Abstract
The estrogen receptor (ER) is expressed on the vast majority of newly diagnosed breast cancers, yet not all ER-positive tumors will respond to endocrine therapy. Selecting patients for endocrine therapy can be considered as a series of predictive tests: does the tumor express the ER and if so, will the endocrine therapy interact with the target to produce a response? These are both challenges to which molecular imaging is functionally suited. Imaging of the ER has been most successful using 16-α[18F]-flouro-17β-estradiol (FES) positron emission tomography (PET). Functional imaging of the ER using FES-PET has been shown to be a predictive tool in determining response to endocrine therapy, and PET imaging of the ER can be used to measure the pharmacodynamic effect of ER-directed endocrine therapy. This article reviews the literature on FES-PET as a functional tool in predicting response to endocrine therapy in breast cancer and discusses future directions.
Introduction
Endocrine therapy is a critical component of breast cancer treatment in estrogen receptor (ER)–positive breast cancer, with fewer side effects than standard chemotherapy and significant improvement in patient outcomes [1]. The ER is expressed on the majority of newly diagnosed breast cancers, and determining ER status is standard clinical care [2, 3]. An important aspect of breast cancer endocrine treatment is patient selection (ie, determining which patients are most likely to respond to endocrine therapy). Although lack of ER expression on breast cancer tumors predicts with some certainty that endocrine therapies will fail, of those patients with newly diagnosed ER-positive tumors, only 50% to 75% will respond, and even fewer (25%) of previously treated patients will respond [4].
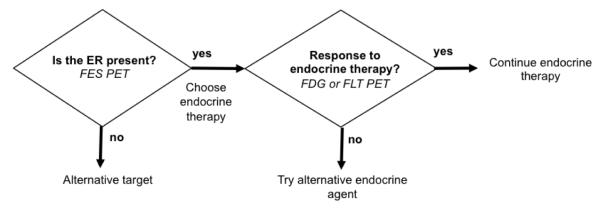
Selecting patients for endocrine therapy can be considered as a series of predictive tests (Fig. 1). First, does the tumor express the target (the ER)? Second, if the target is present, will endocrine therapy interact with the target and produce a tumor response [1, 5•]? Along these lines, current challenges in selecting patients for endocrine therapy include the ability to measure ER expression in more advanced cancer, where there may be multiple sites of disease, as well as some sites such as bone, where biopsy and assay for ER expression may be challenging. In addition, it would be helpful to be able to predict at an early stage of treatment which ER-expressing tumors will respond to endocrine therapy, in opposition to current practice where response is based on a change in tumor size and it take months to see the effect of endocrine therapy on the tumor. These are both clinical challenges to which molecular imaging is ideally suited.
Figure 1.
Schematic approach to determining role of molecular imaging in directing individualized endocrine therapy. ER—estrogen receptor; FDG— fluorodeoxyglucose; FES—16α–[18F]-flouro-17β-estradiol; FLT—18F-fluorothymidine; PET—positron emission tomography. (Reprinted by permission of the Society of Nuclear Medicine from: Mankoff D and Linden HM. Breast Cancer and Hormonal Stimulation. Is Glycolysis the First Sign of Response? J Nucl Med 2010; 51(11):1663–1664. Figure 1)
Estrogen Receptor Imaging
Approximately two thirds of breast carcinomas express the ER [6], and imaging the ER could provide a fairly specific means for identifying these cancers [5•]. Because few normal cells express ER, ER imaging could identify sites of aberrant ER expression and provide a basis for disease localization. Traditionally, knowledge of ER status has been obtained by biopsy, the limitations of which include invasiveness and sampling error. ER imaging also offers complementary information to biopsy, such as the ability to assess entire tumor burden and track changes over time.
Breast cancer ER imaging has been accomplished using radiolabeled estrogen analogs and positron emission tomography (PET). The most successful ER imaging radiopharmaceutical to date has been 16α–[18F]-flouro-17β-estradiol (FES) [7, 8]. The classic study published by Kiesewetter et al. [9] tested binding of the ER for a range of compounds, and found that FES had binding characteristics similar to estradiol for both the ER and sex hormone-binding globulin (SHBG), the transport binding protein [10]. The binding affinity of FES compared with estradiol was approximately 80% for the ER and 10% for the SHBG [9, 11] although in vivo data suggest that FES and estradiol may have similar SHBG binding [10]. Early studies performed on immature female rats found that FES had prolonged retention in rat uteri, with a high target-background ratio (uterus-to-blood 39 ± 16 at 1 hour after injection) [9]. Excess estradiol injected into immature rats was shown to block FES uptake into rat uteri, confirming the specificity of binding and providing an estimate of the relatively modest level of non-specific binding [9, 11, 12]. However, rats lack SHBG [13] and instead use alpha fetal protein (AFP) as the major protein carrier of estradiol. Thus, the kinetics and bio-distribution of estrogen in rats differs from humans. Studies in humans have shown that FES is metabolized through the liver, and blood clearance is rapid, reaching plateau 20 to 30 minutes after injection [14]. Radiation dosimetry studies have shown that the potential radiation risks are well within acceptable limits and that organ doses associated with 18F-FES are comparable to commonly performed nuclear studies [15].
In human breast tumors, FES uptake has been validated as a measure of ER expression in a number of studies [16, 17]. Mintun et al. [16] showed excellent correlation between in vitro tumor ER concentrations and FES uptake within the primary tumor (r = 0.97). ER status was assayed after excision of primary breast tumors using the radioligand method, and FES uptake was quantified from static images taken approximately 90 minutes after injection. In current clinical practice, however, immunohistochemistry (IHC) has supplanted radioligand binding to assess in vitro ER expression. Peterson et al. [17] compared ER expression measured by in vitro IHC with FES uptake. Using a standard uptake value (SUV) of 1.1 as a cut-off for determining ER-positive versus ER-negative tumors, an agreement rate of 94% (16 of 17 patients) was found between IHC results and 18F-FES uptake. FES flux was also measured but was not significantly better than FES-SUV when compared to IHC, suggesting that SUV may be an adequate measure for most studies and that metabolite analysis may not be needed for routine FES imaging [17]. The work by Mintun et al. [16] and Peterson et al. [17] established FES-PET as a quantitative measure of regional ER expression.
Further studies demonstrated the ability of FES-PET to measure ER expression in multiple sites of disease. An early study published by Mintun et al. [16] showed FES uptake not only in primary carcinomas but also in axillary nodes and one distant metastatic site. A later study published by this same group of investigators found good correlation between FES uptake and in vitro ER status in 16 patients with metastatic breast cancer [18]. Specifically, increased FES-SUV was seen in 53 of 57 metastatic lesions, with only two false positives and a sensitivity of 93% [18]. In a larger cohort of 21 patients with metastatic breast cancer, this same group of researchers showed an overall degree of concordance of 88% between in vitro ER status and FES-PET [19]. Additionally, using an SUV > 1.0 to identify ER-positive disease, the sensitivity of FES imaging was 76% and the predictive accuracy of FES negativity was 80%, with no false positives [19]. Kumar et al. [20] compared in vivo 18F-FES with fludeoxyglucose (FDG) uptake in a patient with metastatic ER-positive disease, and found multiple sites of FDG avidity without FES uptake, confirming the potential utility of 18F-FES for identifying phenotypic differences in sites of metastatic breast cancer.
A significant advantage of ER imaging compared to tissue biopsy is the ability to assess the heterogeneity of ER expression (ie, not all ER-positive tumors demonstrate FES uptake). Mortimer et al. [19] found that of 21 patients with ER-positive disease by in vitro assay, 5 patients were negative by FES-PET. Although this study size was small, the data suggest that results of FES-PET imaging may provide information about the functional status of the ER not obtainable by in vitro assay. Similarly, Linden et al. [21] found that 6 of 47 (13%) patients with ER-expressing primary tumors had one or more sites of completely FES-negative disease. The rate of loss of ER expression at metastatic sites was comparable (only slightly lower) to that obtained from tissues samples, which is typically more in the range of 25% [22, 23]. This discrepancy may result from reduced sampling error in imaging versus biopsy.
Although validation studies have shown that FES-PET correlates with in vitro ER status in both regional and metastatic disease, perhaps the most important potential application of PET imaging is as a predictive tool for endocrine therapy. Although FES-PET has not been studied prospectively or in randomized control trials, a number of studies have shown the potential use of FES and FDG-PET in predicting response to endocrine therapy. Mortimer et al. [24] showed the level of FES uptake predicted response to tamoxifen therapy in locally advanced and metastatic breast tumors. Forty women with biopsy proven ER-positive breast cancer underwent FES and FDG-PET before initiating tamoxifen and 7 to 10 days after starting therapy. Fifty-two percent of patients responded to therapy (mean duration, 11.7 months) and 48% showed with disease progression. Responders were found to have higher baseline FES-SUV than non-responders and had a greater decrease in mean SUV post-therapy (decrease 2.5 ± 1.8 SUV units in responders vs decrease 0.5 ± 0.6 SUV in non-responders; P = 0.0003). Responders also had a greater percentage decrease in SUV from baseline FES-PET compared to non-responders (54.8% ± 14.2% in responders vs 19.4% ± 17.3% in non-responders; P = 0.0003) [24]. When an arbitrarily selected cut-off SUV of 2.0 was used to define hormone-sensitive cancers, the positive predictive value was 79% (19 of 24 responded) and the negative predictive value was 88% (14 of 16 patients failed to respond) [24]. Moreover, no patient with a baseline FES-SUV of < 1.5 responded to therapy. Baseline FES-SUV predicted response to tamoxifen as did mean and percentage decrease in FES-SUV after starting therapy.
Linden et al. [21] showed the ability of FES-PET to predict response to endocrine therapy (mostly aromatase inhibitors [AIs] in a group of patients who had been heavily pre-treated (Fig. 2). In this study, 47 patients with ER-positive tumors, most of whom had bone dominant disease and prior exposure to anti-estrogen therapy, underwent FES-PET at a change in therapy and disease assessment 6 months after salvage hormonal therapy. FES-PET was assessed qualitatively and quantitatively using SUV and estradiol binding flux. Objective response to endocrine therapy was observed in 11 of 47 patients (23%). No patient (0 of 15) with an SUV < 1.5 responded to salvage endocrine therapy, compared to 11 of 32 patients (34%) with an SUV higher than 1.5 (P < 0.01) [21]. Similar results were found when flux was used as a measurement of uptake. Like Mortimer et al. [24], Linden et al. [21] found that an FES-SUV < 1.5 to 2.0 predicted a lack of response to hormonal therapy. An additional and highly significant finding by Linden et al. [21] was that no patients whose tumor over-expressed HER2, including those whose tumors had an FES-SUV > 1.5, showed response to treatment. If quantitative FES-SUV had been used to select patients, the overall response rate would have increased from 23% to 34% and from 29% to 46% in the subset of patients without HER2/neu over-expression [21]. The work by both Mortimer et al. [24] and Linden et al. [21] demonstrated the potential role of FES-PET in predicting response to endocrine therapy.
Figure 2.

Imaging examples of Pre-treatment 16α–[18F]-flouro-17β-estradiol (FES; left) and fluorodeoxyglucose (FDG; middle) scans and follow-up FDG post-therapy (right). Dashed arrows show normal liver FES uptake. A, Bone metastasis with robust FES and FDG uptake; response at 3 months. B, Bone metastasis (solid arrow) without FES but with FDG uptake; progressive disease at 6 months. (From Linden, HM et al: J Clin Oncol 24(18), 2006:2793–2799 [21]. Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved.)
Measuring Tumor Response to Endocrine Therapy
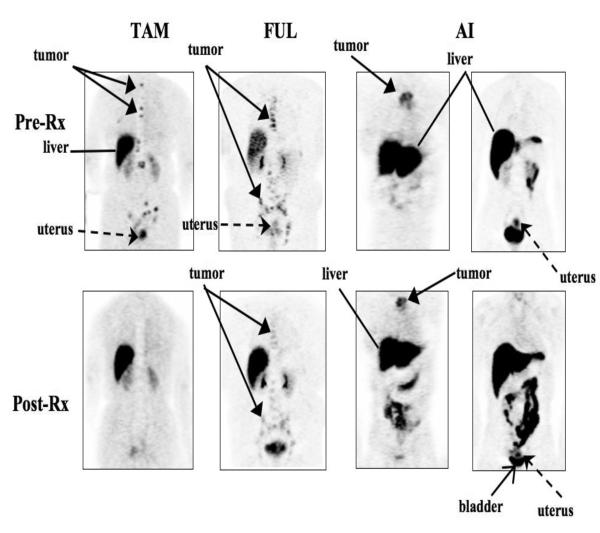
Although PET-ER imaging is useful in predicting response to endocrine therapy, serial PET-ER imaging can be used to measure the pharmacodynamic effect of ER-directed endocrine therapy. Early work published by McGuire et al. [18] showed blockade of the ER by tamoxifen on serial FES-PET scans. Mortimer et al. [24] reported decreased FES uptake in patients 1 week after starting tamoxifen therapy, consistent with tamoxifen and its metabolites binding to the ER. Linden et al. [25••] later demonstrated the ability of FES-PET to measure the pharmacodynamics of ER-targeted therapies, specifically, tamoxifen, fulvestrant, and AIs. In the study by Linden et al. [25••], 30 patients with metastatic breast cancer underwent FES-PET prior to and then during treatment with AI, tamoxifen, or fulvestrant. Tumor FES uptake decreased more with ER-blocking therapies (tamoxifen and fulvestrant, average 54% decline) than with estrogen-depleting therapies (ATs, 14% decline) (Fig. 3). Additionally, complete tumor blockade (SUV ≤ 1.5) was greater following tamoxifen (5 of 5 patients) than with fulvestrant (4 of 11; 2-sided mid P value = 0.019) [25••]. FES uptake was also measured in patients with a uterus, which has high ER expression, to determine the extent to which tumor uptake may match uterine uptake. There was a strong association between percent decrease in FES-SUV after endocrine therapy in the uterus and the breast tumor (rho =0.63; P = 0.01), suggesting that functional imaging of the uterus in patients undergoing endocrine therapy may reflect the activity of endocrine therapy tumor sites [25••]. In sum, serial FES-PET imaging showed that the ER was blocked by ER-blocking agents (tamoxifen and to a lesser extent fulvestrant) and not by estrogen-depleting therapies (AIs). The work of Linden et al. [25••] demonstrated the ability of FES-PET to measure the pharmacodynamic effect of endocrine therapies and is concordant with clinical observations of reduced activity of low-dose fulvestrant and increased activity of a higher loading and maintenance schedule.
Figure 3.
Pre- and post-treatment (Rx) images are shown in representative patients treated with estrogen receptor (ER)-blocking therapy (tamoxifen [TAM] and fulvestrant [FUL]) and estrogen-lowering therapy (aromatase inhibitor [AI]). Tumor is shown with solid arrow, uterus is shown with a dashed arrow. Liver (with diffuse uptake due to metabolism of 16α–[18F]-flouro-17β-estradiol) is shown with a thin line, and the bladder is denoted by a thin arrow. A, Tumor in the upper spine shows baseline uptake and complete ER blockade in tumor and uterus following 21 days of TAM. B, Tumor in mediastinal nodes shows baseline uptake and incomplete ER blockade in tumor and uterus following 68 days of FUL. C, Sternal tumor shows ER uptake and no blockade in the tumor and uterus following 29 days of letrozole (AI). (From Linden et al. [25••].)
Even if the selected endocrine therapy interacts with the ER to either block the receptor or reduce agonist ligands, changing receptor occupancy may not lead to a significant tumor response. Studies have shown that measures of tumor proliferation, based upon serial tumor biopsy and assay of the Ki67 antigen, before and after initiation of endocrine treatment, predicted clinical response and patient outcome. Dowsett et al. [26], using data from the IMPACT trial, evaluated tumor biopsy samples for Ki67 proliferation before and 2 weeks after initiation of endocrine therapy. A lower Ki67 index at the 2-week time-point predicted a reduced risk of relapse. By contrast, baseline Ki67 index, whether low or high, did not predict response to therapy [26]. Ellis et al. [27••] compared changes in Ki-67 levels prior to and after neo-adjuvant AI therapy and found no significant differences in biologic activity between letrozole, anastrozole, or exemestane. An intrinsic subtype analysis utilizing the PAM50 assay demonstrated a higher baseline Ki67 in luminal B compared to luminal A breast cancer, and that all patients with HER2–enriched tumors had persistently elevated Ki67 levels (> 20%). A baseline Ki67 level of 10% or less and luminal A subtype were useful in predicting which patients would have a Preoperative Endocrine Prognostic Index (PEPI) score of zero [27••]. Thus, Ki67 level may be useful in predicting which patients might benefit from neo-adjuvant AI therapy.
Recognizing that alterations in the activation of pathways downstream of the ER produce functional molecular changes, investigators at Washington University in Saint Louis hypothesized that effect of ER activation could be visualized by PET imaging and would be predictive of response. They first investigated 18F FDG-PET as a method to identify a “metabolic flare” reaction to predict response to endocrine therapy. A clinical flare reaction is described as transient disease progression associated with starting anti-estrogen therapy, and it is often characterized by pain over sites of tumor involvement, enlargement of soft tissue foci, and increases in serum markers [28]. However, a clinical flare occurs in fewer than 5% of patients and is difficult to distinguish from actual disease progression [24]. Mortimer et al. [24] used serial FDG-PET imaging to assess for sub-clinical metabolic flare in breast cancer patients starting endocrine therapy with tamoxifen. Forty patients with advanced ER breast cancer underwent FDG-PET prior to and 7 to 10 days after initiating therapy endocrine therapy [24]. Metabolic flare was arbitrarily defined as an increase of tumor FDG-SUV of ≥ 10% between scans. The positive predictive value of metabolic flare was 91% (20 of 22 patients showed clinical response at 4 weeks to tamoxifen) and the negative predictive value was 94% (17 of 18 such patients failed to respond to tamoxifen therapy) [24]. Although this study involved only a small number of patients, it did show that a sub-clinical metabolic flare detectable by serial PET imaging could predict response to tamoxifen.
Dehdashti et al. [28], showed the ability of metabolic flare, induced by estradiol, to predict tumor response to AIs and fulvestrant. Fifty-one postmenopausal women with advanced ER-positive breast cancer underwent baseline FES-PET and FDG-PET followed by administration of estradiol and repeat FDG-PET scan [28]. Patients were then treated with either an AI or fulvestrant. Metabolic flare was defined as a ≥ 12% increase in FDG-SUV, and ER positivity was defined as a FES-SUV ≥ 2. A metabolic flare was observed in 15 of the 17 who responded to therapy (88%) and none of the 34 non-responding patients (P > 0.0001). Longer survival was seen in patients with metabolic flare than in those without a flare reaction. The work of Dehdashti et al. [28] showed that metabolic flare can be predictive of subsequent tumor response to endocrine therapy with AI and fulvestrant (positive predictive value 100%, negative predictive value 94%).
In a follow up study by Ellis et al. [29], estradiol-induced metabolic flare was shown to have predictive value in post-menopausal patients with evidence of prior endocrine sensitivity whose disease had ultimately progressed on aromatase inhibitor therapy. The primary research aim of this study was to determine whether daily estradiol therapy would show clinical benefit, as it has been hypothesized that AIs may sensitize hormone receptor–breast cancer to estradiol therapy. Sixty-six patients were randomized to either 6 mg or 30 mg of daily oral estradiol, and underwent FDG-PET prior to and 24 hours after the initial dose of estradiol. Metabolic flare was considered an increase of ≥ 12% in FDG-SUV between scans. Results showed that both the 6-mg estradiol group and the 30-mg estradiol group demonstrated metabolic flare, with an increase in SUV of 20.9% and 22.1% respectively (P = 0.92). The predictive results of FDG-PET were highly significant, showing that metabolic flare had a positive predictive value for response of 80% (12 of 15; 95% CI, 61%–92%) and a negative predictive value for non-response of 97% (27 of 31; 95% CI, 76%–94%) [29]. Additionally, it was also found that patients who demonstrated metabolic flare had a significantly longer progression-free survival. This study shows how FDG-PET, as a predictive biomarker, has the potential to differentiate which hormone receptor–positive patients may benefit from endocrine therapy.
Hypothesizing that a withdrawal of estrogen would result in a decline in tumor glucose metabolism, Linden et al. [30] compared serial FDG-PET imaging with Ki67 assays from breast tumors in 21 patients with late-stage breast cancer before and after initiating AI therapy. FDG-PET at the 2-week point showed a ≥ 20% decrease in FDG-SUV in 76% of patients (16 of 21). Biopsy at the 2-week point (in the 11 patients in whom repeat biopsy was successful) showed a Ki67 ≤ 5% in all patients [30]. Of the 5 patients who did not have ≥ 20% in FDG-SUV, all 3 of whom underwent a 2-week biopsy had a Ki67 ≥ 20%. Linden et al. [30] also evaluated response to AI therapy at 4 to 6 months and found that treatment responders (5 of 8 patients) had at least a 20% decline in FDG-SUV, whereas 2 of the 3 non-responders did not have significant changes in SUV. Thus, a decline of ≥ 20% in SUV correlated with 2 week Ki67 of ≤ 5%, and a large drop in SUV predicted response in almost all cases [30]. These data suggest that PET may accurately, and without need for biopsy, predict response to endocrine therapy.
Interestingly, some recent studies have suggested that changes in FDG uptake following estrogen challenge in breast cancers may be the result of non-genomic effects of estrogen-ER binding, rather than the classic mechanism of action through ER interaction with DNA, leading to cellular proliferation. Ko et al. [31] showed, in vitro, that increases in FDG uptake following estradiol challenge parallel activation of PI3K and did not require interaction of the estrogen-ER complex with the nucleus, suggesting a non-genomic effect. This raises interesting clinical and biologic questions and suggests that alternative PET tracers, such as 18F-fluorothymidine (FLT), a cellular proliferation tracer, may provide an attractive means for predicting early response, in parallel with Ki67 as a measure of proliferation in serial biopsy [5•]. Ongoing studies may shed further light on this interesting and promising area of investigation.
Conclusions
ER imaging and pharmacodynamic imaging of endocrine therapy using FDG PET have tremendous potential to “personalize” treatment for a given patient and to shed light on tumor metabolic pathways to evaluate the impact of targeted therapies. Other promising modalities, such as FLT and diffusion MRI, are also under study and merit comparison to determine the optimal modality to study molecular pathways of endocrine sensitivity and resistance. Future studies are indicated to determine reproducibility between centers and optimal timing of imaging as well as ideal imaging biomarker and targeted agent combinations.
Thus far, the data supporting FES have come from relatively small, single-center trials. Although these studies have established FES-PET as a useful method for quantitative imaging of ER expression and shown that FES uptake correlates with in vitro ER expression with potential use a predictive assay, larger multi-center trials are needed for further validation and regulatory approval. Future clinical trials will be needed to determine the role that targeted molecular imaging, such as FES-PET, could play in directing individualized therapy in ER-positive breast cancer. An important step in this direction was the creation of an Investigational New Drug approval from the National Cancer Institute (IND79_2005), and forthcoming trials will lead to more widespread availability and clinical use of this valuable imaging tool.
Footnotes
Disclosure E. Currin: none. H.M. Linden is a consultant for Roche-Genentech and GTx and has received grants from Ortho Biotech, Pfizer, Novartis, MGI Pharma, and Merck. D.A. Mankoff has received grants from Merck and Pfizer (to his institution) and owns stock/stock options in Pfizer and Bristol-Myers Squibb.
Contributor Information
Erin Currin, Department of Medicine Box 354760 University of Washington 1959 N.E. Pacific St. Seattle, WA 98195 206-598-8750 (ph) ecurrin@u.washington.edu.
Hannah M. Linden, Department of Oncology University of Washington and Seattle Cancer Care Alliance G3-210, 825 Eastlake Avenue East Seattle WA, 98109 206 288-6710 (ph) 206 288-2054 (fax) hmlinden@u.washington.edu.
David A. Mankoff, Department of Radiology University of Washington and Seattle Cancer Care Alliance G2-600, 825 Eastlake Avenue East Seattle, WA 98109 206-288-2173 (ph) 206-288-6556 (fax) dam@u.washington.edu.
References
- 1.Johnston SR. New strategies in estrogen receptor-positive breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(7):1979–87. doi: 10.1158/1078-0432.CCR-09-1823. [DOI] [PubMed] [Google Scholar]
- 2.Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(1):28–34. doi: 10.1200/JCO.2003.03.088. [DOI] [PubMed] [Google Scholar]
- 3.Carlson RW, A.D., Anderson BO, et al. Breast Cancer Clinical Practice Guidelines in Oncology. 2 Ed. National Comprehensive Cancer Network; 2011. [PubMed] [Google Scholar]
- 4.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annual Review of Medicine. 2011;62:233–47. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5 •.Linden HM, Mankoff DA. Breast cancer and hormonal stimulation: is glycolysis the first sign of response? Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51(11):1663–4. doi: 10.2967/jnumed.110.078329. This article raises important questions regarding hormonal stimulation, FDG-PET, and glycolisis. For instance, are glycolisis and proliferation equivalent predictors of endocrine responsiveness?
- 6.Cigler T, Goss PE. Breast cancer adjuvant endocrine therapy. Cancer Journal. 2007;13(3):148–55. doi: 10.1097/PPO.0b013e318074d363. [DOI] [PubMed] [Google Scholar]
- 7.Cummins CH. Radiolabeled steroidal estrogens in cancer research. Steroids. 1993;58(6):245–59. doi: 10.1016/0039-128x(93)90069-y. [DOI] [PubMed] [Google Scholar]
- 8.Katzenellenbogen JA, Welch MJ, Dehdashti F. The development of estrogen and progestin radiopharmaceuticals for imaging breast cancer. Anticancer Research. 1997;17(3B):1573–6. [PubMed] [Google Scholar]
- 9.Kiesewetter DO, et al. Preparation of four fluorine- 18-labeled estrogens and their selective uptakes in target tissues of immature rats. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1984;25(11):1212–21. [PubMed] [Google Scholar]
- 10.Tewson TJ, et al. Interactions of 16alpha-[18F]-fluoroestradiol (FES) with sex steroid binding protein (SBP) Nuclear Medicine and Biology. 1999;26(8):905–13. doi: 10.1016/s0969-8051(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 11.Pomper MG, et al. Target tissue uptake selectivity of three fluorine-substituted progestins: potential imaging agents for receptor-positive breast tumors. International Journal of Radiation Applications and Instrumentation. Part B, Nuclear Medicine and Biology. 1990;17(3):309–19. doi: 10.1016/0883-2897(90)90058-9. [DOI] [PubMed] [Google Scholar]
- 12.Katzenellenbogen JA. The pharmacology of steroid radiopharmaceuticals: specific and non-specific binding and uptake selectivity. In: Nunn A, editor. Radiopharmaceuticals: Chemistry and Pharmacology. Marcel Dekker; New York: 1992. [Google Scholar]
- 13.Petra PH. The plasma sex steroid binding protein (SBP or SHBG). A critical review of recent developments on the structure, molecular biology and function. The Journal of Steroid Biochemistry and Molecular Biology. 1991;40(4-6):735–53. doi: 10.1016/0960-0760(91)90299-k. [DOI] [PubMed] [Google Scholar]
- 14.Mankoff DA, Tewson TJ, Eary JF. Analysis of blood clearance and labeled metabolites for the estrogen receptor tracer [F-18]-16 alpha-fluoroestradiol (FES) Nuclear Medicine and Biology. 1997;24(4):341–8. doi: 10.1016/s0969-8051(97)00002-4. [DOI] [PubMed] [Google Scholar]
- 15.Mankoff DA, et al. [18F]fluoroestradiol radiation dosimetry in human PET studies. Journal of Nuclear Medicine. 2001;42(4):679–84. [PubMed] [Google Scholar]
- 16.Mintun MA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988;169(1):45–8. doi: 10.1148/radiology.169.1.3262228. [DOI] [PubMed] [Google Scholar]
- 17.Peterson LM, et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008;49(3):367–74. doi: 10.2967/jnumed.107.047506. [DOI] [PubMed] [Google Scholar]
- 18.McGuire AH, et al. Positron tomographic assessment of 16 alpha-[18F] fluoro-17 beta-estradiol uptake in metastatic breast carcinoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1991;32(8):1526–31. [PubMed] [Google Scholar]
- 19.Mortimer JE, et al. Positron emission tomography with 2-[18F]Fluoro-2-deoxy-D-glucose and 16alpha-[18F]fluoro-17beta-estradiol in breast cancer: correlation with estrogen receptor status and response to systemic therapy. Clinical Cancer Research. 1996;2(6):933–9. [PubMed] [Google Scholar]
- 20.Kumar P, et al. Clinical production, stability studies and PET imaging with 16-alpha-[18F]fluoroestradiol ([18F]FES) in ER positive breast cancer patients. Journal of Pharmacy & Pharmaceutical Sciences. 2007;10(2):256s–265s. [PubMed] [Google Scholar]
- 21.Linden HM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. Journal of Clinical Oncology. 2006;24(18):2793–9. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- 22.Kuukasjarvi T, et al. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14(9):2584–9. doi: 10.1200/JCO.1996.14.9.2584. [DOI] [PubMed] [Google Scholar]
- 23.Spataro V, et al. Sequential estrogen receptor determinations from primary breast cancer and at relapse: prognostic and therapeutic relevance. The International Breast Cancer Study Group (formerly Ludwig Group) Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1992;3(9):733–40. doi: 10.1093/oxfordjournals.annonc.a058330. [DOI] [PubMed] [Google Scholar]
- 24.Mortimer JE, et al. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. Journal of Clinical Oncology. 2001;19(11):2797–803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- 25 ••.Linden H, K B, Peterson L, et al. Flouroestradiol (FES) Poistron Emission Tomography (PET) Reveals Differences in Pharmacodynamics Of Aromatase Inhibitors, Tamoxifen and Fulvestrant in Patients with Metastatic Breast Cancer. Clin Cancer Res. 2011 Jul 15;17(14) doi: 10.1158/1078-0432.CCR-10-3321. This article shows that withdrawal of estrogen by AI therapy correlated with a percentage decrease in tumor glucose metabolism by FDG-PET, suggesting that PET may accurately predict response to therapy.
- 26.Dowsett ME, S.I., Ebbs SR, et al. Prognostic Value of Ki67 Expression After Short-Term Presurgical Endocrine Therapy for Primary Breast Cancer. J Natl Cancer Inst. 2007;99(2):167–170. doi: 10.1093/jnci/djk020. [DOI] [PubMed] [Google Scholar]
- 27 ••.Ellis MJ, et al. Randomized Phase II Neoadjuvant Comparison Between Letrozole, Anastrozole, and Exemestane for Postmenopausal Women With Estrogen Receptor-Rich Stage 2 to 3 Breast Cancer: Clinical and Biomarker Outcomes and Predictive Value of the Baseline PAM50-Based Intrinsic Subtype--ACOSOG Z1031. Journal of Clinical Oncology. 2011 doi: 10.1200/JCO.2010.31.6950. This article shows that a baseline Ki67 < 10% and luminal A subtype were predictive of good outcome and PEPI score of zero, and that Ki-67 may be useful in predicting which patients might benefit from neo-adjuvant AI therapy.
- 28.Dehdashti F, et al. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Research and Treatment. 2009;113(3):509–17. doi: 10.1007/s10549-008-9953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis MJ, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302(7):774–80. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linden HM. Early Assessment of Response to Aromatase Inhibiotr (AI) Therapy. American Society of Clinical Oncology 2009 Annual Meeting. 2009;27:15s. e.a. abstract number 11075 (Orlando, Fla) [Google Scholar]
- 31.Ko BH, et al. 17beta-estradiol augments 18F-FDG uptake and glycolysis of T47D breast cancer cells via membrane-initiated rapid PI3K-Akt activation. Journal of Nuclear Medicine. 2010;51(11):1740–7. doi: 10.2967/jnumed.110.074708. [DOI] [PubMed] [Google Scholar]