Abstract
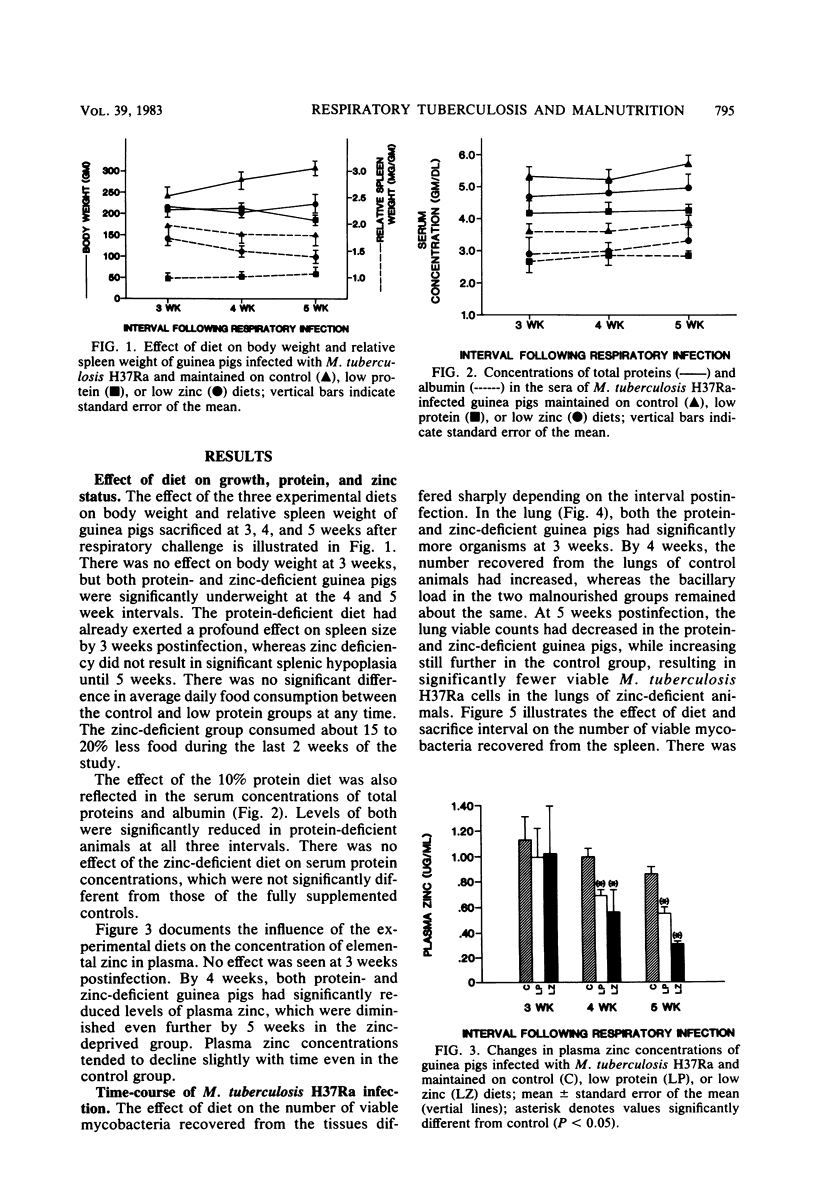
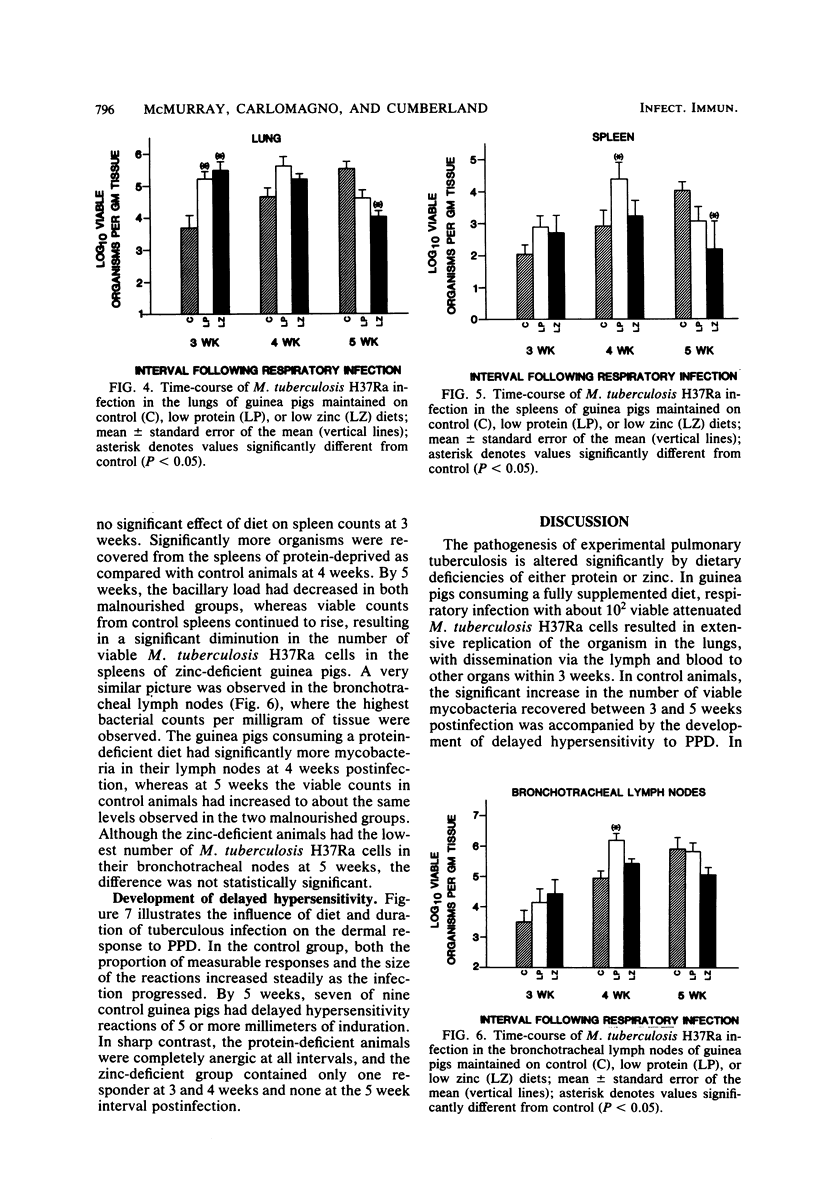
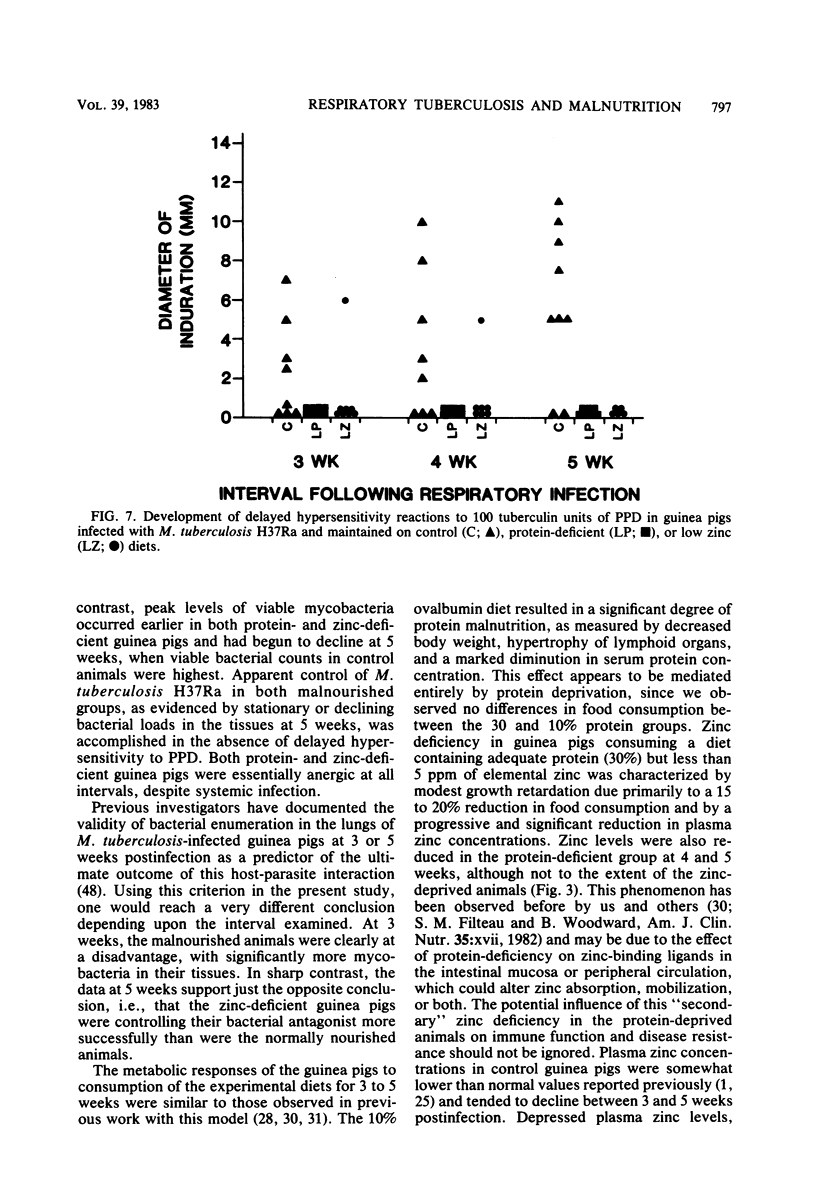
Specific pathogen-free guinea pigs were infected via the respiratory route with viable, attenuated Mycobacterium tuberculosis H37Ra and maintained on purified isocaloric diets. The control diet contained 30% protein (ovalbumin) and 50 ppm of added zinc (50 micrograms/g), the low protein diet contained 10% protein and 50 ppm of added zinc, and the low zinc diet contained 30% protein and no added zinc. Guinea pigs from each diet treatment were skin tested with purified protein derivative 48 h before sacrifice at 3, 4, and 5 weeks postinfection. Protein-deficient animals exhibited significantly reduced body weight, spleen weight, serum total proteins, and serum albumin. Zinc deficiency was characterized by loss of weight and progressive reductions in plasma zinc concentrations. The number of viable M. tuberculosis H37Ra cells was significantly higher in the lungs of both malnourished groups at 3 weeks, but fell below control viable counts by 5 weeks postinfection. A similar pattern was seen in the spleens and bronchotracheal lymph nodes. Both the proportion and intensity of delayed hypersensitivity reactions increased steadily between 3 and 5 weeks in control animals, whereas the two malnourished groups were essentially anergic at all intervals, despite systemic infection. These results demonstrate that both protein and zinc deficiencies exert a significant influence on the development of pulmonary tuberculosis but that the nature of the influence depends upon the interval studied. In both malnourished groups, the pulmonary infection tended to peak early and decline, whereas the disease developed more slowly in control animals. Apparent control of mycobacterial populations in the tissues was accomplished by malnourished animals in the absence of demonstrable delayed hypersensitivity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts J. C., Lang J. A., Reyes P. S., Briggs G. M. Zinc requirement of the young guinea pig. J Nutr. 1977 Aug;107(8):1517–1527. doi: 10.1093/jn/107.8.1517. [DOI] [PubMed] [Google Scholar]
- Allen J. I., Kay N. E., McClain C. J. Severe zinc deficiency in humans: association with a reversible T-lymphocyte dysfunction. Ann Intern Med. 1981 Aug;95(2):154–157. doi: 10.7326/0003-4819-95-2-154. [DOI] [PubMed] [Google Scholar]
- Alsaadi A. I., Smith D. W. The fate of virulent and attenuated Mycobacteria in guinea pigs infected by the respiratory route. Am Rev Respir Dis. 1973 Jun;107(6):1041–1046. doi: 10.1164/arrd.1973.107.6.1041. [DOI] [PubMed] [Google Scholar]
- Beach R. S., Gershwin M. E., Hurley L. S. Altered thymic structure and mitogen responsiveness in postnatally zinc-deprived mice. Dev Comp Immunol. 1979 Fall;3(4):725–738. doi: 10.1016/s0145-305x(79)80065-8. [DOI] [PubMed] [Google Scholar]
- Beisel W. R. Single nutrients and immunity. Am J Clin Nutr. 1982 Feb;35(2 Suppl):417–468. doi: 10.1093/ajcn/35.2.417. [DOI] [PubMed] [Google Scholar]
- Bogden J. D., Lintz D. I., Joselow M. M., Charles J., Salaki J. S. Effect of pulmonary tuberculosis on blood concentrations of copper and zinc. Am J Clin Pathol. 1977 Mar;67(3):251–256. doi: 10.1093/ajcp/67.3.251. [DOI] [PubMed] [Google Scholar]
- Chandra R. K. Cell-mediated immunity in nutritional imbalance. Fed Proc. 1980 Nov;39(13):3088–3092. [PubMed] [Google Scholar]
- Edirisinghe J. S., Fern E. B., Targett G. A. The influence of dietary protein on the development of malaria. Ann Trop Paediatr. 1981 Jun;1(2):87–91. doi: 10.1080/02724936.1981.11748067. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Zwickl C. M., Luecke R. W. Delayed type hypersensitivity in zinc deficient adult mice: impairment and restoration of responsivity to dinitrofluorobenzene. J Nutr. 1982 Feb;112(2):309–313. doi: 10.1093/jn/112.2.309. [DOI] [PubMed] [Google Scholar]
- Golden M. H., Jackson A. A., Golden B. E. Effect of zinc on thymus of recently malnourished children. Lancet. 1977 Nov 19;2(8047):1057–1059. doi: 10.1016/s0140-6736(77)91888-8. [DOI] [PubMed] [Google Scholar]
- Good R. A., Fernandes G. Nutrition, immunity, and cancer-a review. Part I: Influence of protein or protein-calorie malnutrition and zinc deficiency on immunity. Clin Bull. 1979;9(1):3–12. [PubMed] [Google Scholar]
- Grover A. A., Kim H. K., Wiegeshaus E. H., Smith D. W. Host-parasite relationships in experimental airborne tuberculosis. II. Reproducible infection by means of an inoculum preserved at -70 C. J Bacteriol. 1967 Oct;94(4):832–835. doi: 10.1128/jb.94.4.832-835.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland P. S. Tuberculin reactions in malnourished children. Lancet. 1965 Oct 9;2(7415):719–721. doi: 10.1016/s0140-6736(65)90457-5. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Warr G. A., Astry C. L. Alterations of pulmonary defense mechanisms by protein depletion diet. Infect Immun. 1981 Nov;34(2):610–622. doi: 10.1128/iai.34.2.610-622.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardjito T., Donosepoetro M., Grange J. M. The Mantoux test in tuberculosis: correlations between the diameters of the dermal responses and the serum protein levels. Tubercle. 1981 Mar;62(1):31–35. doi: 10.1016/0041-3879(81)90032-5. [DOI] [PubMed] [Google Scholar]
- Karl L., Chvapil M., Zukoski C. F. Effect of zinc on the viability and phagocytic capacity of peritoneal macrophages. Proc Soc Exp Biol Med. 1973 Apr;142(4):1123–1127. doi: 10.3181/00379727-142-37190. [DOI] [PubMed] [Google Scholar]
- Kramer T. R., Good R. A. Increased in vitro cell-mediated immunity in protein-malnourished guinea pigs. Clin Immunol Immunopathol. 1978 Oct;11(2):212–228. doi: 10.1016/0090-1229(78)90045-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefford M. J. Macrophage activation and resistance to pulmonary tuberculosis. Infect Immun. 1980 May;28(2):508–515. doi: 10.1128/iai.28.2.508-515.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRICK J. V., RATCLIFFE H. L. Tuberculosis induced by droplet nuclei infection; its developmental pattern in hamsters in relation to levels of dietary protein. Am J Pathol. 1957 Jan-Feb;33(1):107–129. [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- McBean L. D., Smith J. C., Jr, Halsted J. A. Zinc deficiency in guinea pigs. 1. Proc Soc Exp Biol Med. 1972 Sep;140(4):1207–1209. doi: 10.3181/00379727-140-36642. [DOI] [PubMed] [Google Scholar]
- McMurray D. N., Loomis S. A., Casazza L. J., Rey H., Miranda R. Development of impaired cell-mediated immunity in mild and moderate malnutrition. Am J Clin Nutr. 1981 Jan;34(1):68–77. doi: 10.1093/ajcn/34.1.68. [DOI] [PubMed] [Google Scholar]
- McMurray D. N. Mechanisms of anergy in tuberculosis. Chest. 1980 Jan;77(1):4–5. doi: 10.1378/chest.77.1.4. [DOI] [PubMed] [Google Scholar]
- McMurray D. N., Yetley E. A. Cell-mediated immunity in malnourished guinea pigs after Mycobacterium bovis BCG vaccination. Infect Immun. 1982 Mar;35(3):909–914. doi: 10.1128/iai.35.3.909-914.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray D. N., Yetley E. A. Immune responses in malnourished guinea pigs. J Nutr. 1982 Jan;112(1):167–174. doi: 10.1093/jn/112.1.167. [DOI] [PubMed] [Google Scholar]
- McMurray D. N., Yetley E. A. Response to Mycobacterium bovis BCG vaccination in protein- and zinc-deficient guinea pigs. Infect Immun. 1983 Feb;39(2):755–761. doi: 10.1128/iai.39.2.755-761.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. J., Murray A. B. Starvation suppression and refeeding activation of infection. An ecological necessity? Lancet. 1977 Jan 15;1(8003):123–125. doi: 10.1016/s0140-6736(77)91710-x. [DOI] [PubMed] [Google Scholar]
- Oleske J. M., Westphal M. L., Shore S., Gorden D., Bogden J. D., Nahmias A. Zinc therapy of depressed cellular immunity in acrodermatitis enteropathica. Its correction. Am J Dis Child. 1979 Sep;133(9):915–918. doi: 10.1001/archpedi.1979.02130090043007. [DOI] [PubMed] [Google Scholar]
- RATCLIFFE H. L., MERRICK J. V. Tuberculosis induced by droplet nuclei infection; its developmental pattern in guinea pigs and rats in relation to dietary protein. Am J Pathol. 1957 Nov-Dec;33(6):1121–1135. [PMC free article] [PubMed] [Google Scholar]
- Roth H. P., Kirchgessner M. Zn metalloenzyme activities. Changes and biochemical aspects in Zn deficiency. World Rev Nutr Diet. 1980;34:144–160. [PubMed] [Google Scholar]
- SRIRAMACHARI S., GOPALAN C. Nutrition and tuberculosis. III. Effect of some nutritional factors on resistance to tuberculosis. Indian J Med Res. 1958 Jan;46(1):105–112. [PubMed] [Google Scholar]
- Scrimshaw N. S., Taylor C. E., Gordon J. E. Interactions of nutrition and infection. Monogr Ser World Health Organ. 1968;57:3–329. [PubMed] [Google Scholar]
- Shapiro C. D., Harding G. E., Smith D. W. Relationship of delayed-type hypersensitivity and acquired cellular resistance in experimental airborne tuberculosis. J Infect Dis. 1974 Jul;130(1):8–15. doi: 10.1093/infdis/130.1.8. [DOI] [PubMed] [Google Scholar]
- Sinha D. P., Bang F. B. Protein and calorie malnutrition, cell-mediated immunity, and B.C.G. vaccination in children from rural West Bengal. Lancet. 1976 Sep 11;2(7985):531–534. doi: 10.1016/s0140-6736(76)91791-8. [DOI] [PubMed] [Google Scholar]
- Smith D. W. Editorial: Animal models for study of immunity to infectious disease. J Infect Dis. 1973 Dec;128(6):800–801. doi: 10.1093/infdis/128.6.800. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Harding G. E. Animal model of human disease. Pulmonary tuberculosis. Animal model: Experimental airborne tuberculosis in the guinea pig. Am J Pathol. 1977 Oct;89(1):273–276. [PMC free article] [PubMed] [Google Scholar]
- Smith D. W., McMurray D. N., Wiegeshaus E. H., Grover A. A., Harding G. E. Host-parasite relationships in experimental airborne tuberculosis. IV. Early events in the course of infection in vaccinated and nonvaccinated guinea pigs. Am Rev Respir Dis. 1970 Dec;102(6):937–949. doi: 10.1164/arrd.1970.102.6.937. [DOI] [PubMed] [Google Scholar]
- Smythe P. M., Brereton-Stiles G. G., Grace H. J., Mafoyane A., Schonland M., Coovadia H. M., Loening W. E., Parent M. A., Vos G. H. Thymolymphatic deficiency and depression of cell-mediated immunity in protein-calorie malnutrition. Lancet. 1971 Oct 30;2(7731):939–943. doi: 10.1016/s0140-6736(71)90267-4. [DOI] [PubMed] [Google Scholar]
- Wiegeshaus E. H., McMurray D. N., Grover A. A., Harding G. E., Smith D. W. Host-parasite relationships in experimental airborne tuberculosis. 3. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am Rev Respir Dis. 1970 Sep;102(3):422–429. doi: 10.1164/arrd.1970.102.3.422. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Young J. B. Acute starvation protects mice against Listeria monocytogenes. Infect Immun. 1980 Jun;28(3):771–776. doi: 10.1128/iai.28.3.771-776.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



