Abstract
Background
Supernumerary sex chromosome aneuploidies (X/Y-aneuploidies), the presence of extra X- and/or Y-chromosomes, are associated with heightened rates of language impairments and social difficulties. However, no single study has examined different language domains and social functioning in the same sample of children with tri-, tetra-, and pentasomy X/Y-aneuploidy. The current research sought to fill this gap in the literature and to examine dosage effects of X- and Y-chromosomes on language and social functioning.
Methods
Participants included 110 youth with X/Y-aneuploidies (32 female) and 52 with typical development (25 female) matched on age (mean~12 years; range 4–22) and maternal education. Participants completed the Wechsler intelligence scales and parents completed the Children’s Communication Checklist-2 and the Social Responsiveness Scale to assess language skills and autistic traits, respectively.
Results
Both supernumerary X- and Y-chromosomes were related to depressed structural and pragmatic language skills and increased autistic traits. The addition of a Y-chromosome had a disproportionately greater impact on pragmatic language; the addition of one or more X-chromosomes had a disproportionately greater impact on structural language.
Conclusions
Given that we link extra X-chromosomes with structural language impairments and an extra Y-chromosome with pragmatic language impairments, X/Y-aneuploidies may provide clues to genetic mechanisms contributing to idiopathic language impairment and autism spectrum disorders.
Keywords: Chromosome anomalies, Social cognition, Language disorder, Autistic disorder, Sex differences
Supernumerary sex chromosome aneuploidies (referred to here as X/Y-aneuploidies), the presence of extra X- and/or Y-chromosomes, occur at a collective rate of ~1/475 births (Nielsen & Wohlert, 1990). Sex chromosome trisomies (e.g., XXX, XXY, XYY) occur most frequently, each with rates of ~1/900 births, while tetra- and pentasomies (e.g., XXXX, XXXXX, XXXY, XXXXY) are considerably rarer, each with rates of ~1/85,000 births or fewer (Linden, Bender, & Robinson, 1995). There is longstanding evidence that children with X/Y-aneuploidies have language-learning difficulties. However, only recently, attention also has been drawn to social difficulties, including autism spectrum disorder (ASD) symptomatology, in these groups (for reviews, see Leggett, Jacobs, Nation, Scerif, & Bishop, 2010; Lee, Lopez, Adeyemi, & Giedd, 2011). Given the close connection between idiopathic language impairments and ASDs and the fact no prior studies have examined language and/or social functioning in children with the X/Y tri-, tetra-, and pentasomies, the current study sought to examine both of these domains of functioning in a sample of children with XXX, XXXX, XXXXX, XYY, XXY, XXXY, and XXXXY and typically developing (TD) controls. In particular, we aimed to evaluate dosage effects of X- and Y-chromosomes on language and social functioning in order to shed light not only on the nature of language and social difficulties in children with X/Y aneuploidies, but also on the possible contributions of the X- and Y-chromosomes to idiopathic language impairments and ASDs.
Prospective newborn screening studies of X/Y-aneuploidies (Walzer, Bashir, & Silbert, 1990; Ratcliffe et al., 1982, Bender et al., 1983; Netley & Rovet, 1982) and more recent reports (Bishop et al., 2011; Ross, Zeger, Kushner, Zinn, & Roeltgen, 2009) have noted increased rates of language-based learning disorders, including speech, semantic and syntactic deficits (which we refer to as ‘structural language’ or non-social language deficits) and depressed Verbal IQ scores in XXY and XXX. While these children often have lower nonverbal IQ scores than siblings, significant nonverbal learning difficulties are not commonly reported (Bender, Linden, & Robinson, 1991).
For males with an additional Y-chromosome (XYY), cognitive findings are inconsistent. Some prospective studies reported about a one standard deviation decrease in general cognitive functioning (Ratcliffe et al., 1982; Walzer et al., 1990). However, when data were pooled across early studies, no significant depression in overall cognitive abilities was found (Netley, 1986). Nevertheless, more recent research suggests that at a minimum, males with XYY have depressed verbal cognitive and structural language skills relative to TD peers (Bishop et al., 2011; Ross et al., 2009).
Research on children with X-chromosome tetra- and pentasomies (XXXX, XXXXX, XXXY, XXXXY) suggests decreases in verbal and nonverbal intellectual abilities with each additional X-chromosome (Linden et al., 1995), such that many of these individuals have cognitive abilities in the borderline to intellectually disabled range (Visootsak, Rosner, Dykens, Tartaglia, & Graham, 2007; though Gropman et al., 2010 suggests that nonverbal cognition is relatively preserved in XXXXY despite profound language deficits). Furthermore, studies suggest significant structural language impairments in these groups (Visootsak et al., 2007), including severe dyspraxia resulting in limited to no speech in XXXXY (Gropman et al., 2010).
In summary, there appears to be strong evidence for structural language impairments in X/Y-aneuploidies. However, less is known about pragmatic or more social aspects of language, including discourse, understanding of metaphor and humor, and nonverbal communication. The few studies that have been completed converge in implicating pragmatic language difficulties in X/Y trisomies using standardized (Ross et al., 2009) and experimental cognitive tasks (van Rijn et al., 2007) as well as parent report (Bishop et al., 2011). However, no study has examined pragmatic language skills in X/Y tetra- and pentasomies, or pragmatic language vis-à-vis structural language functioning in any X/Y-aneuploidy.
Even less is known about the social phenotype associated with X/Y-aneuploidies. Recent studies suggest that the addition of one X-chromosome in males (XXY) is associated with heightened rates of ASDs and social-cognitive impairments (Bishop et al., 2011; Bruining, Swaab, Kas, & van Engeland, 2009; van Rijn, Swaab, Aleman, & Kahn, 2006). In contrast, reports of females with XXX and males with XXXY and XXXXY are not indicative of increased ASD risk (Bishop et al., 2011; Visootsak et al., 2007). Reports of males with XYY indicate that an additional Y-chromosome may be associated with social difficulties, as these males appear to have increased rates of ASDs and ASD symptomatology (Bishop et al., 2011; Geerts, Steyaert, & Fryns, 2003).
Thus, the current research sought to examine verbal and nonverbal intellectual skills, structural and pragmatic language abilities, and ASD symptomatology in a large sample of children with sex chromosome tri-, tetra-, and pentasomies and TD controls in order to examine X- and Y-chromosome dosage effects on these phenotypes. Unlike previous studies investigating genotype-specific profiles, we focused on quantity of supernumerary sex chromosomes. Therefore, we collapsed across genotypes (e.g., +0X=XX, XY; +1X=XXX, XXY), and in the case of tetra- and pentasomies, limited sample size necessitated combining these groups (i.e., +2/3X=XXXX, XXXXX, XXXY, XXXXY; consistent with prior work by Visootsak et al., 2007) in order to answer study questions regarding X and Y dosage effects. We predict that:
1- Increased X- and Y-chromosome number will be associated with increased intellectual impairments with a discrepantly stronger impact on verbal relative to nonverbal intelligence.
2-Supernumerary X- and Y-chromosomes will be associated with impairments in both structural and pragmatic language; however, a supernumerary Y-chromosome will be associated with more pronounced pragmatic than structural language deficits.
3-Elevated ASD symptomatology will be associated with X/Y-aneuploidy, but supernumerary X-chromosome dosage effects will not be present.
METHOD
Participants
A total of 162 (110 with X/Y-aneuploidies; 52 TD) youth, ages 4–22 years, participated. The X/Y-aneuploidy group was recruited through advertisements via the NIH website and parent-support groups across North America (see Giedd et al., 2007 for XXY study description). To be included in the study, participants must have had an X/Y-aneuploidy (confirmed by karyotype) and not to have ever had an acquired head injury or condition that would result in gross brain abnormalities. All but 3 participants with X/Y-aneuploidy were non-mosaic. Two participants with XXX and 1 with XXY had a small number of disomy lymphoblast cells (4–14% disomy). Results of analyses run with and without these participants were the same; thus, participants with mosaicism were included in all analyses.
TD participants were recruited from the US and were enrolled in an ongoing brain development study of single and twin births (Giedd et al., 2009). Twenty-one of the 52 controls were unrelated twins. Inclusionary criteria for TD participants included never having required special education services, taken psychiatric medications, received mental health treatment, or having had any condition known to affect gross brain development. TD participants were selected to come from families with similar background characteristics to the X/Y-aneuploidy groups. This was operationalized in terms of group matching on race and maternal educational achievement. While the X/Y-aneuploidy subgroups were not explicitly matched to one another using these criteria, the groups were quite similar on these demographic variables.
Table 1 provides demographic information as well as Wechsler Estimated-IQ scores, Children’s Communication Checklist-II (CCC-2) Composite Scores, and Social Responsiveness Scale (SRS) T-Scores. Note that data are presented in terms of X/Y dosage rather than genotype – i.e., data are reported for +0X, +1X, +2/3X, +0Y, and +1Y chromosomes. Demographic information by genotype can be found in the online appendix-Table A1. Groups did not differ on age, maternal education, or race (ps>.14). The control group had higher Estimated-IQ and CCC-2 scores and lower SRS autism ratings. The finding of reduced IQ in the X/Y-aneuploidy groups is consistent with the observation based on prospective newborn screening studies that each additional X- or Y-chromosome is associated with ~1 standard deviation IQ reduction (Polani, 1977). Thus, we elected not to match cases and controls on IQ, since we wanted to avoid matching participants on a cognitive variable known to be a part of the phenotype. However, follow-up analyses were also completed with an IQ-matched subsample.
TABLE 1.
Demographic Information
| X&Y Dosage: | X-EFFECTS (♂,♀) | Y-EFFECTS (♂) | ||||
|---|---|---|---|---|---|---|
| +0X
|
+1X
|
+2/3X
|
+0Y
|
+1Y
|
||
| N | 52 | 55 | 24 | 27 | 15 | |
| Genotypes | XX (n=25) | XXX(n=28) | XXXX(n=3) | XY(n=27) | XYY(n=15) | |
| XY(n=27) | XXY(n=27) | XXXXX(n=1) | ||||
| XXXY (n=8) | ||||||
| XXXXY(n=12) | ||||||
| AGE | M | 12.24 | 11.83 | 10.31 | 12.36 | 12.3 |
| SD | 3.72 | 3.19 | 4.58 | 3.76 | 3.14 | |
| MALE1 | n (%) | 27 (51.9) | 27 (49.1) | 20 (83.3) | 27 (100) | 15 (100) |
| CAUCAS. | n (%) | 43 (82.7) | 48 (87.3) | 18 (75.0) | 23 (85.2) | 15(100) |
| MAT. ED. | ||||||
| -Grad. | n (%) | 8 (15.4) | 9 (16.4) | 4 (16.7) | 3 (11.1) | 3 (20) |
| -Bach. | n (%) | 20 (38.5) | 29 (52.7) | 9 (37.5) | 12 (44.4) | 5 (33.3) |
| -H.S. | n (%) | 24 (46.2) | 17 (30.9) | 11 (45.8) | 12 (44.4) | 7 (46.7) |
| Estimated-IQ 2,4,6 | M | 113.15 | 96.74 | 69.02 | 114.21 | 97.05 |
| SD | 10.37 | 12.61 | 8.63 | 8.33 | 13.67 | |
| CCC COMPOS.2,4 | M | 112.06 | 88.2 | 72.62 | 111.12 | 79.71 |
| SD | 9.79 | 16.94 | 10.6 | 9.97 | 17.37 | |
| SRS T-SCORE 3,5 | M | 43.4 | 64.35 | 67.54 | 43.65 | 68.47 |
| SD | 6.11 | 14.86 | 12.94 | 7.06 | 16.25 | |
+0X,+1X<+2/3X;
+0X>+1X>+2/3X;
+0X<+1X, +2/3X;
+0Y>+1Y;
+0Y<+1Y;
Estimated-IQ based on mean scores of Vocabulary, Similarities, Block Design, & Matrix Reasoning subtests
Written consent was obtained for participants over the legal age of majority. Verbal or written assent was obtained from minors along with written parental consent. The National Institute of Mental Health Institutional Review Board approved the protocol.
Procedures and Measures
Direct testing of participants was completed at the National Institutes of Health in Bethesda, Maryland. Depending on participant age, either the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) or Wechsler Preschool and Primary Scale of Intelligence–Third Edition (WPPSI-III; Wechsler, 2002) was completed. These scales share four subtests in common. Thus, for comparability across tests, estimated Verbal IQ (Estimated-VIQ) and Performance IQ (Estimated-PIQ) scores were generated based on averaging published, age-normed standardized scores from the Vocabulary and Similarities subtests and the Block Design and Matrix Reasoning subtests, respectively. These averaged scores were converted so that they were on a scale in which the mean was 100 and SD was 15, given that this is a familiar metric to most professionals.
Parents of participants completed the CCC-2 U.S. Edition (Bishop, 2003). It is comprised of four structural language (A-Speech, B-Syntax, C-Semantics, D-Coherence) and four pragmatic language (E-Initiation, F-Scripted Language, G-Context, H-Nonverbal Communication) scales. There are also two scales that assess behavioral difficulties associated with ASD (I-Social Relations and J-Interests). See Norbury, Nash, Baird, and Bishop (2004) for details.
To reduce the number of analyses completed, composite scores were created by averaging the publisher’s age-based standard scores (mean=10; SD=3) on the structural (A–D) and pragmatic (E–H) language subtests, referred to as CCC-StructLang and CCC-PragLang, respectively. Analogous to the Wechsler scales, these composite scores were converted so that they could be on a scale where the mean was 100 and SD was 15. The CCC-2 also yields a single score, the Social-Interaction-Difference-Index, which provides information about a child’s social skills/pragmatic language relative to their structural language. The manual indicates that the Social-Interaction-Difference-Index ‘… was designed to be helpful in identifying children with a communicative profile that might be characteristics of language impairment or ASD’ (Bishop, 2003, p.18). The Social-Interaction-Difference-Index reflects the difference between scales, E+H+I+J and A+B+C+D. Note the scales included in the pragmatic/social component of the Social-Interaction-Difference-Index do not map directly onto the CCC-PragLang composite computed for this study. The Social-Interaction-Difference-Index replaces two pragmatic scales with the two ASD scales. The manual reports that positive scores indicate stronger pragmatic/social skills than structural language abilities, characteristic of language impairments, while negative scores indicate stronger structural language than pragmatic/social skills, characteristic of ASD.
The SRS (Constantino, 2005), a sex-normed measure of behaviors associated with ASD across the full range of severity, was completed by parents. The SRS distinguishes individuals with ASD from controls and is highly correlated with the Autism Diagnostic Interview (Constantino et al., 2003). The total raw score from the SRS was used in primary analyses, as it is the most psychometrically sound index based on factor analytic, reliability, and validity studies (Constantino, 2005; Constantino et al., 2004). However, sex-normed standardized T-scores are reported in Table 1 as a common metric to indicate severity of ASD traits; higher scores indicate more autistic traits.
RESULTS
Separate analyses were run to evaluate X- and Y-chromosome dosage effects. X-dosage effects were evaluated in a sample males and females grouped based on the number of supernumerary X chromosomes present (i.e., +0X=XX, XY; +1X=XXX, XXY; +2/3X=XXXX, XXXXX, XXXY, XXXXY). Note that tetra- and pentasomy X groups were combined due to small sample sizes and lack of significant differences between groups with 2 or 3 Xs on dependent variables (DVs), which included Estimated-VIQ, Estimated-PIQ, VIQ-PIQ-Difference, CCC-StructLang, CCC-PragLang, CCC-Social-Interaction-Difference-Index, SRS Total. Initially, sex effects on X-dosage were evaluated in univariate ANOVA models. However, because there were neither sex main effects nor sex-by-X interactions for the seven DVs, sex was dropped from all models. Y-dosage effects were evaluated by contrasting the performance of males with one extra Y-chromosome (XYY) with typical males (XY).
Follow-up tests were completed in order to contrast X- and Y-chromosome effects on language profiles, evaluate the presence of possible ascertainment and recruitment biases, examine the contributions of group IQ differences to results, and test X-chromosome dosage effects utilizing trend analyses.
Primary Analyses
HYPOTHESIS 1: Increased X- and Y-chromosome number will be associated with increased intellectual impairments with a discrepantly stronger impact on verbal relative to nonverbal intelligence
X Effects
Results of the univariate ANOVAs revealed a significant effect of X-chromosome number on estimated-VIQ scores (F(2,115)=80.9, p<.001) and estimated-PIQ scores (F(2,115)=63.00, p<.001). Tukey-adjusted posthoc tests showed that VIQ and PIQ were negatively impacted by increasing Xs in a step-wise fashion, with +0X>+1X>+2/3 Xs (ps<.001). However, there was no significant effect of X-chromosome number on the VIQ-PIQ difference score (F(2,114)=1.66, p=.19).
Y Effects
Univariate ANOVA results included a significant effect of Y-chromosome number on estimated-VIQ (F(1,39)=25.11, p<.001) and estimated-PIQ (F(1,39)=10.03, p<.01). Furthermore, there was a significant effect of Y-chromosome number on the VIQ-PIQ difference score (F(1,39)=5.96, p<.05) such that VIQ was relatively lower.
See Table 2 for these results and those of all primary analyses.
HYPOTHESIS 2: Supernumerary X- and Y-chromosomes will be associated with impairments in both structural and pragmatic language; however, a supernumerary Y-chromosome will be associated with more pronounced pragmatic than structural language deficits
Table 2.
X and Y Dosage Effects on Language and Social Measures
| X-EFFECTS (♂,♀) | Y-EFFECTS (♂) | |||||
|---|---|---|---|---|---|---|
| X&Y Dosage Genotypes included in analyses | +0X (XX, XY)
|
+1X (XXY, XXX)
|
+2/3X (XXXY, XXXX, XXXXY, XXXXX)
|
+0Y (XY)
|
+1Y (XYY)
|
|
| Estim-VIQ2,4, | N | 52 | 52 | 14 | 27 | 14 |
| M | 113.61 | 95.48 | 67.68 | 113.98 | 91.25 | |
| SD | 12.50 | 13.25 | 9.07 | 10.77 | 18.36) | |
| Estim-PIQ2,4 | N | 52 | 51 | 15 | 27 | 14 |
| M | 112.69 | 98.33 | 71 | 114.44 | 102.86 | |
| SD | 11.01 | 15.19 | 10.08 | 10.1 | 12.89 | |
| Estim-VIQPIQ | N | 52 | 51 | 14 | 27 | 14 |
| DIFF.4 | M | 0.91 | −3.19 | −2.68 | −0.46 | −11.61 |
| SD | 11.17 | 13.10 | 8.46 | 12.60 | 16.10 | |
| STRUCT. | N | 51 | 52 | 21 | 26 | 14 |
| LANG.2,4 | M | 106.67 | 90.34 | 73.33 | 106.74 | 85.8 |
| SD | 6.22 | 14.06 | 9.61 | 5.83 | 16.04 | |
| PRAG. | N | 51 | 52 | 21 | 26 | 14 |
| LANG.2,4 | M | 108.26 | 91.15 | 82.2 | 107.02 | 81.07 |
| SD | 7.32 | 14.27 | 9.86 | 8.08 | 13.81 | |
| SOC. | N | 51 | 52 | 21 | 26 | 14 |
| INTER. | M | 2.14 | 1.37 | 8.33 | 0.81 | −4.64 |
| DEV. IND.1, 4 | SD | 5.73 | 9.3 | 8.24 | 6.45 | 10.04 |
| SRS | N | 50 | 55 | 24 | 26 | 15 |
| TOTAL3, 5 | M | 17.83 | 59.07 | 67.99 | 20.24 | 73.13 |
| SD | 12.42 | 30.04 | 24.83 | 14.64 | 34.99 | |
+0X,+1X<+2/3X;
+0X>+1X>+2/3X;
+0X<+1X, +2/3X;
+0Y>+1Y;
+0Y<+1Y;
X Effects
There was a significant effect of number of Xs on CCC-StructLang (F(2,121)=77.90, p<.001) and CCC-PragLang scores (F(2,121)=51.70, p<001). Tukey-adjusted posthoc analyses show that both CCC-StructLang and CCC-PragLang were impacted negatively by increasing number of Xs in a step-wise fashion, with +0X>+1X>+2/3 Xs (ps<.003). There was also a significant effect of number of Xs on CCC-Social-Interaction-Difference-Index scores (F(2,121)=6.31, p<.01). Tukey-adjusted posthoc tests show that the +2/3X group had a greater CCC-Social-Interaction-Difference-Index score (greater structural language deficits) than the +0X and +1X groups (ps<.01) who did not differ from one another.
Y Effects
There was a significant effect of Y on CCC-StructLang (F(1,38)=36.09, p<.001) and CCC-PragLang (F(1,38)=56.62, p<.001) scores. There was also a significant Y effect on CCC-Social-Interaction-Difference-Index scores (F(1,38)=4.37, p<.05), such that the +1Y group had relatively greater pragmatic deficits than the +0Y group.
HYPOTHESIS 3: Elevated ASD symptomatology will be associated with X/Y-aneuploidy, but supernumerary X-chromosome dosage effects will not be present
X Effects
There was a significant effect of X-chromosome number on SRS Total (F(2,126)=54.07, p<.001). Tukey-adjusted posthoc tests revealed that both the +1X and +2/3X groups had significantly more autistic traits than controls (ps<.001) but their scores did not differ significantly from one another (p>.27).
Y Effects
There was a significant Y effect on SRS Total (F(1,39)=46.12, p<.001), with more autistic traits in the +1Y than control group.
Follow-up Analyses
Contrasting X and Y Effects on Language Profiles
X and Y effects on structural and pragmatic language profiles were also contrasted by examining differences on the CCC-Social-Interaction-Difference-Index for the trisomy groups only. A univariate ANOVA with one between-subjects factor (supernumerary sex chromosome: X or Y) revealed a main effect of sex chromosome, such that supernumerary Y was associated with greater pragmatic language deficits (F(1,64) = 4.45, p < .05) relative to a supernumerary X (X: M = 1.37 ± 9.29; Y: M = 4.64 ± 10.04).
Evaluating Possible Ascertainment and Recruitment Effects
Because of the rarity of X/Y aneuploidies and the fact that the US does not routinely conduct prenatal screening for these conditions, children with either pre- (Trisomies-64%) or postnatal (Trisomies-36%; Tetra- and pentasomies-100%) diagnoses were included in the study. Unlike sex chromosome tetra- and pentasomies, the physical phenotypes associated with X/Y trisomies are not pronounced. As a result, many children go undiagnosed (Boyd, Loane, Garne, Khoshnood, & Dolk, 2010). Thus, trisomy participants who were postnatally diagnosed may not be representative of the greater X/Y trisomy population, as their phenotype may be more severe (i.e., learning/social problems led to postnatal genetic testing and subsequent diagnosis). By including postnatally diagnosed children, our results may be impacted by this ascertainment bias. Furthermore, there is a possibility that even prenatally-diagnosed children in our study are not representative of the greater X/Y trisomy population, as we have included participants of parents who may have joined family support groups (and subsequently signed up for our study) due to concerns about their child’s language and social development. We refer to this as a recruitment bias.
To evaluate the possible effects of ascertain and recruitment biases and to identify a subgroup of trisomy participants who are least likely to be impacted by these biases, several steps were taken. First, parents were asked (a) if they belonged to a support group, and (b) if they did, information was obtained about whether they joined the group before or after their child’s first birthday. Consistent with Bishop et al. (2011), we reasoned that parents who joined support groups prior to their child’s 1st birthday were less likely to be motivated to join the group because of concerns about their child’s language and social development (which often arise after age 1) than were those parents who sought membership after their child’s 1st birthday.
We then divided our trisomy participants into three subgroups: (a) PRE1: Prenatal diagnosis; parent did not belong to support group or joined before child’s 1st birthday, (b) PRE2: Prenatal diagnosis; parent joined group after child’s 1st birthday; (3)POST: Postnatal diagnosis. Ns for the groups were: XXX: PRE1=11; PRE2=12; POST=5; XXY: PRE1=7; PRE2=3; POST= 12. (There were also 5 XXY cases for whom this information was missing.) XYY: PRE1=5; PRE2=2; POST=8.
We then re-ran all analyses with only children in the conservative PRE1 group included in the +1X and +1Y groups. These results are summarized in the online appendix-Table A2. The X effects were maintained. The Y-effects were largely the same with two exceptions – namely, the effects of Y on Estimated-PIQ was no longer statistically significant and the CCC-Social-Interaction-Difference-Index finding was reduced to a trend (p=.051). However, this may have been due to limited power, as the sample size was reduced to 5. Reassuringly, the major pattern of findings is the same – Estimated-VIQ is less than PIQ and pragmatic language is somewhat more impaired than structural language.
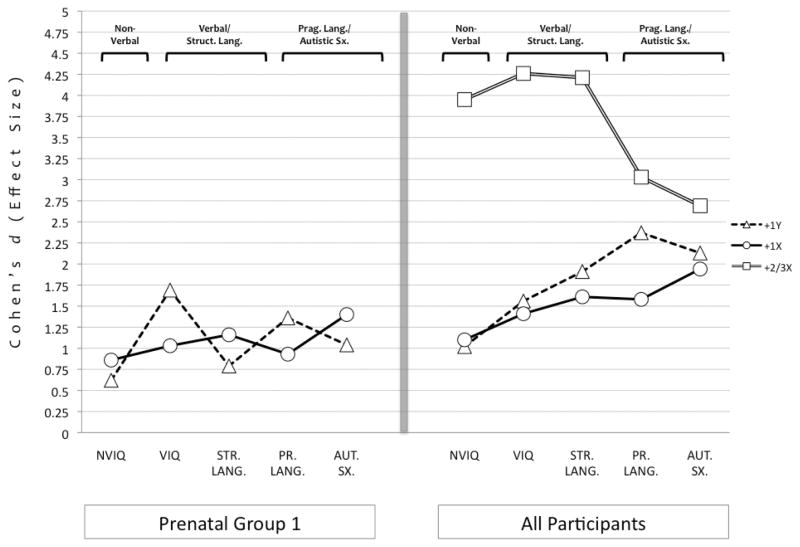
Figure 1 provides graphical presentation of group means on DVs by genotype. It also includes means for the PRE1, PRE2, and POST trisomy subgroups. Figure 2 provides effect sizes on DVs for the PRE1 trisomy subgroups and the complete sample (tri-, tetra-, pentasomies). Even in the PRE1 trisomy subgroups, effect sizes were still medium to large for all DVs. Thus, it seems unlikely that ascertainment or referral biases can account for study findings.
Figure 1.

Scores earned by participants by genotype. Overall group mean is indicated with solid dark line. Means for the XYY, XXY, and XXX PRE1, PRE2, and POST subgroups are indicated with dotted lines. Scores reported in Panels a–f are as follows: (a)WASI Estimated-VIQ, (b)WASI Estimated-PIQ, (c)CCC-2 Structural Language, (d)CCC-2 Pragmatic Language, (e)CCC-2 Social Interaction Difference Index, and (f) SRS Raw Score. Note that PRE1= Prenatally-diagnosed subgroup whose parents were not part of support group or joined when child was <1year; PRE2=Prenatally-diagnosed subgroup whose parents joined support group when child was >1year; POST=Postnatally diagnosed subgroup.
Figure 2.
Cohen’s d effect size scores for primary dependent variables as a function of supernumerary X/Y-chromosome number. Panel (a) Effect sizes for the PRE1 trisomy groups (i.e., prenatally ascertained participants whose parents were not part of a support group or joined before the child’s first birthday) and Panel (b) Effects sizes for all participants regardless of ascertainment/recruitment
IQ Effects
To ensure that language and social deficits associated with X/Y-aneuploidies could not be accounted for by IQ differences alone, univariate analyses were re-run with a subsample of trisomy participants and controls matched on IQ. Results were largely similar. See online appendix- Table A3.
X-Chromosome Dosage Effects examined via Trend Analysis
Dosage effects were evaluated via trend analysis using linear, quadratic, and cubic models. See online appendix-Figure A1.
DISCUSSION
In the present study, we examined X/Y-aneuploidies as models to understand contributions of X- and Y-chromosomes to language and social functioning. In accordance with the extant literature, both X- and Y-aneuploidies were associated with depressed IQ scores. However, only a supernumerary Y-chromosome was associated with a statistically significant verbal IQ disadvantage. Both X- and Y-aneuploidies were found to have lower scores on a parent report of structural language compared to TD peers. Parent report also indicated that X-and Y-aneuploidies were associated with lower pragmatic language skills and heightened ASD symptomatology. However, when comparing the language profile for these groups, a consistent pattern emerges – Y-aneuploidy is associated with discrepantly lower pragmatic versus structural language scores while the opposite is true for X-aneuploidy (most pronounced in the X tetra- and pentasomy groups).
The structural language difficulties documented here are consistent with many previous studies (for a review, see Lee et al., 2011). However, no study has examined these skills in a single sample of children with tri-, tetra-, and pentasomies, thus reducing the ability of prior studies to describe the dosage effects of sex chromosomes on these skills. Our findings suggest that supernumerary X-chromosome dose is associated with increasing structural language impairments, particularly when considered relative to pragmatic language skills. In addition, we find heightened social difficulties, as evidenced by scores on the SRS, associated with supernumerary X- and Y-chromosomes. However, increasing X dosage beyond one extra X did not significantly increase ASD symptomatology. The current findings are consistent with the limited research conducted to date in which an over-representation of ASD diagnoses (Bishop et al., 2011; Bruining et al., 2009) and self-rated autistic traits (van Rijn et al., 2008) are reported for males with XXY and XYY (Bishop et al., 2011; Geerts et al., 2003).
The degree of pragmatic language difficulty was associated with both increasing X- and Y-chromosome dosage; however, a comparison of structural and pragmatic language functioning using the CCC-Social-Interaction-Difference-Index shows a double dissociation. Specifically, relatively greater pragmatic language deficits are associated with increasing Y-chromosome dosage while relatively greater structural language impairments are associated with increasing X chromosome dosage.
When X-chromosome dosage effects on primary DVs were examined using trend analysis (and the +2X and +3X groups were considered separately – See online appendix Figure A1), a linear decrement of additional X appeared to best-capture the Estimated-VIQ and PIQ data. However, quadratric trends were evident for structural and pragmatic language as well as autistic symptoms, such that increasing X dosage was not always associated with reduced abilities. Thus while increasing X dosage may be most detrimental for general cognition, this may not be the case for different language domains and autistic symptoms. This non-linearity is consistent with reports that sex chromosome dosage effects on height are nonlinear (Ottesen et al., 2010).
Research suggests that there are ~1400 genes on the X-chromosome and ~200 on the Y-chromosome (Xu & Disteche, 2006). Approximately 15–20% of genes on the X-chromosome escape inactivation. Thus, excess doses of these genes may contribute to some of the language and social impairments reported for X/Y-aneuploidies. The neuroligin (NLGN) and GTPBP6 (GTP binding protein 6, putative) genes have been identified by others in the field as candidate genes that may be associated with these language and social phenotypes (Bishop et al., 2011; Vawter, Harvey, & DeLisi, 2007) Because these genes are located in the pseudoautosomal region of the X- and Y-chromosomes and expressed in neural tissue, they are potential targets for genetic association and expression studies of language and social phenotypes within X/Y-aneuploidies. For further discussion of potential genetic mechanisms in X/Y-aneuploidies, see Lee et al. (2011).
Though the present study is novel and provides compelling results, there are limitations to consider. The assessment of language and social functioning relied upon parent report, which provides ecological validity, but suffers from limitations inherent in informant reporting (Moskowitz, 1986). However, our direct assessment of intellectual skills confirms weaknesses in verbal cognition providing convergent validity for parent report of structural language skills. Another possible limitation is the small sample sizes of the tetra- and pentasomy X participants which necessitated combining the +2X and +3X participants into one group for primary analyses (though they were separated for follow-up trend analyses). This potential shortcoming should be considered within the context of population base rates of X/Y-aneuploidies with more than one extra X (estimates suggest ~1/100,000 births or fewer) and should be tempered by the fact that our study is the largest to date to examine language and social profiles across these very rare conditions.
Finally, because prenatal screening is not routinely conducted in the US and we recruited many our participants from parent support groups, concerns may arise that our findings, along with those of many recent studies, may be affected by ascertainment and recruitment biases. While we acknowledge that the severity of the language and social phenotype appears to be impacted by ascertainment and recruitment effects, the presence of language and social difficulties relative to TD peers cannot be accounted for by these biases alone. Even when the most conservatively identified trisomy subgroups were compared to TD controls, medium to large effect sizes were evidenced on all primary dependent variables. Furthermore, the chromosomal specificity of our findings (i.e., X associated with greater structural and Y associated with greater pragmatic language difficulties) further bolsters the findings that supernumerary X- and Y-chromosomes are associated with particular language and social phenotypes.
Future studies of X-chromosome dosage could benefit from the inclusion of females with Turner syndrome (X0), a group in which the absence of an X-chromosome is characterized by relatively intact structural language abilities but impaired aspects of social communication (for review, see Lee et al., 2011), in order to extend these dosage effects. Rigorous ASD diagnostic screening utilizing gold-standard instruments and direct testing of structural and pragmatic language could also be completed. Finally, gene expression studies are needed in order to test the dosage of X- and Y-linked genes and their association with these phenotypic markers.
CONCLUSIONS
Both X- and Y-aneuploidies were associated with depressed verbal IQ scores, lower scores on parent-reported structural and pragmatic language abilities, and greater parent endorsement of ASD symptomatology. However, when examining relative strengths and weaknesses in the language profiles of these groups, we find that Y-aneuploidy is associated with discrepantly lower pragmatic versus structural language scores while the opposite pattern is found in X-aneuploidy. Therefore, X/Y-aneuploidies may serve as models for understanding sex chromosome-linked genetic contributions to language and social functioning, particularly idiopathic language disorders and ASDs.
Supplementary Material
KEY POINTS.
Supernumerary sex chromosome aneuploidies (X/Y-aneuploidies) are genetic disorders in which individuals have one or more additional X or Y chromosomes.
X/Y-aneuploidies are associated with language difficulties, but no studies have examined different types of language difficulties (social and non-social) and autism symptoms in children with one, two, or three extra Xs or Ys.
Children with extra X and Y chromosomes had greater social and non-social language difficulties and more autism symptoms than typically-developing children.
However, children with extra Ys had more pronounced social language difficulties, while children with extra Xs had more pronounced non-social language difficulties.
Therefore, studies of X/Y-aneuploidies could inform how X and Y chromosomes impact social and language functioning.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Mental Health. We thank the families for their participation.
Footnotes
Conflict of interest statement: No conflicts declared
Additional supporting information is provided along with the online version of this article.
Table S1. Demographic information by genotype
Table S2. X- and Y-dosage effects on language and social measures in trisomies in PRE1 group only.
Table S3. X- and Y-dosage effects on language and social measures in trisomies in PRE1 group and an IQ- matched control subgroup.
Figure S1. ANOVA trend analyses of X-chromosome dosage effects on dependent variables [PDF file].
References
- Bender B, Fry E, Pennington B, Puck M, Salbenblatt J, Robinson A. Speech and Language-Development in 41 Children with Sex-Chromosome Anomalies. Pediatrics. 1983;71:262–267. [PubMed] [Google Scholar]
- Bender BG, Linden M, Robinson A. Cognitive and Academic Skills in Children with Sex-Chromosome Abnormalities. Reading and Writing. 1991;3:315–327. [Google Scholar]
- Bishop DV, Jacobs PA, Lachlan K, Wellesley D, Barnicoat A, Boyd PA, et al. Autism, language and communication in children with sex chromosome trisomies. Archives of Disease in Childhood. 2011;96:954–959. doi: 10.1136/adc.2009.179747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DVM. Children’s Communication Checklist- 2: United States Edition. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Boyd PA, Loane M, Garne E, Khoshnood B, Dolk H. Sex chromosome trisomies in Europe: Prevalence, prenatal detection and outcome of pregnancy. European Journal of Human Genetics. 2011;19:231–234. doi: 10.1038/ejhg.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruining H, Swaab H, Kas M, van Engeland H. Psychiatric characteristics in a self-selected sample of boys with Klinefelter syndrome. Pediatrics. 2009;123:e865–870. doi: 10.1542/peds.2008-1954. [DOI] [PubMed] [Google Scholar]
- Constantino JN. Social Responsiveness Scale. Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP, Davis S, Hayes S, Passanante N, Przybeck T. The factor structure of autistic traits. Journal of Child Psychology and Psychiatry. 2004;45:719–726. doi: 10.1111/j.1469-7610.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- Geerts M, Steyaert J, Fryns JP. The XYY syndrome: A follow-up study on 38 boys. Genetic Counseling. 2003;14:267–279. [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Wallace GL, Lenroot RK, Lerch JP, Wells EM, et al. XXY (Klinefelter syndrome): A pediatric quantitative brain magnetic resonance imaging case-control study. Pediatrics. 2007;119:E232–E240. doi: 10.1542/peds.2005-2969. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, et al. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropman AL, Rogol A, Fennoy I, Sadeghin T, Sinn S, Jameson R, et al. Clinical Variability and Novel Neurodevelopmental Findings in 49, XXXXY Syndrome. American Journal of Medical Genetics Part A. 2010;152A:1523–1530. doi: 10.1002/ajmg.a.33307. [DOI] [PubMed] [Google Scholar]
- Lee NR, Lopez KC, Adeyemi EI, Giedd JN. Sex chromosome aneuploidies: A window for examining the effects of the X and Y chromosomes on speech, language, and social development. International Review of Research in Developmental Disabilities. 2011;40:139–180. [Google Scholar]
- Leggett V, Jacobs P, Nation K, Scerif G, Bishop DV. Neurocognitive outcomes of individuals with a sex chromosome trisomy: XXX, XYY, or XXY: a systematic review. Developmental Medicine and Child Neurology. 2010;52:119–129. doi: 10.1111/j.1469-8749.2009.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden MG, Bender BG, Robinson A. Sex chromosome tetrasomy and pentasomy. Pediatrics. 1995;96:672–682. [PubMed] [Google Scholar]
- Moskowitz DS. Comparison of self-reports, reports by knowledgeable informants, and behavioral observation data. Journal of Personality. 1986;54:294–317. [Google Scholar]
- Netley C, Rovet J. Verbal deficits in children with 47, XXY and 47, XXX karyotypes: a descriptive and experimental study. Brain and Language. 1982;17:58–72. doi: 10.1016/0093-934x(82)90005-0. [DOI] [PubMed] [Google Scholar]
- Netley CT. Summary overview of behavioural development in individuals with neonatally identified X and Y aneuploidy. Birth Defects Original Articles Series. 1986;22:293–306. [PubMed] [Google Scholar]
- Nielsen J, Wohlert M. Sex chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Birth Defects Original Articles Series. 1990;26:209–223. [PubMed] [Google Scholar]
- Norbury CF, Nash M, Baird G, Bishop DVM. Using a parental checklist to identify diagnostic groups in children with communication impairment: a validation of the Children’s Communication Checklist-2. International Journal of Language and Communication Disorders. 2004;39:345–364. doi: 10.1080/13682820410001654883. [DOI] [PubMed] [Google Scholar]
- Ottesen AM, Aksglaede L, Garn I, Tartaglia N, Tassone F, Gravholt CH, Bojesen A, Juul A. Increased number of sex chromosomes affects height in a nonlinear fashion: A study of 305 patients with sex chromosome aneuploidy. American Journal of Medical Genetics. 2010;152A:1206–12. doi: 10.1002/ajmg.a.33334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polani PE. Abnormal sex chromosomes, behaviour and mental disorder. In: Tanner J, editor. Developments in psychiatric research. London: Hodder and Stoughton; 1977. [Google Scholar]
- Ratcliffe SG, Tierney I, Nshaho J, Smith L, Springbett A, Callan S. The Edinburgh study of growth and development of children with sex chromosome abnormalities. Birth Defects Original Articles Series. 1982;18:41–60. [PubMed] [Google Scholar]
- Ross JL, Zeger MP, Kushner H, Zinn AR, Roeltgen DP. An extra X or Y chromosome: contrasting the cognitive and motor phenotypes in childhood in boys with 47, XYY syndrome or 47, XXY Klinefelter syndrome. Developmental Disabilities Research Reviews. 2009;15:309–317. doi: 10.1002/ddrr.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S, Aleman A, Swaab H, Krijn T, Vingerhoets G, Kahn R. What it is said versus how it is said: comprehension of affective prosody in men with Klinefelter (47, XXY) syndrome. Journal of the International Neuropsychological Society. 2007;13:1065–1070. doi: 10.1017/S1355617707071044. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Swaab H, Aleman A, Kahn RS. X-Chromosomal effects on social cognitive processing and emotion regulation: A study with Klinefelter men (47, XXY) Schizophrenia Research. 2006;84:194–203. doi: 10.1016/j.schres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Swaab H, Aleman A, Kahn RS. Social behavior and autism traits in a sex chromosomal disorder: Klinefelter (47XXY) syndrome. Journal of Autism and Developmental Disorders. 2008;38:1634–1641. doi: 10.1007/s10803-008-0542-1. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Harvey PD, DeLisi LW. Dysregulation of X-Linked Gene Expression in Klinefelter’s Syndrome and Association With Verbal Cognition. American Journal of Medical Genetics: Part B. 2007;144:728–734. doi: 10.1002/ajmg.b.30454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visootsak J, Rosner B, Dykens E, Tartaglia N, Graham JM. Behavioral phenotype of sex chromosome aneuploidies: 48, XXYY, 48, XXXY, and 49, XXXXY. American Journal of Medical Genetics. 2007;143A:1198–1203. doi: 10.1002/ajmg.a.31746. [DOI] [PubMed] [Google Scholar]
- Walzer S, Bashir AS, Silbert AR. Cognitive and behavioral factors in the learning disabilities of 47, XXY and 47, XYY boys. Birth Defects Original Articles Series. 1990;26:45–58. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 3. San Antonio, TX: Psychological Corporation; 2002. [Google Scholar]
- Xu J, Disteche CM. Sex differences in brain expression of X-and-Y-linked genes. Brain Research. 2006;1126:50–55. doi: 10.1016/j.brainres.2006.08.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.