Abstract
A human homologue of high mobility group box 1 (HMGB1) protein was cloned and characterized from the human filarial parasites Wuchereria bancrofti and Brugia malayi. Sequence analysis showed that W. bancrofti HMGB1 (WbHMGB1) and B. malayi HMGB1 (BmHMGB1) proteins share 99 % sequence identity. Filarial HMGB1 showed typical architectural sequence characteristics of HMGB family of proteins and consisted of only a single HMG box domain that had significant sequence similarity to the pro-inflammatory B box domain of human HMGB1. When incubated with mouse peritoneal macrophages and human promyelocytic leukemia cells, rBmHMGB1 induced secretion of significant levels of pro-inflammatory cytokines such as TNF-α, GM-CSF, and IL-6. Functional analysis also showed that the filarial HMGB1 proteins can bind to supercoiled DNA similar to other HMG family of proteins. BmHMGB1 protein is expressed in the adult and microfilarial stages of the parasite and is found in the excretory secretions of the live parasites. These findings suggest that filarial HMGB1 may have a significant role in lymphatic pathology associated with lymphatic filariasis.
Introduction
Lymphatic filariasis is caused mainly by two parasitic worms, Wuchereria bancrofti and Brugia malayi. The disease affects more than 120 million people worldwide and about 40 million of them are seriously incapacitated and or are disfigured (Ottesen 1992; Molyneux and Taylor 2001). Over a billion people living in approximately 80 different countries are at great risk of acquiring the infection from infected mosquitoes that carry the infective stage (L3) of the parasite. The L3s migrate through the skin reach the lymphatic circulation, become L4, and subsequently develop into adult male and female worms.
Host inflammatory responses triggered by dead larvae and adult parasites in infected tissues are believed to be an important triggering mechanism in lymphatic filarial pathogenesis (Dreyer et al. 2000; Taylor et al. 2010). Several previous reports showed that Wolbachia, an intracellular symbiotic bacterium present in lymphatic filarial parasites, can induce inflammatory reactions in the host by activating immune cells including macrophages (Turner et al. 2006; Taylor et al. 2005; Brattig et al. 2000, 2004). However, to our knowledge there are no reports on the proteins produced by the parasite that can function as pro-inflammatory molecules. All studies to date attribute the inflammatory response associated with lymphatic filariasis to the endosymbiont Wolbachia (Taylor et al. 2005).
High mobility group (HMG) proteins are ubiquitous and abundant non-histone nuclear proteins present in eukaryotic organisms. HMG protein family is classified into three subfamilies: HMGB, HMGA, and HMGN. Even though these subfamilies have similar physical characteristics, each has a unique protein signature and a characteristic functional sequence motif (Bustin 1999). HMGB subfamily of proteins have distinctive motif (HMG box) that can bind to DNA (Bustin 1999). In addition to its DNA-binding property, secreted form of HMGB1 is a potent pro-inflammatory molecule that can induce significant levels of tumor necrosis factor alpha (TNF-α) and interleukin IL-6 from human monocytes (Andersson et al. 2000). HMGB1 homologues have been reported from several parasites including trypanosomes (Morales et al. 1992; Cribb et al. 2011), schistosomes (Gnanasekar et al. 2006), and Plasmodium (Briquet et al. 2006). Analysis of the proteomic profile of adult B. malayi excretory/secretory products showed the presence of a variant of HMGB1 protein in the adult parasite secretions (Hewitson et al. 2008). Similarly, a partial sequence of B. malayi HMGB1 protein mRNA is already deposited in the GenBank (accession no. U05269). Based on this sequence, we cloned and expressed the recombinant HMGB1proteins of the lymphatic filarial parasites B. malayi and W. bancrofti in this study and show that the filarial HMGB1 proteins are potent pro-inflammatory molecules similar to human HMGB1.
Materials and methods
Brugia malayi parasites
B. malayi parasites (L3, Mf, and adult) used in this study were obtained from NIH/NIAID Filariasis reagent repository FR3 center, College of Veterinary Medicine, University of Georgia, Athens, GA.
Cloning filarial HMGB1
The BLASTN algorithm of a partial sequence of B. malayi high mobility group protein mRNA deposited in GenBank (accession no. U05269) was performed at the NCBI dbEST nucleotide database. Multiple ESTs with significant similarity were found, permitting a putative full-length sequence to be assembled. The putative sequence was translated and analyzed using Conserved Domain Architecture Retrieval Tool (CDART) from NCBI to confirm the presence of HMG box. Using this sequence, primers were designed to amplify hmgb1 genes from cDNA library. Same set of primers were used to amplify hmgb1 DNA from both W. bancrofti and B. malayi adult stage cDNA using forward primer 5′ GCGGATCCATGGCTAAGACAGGG 3′ and reverse primer 5′ CCGGAATTCTTACTTCTTGTATTTTTTCTTTTCTG 3′ with BamHI and EcoRI restriction sites. Amplified products were ligated to digested pRSETA vector (Invitrogen, Carls-bad, CA, USA) and the DNA insert was sequenced to ensure authenticity of cloned nucleotide sequence on both strands.
Expression and purification of rWbHMGB1
A recombinant construct of Wbhmgb1 in T7 expression vector was maintained in TOP10 E. coli cells (Invitrogen). For expression, recombinant plasmids were transformed into BL21 (DE3) pLysS. When the cultures reached an optical density of 0.6 at 600 nm, 1 mM isopropyl-1-thio-β-D-galactopyranoside was added to the cultures to induce gene expression, and the cultures were incubated for an additional 4 h. Bacterial cell lysate was separated in SDS–PAGE, and the presence of histidine-tagged protein was confirmed using anti-Xpress antibody (Invitrogen). Subsequently, the histidine-tagged recombinant proteins were purified using TALON metal affinity resin (Clontech, Mountain view, CA, USA) as per manufacturer’s recommendations. Contaminating LPS in the samples were removed by passing purified recombinant protein through a polymyxin B affinity column (ThermoFisher Scientific, Rockford, IL, USA) and levels of endotoxin/LPS in the final preparations were determined using E-TOXATE kit (Sigma, St Louis, MO, USA) as per manufacturer’s instructions.
Bioinformatics analysis on filarial HMGB1 sequence
The nucleotide and amino acid sequences of the genes encoding the following HMG proteins were used for bioinformatic analysis. Wb, Wuchereria bancrofti (ABN80426); Bm, Brugia malayi (ABN80425); Ll, Loa loa (XP_003149898); Cs, Clonorchis sinensis (GAA37866); Pf, Plasmodium falciparum (XM_001350402 and XP_001349346); Sj, Schistosoma japonicum (AAP06425), Tc, Trypanosoma cruzi (EFZ27873.1); Tg, Toxoplasma gondii (XP_002371399); As, Ascaris suum (ADY48692.1); and Ce, Caenorhabditis elegans (AAK67238). We also compared the sequences of WbHMGB1 with HMGB1 from other organisms for bioinformatic analysis: Dm, Drosophila melanogaster (Q05783); Ag, Anopheles gambiae str. PEST (XP_311155); Dm, Dermacentor variabilis (AAO92280); Sp, Strongylocentrotus purpuratus (NP_999708); Bt, Branchiostoma belcheri tsingtaunese (AAS91553); Hs, Homo sapiens (AAQ91389); Mus musculus (AAH64790 and AAH46759); Aa, Aedes aegypti (XP_001655323); Xl, Xenopus laevis (NP_001080836); Gg, Gallus gallus (P36194); Om, Oncorhynchus mykiss (ABD73318); Ss, Sus scrofa (P12682); Ag, Anopheles gambiae str. PEST (XP_311155); Sc, Saccharomyces cerevisiae (NP_015377). Accession numbers are indicated in parentheses.
Stage-specific expression of BmHMGB1
Western blot analysis was performed on phosphate buffered saline–soluble protein extract of worm homogenates (L3s, mixed-stage adults, and microfilariae of adult worms after separating the proteins in an SDS–PAGE gel and transferring to nitrocellulose membrane). Worm homogenates were prepared as described previously (Gnanasekar et al. 2002). Polyclonal antibodies against full-length rBmHMGB1 were raised in Balb/c mice using alum as adjuvant. Protocol for the humane use of animals in this study was approved by IACUC at the College of Medicine, University of Illinois. Polyclonal anti-BmHMGB1 was used (1:1,000) as the primary antibodies in the immunoblot analysis and HRP-labeled rabbit anti-mouse IgG (ThermoFisher Scientific) was used as secondary antibodies. Color was developed using OPD (ortho-phenyl-enediamine dihydrochloride) substrate (Sigma).
Gel retardation assay
Gel retardation experiments were carried out by using equimolar mixture of highly purified negatively supercoiled pRSET plasmid and the Hind III linearized pRSET plasmid, as previously described (Stros and Reich 1998), or with the mixture of supercoiled DNA and closed-circular DNA. Briefly, 0.5 μg of plasmid DNA was mixed with increasing amounts of WbHMGB1 in buffer containing 0.14 M NaCl, 20 mM Tris–HCl, pH 7.5, 0.2 mM EDTA, and 5 mM DTT to a final volume of 20 μl and preincubated on ice for 1 h. The DNA–protein complexes were resolved by electrophoresis on 1 % agarose gels in 0.5× TBE buffer at 3 V/cm for 15–18 h at 4°C. The gels were stained with 0.5 μg/ml ethidium bromide, destained in water, and photographed in a UV transilluminator.
Cytokine array analysis
Cytokine secretion by rWbHMGB1-treated mouse peritoneal macrophages or human HL60 cell lines was detected using multiplex sandwich fluorescent immunoassay (ExcelArray Mouse/Human Inflammation Array, ThermoFisher Scientific). Resident mouse peritoneal macrophages (PM) were collected by peritoneal lavage of BALB/c mice with RPMI media as described earlier (Rendon-Mitchell et al. 2003). The human promyelocytic leukemia cells (HL-60) were purchased from the American Type Culture Collection (Manassas, VA, USA). Macrophages or HL-60 cells (5×105 cells/24-well dish) were then cultured in presence of polymyxin B (30 μg/ml) and stimulated for 24 h with 1 μg/ml of rWbHMGB1, 10 μg/ml of rGBF, or 10 ng/ml of LPS. Cells stimulated with purified his-tagged recombinant Schistosoma mansoni G-binding factor (rGBF) that was expressed and purified under similar conditions as rWbHMGB1 served as a negative control. Earlier studies showed that rGBF has no cytokine stimulatory or inhibitory effect on mammalian cells (Gnanasekar et al. 2006).
For cytokine analysis, 100 μl of cell culture supernatants was added to each well of the array and incubated for 2 h at room temperature while shaking at 200 rpm. After washing the wells three times with wash buffer, 75 μl of biotinylated detection reagent was added to each well and for 1 h with shaking at 200 rpm at room temperature. After washing the wells three times with wash buffer, 100 μl of Streptavidin-DyLight 649 Reagent (ThermoFisher Scientific) was adder to each well and incubated for 30 min at room temperature. After washing the wells five times with wash buffer, the slide was spin-dried by centrifugation at 1,500 rpm and scanned by a GenePix 4000B microarray scanner (Axon Instruments, Grand Terrace, CA, USA) and fluorescence was quantitated using a GenePix Pro 3.0 quantitation software. Data was analyzed by using MSI Analyzer software (Molecular Staging, Inc.).
Statistical analysis
All statistical analyses were performed with the GraphPad Prism software version 5.0c (GraphPad Software, Inc., La Jolla, CA, USA) using one-way ANOVA. All data are presented as mean±SEM. P<0.05 was considered statistically significant.
Results
Sequence of filarial HMGB1 proteins
HMG proteins of W. bancrofti and B. malayi are small proteins consisting of 101 amino acids. Sequence analysis of these putative HMG proteins suggested that these proteins belong to the HMGB family. Therefore, these proteins were named WbHMGB1 and BmHMGB1. Unlike most of eukaryotic HMGB proteins which have N- and C-terminal extensions of varied lengths and functional roles, the two filarial proteins had only a short basic extension upstream of the HMGB box domain and lacked the acidic C-terminal tail. Similarly, both the proteins had only one HMG box domain (aa 36–100). Sequence analysis also showed that WbHMGB1 and BmHMGB1 proteins share 99 % identity and differed only in one amino acid (aa 7) which was present outside the HMG box domain.
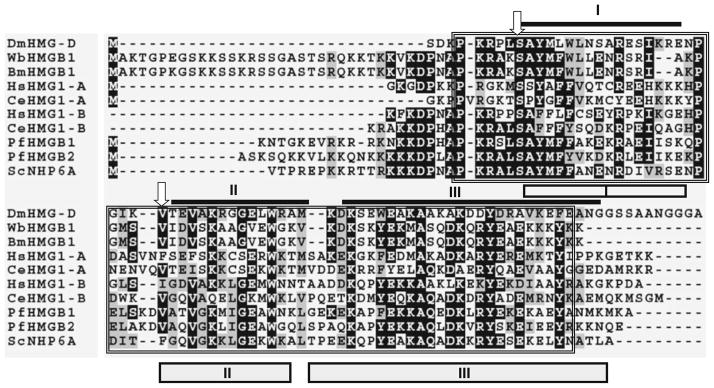
Sequence alignment analysis of filarial HMGB1 proteins with known HMG boxes from other parasitic organisms showed that WbHMGB1 and BmHMGB1 proteins have close sequence similarity with HMG box domain of other parasitic organisms (Fig. 1). In Drosophila, non-sequence-specific binding of HMGB1 box proteins to DNA were confirmed by the presence of serine at amino acid position 10 and hydrophobic residues at position 32 (Murphy et al. 1999). Analysis of the two filarial HMG proteins showed that they both possess these two determinants. Based on this characteristic, WbHMGB1 and BmHMGB1 were assigned to the architectural HMGB family of proteins. PROSITE analysis (Hulo et al. 2008) did not detect the presence of HMG box A DNA-binding domain signature sequence in the filarial HMGB1.
Fig. 1.
Multiple sequence alignment of HMGB 1 of W. bancrofti and B. malayi with HMG proteins from other parasitic organisms. HMG box domains were identified using PROSITE and marked by a box and compared with sequences of human, Saccharomyces NHP6A, and Drosophila HMG-D. Identical residues are represented as asterisks. Amino acids marked by arrows are two crucial determinants that differ between sequence-specific and the non-specific HMG domains. As shown here, a serine and a hydrophobic residue are present in all non-sequence or structural specific HMG proteins, whereas an asparagine and a hydrophilic residue are present at these positions of all sequence-specific HMG proteins. I, II, and III represent the three α-helices of the Drosophila HMG-D [dark lines (Murphy et al. 1999)] and Saccharomyces structure NHP6A [boxes (Allain et al. 1999)]
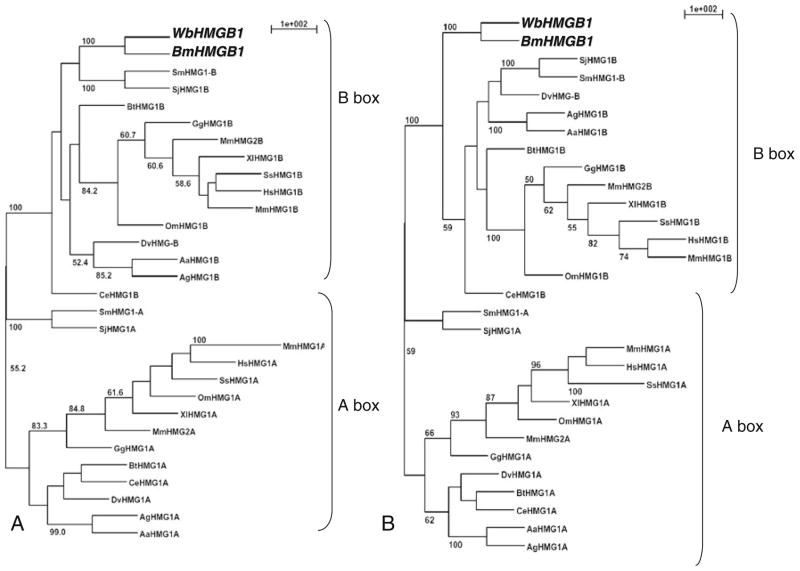
A phylogenetic analysis by parsimony and distance matrix methods (Fig. 2a, b) showed that HMG boxes of filarial HMG proteins were similar to the B box domains reported from other organisms. Specifically, our data supported that 100 % bootstrap values for HMG box domain of filarial HMGB1 inclusion were within the B box domain group.
Fig. 2.
Unrooted phylogenetic tree of A and B box domains of HMGB1 proteins. The tree includes BmHMGB1, WbHMGB1, and homologues found in other eukaryotes. A and B box sequences of several eukaryotic HMGB1 proteins were recognized by PROSITE and used to construct a phylogenetic tree with BmHMGB1 and WbHMGB1. Bootstrap values (>50 %) from 100 re-samplings are indicated prior to the branch points of the tree. a Most parsimonious consensus trees based on parsimony analysis (PROTPARS). b Distance matrix-based tree with proportional branch lengths (PROTDIST and NEIGHBOR)
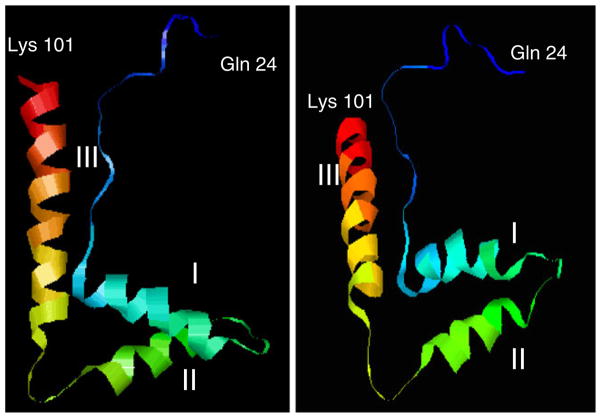
A three-dimensional structure of the HMG box domain of filarial HMGB1 proteins was modeled with help of Phyre, a 3D-PSSM folding server (Bennett-Lovsey et al. 2008) using Saccharomyces cerevisiae NHP6a (PDB file 1LWM) as the template structure. Three α-helices were predicted to fold into an L shape in the HMG box domain of filarial HMGB sequence from glutamine 24 to lysine 101 (Fig. 3). A PDB database search was performed with the modeled structure using FATCAT (Li et al. 2006), and the results suggested that HMG box domain of filarial HMGB1 was similar to the structures of Drosophila HMG-D, S. scrofa HMGB2, and Rattus norvegicus HMGB1. Thus, all of our computational analyses suggested that both WbHMGB1 and BmHMGB1 belonged to the HMGB family of proteins and possess a predicted DNA binding structure.
Fig. 3.
Ribbon cartoon representations of the predicted structure of filarial HMG box showing the relative orientation of the three helices (I, II, and III)
Stage-specific gene expression of filarial hmgb1
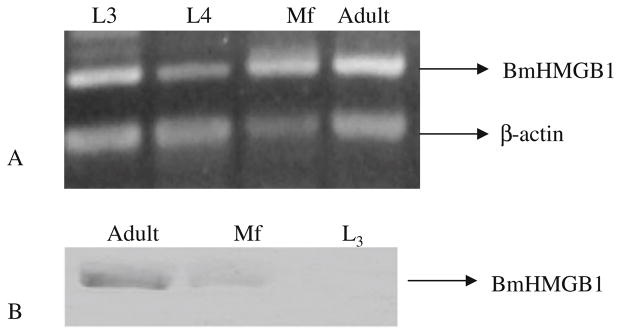
Expression of hmgb1 transcripts were evaluated in various life-cycle stages of B. malayi using RT–PCR analysis. Filarial hmgb1 gene specific primers were used to amplify the transcripts from several stage-specific cDNA libraries obtained from the Filarial Genome Project. Results of these analysis showed that Bmhmgb1 mRNA was present in L3, Mf, and adult stages of the parasite (Fig. 4a).
Fig. 4.
Expression of BmHMGB1 in different life-cycle stages of the parasite. a Library PCR using gene-specific primers on B. malayi cDNA libraries prepared from L3, L4, microfilariae (Mf), and adult parasites. b Western blot analysis was performed on protein extracts prepared from B. malayi L3, microfilaria, and adult worm antigens. Parasite proteins were probed with mouse anti-BmHMGB1 polyclonal antibodies and HRP-labeled rabbit anti-mouse IgG. Color was developed using OPD substrate. Note the presence of BmHMGB1 in adults, microfilaria, and in adult ES secretions
In addition, we also evaluated the expression of BmHMGB1 protein in various life-cycle stages of B. malayi using an immunoblot approach using polyclonal antibodies against rBmHMGB1. These results showed that BmHMGB1 protein was expressed only in adult and Mf life-cycle stages of the parasite. There was no BmHMGB1 expression detected in L3 stages (Fig. 4b). Thus, protein expression pattern of BmHMGB1 was distinctly different from mRNA expression pattern.
WbHMGB1 is a DNA binding protein
HMGB1 proteins in general exhibit a preferential binding affinity for supercoiled DNA over linear double-stranded DNA plasmid (Gnansekar et al.2006; Stros and Reich 1998). Since BmHMGB1 and WbHMGB1 proteins share 99 % amino acid identity, subsequent studies focused on characterizing one of these proteins (WbHMGB1) in detail. Gel retardation assays showed that rWbHMGB1 preferentially bound to supercoiled DNA. When WbHMGB1 was pre-incubated with a mixture of negatively supercoiled and linearized or relaxed closed-circular plasmids and separated in an agarose gel, significant amounts of rWbHMGB1 bound to supercoiled DNA compared to relaxed closed-circular DNA (Fig. 5a) or linear plasmid (Fig. 5b). Binding of rWbHMGB1 to supercoiled DNA was dependent on the concentration. The higher the concentration of rWbHMGB1, the more supercoiled DNA bound to the protein (Fig. 5). However, we did not observe any binding of rWbHMGB1 to linearized DNA or relaxed closed-circular DNA.
Fig. 5.
Preferential binding of WbHMGB1 protein to DNA and super-coiled DNA. a Gel retardation experiments were carried out with mixture of supercoiled DNA and closed-circular DNA. Increasing amount of His-tagged WbHMGB1 (0.5–5 μM, left to right) was added to determine the binding. b An equimolar mixture of supercoiled and linearized plasmid was pre-incubated with increasing amounts of WbHMGB1 (0.1–2 μM, from left to right). DNA–protein complexes were resolved on 1 % agarose gel, followed by staining the gel with ethidium bromide. S supercoiled DNA, L linear DNA, C relaxed open circular DNA
rWbHMGB1 is a pro-inflammatory molecule
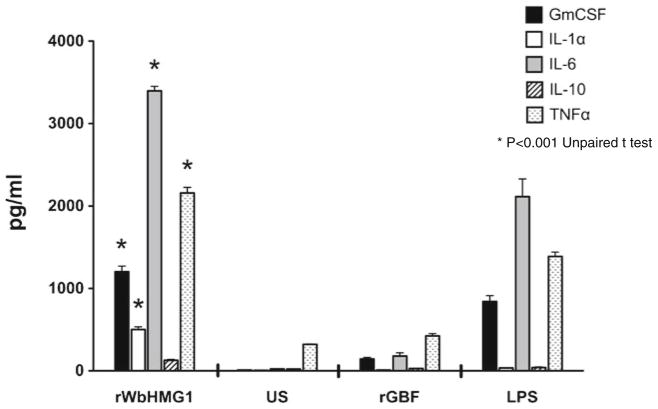
Sequence analysis suggested that the HMG box domain of WbHMGB1 has significant sequence identity to the B box domain of human HMGB1, which has potent pro-inflammatory cytokine-like activity (Erlandsson and Andersson 2004). Therefore, in this study, we wanted to evaluate if rWbHMGB1 also has pro-inflammatory property. Endotoxin levels in the purified rWbHMGB1 protein were below 0.02 EU/ml as determined by the LAL assay. In addition, we also used polymyxin B in all our cell cultures to neutralize any trace endotoxin activity. When mouse peritoneal macrophages were stimulated with rWbHMGB1, the cells secreted significant amounts of TNF-α, GM-CSF, and IL-6 as measured by a protein array (Fig. 6). These levels were significantly higher than the LPS control suggesting that the rWbHMGB1 protein is a potent pro-inflammatory molecule similar to the B box domain of human HMGB1. Levels of IL-10 in the culture supernatants were close to background levels. There was very little GM-CSF, IL-6, and TNF-α in the culture supernatants of cells stimulated with a non-specific recombinant protein, rGBF.
Fig. 6.
WbHMGB1 stimulates cytokines release (GM-CSF, IL1-α, IL-6 IL-10, TNF-α) from mouse peritoneal macrophages. Cells were stimulated with 1 μg/ml of purified rWbHMGB1, in the presence of polymyxin B (30 μg/ml), and the supernatant was assayed for cytokines using a protein array at 24 h after stimulation. LPS and unstimulated cells served as positive and negative control, respectively. Data represent mean ± SEM from three replicate wells. *Significant differences observed between rWbHMGB1 and other groups
rWbHMGB1 induces secretion of pro-inflammatory cytokines from HL-60 cells
We also evaluated the pro-inflammatory effect of rWbHMGB1 on HL-60, a human promyelocytic leukemia cell line. Analysis of the expression profile of cytokine proteins in rWbHMGB1-stimulated HL-60 cells showed that TNF-α, IL-8, MIP-1α, and RANTES were significantly increased (Fig. 7) compared to unstimulated cells and/or cells stimulated with a non-specific recombinant protein, rGBF. These findings thus showed that WbHMGB1 is a potent pro-inflammatory molecule.
Fig. 7.
WbHMGB1 stimulates cytokines release (TNF-α, IL-8, MIP-1α, and RANTES) from HL-60 cells. Cells were stimulated with 1 μg/ml of purified WbHMGB1 and the supernatants were assayed by inflammatory protein array 24 h after stimulation. Control cells were either left unstimulated (US) or treated with 10 μg/ml GBF protein. Cells were cultured in the presence of polymyxin B (30 μg/ml). Data represent mean ± SEM of three wells. *Significant differences observed between rWbHMGB1 and other groups
Discussion
Homologues of high mobility group box 1 (HMGB1) proteins are reported from protozoan and trematode parasites such as Plasmodium (Kumar et al. 2008), Entamoeba (Abhyankar et al. 2008), Trypanosoma (Morales et al. 1992), Toxoplasma (Zhao et al. 2009), and Schistosoma (Gnanasekar et al. 2006). In this manuscript, we show that the lymphatic filarial parasites B. malayi and W. bancrofti also express HMGB1. A variant of BmHMGB1 has been reported from the excretory secretions of adult B. malayi parasites (Hewitson et al. 2008). Subsequently, we cloned the filarial HMGB1 and showed that the filarial HMGB1 can function similar to other HMG family proteins in that they can bind to DNA and induce secretion of pro-inflammatory cytokines from macrophages. These findings thus suggested that the lymphatic filarial organisms can produce proteins with potent pro-inflammatory activity.
Lymphatic filariasis is associated with a range of clinical signs and symptoms, including lymphatic damages such as lymphedema, hydrocele, and lymph scrotum (Dreyer et al. 2000). In addition, some of the patients show extra lymphatic manifestations such as tropical pulmonary eosinophilia and microfilarial granulomata. These clinical symptoms in lymphatic filariasis are characterized by either acute or chronic inflammation (Freedman 1998; Nutman and Kumaraswami 2001). Similarly, in certain highly endemic areas, patients treated with DEC and albendazole develop adverse inflammatory reactions. Antigens released from dead or dying parasites are believed to be responsible for this inflammatory reaction (Cross et al. 2001; Taylor et al. 2000). Thus, inflammatory responses appear to be a predominant reaction in lymphatic filariasis-infected patients. A role for Wolbachia proteins has been established as a potential cause for the inflammatory responses associated with immune-mediated pathology in lymphatic filariasis (Bonofiglio et al. 2007; Brattig et al. 2004; Taylor et al. 2000). However, it is not known if parasites can also produce molecules that are pro-inflammatory. Results presented in this study show that the lymphatic filarial parasites can produce a molecule that has potent pro-inflammatory properties.
The filarial HMG belongs to the HMGB subfamily of proteins. However, they differ from eukaryotic HMGB1 proteins in that the filarial HMGB1 protein has only one HMG box domain. W. bancrofti and B. malayi HMG box domain has 100 % sequence homology. The eukaryotic HMGB1 proteins, however, have two HMG box domains in tandem, HMG box A and HMG box B (Huang et al. 2010). Analysis of the filarial HMGB1 proteins showed that they were similar to the plants, Drosophila, yeast, and Plasmodium HMGB1. All these HMGB1 proteins have only one domain. The presence of Ser-41 and Val-61 residues in filarial HMGB1 showed that the filarial HMGB1 protein belonged to the architectural HMGB family of proteins (Grasser et al. 1998) despite having only one HMG box domain. Similar to other HMG family of proteins, the filarial HMGB1 proteins are also DNA-binding proteins with significant preference for supercoiled DNA. Based on the published functions of these proteins in human (Grasser et al. 1998; Stros and Reich 1998; Teo et al. 1995), it is possible that the WbHMGB1 and BmHMGB1 may function as a DNA chaperon in parasite cells.
Phylogenetic analysis on the HMG domain of filarial HMGB1 confirmed that the filarial proteins are closely related to the B box domains reported in the literature. This was further confirmed by sequence analysis which also showed that the predicted structure of HMG box present in WbHMGB1 and BmHMGB1 is identical to HMG-D of Drosophila (Thomas and Travers 2001) and B box domain of human HMGB1 (Erlandsson Harris and Andersson 2004). Human HMGB1 proteins are actively secreted from inflammatory cells or are released passively from necrotic cells (Erlandsson Harris and Andersson 2004). Irrespective of the source, such secreted HMGB1 acts as a signal for tissue injury and initiate a pro-inflammatory response and/or reparative processes (Peltz et al. 2009). Secreted HMGB1 can efficiently activate macrophages to release TNF-α and other pro-inflammatory cytokines (Erlandsson Harris and Andersson 2004). This TNF-α stimulating activity of HMGB1 is localized to the first 20 amino acids (aa 89 to 108) of the B box domain (Li et al. 2003). A comparison of the human HMGB1 sequence with the filarial HMG protein showed that a short peptide segment in the N-terminal region of the HMG box of filarial HMGB1 protein has 75 % sequence identity with human HMGB1 box B domain (data not shown). This homologous region also has significant sequence similarity to the TNF-α stimulating domain of human HMGB1. This might explain the TNF-α-inducing activities of rBmHMGB1 observed in the present study. Our results confirmed that rWbHMGB1 can induce TNF-α and other pro-inflammatory molecules from mouse peritoneal macrophages and human macrophage cell lines similar to the human HMGB1.
Lymphatic filarial infection is associated with significant increase in the levels of circulating IL-1, IL-6, IFN-γ, and TNF-α. Levels of TNFα is increased several fold in the blood of microfilaremic patients after treatment with anti-filarial drugs. The response is higher with severity of infection and parasite burden (Ottesen 1987). Previous studies showed that development of lymphedema in filariasis patients is associated with local production of pro-inflammatory cytokines such as IL-6, TNF-α, and GM-CSF in parasitized lymph vessels (Rao et al. 1996; Babu et al. 2009). Thus, there is a significant pro-inflammatory response in infected patients largely associated with the parasite. Studies by Taylor et al. (2000) showed that soluble extracts of B. malayi can induce potent inflammatory responses including TNF-α. A role for Wolbachia-derived products such as LPS, DNA, and WSP has been established in these pro-inflammatory responses (Brattig et al. 2000, 2004; Taylor et al. 2000; Cross et al. 2001). However, our studies suggest that the filarial parasites express proteins that can induce potent pro-inflammatory cytokine responses similar to that seen during filarial infections. Studies by Hewitson et al. (2008) showed that a variant of BmHMGB1 is present in the excretory secretions of adult parasites. Given the pro-inflammatory function of BmHMGB1, we believe that this protein released from adult parasites may have a role in the pathology of lymphatic filariasis.
We observed a discrepancy in the expression levels of BmHMGB1 in various life-cycle stages of the parasite. Message levels for BmHMGB1 were present in nearly all stages of the parasite studied. However, expression of BmHMGB1 protein was evident only in the adult and microfilarial stages. These findings were similar to that reported by Gary Weil’s group (Li et al. 2004; Michalski and Weil 1999), where they show that HMGB1 is expressed in adult female worms and in microfilaria.
In conclusion, our studies show that filarial HMGB1 proteins belong to the architectural HMGB family of proteins which can bind to supercoiled DNA. Unlike eukaryotic HMGB1, the filarial HMGB1 has only one HMG domain that is identical to the B box domain of human and other eukaryotes. We show that the B box domain of WbHMGB1 is a potent pro-inflammatory protein similar to other HMGB1 family of proteins. BmHMGB1 protein is secreted by adult and microfilarial stages. Such secreted filarial HMGB1 proteins may have a significant role in the inflammatory immune responses associated with lymphatic pathology in acute lymphatic filariasis.
Acknowledgments
This study was funded by NIH RO1 grant (AI064745). We would like to thank Dr. Steven Williams, Smith College, Northampton, MA for providing us W. bancrofti and B. malayi cDNA libraries. We would also like to thank NIAID/NIH Filariasis Research Reagent Resource Center (FR3) at the University of Georgia, Athens, GA for providing us with parasite materials for this study.
Contributor Information
Sivasakthivel Thirugnanam, Department of Biomedical Sciences, College of Medicine, University of Illinois, Rockford, IL 61107, USA.
Gnanasekar Munirathinam, Department of Biomedical Sciences, College of Medicine, University of Illinois, Rockford, IL 61107, USA.
Anandharaman Veerapathran, Department of Biomedical Sciences, College of Medicine, University of Illinois, Rockford, IL 61107, USA.
Gajalakshmi Dakshinamoorthy, Department of Biomedical Sciences, College of Medicine, University of Illinois, Rockford, IL 61107, USA.
Maryada V. Reddy, Department of Biochemistry, Mahatma Gandhi Institute of Medical Sciences, Wardha, India
Kalyanasundaram Ramaswamy, Email: ramswamy@uic.edu, Department of Biomedical Sciences, College of Medicine, University of Illinois, Rockford, IL 61107, USA.
References
- Abhyankar MM, Hochreiter AE, Hershey J, Evans C, Zhang Y, Crasta O, Sobral BW, Mann BJ, Petri WA, Jr, Gilchrist CA. Characterization of an Entamoeba histolytica high-mobility-group box protein induced during intestinal infection. Eukaryot Cell. 2008;7:1565–1572. doi: 10.1128/EC.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain FH, Yen YM, Masse JE, Schultze P, Dieckmann T, Johnson RC, Feigon J. Solution structure of the HMG protein NHP6A and its interaction with DNA reveals the structural determinants for non-sequence-specific binding. Embo J. 1999;18:2563–2579. doi: 10.1093/emboj/18.9.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, Tracey KJ. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192(4):565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu S, Bhat SQ, Pavan Kumar N, Lipira AB, Kumar S, Karthik C, Kumaraswami V, Nutman TB. Filarial lymphedema is characterized by antigen-specific Th1 and th17 proinflammatory responses and a lack of regulatory T cells. PLoS Negl Trop Dis. 2009;3:e420. doi: 10.1371/journal.pntd.0000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett-Lovsey RM, Herbert AD, Sternberg MJ, Kelley LA. Exploring the extremes of sequence/structure space with ensemble fold recognition in the program Phyre. Proteins. 2008;70:611–625. doi: 10.1002/prot.21688. [DOI] [PubMed] [Google Scholar]
- Bonofiglio M, Hay J, McPherson C. Lymphatic filariasis: inflammatory response to Wolbachia bacteria in filarial worms. Lymphology. 2007;40:191–192. [PubMed] [Google Scholar]
- Brattig NW, Bazzocchi C, Kirschning CJ, Reiling N, Buttner DW, Ceciliani F, Geisinger F, Hochrein H, Ernst M, Wagner H, Bandi C, Hoerauf A. The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J Immunol. 2004;173:437–445. doi: 10.4049/jimmunol.173.1.437. [DOI] [PubMed] [Google Scholar]
- Brattig NW, Rathjens U, Ernst M, Geisinger F, Renz A, Tischendorf FW. Lipopolysaccharide-like molecules derived from Wolbachia endobacteria of the filaria Onchocerca volvulus are candidate mediators in the sequence of inflammatory and antiinflammatory responses of human monocytes. Microbes Infect. 2000;2:1147–1157. doi: 10.1016/s1286-4579(00)01269-7. [DOI] [PubMed] [Google Scholar]
- Briquet S, Boschet C, Gissot M, Tissandie E, Sevilla E, Franetich JF, Thiery I, Hamid Z, Bourgouin C, Vaquero C. High-mobility-group box nuclear factors of Plasmodium falciparum. Eukaryot Cell. 2006;5:672–682. doi: 10.1128/EC.5.4.672-682.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol. 1999;19:5237–5246. doi: 10.1128/mcb.19.8.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross HF, Haarbrink M, Egerton G, Yazdanbakhsh M, Taylor MJ. Severe reactions to filarial chemotherapy and release of Wolbachia endosymbionts into blood. Lancet. 2001;358:1873–1875. doi: 10.1016/S0140-6736(01)06899-4. [DOI] [PubMed] [Google Scholar]
- Cribb P, Perozzi M, Villanova GV, Trochine A, Serra E. Characterization of TcHMGB, a high mobility group B family member protein from Trypanosoma cruzi. Int J Parasitol. 2011;41:1149–1156. doi: 10.1016/j.ijpara.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Dreyer G, Noroes J, Figueredo-Silva J, Piessens WF. Pathogenesis of lymphatic disease in bancroftian filariasis: a clinical perspective. Parasitol Today. 2000;16:544–548. doi: 10.1016/s0169-4758(00)01778-6. [DOI] [PubMed] [Google Scholar]
- Erlandsson Harris H, Andersson U. Mini-review: The nuclear protein HMGB1 as a proinflammatory mediator. Eur J Immunol. 2004;34:1503–1512. doi: 10.1002/eji.200424916. [DOI] [PubMed] [Google Scholar]
- Freedman DO. Immune dynamics in the pathogenesis of human lymphatic filariasis. Parasitol Today. 1998;14:229–234. doi: 10.1016/s0169-4758(98)01244-7. [DOI] [PubMed] [Google Scholar]
- Gnanasekar M, Velusamy R, He YX, Ramaswamy K. Cloning and characterization of a high mobility group box 1 (HMGB1) homologue protein from Schistosoma mansoni. Mol Biochem Parasitol. 2006;145:137–146. doi: 10.1016/j.molbiopara.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Gnanasekar M, Rao KV, Chen L, Narayanan RB, Geetha M, Scott AL, Ramaswamy K, Kaliraj P. Molecular characterization of a calcium binding translationally controlled tumor protein homologue from the filarial parasites Brugia malayi and Wuchereria bancrofti. Mol Biochem Parasitol. 2002;121:107–118. doi: 10.1016/s0166-6851(02)00027-0. [DOI] [PubMed] [Google Scholar]
- Grasser KD, Teo SH, Lee KB, Broadhurst RW, Rees C, Hardman CH, Thomas JO. DNA-binding properties of the tandem HMG boxes of high-mobility-group protein 1 (HMG1) Eur J Biochem. 1998;253:787–795. doi: 10.1046/j.1432-1327.1998.2530787.x. [DOI] [PubMed] [Google Scholar]
- Hewitson JP, Harcus YM, Curwen RS, Dowle AA, Atmadja AK, Ashton PD, Wilson A, Maizels RM. The secretome of the filarial parasite, Brugia malayi: proteomic profile of adult excretory–secretory products. Mol Biochem Parasitol. 2008;160:8–21. doi: 10.1016/j.molbiopara.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Hulo N, Bairoch A, Bulliard V, Cerutti L, Cuche BA, de Castro E, Lachaize C, Langendijk-Genevaux PS, Sigrist CJ. The 20 years of PROSITE. Nucleic Acids Res. 2008;36 (Database issue):D245–249. doi: 10.1093/nar/gkm977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Singal A, Rizvi MM, Chauhan VS. High mobility group box (HMGB) proteins of Plasmodium falciparum: DNA binding proteins with pro-inflammatory activity. Parasitol Int. 2008;57:150–157. doi: 10.1016/j.parint.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Li BW, Rush AC, Tan J, Weil GJ. Quantitative analysis of gender-regulated transcripts in the filarial nematode Brugia malayi by real-time RT–PCR. Mol Biochem Parasitol. 2004;137:329–337. doi: 10.1016/j.molbiopara.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Li J, Kokkola R, Tabibzadeh S, Yang R, Ochani M, Qiang X, Harris HE, Czura CJ, Wang H, Ulloa L, Warren HS, Moldawer LL, Fink MP, Andersson U, Tracey KJ, Yang H. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med. 2003;9:37–45. [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ye Y, Godzik A. Flexible Structural Neighborhood—a database of protein structural similarities and alignments. Nucleic Acids Res. 2006;34 (Database issue):D277–280. doi: 10.1093/nar/gkj124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux DH, Taylor MJ. Current status and future prospects of the Global Lymphatic Filariasis Programme. Curr Opin Infect Dis. 2001;14:155–159. doi: 10.1097/00001432-200104000-00008. [DOI] [PubMed] [Google Scholar]
- Michalski ML, Weil GJ. Gender-specific gene expression in Brugia malayi. Mol Biochem Parasitol. 1999;104:247–257. doi: 10.1016/s0166-6851(99)00149-8. [DOI] [PubMed] [Google Scholar]
- Morales M, Onate E, Imschenetzky M, Galanti N. HMG-like chromosomal proteins in Trypanosoma cruzi. J Cell Biochem. 1992;50:279–284. doi: 10.1002/jcb.240500308. [DOI] [PubMed] [Google Scholar]
- Murphy FVt, Sweet RM, Churchill ME. The structure of a chromosomal high mobility group protein–DNA complex reveals sequence-neutral mechanisms important for non-sequence-specific DNA recognition. Embo J. 1999;18:6610–6618. doi: 10.1093/emboj/18.23.6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutman TB, Kumaraswami V. Regulation of the immune response in lymphatic filariasis: perspectives on acute and chronic infection with Wuchereria bancrofti in South India. Parasite Immunol. 2001;23:389–399. doi: 10.1046/j.1365-3024.2001.00399.x. [DOI] [PubMed] [Google Scholar]
- Ottesen EA. Description, mechanisms and control of reactions to treatment in the human filariases. Ciba Found Symp. 1987;127:265–283. doi: 10.1002/9780470513446.ch18. [DOI] [PubMed] [Google Scholar]
- Ottesen EA. Infection and disease in lymphatic filariasis: an immunological perspective. Parasitology. 1992;104(Suppl):S71–S79. doi: 10.1017/s0031182000075259. [DOI] [PubMed] [Google Scholar]
- Peltz ED, Moore EE, Eckels PC, Damle SS, Tsuruta Y, Johnson JL, Sauaia A, Silliman CC, Banerjee A, Abraham E. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock. 2009;32:17–22. doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao UR, Vickery AC, Kwa BH, Nayar JK. Regulatory cytokines in the lymphatic pathology of athymic mice infected with Brugia malayi. Int J Parasitol. 1996;26:561–565. doi: 10.1016/0020-7519(96)00036-7. [DOI] [PubMed] [Google Scholar]
- Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, Czura C, Mitchell RA, Chen G, Sama AE, Tracey KJ. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol. 2003;170:3890–3897. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- Stros M, Reich J. Formation of large nucleoprotein complexes upon binding of the high-mobility-group (HMG) box B-domain of HMG1 protein to supercoiled DNA. Eur J Biochem. 1998;251:427–434. doi: 10.1046/j.1432-1327.1998.2510427.x. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Bandi C, Hoerauf A. Wolbachia bacterial endo-symbionts of filarial nematodes. Adv Parasitol. 2005;60:245–284. doi: 10.1016/S0065-308X(05)60004-8. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Cross HF, Bilo K. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J Exp Med. 2000;191:1429–1436. doi: 10.1084/jem.191.8.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, Hoerauf A, Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet. 2010;376:1175–1185. doi: 10.1016/S0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- Teo SH, Grasser KD, Thomas JO. Differences in the DNA-binding properties of the HMG-box domains of HMG1 and the sex-determining factor SRY. Eur J Biochem. 1995;230:943–950. doi: 10.1111/j.1432-1033.1995.tb20640.x. [DOI] [PubMed] [Google Scholar]
- Thomas JO, Travers AA. HMG1 and 2, and related ‘architectural’ DNA-binding proteins. Trends Biochem Sci. 2001;26:167–174. doi: 10.1016/s0968-0004(01)01801-1. [DOI] [PubMed] [Google Scholar]
- Turner JD, Langley RS, Johnston KL, Egerton G, Wanji S, Taylor MJ. Wolbachia endosymbiotic bacteria of Brugia malayi mediate macrophage tolerance to TLR- and CD40-specific stimuli in a MyD88/TLR2-dependent manner. J Immunol. 2006;177:1240–1249. doi: 10.4049/jimmunol.177.2.1240. [DOI] [PubMed] [Google Scholar]
- Zhao YO, Khaminets A, Hunn JP, Howard JC. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog. 2009;5:e1000288. doi: 10.1371/journal.ppat.1000288. Epub 2009 Feb 6. [DOI] [PMC free article] [PubMed] [Google Scholar]