40.1 | C |
| 9 | 48.1 | 46.9 | 48.2 | 47.2 | CH |
| 10 | 37.5 | 36.5 | 37.7 | 36.7 | C |
| 11 | 24.6 | 23.6 | 24.6 | 24.1 | CH2 |
| 12 | 123.8 | 122.3 | 123.9 | 122.6 | CH |
| 13 | 144.3 | 143.6 | 144.2 | 143.8 | C |
| 14 | 44.7 | 43.7 | 44.6 | 43.9 | C |
| 15 | 36.5 | 36.3 | 36.6 | 36.6 | CH2 |
| 16 | 67.6 | 66.7 | 67.7 | 66.9 | CH |
| 17 | 41.6 | 40.6 | 41.6 | 39.5 | C |
| 18 | 44.9 | 44.4 | 45.0 | 44.6 | CH |
| 19 | 47.7 | 46.9 | 47.8 | 47.2 | CH2 |
| 20 | 31.7 | 30.7 | 31.7 | 30.9 | C |
| 21 | 33.2 | 33.9 | 33.8 | 33.5 | CH2 |
| 22 | 25.9 | 25.9 | 26.0 | 26.3 | CH2 |
| 23 | 64.7 | 64.7 | 28.4 | 28.1 | CH2/CH3 |
| 24 | 13.4 | 13.1 | 16.8 | 16.5 | CH3 |
| 25 | 16.5 | 16.0 | 15.9 | 15.6 | CH3 |
| 26 | 17.3 | 16.8 | 17.4 | 16.9 | CH3 |
| 27 | 27.4 | 26.9 | 27.6 | 27.0 | CH3 |
| 28 | 68.9 | 68.9 | 68.8 | 69.8 | CH2 |
| 29 | 33.0 | 33.0 | 33.6 | 33.0 | CH3 |
| 30 | 23.9 | 23.9 | 24.2 | 23.0 | CH3 |
| 3-O-Glc/Gal | |||||
| 1′ | 105.8 | 105.7 | 105.4 | 105.4 | CH |
| 2′ | 75.6 | 72.8 | 81.1 | 81.1 | CH |
| 3′ | 78.3 | 74.9 | 77.7 | 71.7 | CH |
| 4′ | 71.5 | 69.7 | 71.9 | 69.8 | CH |
| 5′ | 77.7 | 76.0 | 77.9 | 76.4 | CH |
| 6′ | 62.7 | 61.9 | 63.1 | 62.0 | CH2 |
| 2′-O-Glc | |||||
| 1″ | 104.5 | 105.0 | CH | ||
| 2″ | 76.3 | 75.1 | CH | ||
| 3″ | 78.5 | 77.8 | CH | ||
| 4″ | 71.5 | 71.7 | CH | ||
| 5″ | 78.3 | 77.5 | CH | ||
| 6″ | 62.7 | 62.7 | CH2 |
The 1H- and 13C-NMR spectroscopic data coupled with the molecular formula suggested that 1 is an oleanane-type triterpene bearing one sugar unit at C-3 with two oxygenated methyl groups. The allocations of the double bond to C-12/C-13, the oxygenated methyl groups to C-23 and C-28, the sugar unit to C-3 and the oxygen-bearing methine to C-16 were determined as follows. The clear long-range HMBC correlation observed from the anomeric proton (δ 4.39) of the sugar unit to C-3 (δ 83.3) demonstrated the attachment of the sugar moiety to be at C-3. In addition, the presence of the trans-decalin ring system in 1 was substantiated by the presence of the proton-proton spin systems from H-3/H-2 and H-2/H-1; and H-5/H-6 and H-6/H-7; and the 3J HMBC cross-peaks from H-3 (δ3.64, dd, J= 11.7, 4.9 Hz, axial proton) to C-1, and C-5; from H-7 (δ1.43, m) to C-5 and C-9; and the NOESY correlations between H-5 and CH3-24, and the oxymethylene CH2-23 and the methyl group at C-10. Furthermore, the methyl groups at C-10 and C-4 were assigned by the observation of the 2J and 3J HMBC correlations from CH3-25 (δ 1.01, s) to C-1, C-9 and C-10 and from CH3-24 (δ 0.72, s) to C-3, C-4 and C-5. The double bond was elucidated to be at C-12/C-13 by the long range HMBC cross peaks observed from H-12 (δ 5.24, br t, J = 2.9) and C-9, C-14 and C-18 (Figure 1). Moreover, the attachments of the two hydroxymethylene groups to be at C-4 and C-17, the methyl groups at C-4, C-8, C-14 and C-20 were deduced from careful analysis of the HMBC spectral data. Inspection of the 13C-NMR spectral data combined with the molecular formula obtained by ESIMS demonstrated that the 1 must have an oxygen-bearing methine in its oleanane-type aglycone. In order to confirm the assignment of the sugar moiety and to locate the oxygen-bearing methine, a 2D-H2BC experiment was carried out. Correlations from H-1′ to C-2′, H-2′ to C-3′, H-3′ to C-4′, H-4′ to C-5′ and H-5′ to C-6′ of the glucopyranosyl unit, as well as from H-16 to C-15 (and vice versa) were clearly observed in the H2BC spectrum (Figure 1).
Figure 1.

Important HMBC correlations observed in 1.
Comparison of the spectroscopic data of 1 with those of similar compounds reported in the literature demonstrated that the 13C-NMR data of 1 are superposable with those of 23-hydroxylongispinogenin 3-O-β-D-galactopyranoside (2) (Table 2), previously isolated from Chorchorus acutangulus [13]. The only differences arise from the signal of the carbons of the sugar unit. For compound 1, the carbon chemical shifts of the β-glucopyranosyl ring (δ 105.8, 75.6, 78.3, 71.5, 77.7 and 62.7) are observed instead of those for the β-galactopyranosyl ring of 2 at δ 105.7, 72.8, 74.9, 69.7, 76.0 and 61.9. The absolute configuration of glucose was established as D by comparison of the optical rotation of 1a ([α]D: +61 (c 0.1, H2O) after allowing to stand overnight at room temperature with that of authentic D-glucose ([α]D: +65 (c 0.18, H2O) under the same conditions. The structure of 1 was thus concluded to be 23-hydroxylongispinogenin 3-O-β-D-glucopyranoside.
The molecular formula of 3 was determined to be C42H70O13 by high resolution ESIMS (m/z 805.4741 [M+Na], calculated for C42H70O13Na, 805.4816). The 1H- and 13C-NMR spectral data of 3 were very similar with those of 1 except for the absence of the oxymethylene group at C-4 and the presence of an additional sugar unit in the molecule. In the 1H-NMR spectrum of 3, two anomeric proton signals of two β-glucopyranosyl units at δ 4.46 ppm (d, J = 7.6 Hz, H-1′) and δ4.69 (d, J = 7.6 Hz, H-1″), and seven methyl signals (δ0.89, 0.90, 0.92, 1.00, 1.06, 1.11, 1.26, each a singlet) were observed instead of one anomeric proton and six methyl signals in 1. The assignment of the functionalities present in 3 was achieved by the interpretation of the HMBC spectral data. The second sugar present in 3 was assigned to be attached at C-2′ due to the observation of 3J HMBC correlations from the anomeric H-1″ (δ 4.69) proton to C-2′ (δ 81.1) and C-5″ (δ 78.3) and from H-2′ to C-1″ (δ 104.5) and C-4′ (δ 71.9). The 13C spectroscopic data of the triterpenoid skeleton of 3 were very similar to those of chorchorsin D2 (4), a compound present in Chorchorus acutangulus [14] (Table 2). The D-configurations of the glucoses in 3 were confirmed by comparison of the TLC of the thiazolidine derivative of the sugars obtained from acid hydrolysis of 3 with those of the corresponding derivatives of D- and L-glucose [15]. Thus the structure of compound 3 was deduced to be longispinogenin 3-O-β-D-glucopyranosyl-(1→2)-β-D-glucopyranoside.
Although cycloartane saponins have been isolated from one species of the genus Tarenna [34], this is the first report on the presence of oleanane-type saponins in the genus. Compounds 1 and 3 showed moderate antiproliferative activity (IC50 values of 7.6 and 4 μM, respectively) against the A2780 ovarian cancer cell line.
Experimental
General experimental procedures
Optical rotations were recorded on a JASCO P-2000 polarimeter. IR and UV spectra were measured on MIDAC M-series FTIR and Shimadzu UV-1201 spectrophotometers, respectively. 1H and 13C NMR spectra were recorded on a Bruker 600 spectrometer in CD3OD with TMS as internal standard. Mass spectra were obtained on a Finnigan LTQ LC/MS. Silica-gel and silica gel C-18, both 40–63 μm, were used for open column chromatography.
Antiproliferative Bioassay
The A2780 ovarian cancer cell line assay was performed at Virginia Polytechnic Institute and State University as previously reported [16]. The A2780 cell line is a drug-sensitive ovarian cancer cell line [17].
Plant material
Leaves and fruits of Tarenna grevei (Drake) Homolle were collected in the dry forests of Orangéa, Diana region of the Antsiranana province, Madagascar, on June 11, 2005 at 12°16′43″S 049°23′19″E. The plant was a shrub 3 meters tall, with a diameter at breast height of 4 cm and yellow fruit growing on rocky terrain. Voucher specimens with collection number Razafitsalama & al. 698 have been deposited in herbaria at the Parc Botanique and Zoologique de Tsimbazaza (TAN), at the Centre National d’Application des Recherches Pharmaceutiques in Antananarivo, Madagascar (CNARP), at the Missouri Botanical Garden in St. Louis, Missouri (MO), and at the Muséum National d’Histoire Naturelle in Paris, France (P).
Extraction and Isolation
Dried leaf and fruit of T. grevei (250 g) were ground in a hammer mill, then extracted with EtOH by percolation for 24 hours at room temperature to give the crude extract MG 3249 (26.8 g), of which 9.59 g was available at Virginia Tech) for investigation. Two grams of MG 3249 were dissolved in MeOH and extracted with hexanes (3 × 300 mL) to afford 212 mg of the hexanes soluble fraction. The MeOH layer was then evaporated, suspended in H2O (400 mL) and extracted with EtOAc (3 × 300 mL) to yield 390 mg of EtOAc soluble fraction. The EtOAc fraction was found to be active in the A2780 antiproliferative assay (IC50 7.9 μg/mL) and was divided into six fractions by size exclusion column chromatography by using Sephadex LH-20. Fractions 3 (Fr. 3, 66 mg) and 4 (Fr. 4, 130.1 mg) were active (IC50 8.4 and 6.8 μg/mL, respectively). Reversed-phase C-18 column chromatography of fraction Fr. 4 using 70% MeOH and 100% MeOH as solvent system afforded five fractions. The most active fraction (Fr. 2, 60.9 mg) was subjected to a silica gel column chromatography (solvent system CHCl3:MeOH; 8:2) to give compounds 1 (5.3 mg) and 3 (2.5 mg).
23-Hydroxylongispinogenin 3-O-β-D-glucopyranoside
[α]D: +27 (c 0.2, MeOH).
1H NMR (600 MHz, CD3OD) and 13C NMR (150 MHz, CD3OD): See Tables 1 and 2.
ESIMS: m/z [M+Na+] calcd for C36H60O9: 659.4135; found 659.4098.
Longispinogenin 3-O-β-D-glucopyranosyl (1→2)-β-D-glucopyranoside
[α]D: +16 (c 0.12, MeOH).
1H NMR (600 MHz, CD3OD) and 13C NMR (150 MHz, CD3OD): See Tables 1 and 2.
ESIMS: m/z [M+Na+] calcd for C42H70O13: 805.4816; found 805.4741.
Acid hydrolysis of 1
Five milliliters of HCl (1N) were added to 2 mg of compound 1 and refluxed for 2 h. The reaction mixture was evaporated and dissolved in 10 mL H2O before neutralization with Amberlite IRA 400 (Cl). The sugar fraction was purified on a C-18 SPE column eluted with H2O to afford the carbohydrate component of 1, which was identified as D-glucose by measuring its optical rotation after allowing it to stand overnight at room temperature: [α]D: +61 (c 0.1, H2O).
Acid hydrolysis of 3
Compound 3 (1 mg) was dissolved in 1N HCl (5 mL) and the solution refluxed for 2 h. The reaction mixture was evaporated and dissolved in 7 mL H2O before neutralization with Amberlite IRA 400 (Cl). The sugar fraction was purified on a C-18 SPE column eluted with H2O.
Preparation of thiazolidine derivatives
A pyridine solution (1 mL) of the sugar fraction from the previous experiment and L-cysteine methyl ester hydrochloride (3 mg) were mixed and heated at 60 °C for 1 h as described [15] After removal of the solvent by evaporation, the residue was dissolved in water 2 ml and extracted with n-BuOH (2 ml). The organic layer after evaporation was shown to contain methyl 2-(D-gluco-pentahydroxypentyl)-thiazolidine-4(R)-carboxylate Rf: 0.42, 0.5 (C-2 epimers of thiazolidine) by TLC on silica gel, solvent system: CH2Cl2–MeOH–H2O (25 : 10 : 1.5) [18].
Figure 2.

Structures of compounds 1–2.
Figure 3.
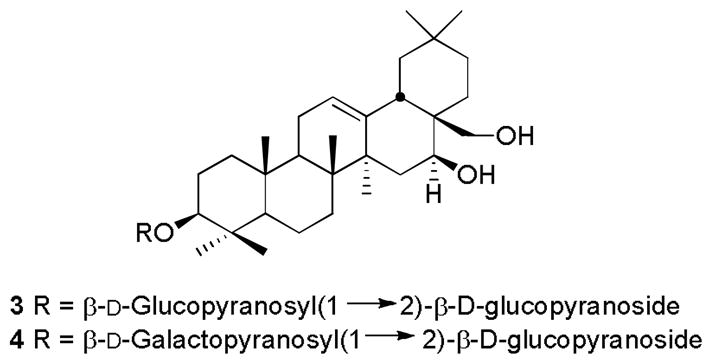
Structures of compounds 3–4.
Acknowledgments
This International Cooperative Biodiversity Group project was supported by the Fogarty International Center, the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart Lung and Blood Institute, the National Center for Complementary and Alternative Medicine, the Office of Dietary Supplements, the National Institute of General Medical Sciences, the Biological Sciences Directorate of the National Science Foundation, and the Office of Biological and Environmental Research of the U.S. Department of Energy under Cooperative Agreement U01 TW00313 with the International Cooperative Biodiversity Groups. This project was also supported by the National Research Initiative of the Cooperative State Research, Education and Extension Service, USDA, Grant #2008-35621-04732. These supports are gratefully acknowledged. Work at Virginia Tech was supported by the National Science Foundation under Grant CHE-0619382 for the purchase of the Bruker Avance 600 NMR spectrometer and Grant CHE-0722638 for the purchase of the Agilent 6220 mass spectrometer. We thank Mr. B. Bebout for obtaining the mass spectra. Fieldwork essential for this project was conducted under a collaborative agreement between the Missouri Botanical Garden and the Parc Botanique et Zoologique de Tsimbazaza and a multilateral agreement between the ICBG partners, including the Centre National d’Application des Recherches Pharmaceutiques. We gratefully acknowledge courtesies extended by the Government of Madagascar (Ministère des Eaux et Forêts). We thank R. Randrianaivo, A. Rakotondrafara, S. Rakotonandrasana, A. S. Manasse, A. Razanakolona, and V. Benjara for assistance with the plant collection.
References
- 1.Biodiversity conservation and drug discovery in Madagascar, Part 51. For Part 50, see Dai Y, Harinantenaina L, Brodie PJ, Callmander MW, Randrianaivo R, Rakotonandrasana S, Rakotobe E, Rasamison VE, Shen Y, TenDyke K, Suh EM, Kingston DGI. Antiproliferative acetogenins from a Uvaria sp. from the Madagascar dry forest. Journal of Natural Products. 2012 doi: 10.1021/np200697j. in press.
- 2.Mabberley DJ. The Plant-Book. A Portable Dictionary of the Higher Plants. Cambridge, England: Cambridge University Press; 1997. [Google Scholar]
- 3.Zhao Z, Matsunami K, Otsuka H, Shinzato T, Takeda Y. Tareciliosides H-M: Further cycloartane glycosides from leaves of Tarenna gracilipes. Chemical and Pharmaceutical Bulletin. 2011;59:902–905. doi: 10.1248/cpb.59.902. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Z, Matsunami K, Otsuka H, Shinzato T, Takeda Y. Tareciliosides A-G: Cycloartane glycosides from leaves of Tarenna gracilipes. Chemical and Pharmaceutical Bulletin. 2008;56:1153–1158. doi: 10.1248/cpb.56.1153. [DOI] [PubMed] [Google Scholar]
- 5.Yang X-W, Zhao P-J, Ma Y-L, Xiao H-T, Zuo Y-Q, He H-P, Li L, Hao X-J. Mixed lignan-neolignans from Tarenna attenuata. Journal of Natural Products. 2007;70:521–525. doi: 10.1021/np0603931. [DOI] [PubMed] [Google Scholar]
- 6.Yang X-W, He H-P, Du Z-Z, Liu H-Y, Y-TD, Ma Y-L, Wang F, Lin H, Zuo Y-Q, Li L, Hao X-J. Tarennanosides A–H, eight new lignan glucosides from Tarenna attenuata and their protective effect on H2O2-induced impairment in PC12 cells. Chemistry and Biodiversity. 2009;6:540–550. doi: 10.1002/cbdv.200800022. [DOI] [PubMed] [Google Scholar]
- 7.Yang X-W, Wang J-S, Wang Y-H, Xiao H-T, Hu X-J, Mu S-Z, Ma Y-L, Lin H, He H-P, Li L, Hao X-J. Tarennane and tarennone, two novel chalcone constituents from Tarenna attenuata. Planta Medica. 2007;73:496–498. doi: 10.1055/s-2007-967165. [DOI] [PubMed] [Google Scholar]
- 8.Yang X-W, Ma Y-L, He H-P, Wang Y-H, Di Y-T, Zhou H, Li L, Hao X-J. Iridoid constituents of Tarenna attenuata. Journal of Natural Products. 2006;69:971–974. doi: 10.1021/np0600301. [DOI] [PubMed] [Google Scholar]
- 9.Salmoun M, Braekman JC, Ranarivelo Y, Rasamoelisendra R, Ralambomanana D, Dewelle J, Darro F, Kiss R. New calamenene sesquiterpenes from Tarenna madagascariensis. Natural Product Research. 2007;21:111–120. doi: 10.1080/14786410600899084. [DOI] [PubMed] [Google Scholar]
- 10.Djoudi R, Bertrand C, Fiasson K, Fiasson J-L, Comte G, Fenet B, Rabesa Z-A. Polyphenolics and iridoid glycosides from Tarenna madagascariensis. Biochemical Systematics and Ecology. 2007;35:315–316. [Google Scholar]
- 11.Boissier JR, Combes G, Efler AH, Klinga K, Schlittler E. Identity of tarennine with dihydroelaeocarpidine. Cellular and Molecular Life Sciences. 1971;27:677. doi: 10.1007/BF02136956. [DOI] [PubMed] [Google Scholar]
- 12.Harinantenaina L, Kasai R, Yamasaki K. Cussosaponins A-E, triterpene saponins from the leaves of Cussonia racemosa, a Malagasy endemic plant. Chemical and Pharmaceutical Bulletin. 2002;50:1290–1293. doi: 10.1248/cpb.50.1290. [DOI] [PubMed] [Google Scholar]
- 13.Mahato SB, Pal BC. Triterpenoid glycosides of Corchorus acutangulus Lam. Journal of the Chemical Society, Perkin Transactions. 1987;1:629–634. [Google Scholar]
- 14.Mahato SB, Pal BC, Sarkar SK. New triterpenoid saponins from Corchorus acutangulus. Phytochemistry. 1988;27:1433–1437. [Google Scholar]
- 15.Hara S, Okabe H, Mihashi K. Gas-liquid chromatographic separation of aldose enantiomers as trimethylsilyl ethers of methyl 2-(polyhydroxyalkyl)-thiazolidine-4(R)-carboxylates) Chemical and Pharmaceutical Bulletin. 1987;35:501–506. [Google Scholar]
- 16.Cao S, Brodie PJ, Miller JS, Randrianaivo R, Ratovoson F, Birkinshaw C, Andriantsiferana R, Rasamison VE, Kingston DGI. Antiproliferative xanthones of Terminalia calcicola from the Madagascar rain forest. Journal of Natural Products. 2007;70:679–681. doi: 10.1021/np060627g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louie KG, Behrens BC, Kinsella TJ, Hamilton TC, Grotzinger KR, McKoy WM, Winker MA, Ozols RF. Radiation survival parameters of antineoplastic drug-sensitive and -resistant human ovarian cancer cell lines and their modification by buthionine sulfoximine. Cancer Research. 1985;45:2110–2115. [PubMed] [Google Scholar]
- 18.Harinantenaina L, Kasai R, Yamasaki K. A new ent-kaurane diterpenoid glycoside from the leaves of Cussonia bojeri, a Malagasy endemic plant. Chemical and Pharmaceutical Bulletin. 2002;50:1122–1123. doi: 10.1248/cpb.50.1122. [DOI] [PubMed] [Google Scholar]


