Abstract
Existing drugs have limited efficacy against the rising threat of drug-resistant TB, have significant side effects, and must be given in combinations of four to six drugs for at least 6 months for drug-sensitive TB and up to 24 months for drug-resistant TB. The long treatment duration has led to increased patient noncompliance with therapy. This, in turn, drives the development of additional drug resistance in a spiral that has resulted in some forms of TB being currently untreatable by existing drugs. New antitubercular drugs in development, particularly those with mechanisms of action that are different from existing first- and second-line TB drugs, are anticipated to be effective against both drug-sensitive and drug-resistant TB. SQ109 is a new TB drug candidate with a novel mechanism of action that was safe and well tolerated in Phase I and early Phase II clinical trials. We describe herein the identification, development and characterization of SQ109 as a promising new antitubercular drug.
Keywords: antitubercular agent, clinical trial, drug discovery, drug resistance, MDR-TB, pharmacology, tuberculosis, XDR-TB
TB is a global health crisis
TB is a systemic disease with typical pulmonary manifestations caused by exposure to Mycobacterium tuberculosis (Mtb), which is transmitted from person to person by airborne bacteria. Approximately a third of the world’s population is currently infected with Mtb (Figure 1). Only 10% of people infected with the bacterium will develop active pulmonary disease in their lifetime, although people coinfected with HIV or suffering from diabetes, rheumatoid arthritis or other pathologies treated with certain drugs [1,2] have a much higher incidence of disease than the general population. TB is the cause of the largest number of human deaths attributable to a single etiologic agent: of approximately 8.8 million incident cases each year, nearly 1.5 million people die [101]. Figure 1 shows the distribution of TB around the world: no country is exempt, but the highest incidence rates are in Asia, parts of South America and much of Africa as a result of the burgeoning HIV epidemic.
Figure 1. WHO estimated TB incidence rates, 2011.
Reproduced with permission from [101].
Treatment of TB is a logistical nightmare with existing drugs: the recommended therapeutic regimen for drug-sensitive (DS)-TB involves four different first-line drugs: isoniazid (INH), rifampicin (RIF), pyrazinamide (PZA) and ethambutol (EMB). The complete four-drug regimen is administered for 2 months, and then both INH and RIF are administered for an additional 4 (WHO) to 6 (CDC) months. These drugs were all discovered more than 40 years ago and have significant safety concerns, including liver, ocular, skin and peripheral nerve toxicities, influenza-like symptoms, rash, headaches and gastrointestinal and neurological complications. These side effects contribute to poor compliance with the standard drug regimens, and outside of clinical trials, such compliance rates are poor, generally between 60 and 80% [3], although some studies demonstrated compliance rates as high as 90% [4,5].
Patients who take the medications erratically can continue to transmit their infection to others for extended periods [6–8]. More importantly, noncompliance with TB treatments can influence development of multidrug-resistant (MDR)-TB, TB that is resistant to INH and RIF [9]. The WHO currently estimates that there are 650,000 prevalent cases of MDR-TB [101]. MDR-TB is even more difficult to treat than DS-TB, with regimens lasting up to 24 months. Even with this long treatment with multiple drugs, a recent systematic review suggested that, worldwide, only 62% of patients treated for MDR-TB were cured or completed treatment [10]. There are now many reports of extensively drug-resistant (XDR)-TB, TB that is resistant not only to INH and RIF, but also to the fluoroquinolones and at least one of the second-line injectable agents, capreomycin (CM), kanamycin (KAN) or amikacin (AMK) [11]. XDR-TB is significantly more expensive to treat and has even poorer outcomes than MDR-TB [12,13]. HIV coinfection worsens these already dismal outcomes [14–16], underscoring the need for a new treatment regimen that could be used in both HIV-negative and HIV-positive patients with DS- and drug-resistant (DR)-TB.
Several new TB drug candidates are in clinical development
After a long hiatus during which no new TB drugs were discovered, pharmaceutical companies restarted programs to develop drugs for both DS-TB and MDR-TB in the 1990s. There are now promising novel, second-generation and repurposed TB drug candidates in clinical trials (see recent reviews [17–21]). Table 1 outlines the TB drug candidates currently in clinical development.
Table 1.
Clinical-stage TB drugs and drug candidates.
| Drug candidate | Class | Intellectual property | Clinical phase |
|---|---|---|---|
| Delamanid (OPC-67683) | Nitroimidazole | Otsuka | III |
| Moxifloxicin | Fluoroquinolone | Bayer AG/TB Alliance | III |
| Gatifloxacin | Fluoroquinolone | WHO/Bristol-Myers Squibb | III |
| Bedaquiline (TMC207) | Diarylquinoline | Janssen Infectious Diseases/TB Alliance | II |
| SQ109 | Ethylenediamine | Sequella | II |
| PA-824 | Nitroimidazole | TB Alliance | II |
| Sutezolid (PNU-100480) | Oxazolidinone | Pfizer | II |
| Linezolid | Oxazolidinone | Pfizer | II |
| Metronidazole | Nitroimidazole | Generic | II |
| AZD5847 | Oxazolidinone | AstraZeneca | I |
SQ109 (Sequella) and bedaquiline (TMC207; Janssen Infectious Diseases/TB Alliance) are two drug candidates with new and distinct mechanisms of action (MOA). Bedaquiline is a diarylquinoline that targets ATP synthase. It has been tested in a 7-day early bactericidal activity (EBA) study [22] and a Phase II clinical trial for the treatment of MDR-TB [23]. SQ109 is a 1,2-ethylenediamine that targets MmpL3 in Mtb [24,25]. MmpL3 is a mycolic acid transporter required for incorporation of mycolic acid into the Mtb cell wall. SQ109 is in Phase II clinical trials for DS-TB and is the subject of this review.
Repurposed TB drug candidates include linezolid, an oxazolidinone antibiotic for treating a variety of serious Gram-positive infections [26] and used off-label to treat MDR- and XDR-TB with some success [27–30]. An EBA study showed minimal killing activity [31], but additional clinical trials to investigate its potential in XDR-TB are ongoing [102]. Metronidazole is a nitroimidazole that has been used to treat certain anaerobic infections for more than 45 years [32]. As Mtb are found in low-oxygen granulomas, investigators thought it might be useful in the eradication of dormant Mtb [33,34]. It is not effective in mouse models of latent TB [33,35–37], but mice do not form granulomas. The National Institute of Allergy and Infectious Diseases (NIAID) at the NIH is sponsoring a Phase II clinical trial to investigate the efficacy of metronidazole to treat MDR-TB [103]. Finally, the fluoroquinolones ofloxacin and levofloxacin are approved for severe bacterial infections, and are also commonly used to treat MDR-TB [17]. The newer fluoroquinolones, gatifloxacin and moxifloxacin (MXF), have activity comparable to INH in mouse models [38], and both are in clinical evaluation for the treatment of TB.
Four TB drug candidates are from known drug classes, but have novel structures optimized for activity against Mtb. Delamanid (Otsuka) and PA-824 (TB Alliance) are novel nitroimidazoles. Delamanid showed activity in an EBA study [39] and is currently being evaluated in a Phase III clinical trial for the treatment of MDR-TB. PA-824 also showed activity in an EBA study [40] and is being evaluated in a Phase II study in combination with MXF and PZA for the treatment of DS- and MDR-TB. Sutezolid (PNU-100480: Pfizer) and AZD5847 (AstraZeneca) are both oxazolidinones, with MOA similar to linezolid. Sutezolid was identified contemporaneously with linezolid, and was noted to have better antimycobacterial activity both in vitro and in a mouse model of TB [41–43]. Sutezolid has an improved safety profile compared with linezolid [44] and shows better time-dependent killing in an ex vivo whole-blood culture test [45]. A Phase II EBA study is ongoing. AstraZeneca completed Phase I clinical trials for AZD5847; a Phase II EBA study is planned but is not yet recruiting.
SQ109 identified by a multistep filtering process
In January 1999, Sequella and the Laboratory of Host Defenses, NIAID, NIH, signed a Collaborative Research and Development Agreement to begin a combinatorial chemistry program to discover a new TB drug. We synthesized and screened a 63,238-member chemical library designed around the active 1,2-ethylenediamine pharmacophore found in EMB, one of the first-line anti-TB drugs. We focused on EMB for several reasons: the lack of combinatorial chemistry and high-throughput screens (HTS) in the 1960s prevented the developers of EMB from a complete evaluation of the structure–activity relationship (SAR) around the pharmacophore [46]; the early clinical trials of EMB at 25 mg/kg showed clear and convincing signs of therapeutic efficacy, even when delivered as monotherapy in patients with severe disease [47,48], but ultimately this dose had to be reduced due to ocular toxicity; and the EMB MIC on Mtb is moderate, and it is difficult to achieve blood levels much higher than the MIC, thereby restricting its efficacy in humans [49]. We hoped that by revisiting this series, we could identify an EMB-like drug candidate with an improved MIC and decreased toxicity.
The library was synthesized with a primary focus on modification at the amine substituents, leaving the ethylenediamine core unaltered. Approximately 300 commercially available amine monomers, NH2R1 and NHR2R3, were used to combinatorially create the ethylenediamine library. Aliphatic, aromatic, open-chain and strained bicyclic amines, amino alcohols and amino acids were selected for significant molecular diversity in size, shape, polarity and charge [46]. The compounds were then filtered through microbiology studies and multiple standard in vitro interspecies absorption, distribution, metabolism, elimination and toxicology (ADMET) assays designed to narrow the number of candidates based on antimicrobial activity, low toxicity and other desirable characteristics for a new TB drug (Figure 2).
Figure 2. Process to identify new drug candidate, SQ109, from a focused combinatorial library of 1,2-ethylenediamine small-molecule compounds.
Compounds were synthesized in groups of ten and evaluated in two in vitro HTS: a broth microdilution assay to determine MIC against Mtb and a bioluminescence HTS in which recombinant Mtb produces light in response to cell wall inhibition [50]. The use of dual assays was important: the microdilution assay allowed us to identify compounds that penetrated the bacterial cell wall to access their target, while the luminescence assay provided MOA specificity to minimize selection of generally toxic compounds. Each active ten-compound pool was resynthesized to enumerate each of the ten discrete compounds to identify the active structures, and our chemists evaluated the SAR of the series. In total, we identified 2796 ten-compound mixtures that were active and 170 individual compounds that had activity in both HTS and MICs in the potentially interesting (<6 μg/ml) range.
The majority of active compounds were lipophilic. The most frequently occurring fragments in the active compounds were: highly α-branched aliphatic moieties, 2,2-diphenylethyl and 3,3-diphenylpropyl fragments, tricyclic skeletons derived from adamantane-containing amine monomers, myrtanylamine and isopinocamphylamine and isoprenoid structures. We used this SAR to create a new 30,000+ diamine library to further develop the structural diversity of our library by modification of the diamine linker, which led to the discovery of novel scaffolds with activity against Mtb [51–53].
The top 27 1,2-ethylenediamine active compounds were evaluated in additional in vitro assays that included an in vitro cytotoxicity assay to determine IC50 on HepG2 cells and a calculation of the selectivity index (SI; IC50/MIC). The SI estimates the therapeutic window of a compound, which is the concentration range at which the compound should be both safe and efficacious. SQ109 had the lowest MIC (0.78 μg/ml) and the highest SI (16.7) of this group. We also determined the minimal bactericidal concentration (MBC), calculated the lipophilicity of each compound (logP) and evaluated the efficacy of the best 13 compounds in Mtb-infected macrophages, since Mtb is a facultatively intracellular organism. The best 11 compounds were advanced to in vivo efficacy studies in mice, and the most promising three compounds from those studies were evaluated in maximum tolerated dose (MTD) and pharmacokinetic (PK) studies in mice.
SQ109 had both the best antibacterial activity and the most promising toxicity, MTD and PK parameters of all of the active drug compounds [54,55], and had the same MIC against DS and MDR Mtb strains (Table 2). Moreover, it was efficacious in both a screening and a chronic model of TB in mice [56] at 10 mg/kg. SQ109 (Figure 2) was selected as the lead TB drug candidate from the library [54] and underwent extensive in vitro and in vivo studies and pharmacological and toxicology (14-, 28- and 90-day) evaluation in rats, dogs and monkeys prior to starting its clinical evaluation.
Table 2.
In vitro activity of SQ109 against Mycobacterium tuberculosis.
| Susceptibility profile | Identification number | Assay | MIC (μg/ml) |
|---|---|---|---|
| H37Rv (pan-susceptible) | ATCC 27294 | BACTEC | ≤0.2 |
| H37Rv (pan-susceptible) | ATCC 27294 | Alamar | ≤0.39† |
| Erdman (pan-susceptible) | ATCC 35801 | Alamar | ≤0.39† |
| EMB-resistant | Not reported | Alamar | 0.78† |
| INH-resistant | ATCC 35822 | Alamar | 0.78† |
| RIF-resistant | ATCC 35838 | Alamar | ≤0.39† |
| CIP-resistant | Not reported | Alamar | ≤0.39† |
| KAN-resistant | Not reported | Alamar | 0.78† |
| XDR, also EMB-resistant | 576W1‡ | Microbroth | 0.20 |
| XDR, also EMB-resistant | 587W1§ | Microbroth | 0.20 |
Work performed by the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF).
Strain from the laboratory of C Barry at the National Institute of Allergy and Infectious Diseases (NIAID): resistant to streptomycin, INH, RIF, EMB, ethionamide, KAN, CIP, capuramycin and cycloserine.
Strain from the laboratory of C Barry at NIAID: resistant to streptomycin, INH, RIF, EMB, ethionamide and KAN; sensitive to CIP, capuramycin and cycloserine.
CIP: Ciprofloxacin; EMB: Ethambutol; INH: Isoniazid; KAN: Kanamycin; RIF: Rifampicin; XDR: Extensively drug-resistant.
Since our selection of SQ109 to move forward, other research groups synthesized and evaluated SQ109 analogs by modifying the adamantane and geranyl fragments [57–59]. However, none of these analogs demonstrated notable improvement over SQ109 MIC or in vivo efficacy, supporting selection of SQ109 as best in class.
It is important to note that, although SQ109 was identified from a focused combinatorial library based on EMB, the chemical structures of EMB and SQ109 are very different (Figure 2) and the potency and activities of both drugs are quite distinct, as are their MOA (see below). We started these studies looking for a better and safer EMB, but we ended up with a substantially better new drug with an entirely new MOA and important new properties.
SQ109 has significant in vitro activity against Mycobacteria
SQ109 is active on all Mtb, including MDR- and XDR-TB clinical strains (Table 2), as well as against other Mycobacteria in the Mtb family (Mycobacteria bovis and M. bovis BCG) and Mycobacteria fortuitum. It has lower activity against other pathogenic Mycobacteria, including Mycobacteria avium, marinum, chelonae and abscessus, with MIC in the range of 4–16 μg/ml. In addition, SQ109 kills Mtb inside macrophages at a level equivalent to INH and superior to EMB [60]. At its MIC, it reduces intracellular Mtb by 99% [54]. Intracellular activity is important because Mtb are facultative intracellular pathogens: they are phagocytosed by pulmonary macrophages, but halt the maturation of the phagosome to create a protected cellular niche for their replication [61].
We also determined MBC to assess whether SQ109 is bacteriostatic or bactericidal. In our tests, SQ109 was bactericidal at a concentration of 0.64 μg/ml, approximately its MIC (range 0.2–0.78 μg/ml). The Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) also identified SQ109 as bactericidal at its MIC (0.78 μg/ml). By contrast, EMB is bacteriostatic at its MIC and at doses that can be used to treat adult pulmonary TB [62,63].
Importantly, Mtb has a very low spontaneous mutation rate (2.55 × 10−11) for SQ109 resistance [Reddy V, Unpublished Data], a rate 100–1000-times better than other antitubercular drugs or drug candidates. If this translates to a low rate of resistance development in vivo, it would be a significant new attribute of this drug.
SQ109 has a novel MOA
Although SQ109 was originally identified by creating analogs of EMB, our data clearly demonstrate that it is not active by the same mechanism as EMB. For example, SQ109 is active against a number of EMB-resistant strains (Table 2) [54]. Boshoff et al. described the transcriptional response of Mtb after exposure to various antibiotics [64], which confirmed that SQ109, similar to other well-characterized mycobacterial cell wall inhibitors, induces the transcription of the iniBAC operon. Consistent with this observation, the overall transcriptional profile of SQ109-treated cells closely resembles other cell wall-active agents such as INH, ethionamide (ETA), thiolactomycin and EMB. However, two genes upregulated by EMB were not upregulated by SQ109, and two genes downregulated by SQ109 were not downregulated by EMB.
In a study conducted by the National Cancer Institute (NCI), NIH, researchers examined postantibiotic protein changes in Mtb after exposure to SQ109, EMB and INH [65]. As with the transcriptional response studies, SQ109 had a signature profile similar to other cell wall inhibitors. However, the profiles of EMB and SQ109 were sufficiently different to suggest a distinct target.
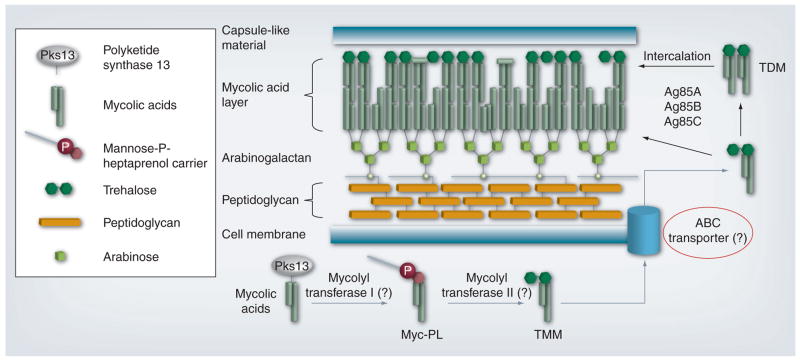
Recently, the molecular target in Mtb of SQ109 was identified by two independent groups, further substantiating the differences in MOA between this diamine and EMB [24,25]. Treatment of Mtb with SQ109 decreased the incorporation of mycolic acids into the cell wall. However, this effect was localized to incorporation into trehalose dimycolate and mycolates attached covalently to the arabinogalactan polymer. Incorporation into trehalose monomyclate (TMM) actually increased, suggesting that SQ109 did not target mycolate synthesis, but rather transport and processing. Although the authors were unable to create spontaneous SQ109-resistant mutants, they did create mutants resistant to similar ethylenediamine compounds that had some cross-resistance to SQ109. The sequencing of these mutants revealed mutations in MmpL3, the putative transporter of TMM, and the precursor of both trehalose dimycolate and cell wall mycolates (Figure 3) [24]. Work by the second group demonstrated that other adamantyl-containing compounds also target MmpL3 and prevent translocation of TMM across the membrane, further supporting the notion that this transporter is likely the target of SQ109 [25].
Figure 3. SQ109 mechanism of action.
The figure depicts stylized components of the cell wall, while primarily focusing on TMM and TDM. Hypothetical or proposed steps are indicated by gray lines, and question marks in parentheses represent hypothetical proteins/enzymes not yet identified. The inset legend represents the shapes used to depict the components shown. Note that the diagram is not to scale and does not include detailed structures or all components of the mycobacterial cell wall. MmpL3, the target of SQ109, is proposed to be the ABC transporter (red circle).
TDM: Trehalose dimycolate; TMM: Trehalose monomycolate.
Adapted with permission from [24].
By contrast, EMB inhibits the synthesis of arabinogalactan and lipoarabinomannan [66,67] and is assumed to act via inhibition of three arabinosyltransferases: EmbA, EmbB and EmbC. Table 3 summarizes the characteristics of SQ109 and EMB and demonstrates that the two drugs have distinct actions and MOA.
Table 3.
Distinct characteristics of ethambutol and SQ109.
| Characteristic | EMB | SQ109 | Ref. |
|---|---|---|---|
| Molecular weight | 204.31 g/mol | 330.55 g/mol | [46] |
| MIC on Mtb | 5–7 μg/ml | 0.12–0.36 μg/ml | [46,54] |
| MIC on EMB-resistant Mtb | 184 μg/ml | 0.12–0.78 μg/ml | [54] |
| Induces Mtb P0341 promoter, which is activated with cell wall disruption | Yes | Yes | [46] |
| Kills nonreplicating Mtb | No | Yes | [Reddy V, Unpublished data] |
| Activity on Mtb gene clusters GC82 and GC120 | Upregulates | Downregulates | [64] |
| Effect on acid-fastness of Mtb | Decreases | No effect | [64] |
| Synergy with RIF | No | Yes | [68] |
| Synergy with INH | No | Yes | [68] |
| Activity against Candida, Helicobacter pylori, Haemophilus influenzae, Streptococcus pneumoniae and Aspergillus fumigatus | No | Yes | [Sequella, Unpublished data] |
| Location of mutations in resistant Mtb | embA, embB and embC | mmpL3 | [24,78,79] |
| Target | Inhibition of AG and LAM biosynthesis | Inhibition of TMM transport | [24,80] |
AG: Arabinogalactan; EMB: Ethambutol; INH: Isoniazid; LAM: Lipoarabinomannan; Mtb: Mycobacterium tuberculosis; RIF: Rifampicin; TMM: Trehalose monomyclate.
SQ109 activity on other pathogens
SQ109 also has activity against organisms with relevance to human health, besides Mtb:
It is bactericidal for Helicobacter pylori: all H. pylori clinical isolates are susceptible to SQ109, with a MIC range of 2.5–15 μg/ml. In addition, SQ109 in time–kill assays is better than amoxicillin and metronidazole, two of the first-line drugs commonly used to treat H. pylori infection [Mokobongo M, Einck L, Merrell D. In vitro characterization of the anti-bacterial activity of SQ109 against Helicobacter pylori. (2012), Manuscript in Preparation]. Sequella filed an SQ109 Investigational New Drug (IND) application for this indication; a Phase II clinical trial is ongoing (2012).
Sequella collaborates on an NIH R01 grant with the Division of Infectious Disease and Global Health, University of Virginia (VA, USA), to investigate SQ109 and SQ109-related compounds for activity to kill Clostridium difficile. SQ109 MIC for this organism is 8–16 μg/ml.
Interestingly, SQ109 also has activity against eukaryotic microorganisms, both filamentous and yeast fungi such as Aspergillus fumigatus (MIC of 8 μg/ml), Candida spp. (MIC of 1–8 μg/ml) and Cryptococcus neoformans (MIC of 0.25–4 μg/ml).
Additional organisms against which SQ109 has in vitro activity include Enterococci spp. (MIC of 8–32 μg/ml), Haemophilus influenzae (MIC of 1–32 μg/ml), Neisseria gonorrhoeae (MIC and MBC of 0.038 μg/ml) and Streptococcus pneumoniae (MIC of 2–4 μg/ml).
It is interesting to note that MmpL3, the identified target of SQ109 in Mtb, only has homologs in other Mycobacteria, and does not have an obvious functional homolog in any of these other susceptible bacteria or fungi. It is possible that SQ109 has a unique target in these organisms that has not yet been identified, or that the inability to generate SQ109-resistant Mtb mutants directly [24] may indicate an as-yet unidentified second (or other) target present in both Mtb and the other susceptible microorganisms.
SQ109 enhances activity of existing & investigational TB drugs
We investigated in vitro interactions of SQ109 with currently used first-line drugs [68]. SQ109 demonstrated synergistic interactions with INH and RIF, and additive effects when combined with EMB or streptomycin. The strongest synergistic combination was SQ109 and RIF, and this synergy worked both ways: <MIC quantities of SQ109 synergistically improved RIF activity eightfold, and <MIC RIF synergistically improved SQ109 activity fourfold. Interestingly, in vitro studies demonstrate that SQ109 can reduce the MIC of RIF in RIF-resistant Mtb strains [68].
Importantly, no in vivo adverse drug–drug interactions or PK changes were found in research and formal pairwise studies of SQ109 with INH, RIF or streptomycin in mice or rats, supporting the value of the incorporation of SQ109 into a first-line treatment regimen.
Because SQ109 has activity against all DR-Mtb (Table 2), it could also be a part of regimens to treat MDR- or XDR-TB. Therefore, we assessed SQ109 in combination with each of the second-line TB drugs in vitro and in vivo to determine any interactions [Reddy V & Nikonenko B, Unpublished Data]. In two-drug combinations in vitro with AMK, ETA, MXF, K AN, cycloserine (CS), para-aminosalicylic acid or CM, SQ109 showed in vitro synergy with some drugs (CM, MXF and AMK) and additive effects with others (ETA, KAN, CS and para-aminosalicylic acid). No antagonistic effects were observed in any of the combinations. In PK evaluations in mice, pharmacology profiles of SQ109 and MXF were not affected by coadministration. In the presence of CS and KAN, exposure of mice to SQ109 was slightly decreased.
Clofazimine (CFZ) is a group 5 drug on the WHO list of drugs to be used in MDR-TB. Group 5 drugs have unclear efficacy or an unclear role in MDR-TB treatment. While there are toxicity concerns with CFZ, new data suggest that it may be a useful drug for combination treatment of TB [69,70]. SQ109 showed synergy with CFZ, both in vitro (Table 4) and in a macrophage intracellular killing assay [Reddy V, Unpublished Data]. Studies in mice are ongoing.
Table 4.
Checkerboard titration of SQ109 and clofazimine.
| Experiment | Drug combination | MIC (μg/ml)
|
ΣFIC | |
|---|---|---|---|---|
| Alone | Combination | |||
| 1 | SQ109 | 0.5 | 0.125 | 0.37 |
| CFZ | 0.5 | 0.062 | ||
|
| ||||
| 2 | SQ109 | 0.5 | 0.125 | 0.37 |
| CFZ | 0.5 | 0.062 | ||
Drug interactions were determined by checkerboard titration of SQ109 and CFZ by a 96-well microdilution assay described previously using Mycobacterium tuberculosis H37Rv [68]. From the MIC values, the FIC of each drug was calculated as (MIC of drug in combination)/(MIC of individual drug). The ΣFIC is the sum of the FICs of the two drugs. ΣFIC ≤0.5 is synergistic; ≥4.0 is antagonistic and in between is additive or indifferent.
CFZ: Clofazimine; ΣFIC: Fractional inhibitory concentration index.
SQ109 has interesting activity when combined with investigational TB drug candidates in development by other companies:
SQ109 has profound synergy with all the rifamycins, including rifapentine (Sanofi) and rifabutin (Pfizer), which are drugs in the same class as RIF that have a longer half-life (rifapentine) or a reduced activation of CYP3A4, a human P450 enzyme that metabolizes many drugs, including drugs to treat HIV (rifabutin). RIF and rifapentine have identical synergy results with SQ109 in vitro. Rifapentine is in clinical trials to evaluate whether it can shorten TB treatment: SQ109 has a similar half-life to rifapentine and, based on mouse studies, could be paired with this drug to both improve efficacy and shorten treatment time [71].
SQ109 and sutezolid have additive effects in vitro, and SQ109 improved synergistically the time to kill intracellular Mtb in an infected macrophage assay [72]. SQ109 and bedaquiline are apparently the two best drugs to pair with sutezolid in a new TB regimen for all forms of DS- and DR-TB [73].
SQ109 and bedaquiline were also synergistic in vitro: small amounts of SQ109 (≤0.5 MIC) improved the MIC of bedaquiline four- to eight-times over its MIC and also improved the rate of Mtb killing when the two drugs were evaluated in combination. Small amounts of bedaquiline (≤0.5 MIC) also improved SQ109 activity fourfold. In addition, SQ109 increased the postantibiotic effect of bedaquiline from 9 to 14 h [74].
SQ109 is active in mouse models of TB
PK studies based on drug serum concentrations in mice suggest that SQ109 has low oral bio-availability, but tissue distribution studies show that SQ109 achieves concentrations well above its MIC, at least 45-fold, in TB murine target organs (lung and spleen) compared with plasma (Figure 4) [60].
Figure 4. SQ109 tissue levels after oral administration to mice for 28 days.
SQ109 at 10 mg/kg; drug levels were measured 1 h after the last administration of the drug.
Reproduced with permission from [60].
Meng et al. investigated esterase-sensitive carbamate prodrugs of SQ109 with the goal of improving bioavailability [75]. They identified a prodrug that improved bioavailability of SQ109 from 21.4 to 91.4% in rats, with a high tissue distribution to the lung and spleen. However, MIC of the carbamate compound was higher than SQ109 in vitro, and the compound was unstable in rodent and human serum, and also in phosphate-buffered saline. In mice, we were unable to reproduce the increases in plasma concentrations that were observed in rats.
In a rapid, high-dose model of TB infection developed for initial screening of drug candidates [56], SQ109 (10 mg/kg) significantly reduced weight loss in treated infected mice compared with untreated infected mice. Similarly, 10 mg/kg SQ109 administered to mice for 6–8 weeks in a model of chronic TB showed better activity than EMB at 100 mg/kg [76]. In dose-ranging experiments, 25 mg/kg SQ109 showed improvement over the 10 mg/kg dose in the spleen, but no difference in lungs [54]; higher concentrations were similar to 25 mg/kg in both lungs and spleens. These efficacious doses are well below the MTD of orally delivered SQ109 in mice (600 mg/kg [54]).
Interestingly, the effects of SQ109 combined with INH and RIF are most evident with >2 weeks of treatment (Table 5): activity is significantly better than EMB at 3 and 4 weeks [76]. This may indicate that SQ109 drug action occurs not on the rapidly replicating bacteria in tissues, but those sequestered in macrophages or in a dormant state.
Table 5.
SQ109 antitubercular activity during intensive-phase drug treatment.
| Drug regimen | Log10 CFU in lung | Log decrease |
|---|---|---|
| 2 weeks | ||
| Untreated | 6.16 ± 0.02 | – |
| INH + RIF + EMB | 4.64 ± 0.23 | 1.52 |
| INH + RIF + SQ109 | 4.46 ± 0.12 | 1.70 |
| 3 weeks | ||
| Untreated | 6.34 ± 0.34 | – |
| INH + RIF + EMB | 4.38 ± 0.05 | 1.96 |
| INH + RIF + SQ109 | 3.80 ± 0.10 | 2.54 |
| 4 weeks | ||
| Untreated | 6.42 ± 0.76 | – |
| INH + RIF + EMB | 3.86 ± 0.14 | 2.56 |
| INH + RIF + SQ109 | 3.26 ± 0.12 | 3.16 |
C57BL/6 mice were inoculated intravenously with 104 Mycobacterium tuberculosis H37Rv and infection was allowed to progress for 21 days. Chemotherapy started on day 21 and continued for 4 weeks; mice were treated with the indicated combinations. Lung CFUs were assessed at 2, 3 and 4 weeks of treatment. Drugs were administered at the following concentrations: INH, 25 mg/kg; RIF, 20 mg/kg; SQ109, 10 mg/kg. At each time-point, one group of mice for each drug combination (six mice per group) was sacrificed; lung homogenates were plated in tenfold serial dilutions on 7H10 agar dishes. CFUs were calculated after 3 weeks of growth, and are expressed ± standard deviation.
EMB: Ethambutol; INH: Isoniazid; RIF: Rifampicin.
Data taken from [76].
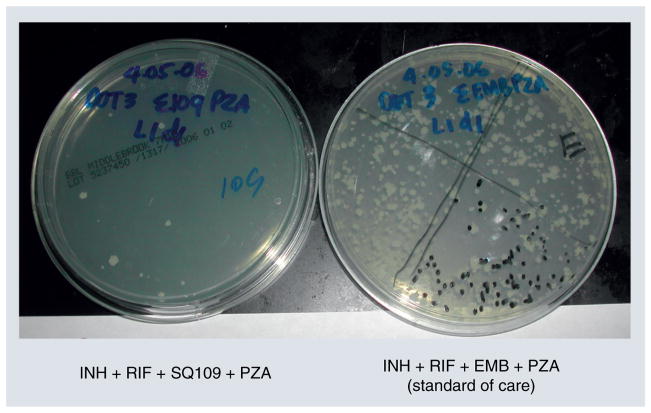
SQ109 improves overall efficacy of the standard four-drug treatment regimen (INH, RIF, PZA and EMB) when it replaces EMB for 8 weeks in a mouse model of TB [76]. In fact, no bacteria remained in lungs of half the mice in the SQ109-containing regimen, and an average 18 CFU remained in the rest; by contrast, mice treated with INH + RIF + PZA + EMB had 32-fold more CFU (average 568 CFU) than in the SQ109-containing regimen (Figure 5). These data suggest that SQ109 works effectively within the existing first-line treatment regimen to replace EMB: it may shorten treatment time and have significant therapeutic benefits over EMB.
Figure 5. SQ109 activity compared with ethambutol in the standard drug regimen.
Estimation of CFUs (white colonies of Mycobacterium tuberculosis on plates of 7H10 agar incubated at 37°C, 5% CO2 in a humidified incubator for 3 weeks) in undiluted lung homogenates of Mycobacterium tuberculosis-infected mice treated for 6 weeks with standard of care TB drugs (right plate: ~568 CFU) or a combination containing SQ109 in place of EMB (left plate: ~18 CFU).
EMB: Ethambutol; INH: Isoniazid; PZA: Pyrazinamide; RIF: Rifampicin.
Data taken from [76].
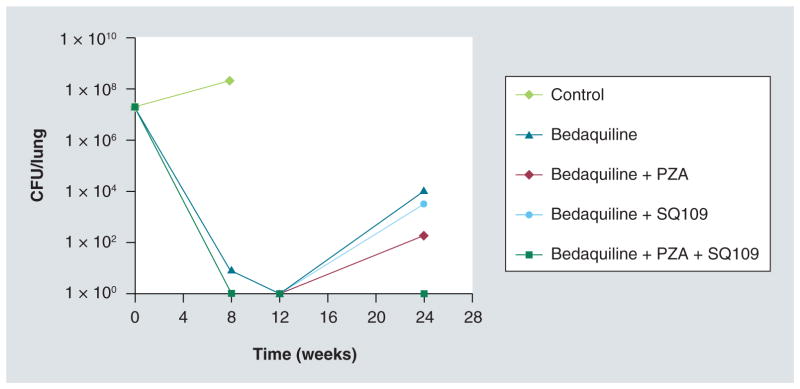
We recently evaluated SQ109 interactions with bedaquiline in vivo in a mouse model of TB: by 3 months of combination drug treatment, the regimen SQ109 + bedaquiline + PZA induced a durable cure in Mtb-infected mice (Figure 6), eliminating lung and spleen CFU better than standard of care [Nikonenko B, Unpublished Data] with a 50% reduction in time (3 vs 6 months). Although such models must be interpreted carefully as predictive ability in humans is, as yet, uncertain, these results support further clinical evaluation of the combination.
Figure 6. Treatment of mice with bedaquiline alone or incombination with SQ109 and/or pyrazinamide.
After 3 weeks of infection with Mycobacterium tuberculosis, female C57BL/6 mice were either treated with nothing (infected control, ten mice) for 3 months or with one of the described regimens in groups of 30 mice/regimen. Drug concentrations were: bedaquiline, 25 mg/kg; SQ109, 25 mg/kg; PZA, 150 mg/kg. Mice were evaluated for CFU/lung at 2 and 3 months. After 3 months of drug therapy, all control mice were dead, and lungs of ten mice in all groups were harvested and plated to evaluate CFU. Lung tissues from all drug-treated groups were culture-negative (no CFU). After therapy termination, 20 additional mice of each drug-treated group were rested (no treatment) for 3 months. Lungs were extracted, homogenized and plated undiluted on 7H10 agar for 3 weeks. CFU/lung 1 × 100 means no detected CFU (limit of detection = 1 bacterium/ml of undiluted lung homogenate).
PZA: Pyrazinamide.
These new in vitro and in vivo drug combination data are particularly exciting because of the toxicity and poor efficacy of current second-line TB drugs. One can now envision an entirely new treatment regimen for MDR-TB composed of several of the current investigational drugs, and this new combination may also prove effective in DS-TB. Sequella has collaborative research agreements with pharmaceutical developers of rifapentine, sutezolid and bedaquiline, and will build on these exciting data to underpin future clinical evaluations of new drug combinations.
IND-directed preclinical development of SQ109
The ADMET profile of SQ109 was determined [77]. Tissue distribution in rats showed the highest concentrations of SQ109 in liver and, similar to the distribution in mice, also high concentrations in the lung and spleen, the target organs for TB treatment. When radioactive SQ109 was orally administered to rats, the highest level of the drug was in liver, suggesting that the drug may undergo a first-pass metabolic step there.
Metabolic profiling of SQ109 in liver microsomes of various animal species, including humans, revealed potential metabolites suggestive of oxidation, epoxidation and N-dealkylation of the drug. CYP450 profile studies of SQ109 on human CYP450 enzymes established the formation of four major metabolites produced primarily by CYP450 2D6 and 2C19 enzymes [77]. The CYP450 enzyme of concern in drug–drug interactions, 3A4, is not activated by SQ109, nor is it metabolized by the enzyme.
Table 6 shows in vitro and in vivo safety and toxicity tests that have been performed on SQ109 to date and the results of these assays.
Table 6.
In vitroand in vivo safety studies of SQ109.
| Assay | Outcome |
|---|---|
| In vitro mutagenicity studies | |
| Salmonella–Escherichia coli/microsome plate incorporation assay | Negative |
| Mouse lymphoma mutation assay | Negative |
| Micronucleus assay in rats | Negative |
| Cardiovascular safety, beagle dogs (single oral dosing at 75, 140 and 300 mg/m2) | |
| Clinical observations | No deaths |
| Body weights | No change |
| Clinical pathology: hematology or clinical chemistry | No change |
| Blood pressure: at 75 and 140 mg/m2 | No change |
| Heart rate/ECG data | No change |
| ECG waveforms (rhythms and morphology) | No change |
| Neurological safety assessment, rats (90-day oral dosing at 30, 120 and 240 mg/m2/day) | |
| Functional observational battery parameters | No change |
| Neurological safety assessment, beagle dogs (90-day oral dosing at 100, 300 and 1000 mg/m2/day) | |
| Functional observational battery parameters | No change |
| Pulmonary safety testing, beagle dogs (single oral dosing at 75, 140 and 300 mg/m2) | |
| Respiratory rate | No change |
| Tidal volume | No change |
| Minute volume | No change |
| Reproductive toxicology studies | |
| Segment 1: effects on male and female gamete maturation, copulatory performance, fertilization, zygote development and embryo implantation | No impact |
| Segment 2: effects on pregnant female and development of embryo and fetus from implantation | No impact |
Extensive IND-directed preclinical safety, toxicology and pharmacology studies were performed on SQ109, many supported by the NCI Developmental Therapeutics Program. SQ109 was evaluated in IND-directed dose-ranging 14- and 28-day and definitive 14- and 90-day studies in dogs, and definitive 90-day studies in rats and nonhuman primates. At 40 mg/kg/day, there were no cardiovascular, hepatic, hematologic, neurological or ocular toxicities, or any adverse events (AEs) at all, except emesis in dogs, in the three animal species. The drug did cause phospholipidosis, similar to virtually all lipophilic drugs and antibiotics. The ‘no observed AEs level’ (NOAEL), depending on species, was from 30 to 40 mg/kg/day. The efficacious dose of SQ109 for treatment of TB in the murine model of acute infection is 10 mg/kg/day (~1 mg/kg/day human-equivalent dose). Based on the toxicology NOAEL, we anticipated that a dose several-fold greater than the human- equivalent dose could be explored in clinical trials to maximize clinical efficacy.
SQ109 safety in humans
Fast-track designation for SQ109 was granted by the US FDA in January 2007 under IND #104,669, and Orphan Drug Status was also granted by the EMA and FDA in 2007.
Safety of SQ109 was evaluated in three Phase I studies (80 healthy humans; Table 7) and one Phase IIa study in TB patients (75 adult patients with sputum-positive, DS pulmonary TB).
Table 7.
SQ109 clinical studies.
| Study | Cohorts | Dose (mg) | Dosing schedule† | Subjects (n)
|
|
|---|---|---|---|---|---|
| SQ109 | Placebo | ||||
| Phase Ia, escalating single dose | Fasting | 5, 10, 20, 50, 100, 200, 300 | Single oral dose after overnight fast | 42 | 14 |
| Food effect | 300 | Single oral dose after high-calorie, high-fat meal | 6 | 0 | |
|
| |||||
| Phase Ib, multiple daily doses | 1B-1 | 75 | Days 1–14 | 8 | 2 |
| 1B-2 | 150 | Days 1–5, 9 and 14 | 8 | 2 | |
| 1B-3 | 150 | Days 1–14 | 8 | 2 | |
|
| |||||
| Phase Ic, multiple daily 300 mg doses | 1C-1 | 300 | Days 1–14 | 8 | 2 |
|
| |||||
| Phase IIa, safety and EBA† | 2A-1 | 75 | Days 1–14 | 15 | 0 |
| 2A-2 | 150 | Days 1–14 | 15 | 0 | |
| 2A-3 | 300 | Days 1–14 | 15 | 0 | |
| 2A-4 | 75 + RIF | Days 1–14 | 15 | 0 | |
| 2A-5 | 150 + RIF | Days 1–14 | 15 | 0 | |
| 2A-6 | 300 + RIF | Days 1–14 | 15 | 0 | |
| 2A-7 | RIF | Days 1–14 | 15 | 0 | |
SQ109 administered in morning after overnight fast for all studies, except Phase Ia – ‘Food effect’ cohort.
EBA: Early bactericidal activity; RIF: Rifampicin 10 mg/kg.
Phase Ia (study conducted by Sequella)
There were no serious AEs (SAEs), and any AEs were not dose-related. All AEs were self-limited and none required intervention. The mean maximum concentration for 10- and 300-mg oral doses of SQ109 administered to fasting human subjects were 0.729 and 20.2 ng/ml, respectively. Both the maximum concentration and area under the curve increased in a dose-dependent manner. Increase in half-life was nonlinear, with substantially larger values for the 200- and 300-mg doses (61.1 h for the 300-mg dose cohort), which is consistent with a large volume of distribution also measured for these doses. Administration of SQ109 with a high-fat, high-calorie meal resulted in a decrease in the rate of absorption with minimal effect on the extent of absorption, resulting in a decreased maximum serum concentration and increased half-life.
Phase Ib (study conducted by NIAID, NIH)
There were no SAEs, and AEs were not dose-related and did not require intervention. SQ109, whether administered as a 75-mg dose daily for 14 days or as a 150-mg dose daily for 5 days followed by single 150-mg doses on days 9 and 14, was well tolerated.
Phase Ic (study conducted by NIAID, NIH)
There were no SAEs, although one subject withdrew due to treatment-emergent AEs of nausea, vomiting and abdominal discomfort. Vital sign, ECG, neurological, mini-mental assessment and ophthalmological data did not reveal any clinically meaningful trends or changes throughout the study.
Steady-state conditions were achieved by day 10 with daily administration of 300 mg SQ109. Based on the preclinical relationship observed between plasma and lung concentrations, steady-state plasma concentrations associated with once-daily dosing of 300 mg SQ109 correlate with lung concentrations approximately 5.8-fold greater than the SQ109 MIC. In addition, based on the plasma concentration–time profile, lung concentrations are expected to be above the MIC for Mtb 12 h after dosing in approximately 50% of subjects.
Phase IIa (study conducted in collaboration with the European Developing Countries Clinical Trial Partnership)
We recently completed enrollment to evaluate safety, tolerance, EBA and PK of 14 days of daily doses of SQ109 at 75, 150 or 300 mg monotherapy, or SQ109 150 or 300 mg plus standard-dose RIF. Standard-dose RIF, the positive control, was administered to one cohort of subjects. The study was conducted at three sites in Cape Town, South Africa. Preliminary safety analysis indicates that all doses of SQ109, with or without concomitant RIF, were safe and well-tolerated. The only AEs were gastric and gastrointestinal complaints, which for all but one patient resolved spontaneously; one patient who was administered SQ109 withdrew because of pain from pre-existing, but undiagnosed, esophageal candidiasis. PK and efficacy data are being evaluated and should be available in mid-2012.
Future perspective
Recent years have shown a rise in interest in the application of modern drug-discovery techniques to the field of TB, leading to an unprecedented number of new TB drug candidates in clinical trials. On the downside, recent years also saw the application of many prejudices of modern drug development to anti-infective and antitubercular drugs. SQ109 does not fit the mold of traditional pharmaceuticals, and its development has been slowed by concern that its basic structure does not fit the norms of other nonantibiotic drug classes. These prejudices slowed new antitubercular clinical evaluation, which has not kept pace with the rapidly expanding worldwide need for new TB drugs. It is important to continue to explore new ideas and new chemical space to combat public health threats such as TB.
The goal of the SQ109 clinical program is to investigate the utility of this agent in both DS and DR strains of Mtb by replacing EMB in the current standard-of-care regimen to treat disease caused by DS strains and adding SQ109 to second-line regimens that are tailored to the specific MDR-TB disease. The low bacterial mutation rate (2.55 × 10−11) observed in vitro combined with the extensive tissue penetration observed in vivo suggest that the drug has significant potential clinical utility to treat pulmonary TB and to decrease relapses/recurrences due to the emergence of antibiotic resistance. Synergy between SQ109 and RIF or INH may also shorten treatment times for uncomplicated TB and could reverse the phenotypic resistance of some MDR-TB strains so that the first-line TB drugs can also be used in certain MDR-TB cases.
While initial SQ109 clinical studies are focused on replacing a single drug or being added to an existing drug regimen, a tantalizing scenario is clinical evaluation of a novel treatment regimen containing multiple TB drug candidates. Because of limited resources, diagnosis of DR-TB often takes place only after treatment failure. This approach leads to an increase in morbidity and mortality, as well as the development of further drug resistance that is difficult or impossible to treat. The FDA recently released draft guidance for the codevelopment of two unmarketed drug candidates, signaling their willingness to consider registration of new drug combinations prior to individual drug registration [104]. This raises the possibility that a completely novel drug regimen could eventually be used to treat both DS and DR-TB. The long half-life of SQ109 may facilitate combination of SQ109 with other TB drug candidates such as bedaquiline (which also has a long half-life) or sutezolid, with which it interacts well in vitro, thereby enabling treatment regimens with less frequent dosing and improved patient compliance.
Executive summary.
TB is a global health crisis
A third of the world’s population is infected with Mycobacterium tuberculosis.
There are 8.8 million incident TB cases each year and more than 1.5 million deaths.
Existing treatment regimens leave significant room for improvement because of toxicity and long durations.
Drug-resistant TB, multidrug-resistant TB and extensively drug-resistant TB threaten our control of this deadly disease.
Several new TB drug candidates are in clinical development
The field of TB drug development has seen a recent resurgence.
Several drugs and drug candidates for TB are in clinical trials.
SQ109 is a novel TB drug candidate & has promising characteristics
-
SQ109 has the following promising characteristics:
Significant in vitro activity against Mycobacterium tuberculosis;
Novel mechanism of action;
Pulmonary accumulation of the drug in preclinical animal studies;
Activity on nontubercular pathogens;
Enhances activity of existing and investigational TB drugs;
Active in mouse models of TB;
Shows promise in Investigational New Drug-directed preclinical studies;
Found to be safe and well tolerated in Phase I and II clinical studies at doses up to 300 mg.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
KA Sacksteder and M Protopopova own stock options in Sequella, Inc.; CA Nacy owns stock and stock options in Sequella, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Stevenson CR, Forouhi NG, Roglic G, et al. Diabetes and tuberculosis: the impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163(9):1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 3.Nolan CM. Beyond directly observed therapy for tuberculosis. Chest. 1997;111(5):1151–1153. doi: 10.1378/chest.111.5.1151. [DOI] [PubMed] [Google Scholar]
- 4.Castelnuovo B. A review of compliance to anti tuberculosis treatment and risk factors for defaulting treatment in sub Saharan Africa. Afr Health Sci. 2010;10(4):320–324. [PMC free article] [PubMed] [Google Scholar]
- 5.Vieira AA, Ribeiro SA. Compliance with tuberculosis treatment after the implementation of the directly observed treatment, short-course strategy in the city of Carapicuiba, Brazil. J Bras Pneumol. 2011;37(2):223–231. doi: 10.1590/s1806-37132011000200013. [DOI] [PubMed] [Google Scholar]
- 6.Small PM, Hopewell PC, Singh SP, et al. The epidemiology of tuberculosis in San Francisco A population-based study using conventional and molecular methods. N Engl J Med. 1994;330(24):1703–1709. doi: 10.1056/NEJM199406163302402. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien JK, Sandman LA, Kreiswirth BN, Rom WN, Schluger NW. DNA fingerprints from Mycobacterium tuberculosis isolates of patients confined for therapy noncompliance show frequent clustering. Chest. 1997;112(2):387–392. doi: 10.1378/chest.112.2.387. [DOI] [PubMed] [Google Scholar]
- 8.Coker RJ. Public health impact of detention of individuals with tuberculosis: systematic literature review. Public Health. 2003;117(4):281–287. doi: 10.1016/S0033-3506(03)00035-0. [DOI] [PubMed] [Google Scholar]
- 9.Weis SE, Slocum PC, Blais FX, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med. 1994;330(17):1179–1184. doi: 10.1056/NEJM199404283301702. [DOI] [PubMed] [Google Scholar]
- 10.Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9(3):153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 11.Zumla A, Abubakar I, Raviglione M, et al. Drug-resistant tuberculosis – current dilemmas, unanswered questions, challenges, and priority needs. J Infect Dis. 2012;205(Suppl 2):S228–S240. doi: 10.1093/infdis/jir858. [DOI] [PubMed] [Google Scholar]
- 12.Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375(9728):1830–1843. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 13.Sotgiu G, Ferrara G, Matteelli A, et al. Epidemiology and clinical management of XDR-TB: a systematic review by TBNET. Eur Respir J. 2009;33(4):871–881. doi: 10.1183/09031936.00168008. [DOI] [PubMed] [Google Scholar]
- 14.Friedland G. Tuberculosis, drug resistance, and HIV/AIDS: a triple threat. Curr Infect Dis Rep. 2007;9(3):252–261. doi: 10.1007/s11908-007-0039-7. [DOI] [PubMed] [Google Scholar]
- 15.Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196(Suppl 1):S86–S107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 16.Farley JE, Ram M, Pan W, et al. Outcomes of multi-drug resistant tuberculosis (MDR-TB) among a cohort of South African patients with high HIV prevalence. PLoS One. 2011;6(7):e20436. doi: 10.1371/journal.pone.0020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsberg AM. Drugs in development for tuberculosis. Drugs. 2010;70(17):2201–2214. doi: 10.2165/11538170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Rivers EC, Mancera RL. New anti-tuberculosis drugs with novel mechanisms of action. Curr Med Chem. 2008;15(19):1956–1967. doi: 10.2174/092986708785132906. [DOI] [PubMed] [Google Scholar]
- 19.Lalloo UG, Ambaram A. New antituberculous drugs in development. Curr HIV/AIDS Rep. 2010;7(3):143–151. doi: 10.1007/s11904-010-0054-4. [DOI] [PubMed] [Google Scholar]
- 20.Dover LG, Coxon GD. Current status and research strategies in tuberculosis drug development. J Med Chem. 2011;54(18):6157–6165. doi: 10.1021/jm200305q. [DOI] [PubMed] [Google Scholar]
- 21.Sala C, Hartkoorn RC. Tuberculosis drugs: new candidates and how to find more. Future Microbiol. 2011;6(6):617–633. doi: 10.2217/fmb.11.46. [DOI] [PubMed] [Google Scholar]
- 22.Rustomjee R, Diacon AH, Allen J, et al. Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob Agents Chemother. 2008;52(8):2831–2835. doi: 10.1128/AAC.01204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360(23):2397–2405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- 24▪.Tahlan K, Wilson R, Kastrinsky DB, et al. SQ109 targets MmpL3, a membrane transporter of trehalose monomycolate involved in mycolic acid donation to the cell wall core of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2012;56(4):1797–1809. doi: 10.1128/AAC.05708-11. Identification of a novel target of SQ109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grzegorzewicz AE, Pham H, Gundi VA, et al. Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nat Chem Biol. 2012;8(4):334–341. doi: 10.1038/nchembio.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leach KL, Brickner SJ, Noe MC, Miller PF. Linezolid, the first oxazolidinone antibacterial agent. Ann NY Acad Sci. 2011;1222:49–54. doi: 10.1111/j.1749-6632.2011.05962.x. [DOI] [PubMed] [Google Scholar]
- 27.Villar M, Sotgiu G, D’Ambrosio L, et al. Linezolid safety, tolerability and efficacy to treat multidrug- and extensively drug-resistant tuberculosis. Eur Respir J. 2011;38(3):730–733. doi: 10.1183/09031936.00195210. [DOI] [PubMed] [Google Scholar]
- 28.Migliori GB, Eker B, Richardson MD, et al. A retrospective TBNET assessment of linezolid safety, tolerability and efficacy in multidrug-resistant tuberculosis. Eur Respir J. 2009;34(2):387–393. doi: 10.1183/09031936.00009509. [DOI] [PubMed] [Google Scholar]
- 29.Von Der Lippe B, Sandven P, Brubakk O. Efficacy and safety of linezolid in multidrug resistant tuberculosis (MDR-TB) – a report of ten cases. J Infect. 2006;52(2):92–96. doi: 10.1016/j.jinf.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Schecter GF, Scott C, True L, Raftery A, Flood J, Mase S. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2010;50(1):49–55. doi: 10.1086/648675. [DOI] [PubMed] [Google Scholar]
- 31.Dietze R, Hadad DJ, McGee B, et al. Early and extended early bactericidal activity of linezolid in pulmonary tuberculosis. Am J Respir Crit Care Med. 2008;178(11):1180–1185. doi: 10.1164/rccm.200806-892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lofmark S, Edlund C, Nord CE. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis. 2010;50(Suppl 1):S16–S23. doi: 10.1086/647939. [DOI] [PubMed] [Google Scholar]
- 33.Brooks JV, Furney SK, Orme IM. Metronidazole therapy in mice infected with tuberculosis. Antimicrob Agents Chemother. 1999;43(5):1285–1288. doi: 10.1128/aac.43.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wayne LG. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur J Clin Microbiol Infect Dis. 1994;13(11):908–914. doi: 10.1007/BF02111491. [DOI] [PubMed] [Google Scholar]
- 35.Paramasivan CN, Kubendiran G, Herbert D. Action of metronidazole in combination with isoniazid & rifampicin on persisting organisms in experimental murine tuberculosis. Indian J Med Res. 1998;108:115–119. [PubMed] [Google Scholar]
- 36.Klinkenberg LG, Sutherland LA, Bishai WR, Karakousis PC. Metronidazole lacks activity against Mycobacterium tuberculosis in an in vivo hypoxic granuloma model of latency. J Infect Dis. 2008;198(2):275–283. doi: 10.1086/589515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhillon J, Allen BW, Hu YM, Coates AR, Mitchison DA. Metronidazole has no antibacterial effect in Cornell model murine tuberculosis. Int J Tuberc Lung Dis. 1998;2(9):736–742. [PubMed] [Google Scholar]
- 38.Alvirez-Freites EJ, Carter JL, Cynamon MH. In vitro and in vivo activities of gatifloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2002;46(4):1022–1025. doi: 10.1128/AAC.46.4.1022-1025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diacon AH, Dawson R, Hanekom M, et al. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2011;15(7):949–954. doi: 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]
- 40.Diacon AH, Dawson R, Hanekom M, et al. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob Agents Chemother. 2010;54(8):3402–3407. doi: 10.1128/AAC.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbachyn MR, Hutchinson DK, Brickner SJ, et al. Identification of a novel oxazolidinone (U-100480) with potent antimycobacterial activity. J Med Chem. 1996;39(3):680–685. doi: 10.1021/jm950956y. [DOI] [PubMed] [Google Scholar]
- 42.Cynamon MH, Klemens SP, Sharpe CA, Chase S. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother. 1999;43(5):1189–1191. doi: 10.1128/aac.43.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw KJ, Barbachyn MR. The oxazolidinones: past, present, and future. Ann NY Acad Sci. 2011;1241(1):48–70. doi: 10.1111/j.1749-6632.2011.06330.x. [DOI] [PubMed] [Google Scholar]
- 44.Wallis RS, Jakubiec W, Kumar V, et al. Biomarker-assisted dose selection for safety and efficacy in early development of PNU-100480 for tuberculosis. Antimicrob Agents Chemother. 2011;55(2):567–574. doi: 10.1128/AAC.01179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallis RS, Jakubiec WM, Kumar V, et al. Pharmacokinetics and whole-blood bactericidal activity against Mycobacterium tuberculosis of single doses of PNU-100480 in healthy volunteers. J Infect Dis. 2010;202(5):745–751. doi: 10.1086/655471. [DOI] [PubMed] [Google Scholar]
- 46.Lee RE, Protopopova M, Crooks E, Slayden RA, Terrot M, Barry CE., 3rd Combinatorial lead optimization of [1,2]-diamines based on ethambutol as potential antituberculosis preclinical candidates. J Comb Chem. 2003;5(2):172–187. doi: 10.1021/cc020071p. [DOI] [PubMed] [Google Scholar]
- 47.Desimoni G. Preliminary observations of ethambutol in pulmonary tuberculosis. Ann NY Acad Sci. 1966;135(2):846–848. doi: 10.1111/j.1749-6632.1966.tb45527.x. [DOI] [PubMed] [Google Scholar]
- 48.Donomae I, Yamamoto K. Clinical evaluation of ethambutol in pulmonary tuberculosis. Ann NY Acad Sci. 1966;135(2):849–881. doi: 10.1111/j.1749-6632.1966.tb45528.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhu M, Burman WJ, Starke JR, et al. Pharmacokinetics of ethambutol in children and adults with tuberculosis. Int J Tuberc Lung Dis. 2004;8(11):1360–1367. [PubMed] [Google Scholar]
- 50.Alland D, Steyn AJ, Weisbrod T, Aldrich K, Jacobs WR., Jr Characterization of the Mycobacterium tuberculosis iniBAC promoter, a promoter that responds to cell wall biosynthesis inhibition. J Bacteriol. 2000;182(7):1802–1811. doi: 10.1128/jb.182.7.1802-1811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bogatcheva E, Hanrahan C, Nikonenko B, et al. Identification of SQ609 as a lead compound from a library of dipiperidines. Bioorg Med Chem Lett. 2011;21(18):5353–5357. doi: 10.1016/j.bmcl.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bogatcheva E, Hanrahan C, Nikonenko B, et al. Identification of new diamine scaffolds with activity against Mycobacterium tuberculosis. J Med Chem. 2006;49(11):3045–3048. doi: 10.1021/jm050948+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bogatcheva E, Hanrahan C, Chen P, et al. Discovery of dipiperidines as new antitubercular agents. Bioorg Med Chem Lett. 2010;20(1):201–205. doi: 10.1016/j.bmcl.2009.10.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54▪▪.Protopopova M, Hanrahan C, Nikonenko B, et al. Identification of a new antitubercular drug candidate, SQ109, from a combinatorial library of 1,2-ethylenediamines. J Antimicrob Chemother. 2005;56(5):968–974. doi: 10.1093/jac/dki319. Demonstration of in vitro and in vivo activity of TB drug candidate SQ109. [DOI] [PubMed] [Google Scholar]
- 55.Jia L, Tomaszewski JE, Noker PE, Gorman GS, Glaze E, Protopopova M. Simultaneous estimation of pharmacokinetic properties in mice of three anti-tubercular ethambutol analogs obtained from combinatorial lead optimization. J Pharm Biomed Anal. 2005;37(4):793–799. doi: 10.1016/j.jpba.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 56.Nikonenko BV, Samala R, Einck L, Nacy CA. Rapid, simple in vivo screen for new drugs active against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2004;48(12):4550–4555. doi: 10.1128/AAC.48.12.4550-4555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Onajole OK, Sosibo S, Govender P, et al. Novel linear diamine disubstituted polycyclic ‘cage’ derivatives as potential antimycobacterial candidates. Chem Biol Drug Des. 2011;78(6):1022–1030. doi: 10.1111/j.1747-0285.2011.01242.x. [DOI] [PubMed] [Google Scholar]
- 58.Onajole OK, Govender P, Van Helden PD, et al. Synthesis and evaluation of SQ109 analogues as potential anti-tuberculosis candidates. Eur J Med Chem. 2010;45(5):2075–2079. doi: 10.1016/j.ejmech.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 59.Meng Q, Luo H, Chen Y, Wang T, Yao Q. Synthesis of novel [1,2]-diamines with antituberculosis activity. Bioorg Med Chem Lett. 2009;19(18):5372–5375. doi: 10.1016/j.bmcl.2009.07.126. [DOI] [PubMed] [Google Scholar]
- 60▪.Jia L, Tomaszewski JE, Hanrahan C, et al. Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug. Br J Pharmacol. 2005;144(1):80–87. doi: 10.1038/sj.bjp.0705984. Demonstrates that SQ109 accumulates in the lung, a target organ for TB treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Armstrong JA, Hart PD. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yendapally R, Lee RE. Design, synthesis, and evaluation of novel ethambutol analogues. Bioorg Med Chem Lett. 2008;18(5):1607–1611. doi: 10.1016/j.bmcl.2008.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Donald PR, Maher D, Maritz JS, Qazi S. Ethambutol dosage for the treatment of children: literature review and recommendations. Int J Tuberc Lung Dis. 2006;10(12):1318–1330. [PubMed] [Google Scholar]
- 64.Boshoff HI, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE., 3rd The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J Biol Chem. 2004;279(38):40174–40184. doi: 10.1074/jbc.M406796200. [DOI] [PubMed] [Google Scholar]
- 65.Jia L, Coward L, Gorman GS, Noker PE, Tomaszewski JE. Pharmacoproteomic effects of isoniazid, ethambutol, and N-geranyl-N′-(2-adamantyl)ethane-1,2-diamine (SQ109) on Mycobacterium tuberculosis H37Rv. J Pharmacol Exp Ther. 2005;315(2):905–911. doi: 10.1124/jpet.105.087817. [DOI] [PubMed] [Google Scholar]
- 66.Goude R, Amin AG, Chatterjee D, Parish T. The arabinosyltransferase EmbC is inhibited by ethambutol in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2009;53(10):4138–4146. doi: 10.1128/AAC.00162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takayama K, Kilburn JO. Inhibition of synthesis of arabinogalactan by ethambutol in Mycobacterium smegmatis. Antimicrob Agents Chemother. 1989;33(9):1493–1499. doi: 10.1128/aac.33.9.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen P, Gearhart J, Protopopova M, Einck L, Nacy CA. Synergistic interactions of SQ109, a new ethylene diamine, with front-line antitubercular drugs in vitro. J Antimicrob Chemother. 2006;58(2):332–337. doi: 10.1093/jac/dkl227. [DOI] [PubMed] [Google Scholar]
- 69.Van Deun A, Maug AK, Salim MA, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182(5):684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 70.Reddy VM, O’Sullivan JF, Gangadharam PR. Antimycobacterial activities of riminophenazines. J Antimicrob Chemother. 1999;43(5):615–623. doi: 10.1093/jac/43.5.615. [DOI] [PubMed] [Google Scholar]
- 71.Langdon G, Wilkins JJ, Smith PJ, McIlleron H. Consecutive-dose pharmacokinetics of rifapentine in patients diagnosed with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2004;8(7):862–867. [PubMed] [Google Scholar]
- 72▪.Reddy VM, Dubuisson T, Einck L, et al. SQ109 and PNU-100480 interact to kill Mycobacterium tuberculosis in vitro. J Antimicrob Chemother. 2012;67(5):1163–1166. doi: 10.1093/jac/dkr589. Demonstration of synergy between two investigational drugs with novel mechanisms of action. [DOI] [PubMed] [Google Scholar]
- 73▪▪.Wallis RS, Jakubiec W, Mitton-Fry M, et al. Rapid evaluation in whole blood culture of regimens for XDR-TB containing PNU-100480 (sutezolid), TMC207, PA-824, SQ109, and pyrazinamide. PLoS One. 2012;7(1):e30479. doi: 10.1371/journal.pone.0030479. Evaluation of multiple regimens found that the most active was a combination of investigational drugs with entirely new mechanisms of action, which could be used to treat both drug-sensitive and drug-resistant TB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74▪.Reddy VM, Einck L, Andries K, Nacy CA. In vitro interactions between new antitubercular drug candidates SQ109 and TMC207. Antimicrob Agents Chemother. 2010;54(7):2840–2846. doi: 10.1128/AAC.01601-09. Demonstration of synergy between two investigational drugs with novel mechanisms of action. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meng Q, Luo H, Liu Y, Li W, Zhang W, Yao Q. Synthesis and evaluation of carbamate prodrugs of SQ109 as antituberculosis agents. Bioorg Med Chem Lett. 2009;19(10):2808–2810. doi: 10.1016/j.bmcl.2009.03.091. [DOI] [PubMed] [Google Scholar]
- 76.Nikonenko BV, Protopopova M, Samala R, Einck L, Nacy CA. Drug therapy of experimental tuberculosis (TB): improved outcome by combining SQ109, a new diamine antibiotic, with existing TB drugs. Antimicrob Agents Chemother. 2007;51(4):1563–1565. doi: 10.1128/AAC.01326-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jia L, Noker PE, Coward L, Gorman GS, Protopopova M, Tomaszewski JE. Interspecies pharmacokinetics and in vitro metabolism of SQ109. Br J Pharmacol. 2006;147(5):476–485. doi: 10.1038/sj.bjp.0706650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sreevatsan S, Stockbauer KE, Pan X, et al. Ethambutol resistance in Mycobacterium tuberculosis: critical role of embB mutations. Antimicrob Agents Chemother. 1997;41(8):1677–1681. doi: 10.1128/aac.41.8.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plinke C, Cox HS, Zarkua N, et al. embCAB sequence variation among ethambutol-resistant Mycobacterium tuberculosis isolates without embB306 mutation. J Antimicrob Chemother. 2010;65(7):1359–1367. doi: 10.1093/jac/dkq120. [DOI] [PubMed] [Google Scholar]
- 80.Belanger AE, Besra GS, Ford ME, et al. The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc Natl Acad Sci USA. 1996;93(21):11919–11924. doi: 10.1073/pnas.93.21.11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.WHO. Global Tuberculosis Control 2011. 2011 www.who.int/tb/publications/global_report/en.
- 102.Linezolid to Treat Extensively-Drug Resistant Tuberculosis. http://clinicaltrials.gov/ct2/show/NCT00727844.
- 103.Metronidazole for Pulmonary Tuberculosis (South Korea) http://clinicaltrials.gov/ct2/show/NCT00425113.
- 104.US Department of Health and Human Services, US FDA, Center for Drug Evaluation and Research (CDER) New Guidance for Industry: Codevelopment of Two or More Unmarketed Investigational Drugs for Use in Combination. 2010 Dec; www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/ucm237264.htm.