Abstract
Ginger is frequently consumed as a spice and has numerous medicinal properties. Extensive research has characterized the anti-inflammatory, antioxidant, and antitumor activities of ginger. Previously, we reported the mercapturic acid pathway as a major metabolic route of [6]-shogaol in mice and the thiol conjugates of [6]-shogaol existed in the glucuronidated and sulfated forms in mouse urine. However, their structures are still unknown. In the present study, we further investigated the phase II metabolism of thiol-conjugated [6]-shogaol in mouse urine, in which we identified sixteen phase II metabolites of thiol-conjugated [6]-shogaol: 5-cysteinyl-[6]-shogaol glucuronide (9), 5-N-acetylcysteinyl-[6]-shogaol glucuronide (10), 5-cysteinylglycinyl-[6]-shogaol glucuronide (11), 5-methylthio-[6]-shogaol glucuronide (12), 5-cysteinyl-M6 glucuronide (13 and 14), 5-cysteinyl-M6 sulfate (15 and 16), 5-N-acetylcysteinyl-M6 glucuronide (17 and 18), 5-cysteinylglycinyl-M6 glucuronide (19 and 20), 5-cysteinylglycinyl-M6 sulfate (21 and 22), and 5-methylthio-M6 glucuronide (23 and 24) using liquid chromatography/electrospray ionization tandem mass spectrometry. The structures of these metabolites were confirmed by analyzing their MSn (n =1! 4) spectra as well as comparing with the tandem mass spectra of authentic standards. To our knowledge, this is the first report involving identification of phase II urinary metabolites of [6]-shogaol in mice.
Keywords: [6]-shogaol, thiol-conjugated phase II metabolites, HPLC-MS/MS, mouse urine
1. Introduction
Ginger (Zngiber officinale Rosc.), a tropical and subtropical cultivated plant, has been widely used as a spice, dietary supplement, and traditional medicine for centuries [1]. In recent times, there has been much research conducted to test the validity of the medicinal claims made about ginger, including anti-inflammatory, anti-microbial, anti-allergic or control of nausea and vomiting activities [2]. [6]-shogaol is one of the predominant pungent constituents in dried ginger [3–6]. A number of in vitro and in vivo studies have found that [6]-shogaol possesses many medical and biological effects including antiemetic, vasodilating, antibacterial, antifouling, antitussive, and anti-hepatotoxic properties [7–12]. Recently, [6]-shogaol has drawn more attention than [6]-gingerol, another major component in ginger, due to its higher potency. Pan et al. showed that [6]-shogaol suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages by inhibiting the activation of NF! B [13]. Dugasani et al. found that [6]-shogaol exhibited more potent antioxidant and anti-inflammatory properties than [6]-, [8]-, and [10]-gingerols [14]. More recently, along with our collaborators, we have demonstrated that [6]-, [8]-, and [10]-shogaols exhibited much higher anti-proliferative potency than [6]-, [8]-, and [10]-gingerols against human lung cancer cells (H-1299) [5], and [6]-shogaol was more effective than [6]-gingerol in inhibiting 12-O-tetradecanoylphorbol 13-acetate-induced tumor promotion in mice [6].
Due to the prospective health benefits of [6]-shogaol, characterization, identification and structure elucidation of its metabolites is an important step for thorough understanding of its biological activities. Recently, we have identified mercapturic acid pathway as one of the major metabolic routes of [6]-shogaol and found that the thiol-conjugated metabolites of [6]-shogaol existed mostly in the glucuronidated and sulfated form in mouse urine [15]. In the present study, we analyzed the phase II metabolites of thiol-conjugated [6]-shogaol in mouse urine using high-performance liquid chromatography tandem mass spectrometry. The structures of the major metabolites were identified by analyzing their MS2 ! MS4 spectra as well as comparing with the tandem mass spectra of authentic standards.
2. Experimental
2.1. Chemicals and reagents
[6]-Shogaol was purified from ginger extract in our laboratory [5]. 1-(4!-Hydroxy-3!-methoxyphenyl)-4-decen-3-ol (M6), 5-methylthio-6S, and 5-Methylthio-M6 were purified from fecal samples collected from [6]-shogaol treated mice [15]. HPLC-grade solvents and other reagents were obtained from VWR International (South Plainfield, NJ). LC-MS grade solvents and other reagents were obtained from Thermo Fisher Scientific (Pittsburgh, PA). Sulfatase from Aerobacter aerogenes and ! -glucuronidase from Helix aspersa were obtained from Sigma (St. Louis, MN).
2.2. Treatment of mice and urine collection
Experiments with mice were carried out according to a protocol described previously [15]. In brief, female C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and allowed to acclimate for at least 1 week prior to the start of the experiment. The mice were housed 5 per cage and maintained in air-conditioned quarters with a room temperature of 20 ! 2 °C, relative humidity of 50 ! 10 %, and an alternating 12-h light/dark cycle. Mice were fed Purina Rodent Chow #5001 (Research Diets) and water, and were allowed to eat and drink ad libitum. [6]-Shogaol in dimethyl sulfoxide (DMSO) was administered to mice by oral gavage (200 mg/kg). Urine samples were collected in metabolism cages (5 mice per cage) 24 h after administration of vehicle (control group, n = 5) or [6]-shogaol (treated group, n = 5). These samples were stored at −80 °C before analysis.
2.3. Urine sample preparation
The urine samples (50 ! L from each group, control group and [6]-shogaol-treated group) were added to 1200 ! L methanol to precipitate proteins. After centrifugation at 17 ! 1000 rpm for 5 min, the supernatants were transferred into vials for LC/MS analysis. Enzymatic deconjugation was performed as described previously with slight modification [16]. In brief, duplicate samples were prepared in the presence of ! -glucuronidase (250 U) and sulfatase (3 U) for 24 h at 37 • and then extracted twice with ethyl acetate. The ethyl acetate fraction was dried under vacuum, and the solid was resuspended in 250 mL of 80% aqueous methanol with 0.1% acetic acid for further LC/MS analysis.
2.4. LC/ESI-MS method
LC/MS analysis was carried out with a Thermo-Finnigan Spectra System which consisted of an Accela high-speed MS pump, an Accela refrigerated autosampler, and an LTQ Velos ion trap mass detector (Thermo Electron, San Jose, CA) incorporated with heated electrospray ionization (H-ESI) interfaces. A Gemini C18 column (50 ! 2.0 mm i.d., 3 ! m; Phenomenex, Torrance, CA, USA) was used for separation at a flow rate of 0.2 mL/min. The column was eluted with 100% solvent A (5% aqueous methanol with 0.2% acetic acid) for 3 min, followed by linear increases in B (95% aqueous methanol with 0.2% acetic acid) to 40% from 3 to 15 min, to 85% from 15 to 45 min, to 100% from 45 to 50 min, and then with 100% B from 50 to 55 min. The column was then re-equilibrated with 100% A for 5 min. The LC eluent was introduced into the ESI interface. The positive ion polarity mode was set for the H-ESI source with the voltage on the HESI interface maintained at approximately 4.5 kV. Nitrogen gas was used as the sheath gas and auxiliary gas. Optimized source parameters, including ESI capillary temperature (300 °C), capillary voltage (50 V), ion spray voltage (3.6 kV), sheath gas flow rate (30 units), auxiliary gas flow rate (5 units), and tube lens (120 V), were tuned using authentic 5-N-acetylcysteinyl-[6]-shogaol. The negative ion polarity mode was set for the H-ESI source with the voltage on the HESI interface maintained at approximately 4.5 kV. Nitrogen gas was used as the sheath gas and auxiliary gas. Optimized source parameters, including ESI capillary temperature (300 °C), capillary voltage (50 V), ion spray voltage (3.6 kV), sheath gas flow rate (30 units), auxiliary gas flow rate (5 units), and tube lens (120 V), were tuned using authentic 5-N-acetylcysteinyl-5-shogaol. The collision-induced dissociation (CID) for both positive and negative was conducted with an isolation width of 2 Da and normalized collision energy of 35 eV for MS2, MS3 and MS4. Default automated gain control target ion values were used for MS• MS4 analyses. The mass range was measured from 50 to 1000 m/z. Data acquisition was performed with Xcalibur 2.0 version (Thermo Electron, San Jose, CA).
3. Results and Discussion
3.1. Strategies for data analysis
Previously, we have reported that the mercapturic acid pathway is one of the major metabolic routes of [6]-shogaol and have identified seven thiol-conjugated metabolites of [6]-shogaol and its ketone-reduced metabolite 1-(4!-hydroxy-3!-methoxyphenyl)-4-decen-3-ol (M6) (Fig. 1). It was determined that the thiol-conjugated metabolites of [6]-shogaol were undetectable without incubation with glucuronidase and sulfatase in mouse urine and plasma indicating that these thiol-conjugated metabolites exist in the glucuronidated and/or sulfated forms [15]. The current study further investigated the phase II metabolites of these thiol-conjugated compounds in mouse urine. In this study, we used selected-ion monitoring (SIM) mode to search all the potential phase II metabolites of the thiol-conjugated metabolites of [6]-shogaol from urine samples collected from [6]-shogaol treated mice. The possible glucuronidated metabolites included 5-cysteinyl-[6]-shogaol glucuronide (5-Cys-6S-Glu, m/z 573), 5-N-acetylcysteinyl-[6]-shogaol glucuronide (5-NAC-6S-Glu, m/z 615), 5-cysteinylglycinyl-[6]-shogaol glucuronide (5-Cys-Gly-6S-Glu, m/z 630), 5-glutathione-[6]-shogaol glucuronide (5-GSH-6S-Glu, m/z 759), 5-methylthio-[6]-shogaol glucuronide (5-Methylthio-6S-Glu, m/z 500), 5-cysteinyl-1-(4!-hydroxy-3!-methoxyphenyl)-4-decan-3-ol glucuronide (5-Cys-M6-Glu, m/z 575), 5-N-acetylcysteinyl-1-(4!-hydroxy-3!-methoxyphenyl)-4-decan-3-ol glucuronide (5-NAC-M6-Glu, m/z 617), 5-cysteinylglycinyl-1-(4!-hydroxy-3!-methoxyphenyl)-4-decan-3-ol glucuronide (5-Cys-Gly-M6-Glu, m/z 632), 5-glutathione-1-(4!-hydroxy-3!-methoxyphenyl)-4-decan-3-ol glucuronide (5-GSH-M6-Glu, m/z 761), and 5-methylthio-1-(4!-hydroxy-3!-methoxyphenyl)-4-decan-3-ol glucuronide (5-Methylthio-M6-Glu, m/z 502). The possible sulfated metabolites included 5-cysteinyl-[6]-shogaol sulfate (5-Cys-6S-Sul, m/z 477), 5-N-acetylcysteinyl-[6]-shogaol sulfate (5-NAC-6S-Sul, m/z 519), 5-cysteinylglycinyl-[6]-shogaol sulfate (5-Cys-Gly-6S-Sul, m/z 534), 5-glutathione-[6]-shogaol sulfate (5-GSH-6S-Sul, m/z 663), 5-methylthio-[6]-shogaol sulfate (5-Methylthio-6S-Sul, m/z 404), 5-cysteinyl-1-(4!-hydroxy-3!-methoxyphenyl)-4-decan-3-ol sulfate (5-Cys-M6-Sul, m/z 479), 5-N-acetylcysteinyl-1-(4!-hydroxy-3!-methoxyphenyl)-4-decan-3-ol sulfate (5-NAC-M6-Sul, m/z 521), 5-cysteinylglycinyl-1-(4!-hydroxy-3!-methoxyphenyl)-4-decan-3-ol sulfate (5-Cys-Gly-M6-Sul, m/z 536), 5-glutathione-1-(4!-hydroxy-3!-methoxyphenyl)-4-decan-3-ol sulfate (5-GSH-M6-Sul, m/z 665), and 5-methylthio-1-(4!-hydroxy-3!-methoxyphenyl)-4-decan-3-ol sulfate (5-Methylthio-M6-Sul, m/z 406).
Figure 1.
Structures of [6]-shogaol, thiol-conjugated [6]-shogaol and their phase II metabolites, and thiol-conjugated M6 and their phase II metabolites.
We analyzed the urine samples collected from both control mice and mice treated with 200 mg/kg [6]-shogaol through oral gavage using both positive and negative ESI-MS (Fig. 2). Among all the possible metabolites, we identified 5-Cys-6S-Glu (9), 5-NAC-6S-Glu (10), 5-Cys-Gly-6S-Glu (11), 5-Methylthio-6S-Glu (12),5-Cys-M6-Glu (13 and 14), 5-NAC-M6-Glu (15 and 16), 5-Cys-Gly-M6-Glu (17 and 18), 5-Cys-M6-Sul (19 and 20), 5-Cys-Gly-M6-Sul (21 and 22), and 5-Methylthio-M6-Glu (23 and 24) as the phase II metabolites of thiol-conjugated [6]-shogaol in mouse urine (Fig. 1 and Table 1).
Figure 2.
Extracted ion chromatograms of urine samples collected from [6]-shogaol-treated mice obtained by positive ESI-MS interface: (A) before and (B) after enzymatic hydrolysis; and by negative ESI-MS interface: (C) before and (D) after enzymatic hydrolysis.
Table 1.
Major phase II metabolites of thiol-conjugated [6]-shogaol in mouse urine.
| Compound/metabolite name | Peak# | Retention time (min) |
|---|---|---|
| 5-Cys-6S | 1 | 23.10 |
| 5-NAC-6S | 2 | 33.50 |
| 5-Cys-Gly-6S | 3 | 19.52 |
| 5-Methylthio-6S | 4 | 35.34 |
| 5-Cys-M6 | 5 | 25.48 |
| 5-NAC-M6 | 6 | 35.39 |
| 5-Cys-Gly-M6 | 7 | 21.30 |
| 5-Methylthio-M6 | 8 | 35.73 |
| 5-Cys-6S-Glu | 9 | 25.77 |
| 5-NAC-6S-Glu | 10 | 35.89 |
| 5-Cys-Gly-6S-Glu | 11 | 22.05 |
| 5-Methylthio-6S-Glu | 12 | 40.42 |
| 5-Cys-M6-Glu | 13 | 24.48 |
| 14 | 28.29 | |
| 5-Cys-M6-Sul | 15 | 36.46 |
| 16 | 39.30 | |
| 5-NAC-M6-Glu | 17 | 34.89 |
| 18 | 36.87 | |
| 5-Cys-Gly-M6-Glu | 19 | 20.91 |
| 20 | 23.98 | |
| 5-Cys-Gly-M6-Sul | 21 | 27.09 |
| 22 | 30.57 | |
| 5-Methylthio-M6-Glu | 23 | 36.79 |
| 24 | 40.60 |
3.2. Identification of the glucuronidated metabolites of thiol-conjugated [6]-shogaol
In the extracted ion chromatogram of m/z 574 [M + H]+ (5-cysteinyl-[6]-shogaol glucuronide under positive mode), one new peak (peak 9 at 25.77 min) was observed from urine samples collected from [6]-shogaol treated mice (Figs 2(A) and 3(A)). This peak showed tandem mass spectrum with m/z 398 (molecular ion of 5-cysteinyl-[6]-shogaol under positive mode) and 277 (molecular ion of [6]-shogaol under positive mode) as the major product ions, indicating that 9 was the potential mono-glucuronidated metabolite of 5-cysteinyl-[6]-shogaol. The tandem mass spectrum of product ion 398 (MS3: m/z 398/574) of 9 (Fig. 3(A)) was almost identical to the MS2 spectrum of 5-cysteinyl-[6]-shogaol (MS2: m/z 398) [15], suggesting that 9 was the mono-glucuronidated metabolite of 5-cysteinyl-[6]-shogaol. The MS4 spectrum of the product ion m/z 277 (MS4: 277/398/574) of 9 (Fig. 3(A)) showed a major product ion at m/z 137 which was almost identical to the MS3 spectrum of 5-cysteinyl-[6]-shogaol (MS3: 277/398) [15], confirming our definition of 9. The presence of the glucuronidated metabolite of 5-cysteinyl-[6]-shogaol was also verified by the observation that peak 9 disappeared and a new peak (peak 1 at 23.10 min) appeared in the extracted ion chromatogram of urine samples treated with ! -glucuronidase and sulfatase (Figs 2(A) and 2(B)). Peak 1 had almost the same retention time and tandem mass spectrum as those of the authentic 5-cysteinyl-[6]-shogaol (data not shown), suggesting that peak 9 was the mono-glucuronidated metabolite of 5-cysteinyl-[6]-shogaol. Since 5-cysteinyl-[6]-shogaol has only one hydroxyl group, we then identified peak 9 as 4!-glucuronide-5-cysteinyl-[6]-shogaol (Fig. 1).
Figure 3.
LC-MS2, MS3 and MS4 (positive) spectra of (A) 5-Cys-6S-Glu (9), (B) 5-NAC-6S-Glu (10), and (C) 5-Cys-Gly-6S-Glu (11); and (D) LC-MS2 and MS3 (positive) spectra of 5-methylthio-6S-Glu (12) and MS2 spectra of authentic 5-methylthio-6S (4).
One new peak was observed (peak 10 at 35.89 min) in the extracted ion chromatogram of m/z 616 [M + H]+ (molecular ion of mono-glucuronidated 5-N-acetylcysteinyl-[6]-shogaol under positive mode) in the urine samples collected from [6]-shogaol treated mice (Figs 2(A) and 3(B)). This peak showed 176 mass units higher than that of 5-N-acetylcysteinyl-[6]-shogaol indicating it was the mono-glucuronidated 5-N-acetylcysteinyl-[6]-shogaol. Similar to 5-cysteinyl-[6]-shogaol glucuronide, 10 showed tandem mass spectrum with m/z 440 (molecular ion of 5-N-acetylcysteinyl-[6]-shogaol under positive mode) and 277 (molecular ion of [6]-shogaol under positive mode) as the major product ions and the tandem mass of m/z 440 (MS3: m/z 440/616) (Fig. 3(B)) was almost identical to the MS2 spectrum (MS2: m/z 440) of 5-N-acetylcysteinyl-[6]-shogaol [15]. Besides, the presence of peak 10 was also confirmed by the disappearance of peak 10 and the appearance of a new peak (peak 2 at 33.50 min) in the extracted ion chromatogram of urine samples treated with ! -glucuronidase and sulfatase (Fig. 2(B)). Peak 2 had almost the same retention time and tandem mass spectrum as those of the authentic 5-N-acetylcysteinyl-[6]-shogaol (data not shown), indicating that peak 10 was the mono-glucuronidated metabolite of 5-N-acetylcysteinyl-[6]-shogaol. There is only one hydroxyl group in 5-N-acetylcysteinyl-[6]-shogaol, we then identified peak 10 as 4!-glucuronide-5-N-acetylcysteinyl-[6]-shogaol (Fig. 1).
Additionally, in the SIM mode for 5-cysteinylglycinyl-[6]-shogaol glucuronide (m/z 631 [M + H]+ under ESI positive mode), one major peak (peak 11 at 20.05 min) was observed from mouse urine treated with [6]-shogaol (Figs 2(A) and 3(C)). This peak showed 176 mass units higher than that of 5-cysteinylglycinyl-[6]-shogaol, indicating it was the mono-glucuronidated 5-cysteinylglycinyl-[6]-shogaol. Peak 11 showed tandem mass spectrum with m/z 613 (molecular ion of dehydrated 11 under positive mode), 455 (molecular ion of 5-cysteinylglycinyl-[6]-shogaol under positive mode), and 437 (molecular ion of dehydrated 5-cysteinylglycinyl-[6]-shogaol under positive mode) as the major product ions. After treatment with ! -glucuronidase and sulfatase, peak 11 disappeared and one new peak (peak 3 at 19.52 min) was shown in the extracted ion chromatogram of urine samples collected from [6]-shogaol treated mice (Figs 2(A) and 2(B)). Peak 3 had a molecular weight of 454 as determined by the mass ion at m/z 455 [M + H]+, which gave 178 mass units higher than that of [6]-shogaol, indicating that 3 was possible the cysteinylglycine conjugated metabolite of [6]-shogaol. The MS2 spectrum (MS2: m/z 455) of 3 showed a dominant fragment ion at m/z 437 (Fig. 4(B)), which was the loss of H2O from 3, and the tandem mass spectrum of this product ion [MS3: m/z 437/455] gave a major product ion at m/z 301, which was the loss of m/z 137 (the major product ion of [6]-shogaol) from the molecular ion at m/z 437(Figs 4(B) and 4(C)). In addition, under negative ESI-MS detection, peak 3 showed a major product ion at m/z 177, which was the deprotonated cysteineglycyine (Figs 4(E) and 4(F)). All of these spectral features identified 3 as 5-cysteinylglycinyl-[6]-shogaol (Fig. 1). The tandem mass of the product ion m/z 455 of 11 (MS3: m/z 455/631) (Fig. 3(C)) was almost identical to the MS2 spectrum of peak 3 (m/z 455) (Fig. 4(B)), indicating that 11 was 5-cysteinylglycinyl-[6]-shogaol glucuronide. Since there is only one hydroxyl group in 5-cysteinylglycinyl-[6]-shogaol, 11 was thus confirmed as 4!-glucuronide-5-cysteinylglycinyl-[6]-shogaol (Fig. 1).
Figure 4.
LC-MS2 and MS3 spectra of 5-Cys-Gly-6S (3) and its potential fragmentation pathways in both positive (A–C) and negative (D–F) ESI detection.
Furthermore, in the extracted chromatogram of m/z 501 [M + H]+ (molecular ion of mono-glucuronidated 5-methylthio-[6]-shogaol under positive mode), one new peak (peak 12 at 40.42 min) was observed from urine samples collected from [6]-shogaol treated mice (Figs 2(A) and 3(D)). Peak 12 showed 176 mass units higher than that of 5-methylthio-[6]-shogaol, indicating that it was the mono-glucuronidated 5-methylthio-[6]-shogaol. Its MS2 spectrum showed product ions of m/z 325 (−176 Da, neutral loss of one glucuronide moiety) and m/z 307 (−194 Da, neutral loss of one glucuronide moiety and one water moiety). The MS3 spectrum of the product ion m/z 307 of 12 was almost identical to the MS2 spectrum of m/z 307 [M−18+H]+ of the authentic 5-methylthio-[6]-shogaol (Fig. 3(D)), indicating 12 was the mono-glucuronidated metabolite of 5-methylthio-[6]-shogaol. The presence of the glucuronide metabolite of 5-methylthio-[6]-shogaol was also confirmed by the observation that peak 12 disappeared and one new peak (peak 4) was shown in the extracted ion chromatogram of the urine samples treated with β-glucuronidase and sulfatase (Figs 2(A) and 2(B)). Peak 4 and authentic 5-methylthio-[6]-shogaol had almost identical retention time and MS/MS spectrum (data not shown), further suggesting that peak 12 was the mono-glucuronidated 5-methylthio-[6]-shogaol. Therefore, peak 12 was identified as 4!-glucuronide-5-methylthio-[6]-shogaol (Fig. 1).
3.3. Identification of glucuronidated and sulfated metabolites of 5-cysteinyl-M6
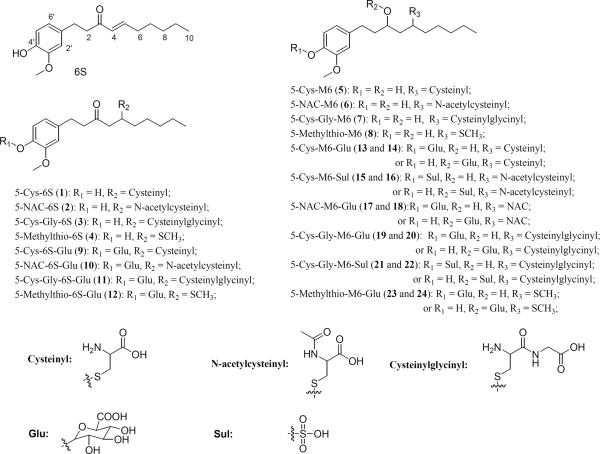
We observed two new peaks (peaks 13 and 14 at 24.48 and 28.29 min, respectively) in the extracted ion chromatogram obtained from positive ESI-MS detection with the molecular ion m/z 576 [M + H]+ (400 + 176), indicating that these two peaks were the glucuronidated metabolites of 5-cysteinyl-M6 (Molecular weight: m/z 399). Both 13 and 14 showed tandem mass spectra (Figs 5(B) and 5(C)) with m/z 400 (molecular ion of 5-cysteinyl-M6 under positive mode) and 261 (dehydrated molecular ion of M6 under positive mode) as the major product ions, indicating that the two compounds were feasible mono-glucuronidated metabolites of 5-cysteinyl-M6. In order to corroborate this proposition, we compared the tandem mass spectra of m/z 400 (MS3: m/z 400/476) of these two peaks (Figs 5(B) and 5(C)) with the MS2 spectrum of authentic 5-cysteinyl-M6 (MS2: m/z 400) [15]. Our results clearly indicated that the tandem mass spectra of this product ion of both peaks were almost identical to the MS2 spectrum of 5-cysteinyl-M6, suggesting that these two compounds were the mono-glucuronidated metabolites of 5-cysteinyl-M6. In addition, the MS4 spectrum of the product ion m/z 261 (MS4: m/z 261/400/576) of 13 and 14 (Figs 5(B) and 5(C)) demonstrated almost identical readings to the MS3 spectrum of 5-cysteinyl-M6 (MS3: m/z 261/400) [15], which also confirmed our conclusion. The presence of the glucuronidated metabolites of 5-cysteinyl-M6 was also verified by the observation that peaks 13 and 14 disappeared and a new peak (peak 5 at 25.48 min) appeared in the extracted ion chromatogram of the urine samples after treatment with ! -glucuronidase and sulfatase (Fig. 2(B)). All of these features suggested that peaks 13 and 14 were 5-cysteinyl-M6 glucuronide. Since the active sites of 5-cysteinyl-M6 for glucuronidation have not been identified and metabolites 13 and 14 had identical tandem mass spectra, we could not determine the exact structures of the two mono-glucuronidated 5-cysteinyl-M6 (Fig. 1). It has been reported that the hydroxyl group at the benzene ring of [6]-gingerol, an analogue of [6]-shogaol, is more active for glucuronidation than the hydroxyl group on the side chain [17]. Therefore, we tentatively identified the major peak (14, RT: 28.29 min) as 4!-glucuronide-5-cysteinyl-M6 and the minor peak (13, 24.48 min) as 3-glucuronide-5-cysteinyl-M6. However, this deduction needs to be further confirmed by corresponding authentic standards.
Figure 5.
(A) Extracted ion chromatogram and (B–C) ESI-MS2, MS3 and MS4 (positive ion) spectra of 5-Cys-M6-Glu (13 and 14).
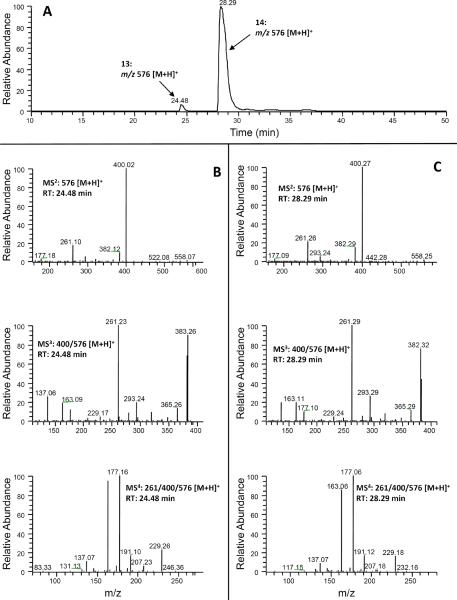
Similarly, in the extracted ion chromatogram of m/z 480 [M + H]+ (molecular ion of mono-sulfated 5-cysteinyl-M6 under positive mode), two new peaks (peaks 15 and 16 at 36.46 and 39.30 min, respectively) were observed from urine samples collected from [6]-shogaol treated mice (Fig. 6(A)). The molecular weight of these two peaks showed 80 mass units higher than that of 5-cysteinyl-M6 and both peaks had product ion m/z 400 [M − 80 + H]+ in their tandem mass spectra, suggesting that they were potential mono-sulfated metabolites of 5-cysteinyl-M6. The tandem mass spectra of the product ion m/z 400 (MS3: m/z 400/480) of those two compounds (Figs 6(B) and 6(C)) and the MS2 spectrum of 5-cysteinyl-M6 [15] showed the same fragment ion mass spectra, indicating that both of them were the mono-sulfated 5-cysteinyl-M6. Similar to the mono-glucuronidated metabolites of 5-cysteinyl-M6, we tentatively identified the major peak (16, RT: 39.30 min) as 4!-sulfate-5-cysteinyl-M6 and the minor peak (15, 36.41 min) as 3-sulfate-5-cysteinyl-M6..
Figure 6.
(A) Extracted ion chromatogram and (B–C) ESI-MS2, MS3 and MS4 (positive ion) spectra of 5-Cys-M6-Sul (15 and 16).
3.4. Identification of glucuronidated metabolite of 5-N-acetylcysteinyl-M6
In the extracted ion chromatogram of m/z 442 [M + H]+ taken from the MS2 data set obtained from m/z 618 [M + H]+ (molecular ion of mono-sulfated 5-N-acetylcysteinyl-M6 under positive mode), two new peaks (peaks 17 and 18 at 34.89 and 36.87 min, respectively) were observed from the urine samples collected from [6]-shogaol treated mice. This suggested that they were likely the mono-glucuronidated metabolites of 5-N-acetylcysteinyl-M6. The tandem mass spectra of the product ion m/z 442 (MS3: m/z 442/618) of these two peaks (Figs 7(B) and 7(C)) were almost identical to the MS2 spectrum of 5-N-acetylcysteinyl [15], confirming that they were the mono-glucuronidated metabolites of 5-N-acetylcysteinyl-M6. Similar to the mono-glucuronidated metabolites of 5-cysteinyl-M6, we tentatively identified the major peak (18, RT: 36.52 min) as 4!-glucuronide-5-N-acetylcysteinyl-M6 and the minor peak (17, 33.90 min) as 3-glucuronide-5-N-acetylcysteinyl-M6..
Figure 7.
(A) Extracted ion chromatogram and (B–C) ESI-MS2, MS3 and MS4 (positive ion) spectra of 5-NAC-M6-Glu (17 and 18).
3.5. Identification of glucuronidated and sulfated metabolites of 5-cysteinylglycinyl-M6
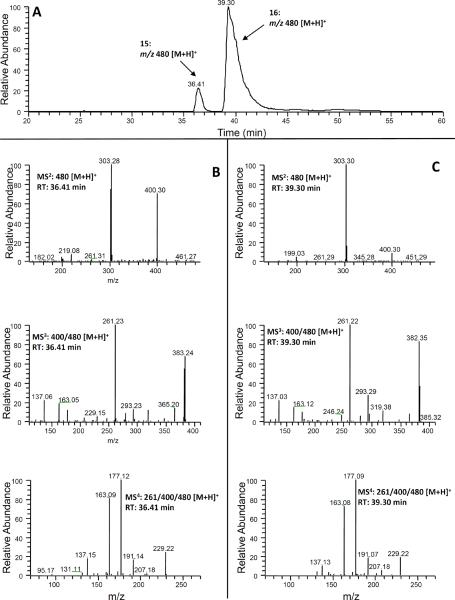
In the SIM mode for mono-glucuronidated 5-cysteinylglycinyl-M6 (m/z 633 [M + H]+ under ESI positive mode), two new peaks (peaks 19 and 20 at 20.91 and 23.98 min, respectively) were detected in the urine samples collected from [6]-shogaol treated mice (Figs 2(A) and 8 (A)). Both 19 and 20 showed similar tandem mass spectra with m/z 457 (molecular ion of 5-cysteinylglycinyl-M6 under positive mode) as the major product ion, indicating that they were potential mono-glucuronidated metabolites of 5-cysteinylglycinyl-M6. In order to further confirm this deduction, we compared the MS3 (MS3: m/z 457/633) and MS4 (MS4: m/z 301/457/633) spectra of 19 and 20 (Figs 8(B) and 8(C)) with the MS2 (MS2: m/z 457) and MS3 (MS3: m/z 301/457) spectra of 5-cysteinylglycinyl-M6 [15], respectively. Our results clearly indicated that 19 and 20 were mono-glucuronidated 5-cysteinylglycinyl-M6. Similar to the mono-glucuronidated metabolites of 5-cysteinyl-M6, we tentatively identified the major peak (20, RT: 23.98 min) as 4!-glucuronide-5-cysteinylglycinyl-M6 and the minor peak (19, 20.91 min) as 3-glucuronide-5-cysteinylglycinyl-M6..
Figure 8.
(A) Extracted ion chromatogram and (B–C) ESI-MS2, MS3 and MS4 (positive ion) spectra of 5-Cys-Gly-Glu (19 and 20).
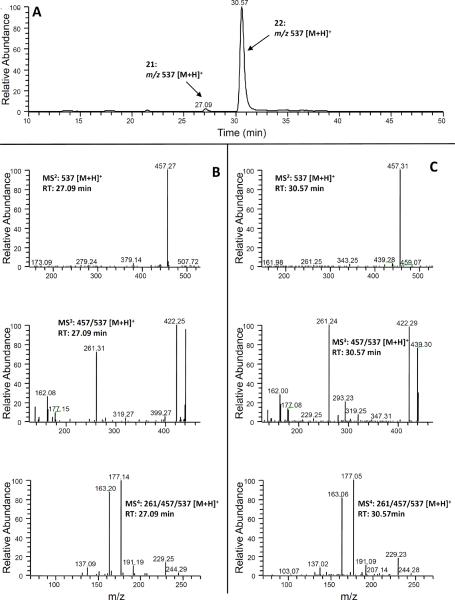
Similarly, two new peaks (peaks 21 and 22 at 20.09 and 30.57 min, respectively) were observed at m/z 537 (mono-sulfated 5-cysteinylglycinyl-M6 under positive mode), which was 80 mass units higher than that of 5-cysteinylglycinyl-M6, indicating that they were the mono-sulfate conjugated 5-cysteinylglycinyl-M6. We then compared the tandem mass spectrum of the major product ion m/z 457 (MS3: m/z 457/537) (Figs 9(B) and 9(C)) of these two peaks with that of 5-cysteinylglycinyl-M6 (MS2: m/z 457) [15]. Our results indicated that they had almost identical mass fragments. Hence, we established these metabolites as mono-sulfated 5-cysteinylglycinyl-M6 (Fig. 1). Similar to the mono-glucuronidated metabolites of 5-cysteinyl-M6, we tentatively identified the major peak (22, RT: 30.57 min) as 4!-sulfate-5-cysteinylglycinyl-M6 and the minor peak (21, 27.09 min) as 3-sulfate-5-cysteinylglycinyl-M6.
Figure 9.
(A) Extracted ion chromatogram and (B–C) ESI-MS2, MS3 and MS4 (positive ion) spectra of 5-Cys-Gly-Sul (21 and 22).
3.6. Identification of glucuronidated metabolites of 5-methylthio-M6
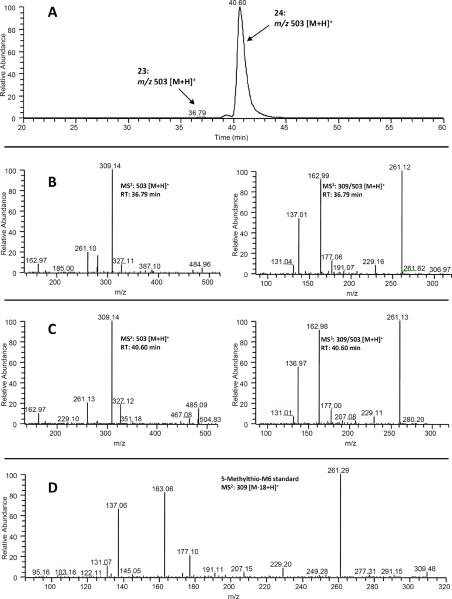
In the extracted ion chromatogram of m/z 503 [M + H]+ (molecular ion of mono-glucuronidated 5-methylthio-M6 under positive mode), two new peaks (peaks 23 and 24 at 36.76 and 40.60 min, respectively) were observed in the urine samples collected from [6]-shogaol treated mice (Figs 2(A) and 10 (A)). These two peaks showed 176 mass units higher than that of 5-methylthio-M6, indicating that they were 5-methylthio-M6 glucuronide. Both 23 and 24 showed the same product ion m/z 309 (Figs 10 (B) and 10 (C)) and the tandem mass of this product ion was almost identical to the tandem mass of authentic 5-methylthio-M6 (Fig. 10 (D)). Similar to the mono-glucuronidated metabolites of 5-cysteinyl-M6, we tentatively identified the major peak (24, RT: 40.60 min) as 4!-glucuronide-5-methylthio-M6 and the minor peak (23, 36.79 min) as 3-glucuronide-5-methylthio-M6.
Figure 10.
(A) Extracted ion chromatogram and (B–C) ESI-MS2 and MS3 (positive ion) spectra of 5-methylthio-M6-Glu (23 and 24), and (D) MS2 spectrum of authentic 5-methylthio-M6 (8).
4. Conclusions
In this study, using LC/ESI-MSn analysis we successfully identified sixteen phase II metabolites of thiol-conjugated [6]-shogaol from mouse urine collected 24 h after administration of 200 mg/kg [6]-shogaol through oral gavage (Fig. 2 and Table 1). To our knowledge, this is the first study to establish the phase II metabolites of thiol-conjugated [6]-shogaol in mouse urine using multi-stage tandem mass spectrometry. Our results indicated that all the thiol conjugated metabolites of [6]-shogaol are the prime substrates for glucuronidation, and some of them are also the substrates for sulfation. Besides the thiol-conjugated metabolites of [6]-shogaol, our previous study has also identified several non-thiol conjugated metabolites of [6]-shogaol (M6–M11) [15]. These non-thiol conjugated metabolites of [6]-shogaol exist in phase II conjugated form since they are barely detectable in mouse urine samples before treatment with glucuronidase and sulfatase (data not shown). However, these phase II metabolites are poorly ionized under ESI detection; we could not get publishable LC/MS spectra of these metabolites. New method is needed to analyze these phase II metabolites.
Our results suggested that the metabolites of [6]-shogaol are in the phase II conjugated form in mice. Glucuronidation and sulfation are typically considered as the means of inactivating biologically active compounds and facilitating excretion. However, examples of biologically active metabolites have also been reported. The most prominent example is that morphine-6-glucuronide has a more potent analgesic action than morphine [18]. Whether the thiol conjugates and their glucuronidated and sulfated metabolites are bioactive remains to be determined. The results would help us assess the relative contribution of [6]-shogaol metabolites to the disease preventive effects of [6]-shogaol. Results from this work are important for understanding the metabolism of [6]-shogaol and related analogs in humans and to provide useful information that may act as a reference in clinical pharmacology. Knowledge of the metabolism of [6]-shogaol may also help in comprehending the mechanism of action and therapeutic effects of [6]-shogaol, as well as ginger extract.
Highlights
Mouse urinary metabolic profile of thiol-conjugated [6]-shogaol was established.
Sixteen phase II metabolites of thiol-conjugated [6]-shogaol were identified.
Thiol conjugated [6]-shogaol are the substrates for glucuronidation and sulfation.
Acknowledgement
Funding for this investigation was provided by grants CA138277 (S. Sang) from the National Cancer Institute and CA138277S1 (S. Sang) from National Cancer Institute and Office of Dietary Supplement of National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Leung AY, Foster S. Encyclopedia of Common Natural Ingredients used in Food, Drugs and Cosmetics. 2nd ed. Wiley; New York: 1996. p. 271. [Google Scholar]
- [2].Kubra IR, Rao LJ. An impression on current developments in the technology, chemistry, and biological activities of ginger (Zingiber officinale Roscoe) Crit Rev Food Sci Nutr. 2012;52:651. doi: 10.1080/10408398.2010.505689. [DOI] [PubMed] [Google Scholar]
- [3].Govindarajan VS. Ginger--chemistry, technology, and quality evaluation: part 1. Crit Rev Food Sci Nutr. 1982;17:1. doi: 10.1080/10408398209527343. [DOI] [PubMed] [Google Scholar]
- [4].Govindarajan VS. Ginger-chemistry, technology, and quality evaluation: part 2. Crit Rev Food Sci Nutr. 1982;17:189. doi: 10.1080/10408398209527348. [DOI] [PubMed] [Google Scholar]
- [5].Sang S, Hong J, Wu H, Liu J, Yang CS, Pan MH, Badmaev V, Ho CT. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J Agric Food Chem. 2009;57:10645. doi: 10.1021/jf9027443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wu H, Hsieh MC, Lo CY, Liu CB, Sang S, Ho CT, Pan MH. 6-Shogaol is more effective than 6-gingerol and curcumin in inhibiting 12-O-tetradecanoylphorbol 13-acetate-induced tumor promotion in mice. Mol Nutr Food Res. 2010;54:1296. doi: 10.1002/mnfr.200900409. [DOI] [PubMed] [Google Scholar]
- [7].Ghayur MN, Gilani AH, Afridi MB, Houghton PJ. Cardiovascular effects of ginger aqueous extract and its phenolic constituents are mediated through multiple pathways. Vascul Pharmacol. 2005;43:234. doi: 10.1016/j.vph.2005.07.003. [DOI] [PubMed] [Google Scholar]
- [8].Mahady GB, Pendland SL, Yun GS, Lu ZZ, Stoia A. Ginger (Zingiber officinale Roscoe) and the gingerols inhibit the growth of Cag A+ strains of Helicobacter pylori. Anticancer Res. 2003;23:3699. [PMC free article] [PubMed] [Google Scholar]
- [9].Etoh H, Kondoh T, Noda R, Singh IP, Sekiwa Y, Morimitsu K, Kubota K. Shogaols from Zingiber officinale as promising antifouling agents. Biosci Biotechnol Biochem. 2002;66:1748. doi: 10.1271/bbb.66.1748. [DOI] [PubMed] [Google Scholar]
- [10].Hikino H, Kiso Y, Kato N, Hamada Y, Shioiri T, Aiyama R, Itokawa H, Kiuchi F, Sankawa U. Antihepatotoxic actions of gingerols and diarylheptanoids. J Ethnopharmacol. 1985;14:31. doi: 10.1016/0378-8741(85)90025-x. [DOI] [PubMed] [Google Scholar]
- [11].Suekawa M, Ishige A, Yuasa K, Sudo K, Aburada M, Hosoya E. Pharmacological studies on ginger. I. Pharmacological actions of pungent constitutents, (6)-gingerol and (6)-shogaol. J Pharmacobiodyn. 1984;7:836. doi: 10.1248/bpb1978.7.836. [DOI] [PubMed] [Google Scholar]
- [12].Abdel-Aziz H, Windeck T, Ploch M, Verspohl EJ. Mode of action of gingerols and shogaols on 5-HT3 receptors: binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur J Pharmacol. 2006;530:136. doi: 10.1016/j.ejphar.2005.10.049. [DOI] [PubMed] [Google Scholar]
- [13].Pan MH, Hsieh MC, Hsu PC, Ho SY, Lai CS, Wu H, Sang S, Ho CT. 6-Shogaol suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. Mol Nutr Food Res. 2008;52:1467. doi: 10.1002/mnfr.200700515. [DOI] [PubMed] [Google Scholar]
- [14].Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol. 2010;127:515. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- [15].Chen H, Lv L, Soroka D, Warin RF, Parks TA, Hu Y, Zhu Y, Chen X, Sang S. Metabolism of [6]-shogaol in mice and in cancer cells. Drug Metab Dispos. 2012;40:742. doi: 10.1124/dmd.111.043331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lambert JD, Sang SM, Hong J, Kwon SJ, Lee MJ, Ho CT, Yang CS. Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate. Drug Metab Dispos. 2006;34:2111. doi: 10.1124/dmd.106.011460. [DOI] [PubMed] [Google Scholar]
- [17].Pfeiffer E, Heuschmid FF, Kranz S, Metzler M. Microsomal hydroxylation and glucuronidation of [6]-gingerol. J Agric Food Chem. 2006;54:8769. doi: 10.1021/jf062235l. [DOI] [PubMed] [Google Scholar]
- [18].Kroemer HK, Klotz U. Glucuronidation of drugs. A re-evaluation of the pharmacological significance of the conjugates and modulating factors. Clin Pharmacokinet. 1992;23:292. doi: 10.2165/00003088-199223040-00005. [DOI] [PubMed] [Google Scholar]