Abstract
Plasma membrane ghosts form when plant protoplasts attached to a substrate are lysed to leave a small patch of plasma membrane. We have identified several factors, including the use of a mildly acidic actin stabilization buffer and the inclusion of glutaraldehyde in the fixative, that allow immunofluorescent visualization of extensive cortical actin arrays retained on membrane ghosts made from tobacco (Nicotiana tabacum L.) suspension-cultured cells (line Bright Yellow 2). Normal microtubule arrays were also retained using these conditions. Membrane-associated actin is random; it exhibits only limited coalignment with the microtubules, and microtubule depolymerization in whole cells before wall digestion and ghost formation has little effect on actin retention. Actin and microtubules also exhibit different sensitivities to the pH and K+ and Ca2+ concentrations of the lysis buffer. There is, however, strong evidence for interactions between actin and the microtubules at or near the plasma membrane, because both ghosts and protoplasts prepared from taxol-pretreated cells have microtubules arranged in parallel arrays and an increased amount of actin coaligned with the microtubules. These experiments suggest that the organization of the cortical actin arrays may be dependent on the localization and organization of the microtubules.
Animal and lower eukaryotic cells contain an F-actin cytoskeleton, which associates with the plasma membrane through the action of various actin-binding proteins and protein complexes. This plasma membrane-associated actin functions in numerous fundamental cellular processes, including cell-shape regulation, cell motility, and regulation of membrane-transport events. F-actin also forms a component of cell regulation and signaling pathways by its indirect linkages with proteins such as the membrane-spanning integrins and matrix-bound fibronectin and vitronectin (Arpin et al., 1994; Hitt and Luna, 1994; Mills and Mandel, 1994).
Whereas an extensive cortical actin cytoskeleton has been characterized in plant cells labeled with rhodamine-phalloidin (Traas et al., 1987), there have been few reports that unequivocally show the interaction of this actin with the plasma membrane. However, indirect evidence does suggest that this type of interaction occurs. Purified plant plasma membrane vesicles retain both actin (Tan and Boss, 1992; Sonesson and Widell, 1993; Cox and Muday, 1994) and a spectrin-related protein on their cytoplasmic surfaces (Faraday and Spanswick, 1993). This spectrin-like protein localizes to the plasma membrane of whole cells by immunofluorescence microscopy (Michaud et al., 1991; de Ruijter and Emons, 1993). Furthermore, if the plant cell wall is fully digested, then the shape of the resulting protoplasts deviates from spherical with slight indentations, where the transvacuolar network meets the cortical cytoplasm. Because these deviations are cytochalasin sensitive, the actin cytoskeleton can impart tension on the plasma membrane (Hahne and Hoffmann, 1984). Further indirect evidence also suggests that at least some of the functions of membrane-associated actin in animal cells are conserved in plants: peptides containing the sequence motif Arg-Gly-Asp (RGD) that mimic integrin-binding proteins can disrupt plant cell functions (Schindler et al., 1989) and, in particular, actin-mediated cytoplasmic streaming (Wayne et al., 1992; Ryu et al., 1997), and vitronectin- and fibronectin-like proteins also occur in plant cells (Sanders et al., 1991; Zhu et al., 1993; Wang et al., 1994). By analogy with animal cells, these results suggest that plant extracellular matrix proteins might interact with the actin cytoskeleton through integrin connections (Wyatt and Carpita, 1993). Furthermore, it was recently reported that the actin modulates the activity of plasma membrane potassium channels, again suggesting a direct interaction of the actin cytoskeleton with the membrane (Hwang et al., 1997).
Because some functions of membrane-associated actin in animal cells, such as cell-shape regulation and cell motility, would not be required by plant cells, there is no reason to assume that the actin structures found in animal cells will occur in plants, nor is there any reason why membrane-associated actin should not perform functions in plants that are different from those found in animal cells. One example of the latter is the possible interaction with, or even the organization of, the cortical microtubule cytoskeleton. Because cortical microtubules interact with the plant plasma membrane (Marchant, 1978), any interaction between actin and the cortical microtubules (indicated by several studies, including those by Kobayashi et al., 1988; Seagull, 1990; Kadota and Wada, 1992; Wernicke and Jung, 1992; Chu et al., 1993) would also suggest that actin interacts with the plasma membrane, even if indirectly. By immunofluorescence microscopy, fine F-actin aligns transversely in elongating cells (Traas et al., 1987), parallel to transversely aligned microtubules (Sonobe and Shibaoka, 1989), but this does not prove that any interaction occurs. As seen by electron microscopy, fine filaments often accompany microtubules (Franke et al., 1972; subsequent refs. cited by Lancelle and Hepler, 1991) and these have been reported to be actin, based on immunogold labeling (Lancelle and Hepler, 1991). Although this demonstrates some form of interaction between actin and microtubules, its nature is unknown.
Our aim was to investigate whether there is plasma membrane-associated actin in plant cells and, if so, to determine whether this actin interacts with the cortical microtubules. We adapted the membrane-ghost technique, which has been widely used to characterize cortical microtubules (Marchant, 1978; van der Valk et al., 1980), to study cortical actin. This approach was independently taken by Kobayashi (1996). In this method, the cell wall is enzymatically digested to make protoplasts, which are attached to a substrate. The protoplasts are then lysed with a hypotonic microtubule-stabilizing buffer and the round piece of plasma membrane that was the contact site remains attached to the substrate, along with the associated cortical microtubules, while the cortical cytoplasm is washed away by the lysis buffer. Because the microtubules survive fixation and labeling well, two types of experiments are possible. Whole protoplasts can be treated with propyzamide (Akashi et al., 1988), extensin (Akashi et al., 1990), trypsin (Akashi and Shibaoka, 1991), cold (Mizuno, 1992), or taxol (Marc et al., 1996), and the effects of these treatments on the cytoskeleton observed. Alternatively, ghosts can be made and then washed with different solutions to probe the cytoskeleton (Kakimoto and Shibaoka, 1986; Cyr et al., 1987; Cyr, 1991). The possibility of performing these types of experiments was the major impetus to use the membrane-ghost system, because if actin can be identified on ghosts, it will provide a convenient way to investigate actin and microtubule interactions.
Although ghosting was initially developed to observe membrane-associated actin in Dictyostelium discoideum (Clarke et al., 1975), and actin-like filaments have been observed on negatively stained ghosts made from onion (Doohan and Palevitz, 1980) and tobacco (Nicotiana tabacum L.) cells (van der Valk and Fowke, 1981; Fowke et al., 1983), only recently has actin been observed on plasma membrane ghosts prepared from plant cells. Kobayashi (1996) prepared ghosts from cultured zinnia mesophyll cells at various times after the induction of tracheary elements and observed variations in actin patterns, but did not label microtubules on the same ghosts or study interactions between actin and microtubules. In this study we demonstrate membrane-associated actin on ghosts from tobacco BY-2 cells by immunofluorescence microscopy, and also demonstrate that this actin can interact with the cortical microtubules.
MATERIALS AND METHODS
Chemicals and Reagents
BSA, MBS, poly-l-Lys, and PMSF were purchased from Sigma, taxol was supplied by Wako Pure Chemicals (Osaka, Japan), sumizyme by Shin-Nihon Kagaku Kogyo (Aichi, Japan), and pectolyase Y-23 was purchased from Seishin Pharmaceuticals (Tokyo, Japan). Monoclonal anti-actin clone C4 (Lessard, 1989) was obtained from ICN, monoclonal anti-α-tubulin was from Amersham, and polyclonal anti-soybean tubulin was kindly provided by Dr. Koichi Mizuno (Osaka University, Japan). Polyclonal anti-maize actin was a kind gift of Dr. Chris Staiger (Purdue University, West Lafayette, IN). Cy-2-labeled goat anti-mouse IgG and Cy-3-labeled goat anti-rabbit IgG were from Jackson (West Grove, PA); Cy-2 and Cy-3 are fluorescent dyes analogous to FITC and rhodamine, respectively. Rhodamine-phalloidin and FITC-labeled goat anti-mouse IgG were from Molecular Probes (Eugene, OR), and rhodamine-labeled goat anti-rabbit IgG was from Organon Teknika (Durham, NC).
Plant Material
The tobacco (Nicotiana tabacum L.) suspension-cultured line Bright Yellow 2 (BY-2) (Nagata et al., 1981) was subcultured weekly in modified Linsmaier-Skoog medium as described previously (Akashi et al., 1988). For taxol treatments, including all experiments done on whole cells, protoplasts, and ghosts, whole cells were incubated with taxol (10 μm) for 30 min before wall digestion, with taxol included in the subsequent digest and in the wash buffer in which the protoplasts were resuspended (see below). Microtubules were depolymerized in whole cells by the addition of 100 μm propyzamide for 3 h before wall digestion, with propyzamide included in the subsequent digest and wash buffers. DMSO controls were run at appropriate concentrations (1.0% and 1.6%) for all taxol and propyzamide treatments, and gave results identical to controls lacking DMSO.
Fixing and Labeling of Whole Cells
Whole cells were attached to multiwell slides coated with poly-l-Lys (1 mg mL−1). After several minutes, cells were treated with 400 μm MBS in microtubule-stabilizing buffer (50 mm Pipes, 5 mm EGTA, 2 mm MgSO4, pH 6.9; Table I; based on Cyr et al., 1987) for 15 min, and then fixed in this solution with 0.05% Triton X-100, 200 μm PMSF, 4.0% formaldehyde, and 1% glutaraldehyde for 30 min. Cells were washed in PBS (2.68 mm KCl, 1.47 mm KH2PO4, 13.69 mm NaCl, and 0.81 mm Na2HPO4, pH 7.4) (2 × 10 min), and digested with protoplast-digest solution (see below) for 3 min before being washed again with PBS (2 × 10 min). Aldehyde-induced autofluorescence was reduced by treatment with sodium borohydride (5 min; 10 mg mL−1 in PBS) followed by PBS washes (2 × 10 min). Cells were blocked in incubation buffer (1% BSA, 0.05% Tween 20 in PBS) for 10 min, labeled with monoclonal anti-tubulin and polyclonal anti-actin (1:40 and 1:400 in incubation buffer) for 2 h, washed in PBS (30 min), and incubated in secondary antibodies (Cy-2-labeled goat anti-mouse IgG 1:200 and Cy-5-labeled goat anti-rabbit IgG 1:200 in incubation buffer) for 2 h. After washing in PBS (30 min), cells were stained with 4′,6-diamidino-2-phenylindole (1 μg mL−1, 30 s), washed in PBS, and mounted in PBS containing 0.1% p-phenylenediamine as an antifade agent.
Table I.
Buffers and fixatives
| Buffer Name | Buffer Contents |
|---|---|
| Microtubule-stabilizing | 50 mm Pipes, 5 mm EGTA, 2 mm MgSO4, pH 6.9 (K+), also included were 1.0% DMSO, 0.1% Triton X-100, and 200 μm PMSF |
| (400 μm MBS was added to this buffer as a prefixative for whole cells; 4% formaldehyde was included in this buffer as fixative; 200 mm mannitol was included for work with protoplasts) | |
| Actin-stabilization | 7 mm Pipes and 2 mm EGTA, buffered with 14.4 mm KOH to pH 6.4, 5.6 mm KCl (total K+, 20 mm), 10 mm MgCl2; also included were 3 mm DTT, 1.0% DMSO, and 300 μm PMSF |
| (ghosts fixed in this buffer with 4% formaldehyde and 1% glutaraldehyde, except as noted in Results) | |
| Modified pH lysis | pH varied by using different buffers all countered with 20 mm K+ (as KOH/KCl): pH 5.5, 20 mm Mes, 9.2 mm KOH, 10.8 mm KCl; pH 6.0, 20 mm Mes, 14.4 mm KOH 5.6 mm KCl; pH 6.9, 7 mm Pipes, 17.0 mm KOH, 3.0 mm KCl; pH 7.4, 20 mm Hepes, 15.6 mm KOH, 4.4 mm KCl; and pH 8.0, 20 mm Hepes, 20.4 mm KOH; all buffers included 10 mm MgCl2 and 2 mm EGTA; also included were 3 mm DTT, 1.0% DMSO, and 300 μm PMSF |
| (ghosts fixed in these buffers with 4% formaldehyde and 1% glutaraldehyde) | |
| Modified K+ | As with the actin-stabilization buffer, but with KCl increased to either 45.6 or 85.6 mm, giving final K+ concentrations of 60 and 100 mm |
| (ghosts fixed in this buffer with 4% formaldehyde and 1% glutaraldehyde) | |
| Ca2+ lysis | As with the actin-stabilization buffer, but with 10 mm CaCl2 replacing the EGTA; final K+ was kept at 20 mm with 9.0 mm KOH and 11.0 mm KCl |
| (ghosts fixed in this buffer with 4% formaldehyde and 1% glutaraldehyde) |
Numerous different buffers were used for the generation and fixation of whole cells and ghosts. Variations on each of the buffers used are indicated in parentheses.
Making, Fixing, and Labeling Protoplasts
To make protoplasts, whole cells were used 2 to 6 d after subculturing. The cell walls were digested in a solution containing 1% sumizyme, 0.1% pectolyase Y-23, 0.5% BSA, and 0.3 m mannitol dissolved in modified Linsmaier-Skoog-based buffer at pH 5.5 at 30°C for 60 to 70 min. The resulting protoplasts were pelleted, washed twice in wash buffer (10 mm Pipes, pH 6.8, 100 mm KCl, and 285 mm mannitol), and resuspended in wash buffer.
Protoplasts were attached to poly-l-Lys-coated multiwell slides and after several minutes treated with 400 μm MBS in microtubule-stabilizing buffer that contained 200 mm mannitol, 0.1% Triton X-100, and 200 μm PMSF for 5 min, and then fixed in this solution containing 4.0% formaldehyde for 30 min. Protoplasts were washed in PBS for 20 min, blocked, and labeled with monoclonal anti-tubulin (1:40 in incubation buffer) for 1 h, washed in PBS (30 min), and incubated in secondary antibodies (FITC-labeled goat anti-mouse IgG 1:200 in incubation buffer) for 1 h. After washing in PBS (30 min), cells were stained with 4′,6-diamidino-2-phenylindole (1 μg mL−1, 30 s) and then labeled with rhodamine-phalloidin (0.08 μm in PBS, 1 h). After a brief wash in PBS, cells were mounted in 50% glycerol/PBS containing 0.1% p-phenylenediamine as an antifade agent.
Making and Fixing Plasma Membrane Ghosts
Protoplasts were attached to poly-l-Lys-coated multiwell slides. Although 100 μm MBS was then applied for several minutes in some experiments, this was not generally required for actin preservation. The ghosting procedure was modified from the methods of Cyr (1991). After wash buffer was removed, the buffer for protoplast lysis, 15 μL of an actin-stabilization buffer (7 mm Pipes, 2 mm EGTA, 10 mm MgCl2, buffered to pH 6.4 with KOH/KCl to give a final K+ concentration of 20 mm, 1.0% DMSO, 6 mm DTT, and 300 μm PMSF) (Table I) was added. Protoplasts were lysed by a quick flick of the glass slide, and a high rate of ghost formation was ensured by repeating the flicking procedure. The resulting ghosts were then washed in stabilization buffer (15 μL) for another 5 min. Ghosts were fixed for 30 min using 4% formaldehyde and 1% glutaraldehyde and washed in PBS for 30 min. Variations in buffers and fixatives used in these experiments are described in Table I and in Results.
For double-antibody labeling of ghosts, material was air dried for 10 min, rehydrated in PBS, washed in cold methanol (−20°C, 5 min), rehydrated in PBS, and then blocked in incubation buffer. Primary antibodies (C4 anti-actin 1:150 and polyclonal anti-soybean tubulin 1:500) were applied concurrently in incubation buffer for 1 h. Ghosts were then washed in PBS for 30 min and incubated in secondary antibodies (FITC-labeled goat anti-mouse IgG 1:200 and rhodamine-labeled goat anti-rabbit IgG 1:500) applied concurrently in incubation buffer for 1 h. Slides were then washed in PBS for 30 min and mounted as described above.
Observation of Samples
For observation of membrane ghosts, a conventional microscope (Olympus) with epifluorescence optics was used, with data recorded on ASA 400 T-Max film (Kodak) pushed to 1600 ASA, which was digitized at 1350 pixels per inch. For immunofluorescence observations of whole cells, protoplasts, and some ghosts, two systems were used. These were an inverted microscope (Olympus) fitted with epifluorescence optics and equipped with a cooled CCD camera, with optical stacks deconvolved using the Delta Vision system (Applied Precision, Mercer Island, WA), and a confocal microscope using excitation at 488 and 568 nm from argon and krypton lasers, with optical stacks recorded simultaneously through band-pass 515- to 545-nm and long-pass 590-nm filters (Leica, Wetzlar, Germany). All images were processed with Adobe Photoshop software (Grand Prairie, TX).
RESULTS
Whole Cells and Protoplasts
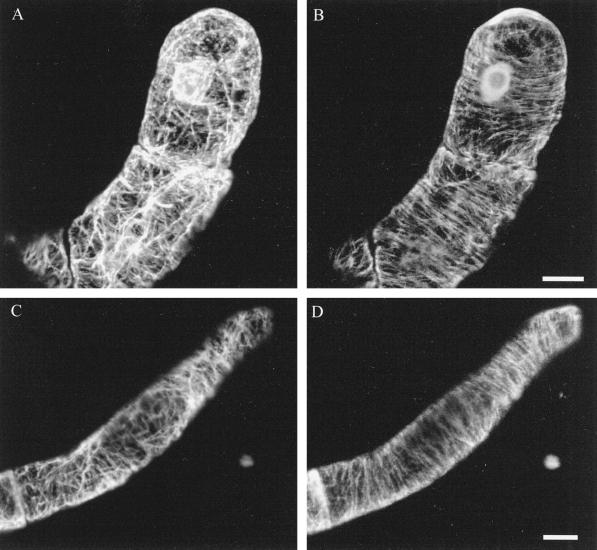
Immunofluorescence observations of MBS-stabilized BY-2 tobacco cells confirmed previous reports that both actin and microtubules are transversely arranged in the cortex of elongating cells (Fig. 1, A and B). Additional actin occurred in the form of subcortical bundles and around the nucleus (Fig. 1A). Incubation with 10 μm taxol for 3 h did not modify the cytoskeleton; both cortical actin and microtubules remained transverse (Fig. 1, C and D).
Figure 1.
Cytoskeletal organization was unaffected when whole cells were pretreated with taxol (10 μm; 3 h) before fixation (C and D) compared with the controls (A and B). In both taxol and control treatments, actin (A and C) occurred as faint, transversely aligned cortical microfilaments and as brighter, subcortical bundles that were more randomly arranged. Actin was also present around the nucleus (A). Microtubules (B and D) were exclusively cortical and transversely arranged, parallel to the cortical actin. Images are projections of optical stacks produced with a confocal microscope; six images covering a depth of 6.9 μm are projected for A and B, and five images over a depth of 4 μm are projected for C and D. Bars = 10 μm.
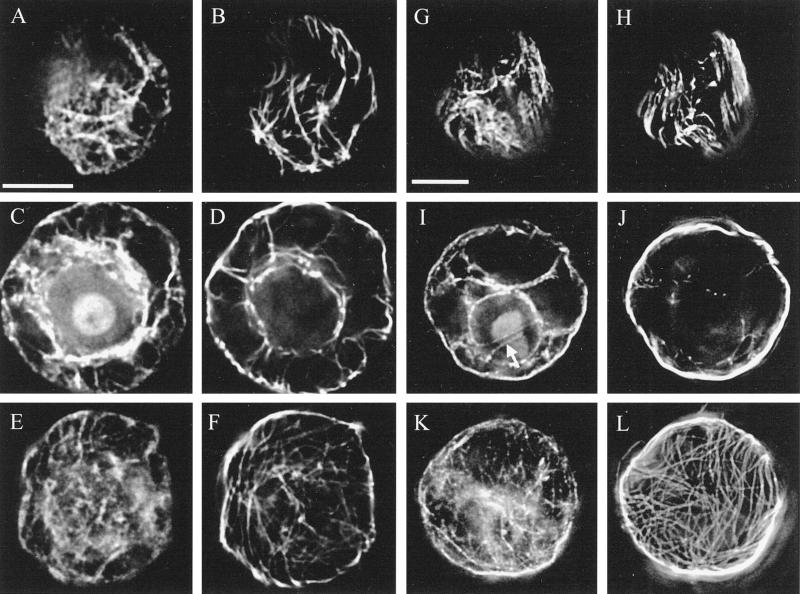
Optical sections of MBS-stabilized protoplasts double labeled for actin and microtubules showed that wall digestion modified the cytoskeleton (Fig. 2, A–F). Protoplasts had random actin (Fig. 2, A and E) and microtubules (Fig. 2, B and F) when viewed at either the top or bottom, and the nucleus was suspended in the center of these protoplasts, encased in a basket of actin and microtubules (Fig. 2, C and D). Pretreatment of whole cells with taxol (10 μm; 3 h) before wall digestion caused modifications to the cytoskeleton of the protoplasts (Fig. 2, G–L). Many of these protoplasts retained some parallel microtubules, often visible at the top of the cells (Fig. 2H) and accompanied by aligned actin, a pattern not seen in control protoplasts (Fig. 2G). Furthermore, the nucleus of these protoplasts was surrounded primarily by actin (Fig. 2I) and not by microtubules (Fig. 2J).
Figure 2.
Pretreatment of whole cells with taxol promoted the alignment of microtubules and actin in protoplasts. Actin (A, C, E, G, I, and K) and microtubules (B, D, F, H, J, and L) were labeled in control protoplasts (A–F) and in protoplasts produced from cells pretreated with taxol (10 μm; 3 h) before and during wall digestion (G–L). Optical stacks of images taken at 0.5-μm intervals were deconvolved using the Delta Vision system. For the top of the protoplasts (A, B, G, and H), three sections were projected together; for the center, two images were projected (C, D, I, and J); a single image was used for the base of the protoplasts (E, F, K, and L). Whereas actin and microtubules were randomly organized in the control protoplasts, considerably more alignment of actin and microtubules was found in taxol treatments, notably at the top of the cell (G and H). The actin strands visible through the nucleus of the taxol-treated protoplast (I, arrow) were rare but were also seen occasionally in control cells. However, there was a considerable reduction in the number of microtubules found around the nucleus in taxol-treated protoplasts (J) compared with controls (D). Bars = 10 μm.
A Plasma Membrane-Associated Actin Cytoskeleton
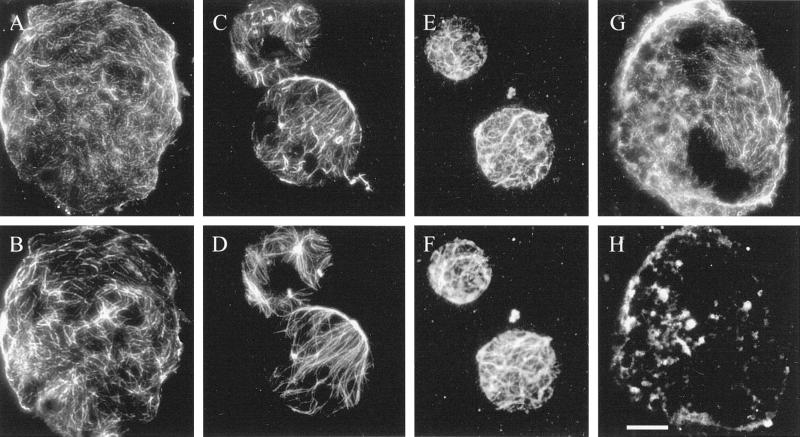
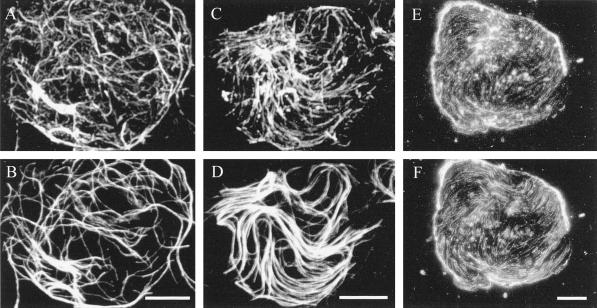
When protoplasts are attached to a poly-l-Lys-coated slide and then lysed, a round piece of plasma membrane, originally the contact site of the protoplast with the slide, remains on the slide. The rest of the protoplast, including the cortical cytoplasm, washes away while the plasma membrane-attached microtubules are retained (Marchant, 1978). We used double-immunofluorescence microscopy to visualize the cytoskeleton on membrane ghosts; all secondary antibody controls were negative (data not presented). If ghosts were prepared and washed for 5 min in an actin-stabilizing buffer and then glutaraldehyde fixed, both actin and microtubules could be visualized (Fig. 3, A–D). The microtubules were random (Fig. 3, B and D), as was the actin, although the intensity of actin labeling was considerably weaker than that of the microtubules (Fig. 3, A and C) and the amount of coalignment was variable. The majority of cells showed little alignment between the actin and the microtubules, so that in some areas the actin and microtubules were co-localized but in other areas they remained separate from each other (Fig. 3, A and B). On rare occasions, however, the random patterns of the actin and the microtubules coincided to a higher degree (Fig. 3, C and D). The actin on the ghosts occurred in two forms. Thick bundles were more random, but thin bundles (or possibly single filaments) showed somewhat more alignment with the microtubules. In actin-stabilization buffer, both the actin and the microtubules showed little disruption over time. Whereas the standard wash period was 5 min, both the actin and the microtubules could survive 30-min washes (Fig. 3, E and F).
Figure 3.
Both actin (A, C, E, and G) and microtubules (B, D, F, and H) were retained on membrane ghosts made using actin-stabilization buffer and viewed with conventional fluorescence techniques. Although co-localization was usually low (A and B), there was considerable overlap on occasions (C and D). Membrane-associated actin (E) and microtubules (F) were stable in actin-stabilization buffer for as long as 30 min. Microtubule depolymerization did not affect the retention of membrane-associated actin: if whole BY-2 cells were treated for 3 h with 100 μm propyzamide before cell wall digestion and ghosting, then all microtubules were depolymerized (H), whereas actin was retained (G). Bar = 10 μm.
That actin remains attached to the membrane ghosts did not indicate that the actin binds directly to the membrane; it would be possible for actin to bind indirectly through the microtubule cytoskeleton. To demonstrate that microtubules are not required for the binding of actin to the plasma membrane, microtubules in whole cells were depolymerized with 100 μm propyzamide (a microtubule-de-polymerizing herbicide) for 3 h before and during wall digestion. Actin was retained on the plasma membrane of ghosts made from these microtubule-free cells (Fig. 3G) even in the absence of microtubules (Fig. 3H).
Testing the Membrane-Associated Cytoskeleton of Ghosts
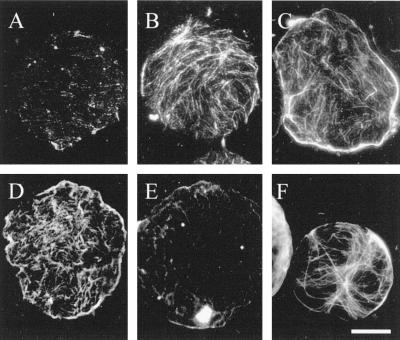
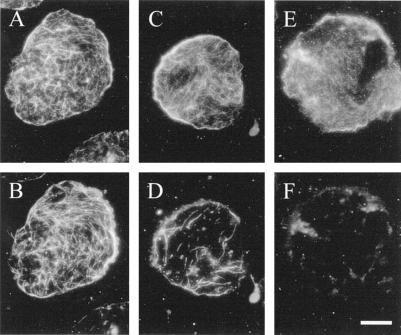
Optimization of the actin-stabilization buffer and fixatives was critical for the successful preservation of both actin and microtubules on ghosts. Compared with the successful labeling of actin on ghosts with anti-actin antibodies (Fig. 3A), protoplasts lysed with the microtubule-stabilizing buffer used for whole cells and protoplasts did not retain actin when they were formaldehyde fixed and stained with rhodamine-phalloidin (Fig. 4A). Three factors were identified that increased the amount of preserved actin but that generally had little influence on the microtubules. First, pretreatment of protoplasts with the protein cross-linker MBS, widely used for actin stabilization (Sonobe and Shibaoka, 1989), increased the amount of actin preserved using the same labeling conditions (Fig. 4B). Second, modifying the lysis buffer by decreasing the ionic strength, increasing the Mg2+ concentration, and decreasing the pH to 6.4 (Table I, actin-stabilization buffer) also promoted actin preservation, even in the absence of MBS, so that it could be labeled with phalloidin on formaldehyde-fixed (Fig. 4C) or freshly prepared ghosts (Fig. 4D). The third factor that promoted actin preservation was the inclusion of 1% glutaraldehyde in the fixative, although this necessitated labeling with actin antibodies rather than staining with rhodamine-phalloidin. Whereas little actin was visible on anti-actin-labeled formaldehyde-fixed ghosts lysed in the microtubule-stabilizing buffer (Fig. 4E), similarly lysed ghosts that were fixed with 1% glutaraldehyde and 4% formaldehyde retained actin (Fig. 4F).
Figure 4.
Lysis and fixation conditions markedly affected the amount of actin observed by conventional fluorescence (A, B, C, E, and F) or confocal microscopy (D) on membrane ghosts. Actin retention on ghosts fixed with 4% formaldehyde in a traditional microtubule-stabilizing buffer (Table I) and labeled with rhodamine-phalloidin (0.08 μm in PBS; 1 h) was higher when protoplasts were pretreated with 100 μm MBS for several minutes before lysis (B) compared with MBS-free controls (A). Use of an optimized actin-stabilization buffer (Table I) in the absence of MBS also allowed for the retention and phalloidin labeling of membrane-associated actin on either formaldehyde-fixed (C) or freshly prepared, unfixed ghosts (D). Monoclonal anti-actin labeling of ghosts in traditional microtubule-stabilizing buffer was poor when 4% formaldehyde was used as a fixative (E) but improved with the inclusion of 1% glutaraldehyde (F). Subsequent experiments used actin-stabilization buffer and fixation with formaldehyde and glutaraldehyde, but did not include prestabilization with MBS. Bar = 10 μm.
For optimal preservation of actin and microtubules, both actin-stabilizing buffer and the inclusion of glutaraldehyde were necessary. Stabilization with MBS did not reveal further actin, and was not used for further ghost experiments because of two problems. First, MBS treatments of whole protoplasts prevented direct experiments on the stability of actin and microtubules. Second, cross-linking also occurred in the cortical cytoplasm, making this more difficult to wash away. Trapped cytoplasm obscured the plasma membrane-associated cytoskeleton because of glutaraldehyde-induced autofluorescence, but in the absence of MBS, cytoplasm was washed away and glutaraldehyde fixation could be used without subsequent borohydride reduction.
Standard actin-stabilization buffer was pH 6.4: if the pH of this lysis buffer was varied from 5.5 to 8.0 while all other components, including the K+ concentration, were kept constant (Table I), then differences in both actin and microtubule stability could be observed (Fig. 5). Actin was most stable at pH 6.0 and 6.4 (Fig. 5, C and E), whereas the microtubules were stable at more alkaline pH values (6.9 and 7.4; Fig. 5, H and J). The choice of pH 6.4 for the lysis buffer in all of the other experiments reflected this being the optimal pH for preservation of actin and microtubules at these K+ and Mg2+ concentrations. Differences in stability between the actin and microtubules were also observed when the Mg2+ concentration of the lysis buffer varied. In the absence of Mg2+, only the microtubules and not the actin were stable, whereas at 10 mm Mg2+ the cytoskeleton was well preserved, and at 35 mm Mg2+ there was disruption in both the actin and microtubules (data not presented). When the K+ concentration of the stabilization buffer was varied, further differences in the stability between the actin and the microtubules occurred. At 20 mm K+, the standard conditions, both the actin and the microtubules were preserved, but when K+ was increased, the disruptive effects were more pronounced on the microtubules, so that by 60 mm significant disruption of the microtubules was apparent, although there was no effect on the actin (Fig. 6, A and B), and by 100 mm few microtubules remained, yet the actin remained in good condition (Fig. 6, C and D). It is interesting that when the EGTA in the actin-stabilization buffer was replaced with 10 mm free Ca2+, both the microtubules and the thicker actin bundles were destabilized, but the thinner actin structures showed some degree of stability (Fig. 6, E and F).
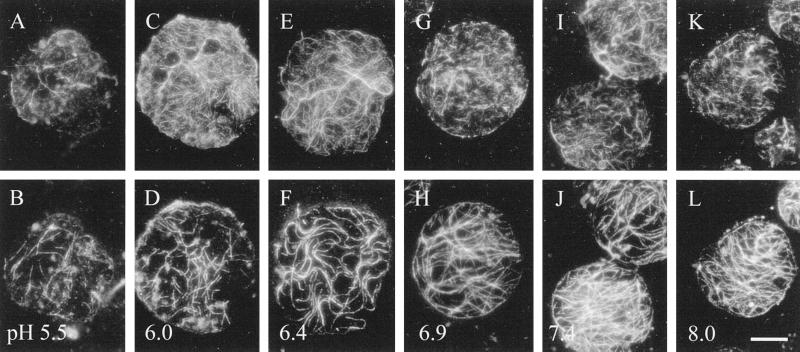
Figure 5.
Stability tests were conducted on membrane-associated actin and microtubules, varying the pH of the actin-stabilization buffer, keeping total K+ (including that acting as a counter ion to the buffer) constant at 20 mm (Table I). Actin (A, C, E, G, I, and K) was more stable at acidic pH values, whereas the microtubules (B, D, F, H, J, and L) were more stable at alkaline pH values. The pH values tested were 5.5 (A and B), 6.0 (C and D), the normal conditions of pH 6.4 (E and F), 6.9 (G and H), 7.4 (I and J), and 8.0 (K and L). Bar = 10 μm.
Figure 6.
Stability tests were conducted on membrane-associated actin (A, C, and E) and microtubules (B, D, and F), varying either the K+ or free Ca2+ concentrations of the actin-stabilization buffer. Compared with pH 6.4 controls with 20 mm K+ and EGTA (see Fig. 4, E and F), increasing total K+ to 60 (A and B) and 100 mm (C and D) caused increasing disruption to the microtubule cytoskeleton but had little effect on the actin. When 10 mm free Ca2+ replaced EGTA in the actin-stabilizing buffer (Table I), all microtubules were removed but some fine actin was retained (E and F). Bar = 10 μm.
Taxol Pretreatments Affect Both Actin and Microtubule Patterns
Whereas microtubule and actin alignment was low on control ghosts, pretreatment of whole cells with taxol, a microtubule-stabilizing agent, promoted microtubule bundling and alignment on a large proportion of ghosts and an accompanying increase in the alignment of the actin cytoskeleton (Fig. 7; Table II). If microtubule alignment was quantified by classification as either random or partially/fully aligned (equivalent to the regional and global order classifications of Wymer et al., 1996a), microtubules were randomly arranged on 85% of the control ghosts (Table II; Fig. 7B), with actin also being random on all of these ghosts (Fig. 7A). The remaining ghosts had partially or fully aligned microtubules, with the majority also having aligned actin. Pretreatment of whole cells and subsequently formed protoplasts with 10 μm taxol gave microtubules in “fingerprint-like” patterns (Fig. 7D) on more than 80% of ghosts (Table II). Such ghosts showed a considerable increase in the amount of alignment of the actin pattern with the microtubules (Fig. 7C). However, this effect occurred only if whole cells had been treated with taxol before wall digestion.
Figure 7.
Taxol treatments before and during wall digestion modified both membrane-associated actin (A, C, and E) and membrane-associated microtubules (B, D, and F) on ghosts. Compared with controls in which confocal microscopy showed random actin and random microtubules in a single optical section (A and B), ghosts produced from cells treated with taxol (10 μm) for 3 h before and during wall digestion retained highly aligned microtubules that were parallel to highly aligned actin (C and D). Unlike controls (see Fig. 5, E and F), ghosts made from taxol-treated protoplasts (viewed with conventional fluorescence) had Ca2+ resistant microtubules, and retained highly aligned actin similar to taxol-free controls (E and F). Bars = 10 μm.
Table II.
Taxol promotion of the alignment of actin with microtubules (MT)
| Type of Actin | Untreated (n =
47)
|
Pretreated with Taxol (n = 46)
|
||
|---|---|---|---|---|
| Random MT | Partially or fully aligned MT | Random MT | Partially or fully aligned MT | |
| Random | 85 | 2 | 11 | 7 |
| Partially or fully aligned | 0 | 13 | 4 | 78 |
Taxol pretreatment of whole cells (10 μm) for 3 h before and during protoplasting increased the percentages of ghosts in which actin and microtubules were partially or fully aligned.
That actin arrangement was modified by taxol strongly suggests an interaction between actin and the microtubules. This interaction, however, is limited. Whereas ghosts from taxol-treated protoplasts have microtubules that are stable to Ca2+ (Fig. 7E), there is no increase in the stability of actin, with only fine actin traces being present, similar to those found in controls (Fig. 7F).
DISCUSSION
Protoplasts produced from suspension-cultured cells are an artificial plant system, with their production inducing metabolic changes (Tan and Boss, 1992) and changes in the microtubule cytoskeleton (Marchant and Hines, 1979; Simmonds, 1992). Therefore, observations of protoplasts and ghosts do not necessarily correspond to what occurs in planta. The data presented in this paper demonstrate that cortical actin microfilaments interact with both the plasma membrane and microtubules in protoplasts. By understanding how membrane-associated actin behaves in a model system such as this, it may be possible to understand the roles that plasma membrane-associated actin might play in the whole plant.
Plasma Membrane-Associated Actin
Plasma membrane ghosts from BY-2 cells retain random microtubules under a wide range of conditions, with these microtubules similar to those found in many previous studies of BY-2 and other protoplasts (Marchant, 1978; Marchant and Hines, 1979; Doohan and Palevitz, 1980; van der Valk et al., 1980; van der Valk and Fowke, 1981; Fowke et al., 1983; Kakimoto and Shibaoka, 1986; Cyr et al., 1987; Akashi et al., 1988, 1990; Akashi and Shibaoka, 1991; Cyr, 1991; Katsuta and Shibaoka, 1992; Mizuno, 1992; Sonobe and Takahashi, 1994; Marc et al., 1996; Wymer et al., 1996a, 1996b; Collings et al., 1998). Traditionally, microtubule retention by ghosts is accepted to mean that direct binding of the microtubules to the plasma membrane occurs, mediated by microtubule-associated proteins. Although several of these reports also indicated the presence of occasional actin-like structures (Doohan and Palevitz, 1980; van der Valk and Fowke, 1981; Fowke et al., 1983), it was not until recently that Kobayashi (1996) demonstrated actin on a plant plasma membrane ghost.
The current study shows that membrane-associated actin occurs in two forms. Random bundles appear brighter by immunofluorescence and might derive from the thick, subcortical actin bundles of plant cells. The second form of membrane-associated actin appears to be smaller bundles or single filaments. Because this actin remains on ghosts in close-to-normal patterns when microtubules are depolymerized by propyzamide, this binding is direct and is not mediated by the microtubules. There are some differences, however, between the actin patterns of microtubule-free and microtubule-containing protoplasts (Collings et al., 1998). That actin binds independently to the membrane is not surprising, considering that there is only partial co-localization of the actin and microtubules. This type of organization is consistent with previous immunolabeling experiments on whole cells and protoplasts (Sonobe and Shibaoka, 1989; and numerous subsequent studies), and also with the labeling of whole cells and protoplasts in this study, where F-actin is present in fine transverse filaments and bundles, coaligned with microtubules.
The cortical cytoskeleton in BY-2 cells consists of both actin and microtubules and shows major structural differences from the membrane-associated cytoskeleton of Dictyostelium discoideum, which is composed of actin (Clarke et al., 1975), and the actin/spectrin membrane cytoskeleton of erythrocytes and other cell types (Bennett and Gilligan, 1993). These structural differences undoubtedly reflect differences in function.
Stabilization of Membrane-Associated Actin
The aims of this investigation were to localize membrane-associated actin and to determine what, if any, interaction this actin has with the cortical microtubules. Initial optimization of buffers and fixatives was required, because little actin remained under normal ghosting conditions optimized for microtubule preservation. Previous studies by Abe and Davies (1991) and Andersland and Parthasarathy (1993) showed that stabilization of plant F-actin in vitro was promoted by a slightly acidic pH and by increased Mg2+ and K+ concentrations. Variations based on their observations generated an acidic (pH 6.4) actin-stabilization buffer, which allowed for visualization of both microtubules and actin in the absence of stabilization agents such as phalloidin (used by Andersland and Parthasarathy, 1993) or the chemical cross-linker MBS (used by Kobayashi, 1996). Kobayashi also observed that membrane-associated actin was stable in the presence of 100 μm free Ca2+, whereas in this study a similar effect with the finer actin filaments was also observed at a higher Ca2+ concentration. High free Ca2+ is normally considered to depolymerize/destabilize plant actin filaments in vivo (Kohno and Shimmen, 1987), but were a stabilization agent present in the membrane this need not always be the case.
The observation that glutaraldehyde was beneficial for the fixation of actin was initially surprising, because strong aldehyde fixatives generally cause disruption to actin both in vitro (Lehrer, 1981; Boyles et al., 1985) and in vivo (Parthasarathy et al., 1985). Several factors may contribute to the extra stability of actin on ghosts. For example, the rapid removal of the vacuole limits disruption and proteolysis. Furthermore, the plasma membrane itself may contribute to the stabilization of actin, a conclusion that Kobayashi (1996) also reached after observing that ghosts stripped of their own actin could polymerize and retain actin filaments composed of animal G-actin. Another factor possibly involved in actin preservation is that glutaraldehyde, which has low membrane permeability (Karnovsky, 1965), has direct access to the inner face of the plasma membrane, and does not have to penetrate it.
The Interaction between Actin and Microtubules
Pretreatment of whole cells with taxol, a microtubule-stabilizing agent, caused apparent changes in actin alignment on microtubule ghosts, implying that cortical actin and microtubules can interact with one another. In fact, we suggest that taxol does not directly change the pattern of the actin array. Instead, taxol stabilizes the microtubule array present in whole cells during protoplast formation, and in doing this, it prevents modifications to the actin arrays already in existence.
Whereas most BY-2 tobacco cells undergo elongation and have transversely aligned microtubules (Hasezawa et al., 1988; Sonobe and Shibaoka, 1989; this study, Fig. 1), BY-2 protoplasts have randomly arranged microtubules (Hasezawa et al., 1988; this study, Fig. 2), possibly because of the dissociation of the microtubule cytoskeleton from the plasma membrane during protoplast formation (Marchant and Hines, 1979). It is also possible, however, that microtubule disorganization occurs because of the rapid cycling of cortical microtubules in plants (Hush et al., 1994) in the absence of wall factors that cause microtubule alignment (Fisher and Cyr, 1998). Taxol does not affect transverse microtubule alignment in elongating cells, but promotes the presence of parallel arrays of microtubules in protoplasts (Melan, 1990; this study, Figs. 1 and 2). Because taxol promotes the association of exogenous microtubules with membrane ghosts from which native microtubules have been stripped (Sonobe and Takahashi, 1994), microtubule stabilization in protoplasts by taxol can be interpreted as taxol preventing the dissociation of microtubules from the plasma membrane, and eliminating any microtubule turnover that might occur (Shibaoka, 1994). One indication that some microtubule turnover does occur in ghosts is that taxol reduces the number of microtubules found around the nucleus and in the transvacuolar strands of protoplasts. Whereas whole BY-2 cells lack microtubules around the nucleus and in the transvacuolar network, with these developing only in the G2 phase before cell division (Katsuta et al., 1990), microtubules are common in strands and around the nucleus of protoplasts, showing that wall digestion induces the formation of new microtubules. Taxol-treated protoplasts lack, or have fewer of, these new microtubules, indicating that taxol might be preventing the formation of new microtubules by reducing the pool of unpolymerized tubulin dimers.
We propose that cortical actin alignment is dependent, at least in part, on the alignment of cortical microtubules. Elongating BY-2 cells have transversely aligned cortical actin and microtubules (Sonobe and Shibaoka, 1989; this study, Fig. 1), the arrangement of which is unaffected by taxol. Yet on wall digestion, both the actin and the microtubules become randomized, except in cases in which taxol is present to stabilize the microtubules. Therefore, membrane ghosts formed from taxol-treated cells retain the aligned actin and microtubules found in whole cells.
In several cases, the dependence of the actin cytoskeleton on microtubules for alignment or stability has been observed. Kobayashi et al. (1988) demonstrated that disruption of microtubules in differentiating tracheary elements of zinnia resulted in the dispersal of the cortical actin arrays, whereas microtubule stabilization with taxol in rye root-tip cells increased the stability of actin microfilaments at low temperatures (Chu et al., 1993). However, there are also several examples in plant cells of microtubules being dependent on the integrity of the actin cytoskeleton (Kobayashi et al., 1988; Seagull, 1990; Wernicke and Jung, 1992). For example, Kadota and Wada (1992) demonstrated that although disruption of the microtubules did not affect actin in the fern Adiantum, cytochalasin caused a marked change in both the actin and the microtubule cytoskeletons. In this study we also attempted to determine whether the microtubule pattern was dependent on the actin cytoskeleton, but we observed no changes in the microtubule arrays on actin disruption with cytochalasin (data not shown). This does not, however, prove that microtubules do not depend on actin for their alignment; a better test for that would require actin stabilization during wall digestion. Whereas phalloidin might do this, its low membrane permeability precludes this experiment. Of more use potentially is jasplakinolide, a compound from marine sponges that binds to and stabilizes actin in a manner similar to phalloidin, and which is reported to be at least partially membrane permeant (Bubb et al., 1994), although there are no reports yet on its effects in plants.
Possible Functions of Actin at the Plasma Membrane
Apart from possibly interacting with the cortical microtubules, what functions might plasma membrane-bound actin have? Although we have demonstrated the presence of membrane-associated actin, this study did not directly address its possible functions. However, indirect evidence from other studies suggests roles for membrane-associated actin in numerous cellular activities. These include involvement in integrin links to the extracellular matrix (Schindler et al., 1989; Wayne et al., 1992, Ryu et al., 1997) and in cell signaling and the phosphoinositol metabolism (Tan and Boss, 1992). Several studies also suggest that actin may be important in stabilizing the structure of the plasma membrane (Abe and Takeda, 1989; Sonobe, 1990), and because plant annexins have been implicated in exocytosis (Clark and Roux, 1995), the identification of some plant annexins as actin-binding proteins (Calvert et al., 1996) suggests that actin may also play a role, although this may not necessarily be at the plasma membrane.
Another function in which membrane-associated actin is of critical importance is the regulation of ion channels and pumps. Actin appears to control the localization of the naphthylphthalamic acid-binding protein, a regulator of the auxin efflux transporter (Cox and Muday, 1994). Furthermore, recent studies also show that actin modulates K+-channel activity in fava bean, controlling the opening of stomata. Regulation of other plant membrane channels might be expected because the plasma membrane-associated actin in animal cells plays a major role in determining channel activity (Mills and Mandel 1994).
In conclusion, we have developed methods for the identification of a membrane-associated actin cytoskeleton on membrane ghosts produced from BY-2 tobacco protoplasts. This cortical actin array associates directly with both the plasma membrane and the cortical microtubules. The methods used in this study may also prove useful in the identification and characterization of additional functions of actin at the plasma membrane.
ACKNOWLEDGMENTS
The authors gratefully acknowledge Koichi Mizuno and Chris Staiger for gifts of antibodies, and John Harper for comments on the manuscript.
Abbreviations:
- FITC
fluorescein isothiocyanate
- MBS
3-maleimidobenzoyl-N-hydroxy-succinimide ester
Footnotes
This work was supported by a Foreign Researchers Fellowship from the Japanese Society for the Promotion of Science to D.A.C. and the North Carolina State University (National Aeronautics and Space Administration Specialized Center of Research and Training grant no. NAGW-4984).
LITERATURE CITED
- Abe S, Davies E. Isolation of F-actin from pea stems. Protoplasma. 1991;163:51–61. [Google Scholar]
- Abe S, Takeda J. Promotion by calcium ions and cytochalasin of the rounding up process (spherulation) of electrofused barley protoplasts and its relation to the cytoskeleton. J Exp Bot. 1989;40:819–826. [Google Scholar]
- Akashi T, Izumi K, Nagano E, Enomoto M, Mizuno K, Shibaoka H. Effects of propyzamide on tobacco cell microtubules in vivo and in vitro. Plant Cell Physiol. 1988;26:1053–1062. [Google Scholar]
- Akashi T, Kawasaki S, Shibaoka H. Stabilization of cortical microtubules by the cell wall in cultured tobacco cells. Planta. 1990;182:363–369. doi: 10.1007/BF02411386. [DOI] [PubMed] [Google Scholar]
- Akashi T, Shibaoka H. Involvement of transmembrane proteins in the association of cortical microtubules with the plasma membrane in tobacco BY-2 cells. J Cell Sci. 1991;98:169–174. [Google Scholar]
- Andersland JM, Parthasarathy MV. Conditions affecting depolymerization of actin in plant homogenates. J Cell Sci. 1993;104:1273–1279. [Google Scholar]
- Arpin M, Algrain M, Louvard D. Membrane-actin microfilament connections: an increasing diversity of players related to band 4.1. Curr Opin Cell Biol. 1994;6:136–141. doi: 10.1016/0955-0674(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- Boyles J, Anderson L, Hutcherson P. A new fixative for the preservation of actin filaments. J Histochem Cytochem. 1985;33:1116–1128. doi: 10.1177/33.11.3902963. [DOI] [PubMed] [Google Scholar]
- Bubb MR, Senderowicz AMJ, Sausville EA, Duncan KLK, Korn ED. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J Biol Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- Calvert CM, Gant SJ, Bowles DJ. Tomato annexins p34 and p35 bind to F-actin and display nucleotide phosphodiesterase activity inhibited by phospholipid binding. Plant Cell. 1996;8:333–342. doi: 10.1105/tpc.8.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B, Kerr P, Carter JV. Stabilizing microtubules with taxol increases microfilament stability during freezing of rye root tips. Plant Cell Environ. 1993;16:883–889. [Google Scholar]
- Clark GB, Roux SJ. Annexins of plant cells. Plant Physiol. 1995;109:1133–1139. doi: 10.1104/pp.109.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M, Schatten G, Mazia D, Spudich JA. Visualization of actin fibers associated with the cell membrane in amoebae of Dictyostelium discoideum. Proc Natl Acad Sci USA. 1975;72:1758–1762. doi: 10.1073/pnas.72.5.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collings DA, Asada T, Shibaoka H (1998) Plasma membrane ghosts form differently when produced from microtubule-free tobacco BY-2 cells. Plant Cell Physiol (in press)
- Cox DN, Muday GK. NPA binding activity is peripheral to the plasma membrane and is associated with the cytoskeleton. Plant Cell. 1994;6:1941–1953. doi: 10.1105/tpc.6.12.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr RJ. Calcium/calmodulin affects microtubule stability in lysed protoplasts. J Cell Sci. 1991;100:311–317. [Google Scholar]
- Cyr RJ, Bustos MM, Guiltinan MJ, Fosket DE. Developmental modulation of tubulin protein and mRNA levels during somatic embryogenesis in cultured carrot cells. Planta. 1987;171:365–376. doi: 10.1007/BF00398682. [DOI] [PubMed] [Google Scholar]
- de Ruijter N, Emons A. Immunodetection of spectrin antigens in plant cells. Cell Biol Int. 1993;17:169–182. [Google Scholar]
- Doohan ME, Palevitz BA. Microtubules and coated vesicles in guard-cell protoplasts of Allium cepa L. Planta. 1980;149:389–401. doi: 10.1007/BF00571175. [DOI] [PubMed] [Google Scholar]
- Faraday CD, Spanswick RM. Evidence for a membrane skeleton in higher plants. FEBS Lett. 1993;318:313–316. doi: 10.1016/0014-5793(93)80536-4. [DOI] [PubMed] [Google Scholar]
- Fisher DD, Cyr RJ. Extending the microtubule/microfibril paradigm. Plant Physiol. 1998;116:1043–1051. doi: 10.1104/pp.116.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowke LC, Griffing LR, Mersey BG, van der Valk P (1983) Protoplasts for studies of the plasma membrane and associated cell organelles. Experientia s46: 101–110
- Franke WW, Herth W, Van der Woude WJ, Morre DJ. Tubular and filamentous structures in pollen tubes: possible involvement as guide elements in protoplasmic streaming and vectorial migration of secretory vesicles. Planta. 1972;105:317–341. doi: 10.1007/BF00386769. [DOI] [PubMed] [Google Scholar]
- Hahne G, Hoffmann F. The effect of laser microsurgery on cytoplasmic strands and cytoplasmic streaming in isolated plant protoplasts. Eur J Cell Biol. 1984;33:175–179. [PubMed] [Google Scholar]
- Hasezawa S, Hogetsu T, Syono K. Rearrangement of cortical microtubules in elongating cells derived from tobacco protoplasts: a time-course observation by immunofluorescence microscopy. J Plant Physiol. 1988;133:46–51. [Google Scholar]
- Hitt AL, Luna EJ. Membrane interactions with the actin cytoskeleton. Curr Opin Cell Biol. 1994;6:120–130. doi: 10.1016/0955-0674(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Hush JM, Wadsworth P, Callaham DA, Hepler PK. Quantification of microtubule dynamics in living plant cells using fluorescence redistribution after photobleaching. J Cell Sci. 1994;107:775–784. doi: 10.1242/jcs.107.4.775. [DOI] [PubMed] [Google Scholar]
- Hwang J-U, Suh S, Yi H, Kim J, Lee Y. Actin filaments modulate both stomatal opening and inward K+ channel activities in guard cells of Vicia faba L. Plant Physiol. 1997;115:335–342. doi: 10.1104/pp.115.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota A, Wada M. The circular arrangement of cortical microtubules around the subapex of tip-growing fern protonemata is sensitive to cytochalasin B. Plant Cell Physiol. 1992;33:99–102. [Google Scholar]
- Kakimoto T, Shibaoka H. Calcium-sensitivity of cortical microtubules in the green alga Mougeotia. Plant Cell Physiol. 1986;27:94–101. [Google Scholar]
- Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol. 1965;27:137a–138a. [Google Scholar]
- Katsuta J, Hashiguchi Y, Shibaoka H. The role of the cytoskeleton in positioning of the nucleus in premitotic tobacco BY-2 cells. J Cell Sci. 1990;95:413–422. [Google Scholar]
- Katsuta J, Shibaoka H. Inhibition by kinase inhibitors of the development and the disappearance of the preprophase band of microtubules in tobacco BY-2 cells. J Cell Sci. 1992;103:397–405. [Google Scholar]
- Kobayashi H. Changes in the relationship between actin filaments and the plasma membrane in cultured Zinnia cells during tracheary element differentiation investigated by using plasma membrane ghosts. J Plant Res. 1996;109:61–65. [Google Scholar]
- Kobayashi H, Fukuda H, Shibaoka H. Interrelation between the spatial disposition of actin filaments and microtubules during the differentiation of tracheary elements in cultured Zinnia cells. Protoplasma. 1988;143:29–37. [Google Scholar]
- Kohno T, Shimmen T. Ca2+-induced fragmentation of actin filaments in pollen tubes. Protoplasma. 1987;141:177–179. [Google Scholar]
- Lancelle SA, Hepler PK. Association of actin with cortical microtubules revealed by immunogold localization in Nicotiana pollen tubes. Protoplasma. 1991;165:167–172. [Google Scholar]
- Lehrer SS. Damage to actin filaments by glutaraldehyde: protection by tropomyosin. J Cell Biol. 1981;90:459–466. doi: 10.1083/jcb.90.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard JL. Two monoclonal antibodies to actin: one muscle selective and one generally reactive. Cell Motil Cytoskel. 1989;10:349–362. doi: 10.1002/cm.970100302. [DOI] [PubMed] [Google Scholar]
- Marc J, Sharkey DE, Durso NA, Zhang M, Cyr RJ. Isolation of a 90-kDa microtubule-associated protein from tobacco membranes. Plant Cell. 1996;8:2127–2138. doi: 10.1105/tpc.8.11.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant HJ. Microtubules associated with the plasma membrane isolated from protoplasts of the green alga Mougeotia. Exp Cell Res. 1978;115:25–30. doi: 10.1016/0014-4827(78)90397-x. [DOI] [PubMed] [Google Scholar]
- Marchant HJ, Hines ER. The role of microtubules and cell-wall deposition in elongation of regenerating protoplasts of Mougeotia. Planta. 1979;146:41–48. doi: 10.1007/BF00381253. [DOI] [PubMed] [Google Scholar]
- Melan MA. Taxol maintains organized microtubule patterns in protoplasts which lead to the resynthesis of organized cell wall microfibrils. Protoplasma. 1990;153:169–177. [Google Scholar]
- Michaud D, Guillet G, Rogers PA, Charest PM. Identification of a 220 kDa membrane-associated plant cell protein immunologically related to human beta-spectrin. FEBS Lett. 1991;294:77–80. doi: 10.1016/0014-5793(91)81347-b. [DOI] [PubMed] [Google Scholar]
- Mills JW, Mandel LJ. Cytoskeletal regulation of membrane transport events. FASEB J. 1994;8:1161–1165. doi: 10.1096/fasebj.8.14.7958622. [DOI] [PubMed] [Google Scholar]
- Mizuno K. Induction of cold stability of microtubules in cultured tobacco cells. Plant Physiol. 1992;100:740–748. doi: 10.1104/pp.100.2.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T, Okada K, Takebe I, Matsui C. Delivery of tobacco mosaic virus RNA into plant protoplasts mediated by reverse-phase evaporation vesicles (liposomes) Mol Gen Genet. 1981;184:161–165. [Google Scholar]
- Parthasarathy MV, Perdue TD, Witztum A, Alvernaz J. Actin network as a normal component of the cytoskeleton in many vascular plant cells. Am J Bot. 1985;72:1318–1323. [Google Scholar]
- Ryu J, Mizuno K, Takagi S, Nagai R. Extracellular components implicated in the stationary organization of the actin cytoskeleton in mesophyll cells of Vallisneria. Plant Cell Physiol. 1997;38:420–432. doi: 10.1093/oxfordjournals.pcp.a029185. [DOI] [PubMed] [Google Scholar]
- Sanders LC, Wang C, Walling LL, Lord EM. A homolog of the substrate adhesion molecule vitronectin occurs in four species of flowering plants. Plant Cell. 1991;3:629–635. doi: 10.1105/tpc.3.6.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M, Meiners M, Cheresh DA. RGD-dependent linkage between cell wall and plasma membrane: consequences for growth. J Cell Biol. 1989;108:1955–1965. doi: 10.1083/jcb.108.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagull RW. The effects of microtubule and microfilament disrupting agents on cytoskeletal arrays and wall deposition in developing cotton fibres. Protoplasma. 1990;159:44–59. [Google Scholar]
- Shibaoka H. Plant hormone-induced changes in the orientation of cortical microtubules: alterations in the cross-linking between microtubules and the plasma membrane. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:527–544. [Google Scholar]
- Simmonds DH. Plant cell wall removal: cause for microtubule instability and division abnormalities in protoplast cultures? Physiol Plant. 1992;85:387–390. [Google Scholar]
- Sonesson A, Widell S. Cytoskeleton components of inside-out and right-side-out plasma membrane vesicles from plants. Protoplasma. 1993;177:45–52. [Google Scholar]
- Sonobe S. Cytochalasin B enhances cytokinetic cleavage in miniprotoplasts isolated from cultured tobacco cells. Protoplasma. 1990;155:239–242. [Google Scholar]
- Sonobe S, Shibaoka H. Cortical fine actin filaments in higher plant cells visualized by rhodamine-phalloidin after pretreatment with m-maleimidobenzoyl N-hydroxysuccinimide ester. Protoplasma. 1989;148:80–86. [Google Scholar]
- Sonobe S, Takahashi S. Association of microtubules with the plasma membrane of tobacco BY-2 cells in vitro. Plant Cell Physiol. 1994;35:451–460. [Google Scholar]
- Tan Z, Boss WF. Association of phosphatidylinositol kinase, phosphatidylinositol monophosphate kinase, and diacylglycerol kinase with the cytoskeleton and F-actin fractions of carrot (Daucus carota L.) cells grown in suspension culture. Plant Physiol. 1992;100:2116–2120. doi: 10.1104/pp.100.4.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traas JA, Doonan JH, Rawlins DJ, Shaw PJ, Watts J, Lloyd CW. An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J Cell Biol. 1987;105:387–395. doi: 10.1083/jcb.105.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Valk P, Fowke LC. Ultrastructural aspects of coated vesicles in tobacco protoplasts. Can J Bot. 1981;59:1307–1313. [Google Scholar]
- van der Valk P, Rennie PJ, Connolly JA, Fowke LC. Distribution of cortical microtubules in tobacco protoplasts: an immunofluorescence microscopic and ultrastructural study. Protoplasma. 1980;105:27–43. [Google Scholar]
- Wang C, Walling LL, Gu YQ, Ware CF, Lord EM. Two classes of proteins and mRNAs in Lilium longiflorum L. identified by human vitronectin probes. Plant Physiol. 1994;104:711–717. doi: 10.1104/pp.104.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R, Staves MP, Leopold AC. The contribution of the extracellular matrix to gravisensing in characean cells. J Cell Sci. 1992;101:611–623. doi: 10.1242/jcs.101.3.611. [DOI] [PubMed] [Google Scholar]
- Wernicke W, Jung G. Role of cytoskeleton in cell shaping of developing mesophyll of wheat (Triticum aestivum L.) Eur J Cell Biol. 1992;57:88–94. [PubMed] [Google Scholar]
- Wyatt SE, Carpita NC. The plant cytoskeleton-cell-wall continuum. Trends Cell Biol. 1993;3:413–417. doi: 10.1016/0962-8924(93)90022-s. [DOI] [PubMed] [Google Scholar]
- Wymer CL, Fisher DD, Moore RC, Cyr RJ. Elucidating the mechanism of cortical microtubule reorientation in plant cells. Cell Motil Cytoskel. 1996a;35:162–173. doi: 10.1002/(SICI)1097-0169(1996)35:2<162::AID-CM8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wymer CL, Wymer SA, Cosgrove DJ, Cyr RJ. Plant cell growth responds to external forces and the response requires intact microtubules. Plant Physiol. 1996b;110:425–430. doi: 10.1104/pp.110.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Shi J, Singh U, Wyatt SE, Bressan RA, Hasegawa PM, Carpita NC. Enrichment of vitronectin- and fibronectin-like proteins in NaCl-adapted plant cells and evidence for their involvement in plasma membrane-cell wall adhesion. Plant J. 1993;3:637–646. [PubMed] [Google Scholar]