Intraneural microstimulation (INMS) was developed independently by Torebjörk and Ochoa [20] and by Vallbo [23]. As pioneers, we often wonder whether the awesome yield of the method, as applied to discern subjective attributes of pure evoked sensations in human subjects, has been a mirage. Cultural nihilism resists the proposition that certain single sensory units from skin of the human hand may normally be able to evoke a conscious sensation endowed with specific and consistent subjective quality, measurable magnitude, and chartable location. But, of course, depending on the physical circumstances prevailing in the neighborhood of a given intraneural site, an electrode tip can regularly record anywhere from between zero and multiple nerve fibers, and usually a few. And why not even record one single fiber in focus? The usual situation in which zero to multiple fibers are recordable from the same intra-neural microelectrode site, certainly does not negate that it may be possible also to microstimulate zero, a few, or the whole fiber content of a fascicle at stimulus intensity is gradually increased. And why not even stimulate one single fiber? Indeed, it is more plausible to accept viability of single fiber stimulation than mandatory multi-fiber stimulation at stimulus intensity at threshold for the first detectable sensation. Therefore, after the test of time, we do believe it is possible to excite single sensory nerve fibers through intra-neural microstimulation in humans [10,15,17,18]. Nobody does intraneural microstimulation (INMS) these days. It is extremely demanding.
But a more challenging question is: can excitation of single sensory units (serving the human hand) evoke a conscious percept? The answer given by those who pioneered INMS is affirmative, and the criteria are as follows:
INMS, using weak electrical stimulus trains at threshold for sensation evokes a monofocal unitary sensation projected spatially to the receptive field of the recorded unit.
Subjective quality of the INMS-evoked unitary sensation matches physiological type of unit recorded at the monofocal receptive field.
Quality of sensation is distinct, pure and simple, and immutable for same kinds of units in different individuals.
Some units consistently evoke no sensation at all (SAII units).
Increasing INMS intensity incrementally evokes different quantal sensations of random subjective qualities and multifocal localizations projected within a fascicular territory.
Through a marking method [10], it is possible to prove that a pre-recorded unit was indeed activated during stimulation. However, this does not necessarily mean that such unit was the only unit stimulated (Fig. 1).
Fig. 1.
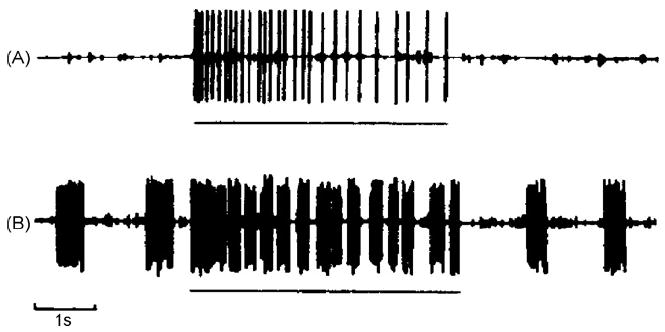
Response of SA I unit to pressure (horizontal line) before (A) and after (B) INMS at 200 Hz for 10 min. In (B), post-tetanic spontaneous and triggered bursts of impulses provide evidence of prior activation via INMS of this recorded unit.
Other evidence supports the notion that afferent impulse activity in single human nerve fibers supplying skin areas with priority sensory acuity, like the hand, may reach consciousness. For example, in spontaneous paresthesias induced during the hyperexcitable post ischemic state, in which individual nerve fibers of various anatomophysiological types, with receptive/projected fields disseminated in the innervated skin, fire chaotically, the anti-natural sensory percept reflects a composite of sensations projected randomly in time and space. Unlike the blended sensations evoked by orderly co-activation of different units during natural stimulation of innervated skin, the paresthetic chaos can be recognized, with attention, as made of unitary sensations that individually have qualities much like those evoked by INMS [9]. Also, an individual may occasionally experience a faint spontaneous sensation, monofocally projected to the skin, which may last for many seconds. Its quality may be perfectly distinct: a pressure, tapping, vibration, pain (or itch). It may be continuous or my interrupt and recur cyclically. Fig. 2 reproduces an analog of the perceived subjective temporal features of transient spells of a faint monofocal paresthesia which now and then visits the author’s Right thumb in median nerve territory. Its quality is buzzing/vibration. Each spell of buzzing lasts for close to half a minute, and is typically broken into several brief cycles of decreasing duration, each lasting between approximately 3 s and a fraction of a second. Subjective intracycle spike frequency decreases with time and intervals between the repetitive cycles of buzzing sensation increase, while successive cycles become shorter with repetition. This temporal psychophysical profile mirrors perfectly the spontaneous bursts of unitary nerve impulse discharge directly recorded during post ischemic paresthesias, as illustrated in Fig. 3 of [9] (Fig. 3). The cyclic unitary bursting behavior is also similar to what has been illustrated behind the sign of Spurling in cervical radiculopathy (Fig. 1 in [13]) (Fig. 4). We have all experienced these phenomena at some point in our lives.
Fig. 2.
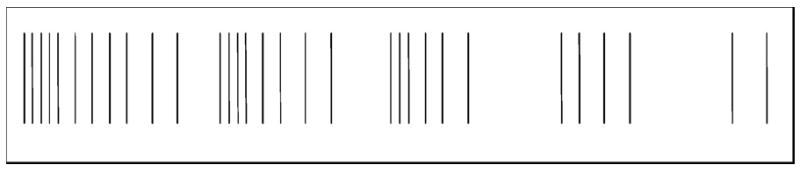
Analog of spontaneous monofocal buzzing paresthesia and its cyclic tempo.
Fig. 3.
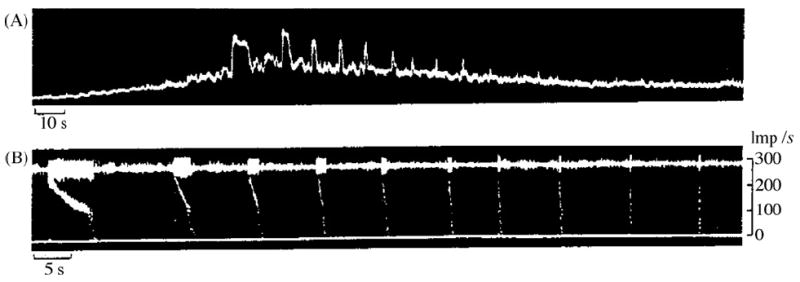
Series of 10 high-frequency discharges from a single unit recorded from the ulnar nerve at elbow level during hyperventilation. (A) Integrated neurogram, dominated by single unit bursts. Recording started on release of cuff in upper arm after 15 min of ischaemia. (B) Part of the sequence shown in (A) displayed at an extended time scale. Upper trace is original neurogram, lower trace displays “instantaneous” firing frequency of the unit. Note the fairly regular repeats of bursts, and their decreasing duration. Note also the uniform impulse frequency at the onset of bursts and the progressive steepness of the frequency decay in subsequent bursts. As in Fig. 2, there seems to be a critical firing frequency of about 130 Hz, below which the impulse frequency decays abruptly.
Fig. 4.
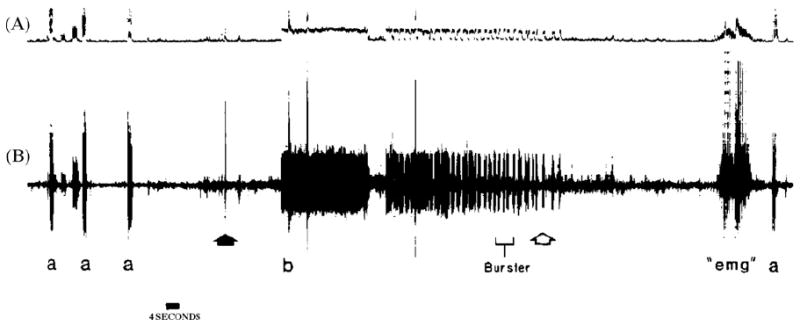
Recording from left ulnar nerve at wrist level. A = integrated neurogram; B = simultaneous discriminated neurogram. Symbols: a = stimulation of receptive field in little finger; solid arrow = head flexed to left and extended; b = onset of abnormal unitary bursting, temporally correlating to verbalized report of paresthesias reaching the hand; open arrow = head returned to neutral position; “emg” = voluntary muscle artifact; a = original receptive field reconfirmed.
The following are reasons why the psychophysical behavior illustrated in Fig. 2 reflects nerve impulse activity in a spontaneously bursting single RA (or perhaps Pacini) unit, rather than reflecting multiple units firing simultaneously:
The sensation projected to a discrete virtual point on the skin is monofocal.
Its subjective quality is pure, not a blend.
Each discharge cycle obviously reflects a brisk unitary burst which could not be transparently discriminable if more than one unit, with their private discharge pattern, evoked subjective quality and monofocal projection, were discharging at the same time.
One caveat can be brought up against the feasibility of single fiber excitation through INMS. Hallin and Wu [5] have proposed that clusters of sensory units, of the same anatomophysiological type, travel as neighbors in the endoneurium and terminate in neighboring cutaneous receptors. If so, the qualitatively pure and simple, monofocal, “unitary,” projected sensation evoked during INMS might in reality derive from excitation of multiple units of the same type.
Indeed, it might be argued that if a group of separate units of the same anatomophysiological type were to run as close intrafascicular neighbors and were to have overlapping receptive fields in the skin, and were to be stimulated to discharge synchronously during INMS, or were to discharge spontaneously and synchronously by coincidence during spontaneous paresthesias, then, through spatial summation, the afferent input might reach conscious levels as a monofocal, pure specific sensation. But this is unlikely on several scores. Even if those extraordinary conditions were met, different individual conduction velocities of the neighboring fibers would unavoidably desynchronize their afferent impulses between site of INMS and brain, and the crisp subjective frequency of the intermittent evoked percept would become blurred, and the cyclic bursts would not be regularly intervalled. In fact, the norm for pure sensations perceived during INMS of afferent units that evoke intermittent percepts, is that such percept is clearly discontinuous and, therefore, uninterfered by multi-unit desynchronization. It would be more difficult to discern unitary features from a subjective percept if the natural tempo of its subjective quality were not intermittent, as it happens with RA and PC units, but were continuous, as it happens with the steady monofocal pressure sensation evoked by SAI units, or with the continuous monofocal pains evoked by A delta or C nociceptors.
One most powerful argument supports the basic claim that, under stringent experimental circumstances, intraneural microstimulation given into human nerve fascicles innervating skin of the hand may activate single sensory units and that such afferent activity may evoke a specific conscious percept projected monofocally. It is the spectacular matching between anatomophysiologically identified receptive field and fiber type of an afferent unit in focus for intraneural recording compared to the subjective quality, temporal profile and projected localization of the unitary sensation evoked at threshold during stimulation from the intraneural site. Indeed, RA units evoke tapping-flutter; PC units evoke higher frequency vibration; SA1 units evoke sustained pressure; A delta nociceptors evoke pricking pain; C nociceptors evoke dull or burning pain (and “C itch units” evoke itch).”
A glance at the idiosyncratic behavior of SAII units, which consistently fail to evoke a conscious unitary monofocal and qualitatively distinct sensation during INMS, adds credibility to the claim that other A fiber mechanoreceptor units consistently succeed in evoking unitary sensations (Fig. 8). During poster presentation of our INMS results at the World Congress of Pain in Edinburgh (September 1981), one participant, the insightful Professor Robert Schmidt from Würzburg, asked “What do you feel when you microstimulate identified SAII units?” To the answer “nothing,” Schmidt exclaimed “In that case I believe you can stimulate single units and evoke unitary sensations, because right now you and I have many thousands SAII units firing at rest (as they often normally do), and I can feel nothing.”
Fig. 8.
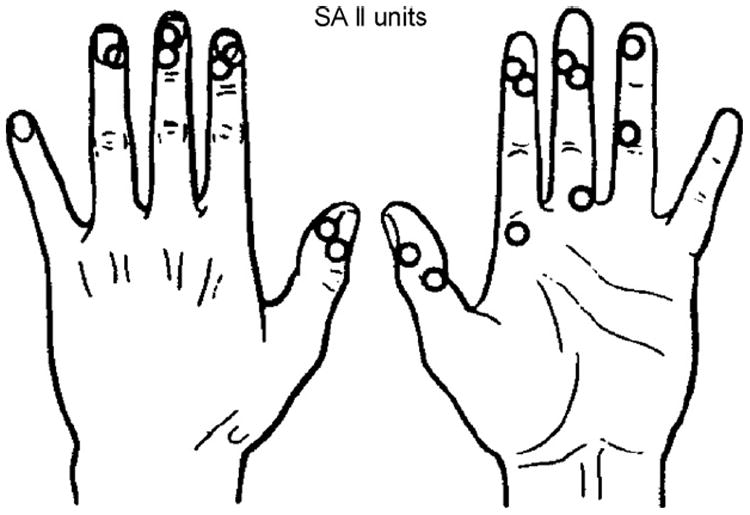
Location of receptive fields of 17 SA II units. Note clustering close to nails and joints. INMS failed to evoke sensation from any of these units.
Figs. 5-9 illustrate the evocation of different specific unitary sensations in multiple experiments in which identified A mechanoreceptors and C nociceptors were proven to have been excited during weak intensity INMS at threshold for first unitary sensation [10,15,22].
Fig. 5.
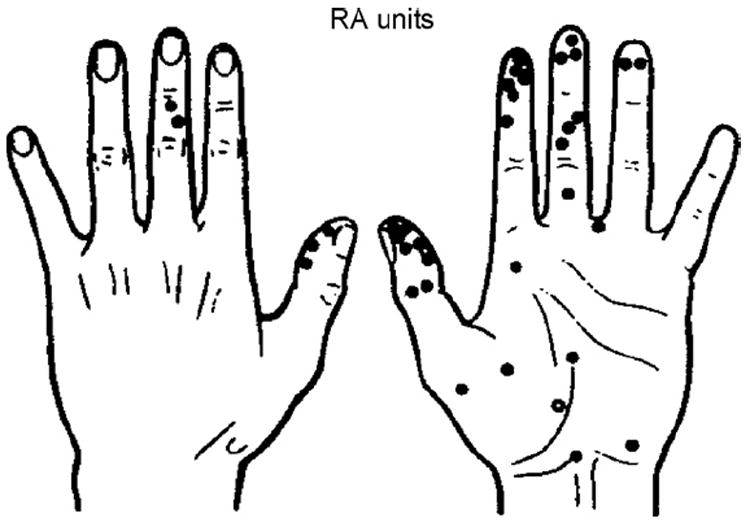
Location of receptive fields of 38 RA units. Note clustering in finger pulps. INMS evoked a conscious sensation from most of the units (●); only three units had no cognitive correlate (○).
Fig. 9.
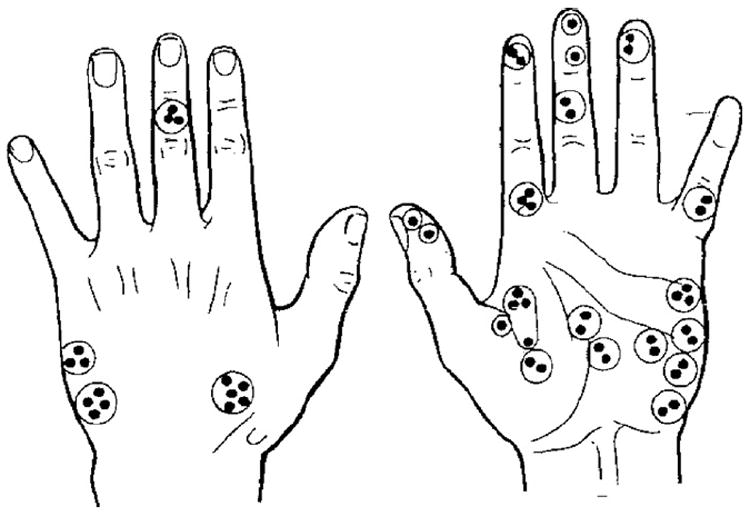
Location of receptive fields (●) of 52 C polymodal nociceptors in the hand. Units recorded together in any one recording site are encircled.
“Specific sensations evoked by activity in single identified sensory units in man” was the title of the original article on intraneural microstimulation [20]. Not surprisingly, this candid claim disturbed Professor P.D. Wall, an inspired theorist, in the measure that it contradicted the Gate Control Theory [8]. The specificity connotation that we rationalized from INMS rested mainly upon the consistent observation that the qualitatively distinct, pure and simple, monofocal projected sensations evoked by INMS not only matched spatially the receptive field of the particular units recorded from the INMS site, but also matched the unit’s physiological type. With McMahon, Wall, then chief editor of “Pain,” perused the journal to publish a critical review article against the proposal that “perceived sensation has modality and spatial and temporal characteristics entirely determined by the characteristics of the individual types of peripheral fibre” [25]. Torebjörk et al. [21] published a rebuttal which, first and foremost, reminded the reader that the Gate Control Theory of Melzack and Wall [8] was based on assumptions rather than evidence and that the authors had conceded “knowledge of how this occurs is meager.” We then addressed Wall and McMahon’s itemized criticisms of INMS and its contribution to understanding somatosensory function in the human hand. We first noted that Wall and McMahon had acknowledged that they had themselves performed neither microneurography nor intra-neural microstimulation as part of their research efforts. Wall and McMahon spelled out four objections to our thesis:
They assumed that the intraneural electrode caused impulse conduction block in most fibers, which would filter out an ongoing “afferent barrage,” thus disturbing central processing and rendering an artifactual-evoked sensation.
They felt that the stimulus intensities employed during INMS were huge and, therefore, our monofocal, simple, pure, and qualitatively distinct “unitary” sensations must derive from multi-fiber activation.
They reasoned that the psychophysical estimate of evoked unitary sensory qualities would be biased in favor of “specificity,” thus questioning our claimed match between quality and physiological type of stimulated and recorded unit.
They arbitrated that our claimed match between anatomical unitary receptive field and projected localization of the unitary evoked sensation was “not supported by the data.”
Based upon those four objections, Wall and McMahon not only rejected our results and neuroscientific thesis thereof, but they argued, without experimental support, that our published results in fact supported Melzack and Wall’s modified pattern theory.
Torebjörk et al. [21] countered each of those four objections on a scientific evidential basis. We documented that “the vast majority of the fibers” are not blocked. We also clarified “we do not need to rely on speculations to conclude that the stimulus intensity is adequate, because direct proof is available.” Actually, the measured stimulus current intensity and pulse polarity we used for INMS are optimal for selective activation of single myelinated fibers. With regard to objections (3) and (4), our published data spoke for itself (see [21, pp. 1521, 1524]).
Our results certainly did not support Melzack and Wall’s modified pattern theory of sensation. Furthermore, changing the pattern of pulse stimuli did not change the basic quality of the evoked sensations. When Ochoa et al. [11] processed patterns of abnormal neural discharges recorded during post ischemic paresthesias, and transformed them into trains of intraneural patterns of stimuli, the specific sensation evoked by intraneural microstimulation retained its specific quality while its temporal profile followed faithfully the patterned stimuli. For all those reasons, in 1987, we concluded “our findings support the view that the tactile modality of the somatosensory system is organized with a high degree of specificity.” [21, p. 1525]
In a separate assault, Wall [24] rationalized the “hyperpathic syndrome” as “a challenge to specificity,” and claimed natural incompatibility between clinical sensory phenomena displayed by some neurological patients and specificity theory. This was robustly countered by [3]. For example, Wall noted “No recordings from normal or damaged nociceptors have shown the expected signs of summation or long latency and prolonged after-discharge, which would be required to explain the sensory experience.” Cline et al. argued: “Pain which outlasts the stimulus has a peripheral explanation in our patient, in light of the observed prolonged after-discharge, which would be required to explain the sensory experience.” Wall continued: “The rapidity with which the syndrome occurs following trauma has no peripheral correlate.” Cline et al. countered: “It is no surprise that the onset of symptoms following injury may be quite rapid because C nociceptors are capable of becoming sensitized within minutes following noxious stimulation.” Wall went on: “The failure of nerve grafts to cure the symptoms once the graft has reinnervated its target tissue …” Cline et al. clarified: “The idea that delayed failure of nerve graft therapy indicates a central dysfunction ignores a reasonable peripheral explanation: Nociceptors whose sensitized state cannot be expressed symptomatically when the stimulated skin is denervated might recreate painful symptoms when reinnervating the target.”
Strong intraneural electrical stimulation of human cutaneous nerve fascicles is painful. This also applies to muscle nerves [7,19]. Obviously, during intraneural stimulation at intensities that activate unmyelinated C fibers, both afferent somatic and efferent sympathetic C fibers discharge. Many primary C nociceptors with unmyelinated fibers are equipped with terminal neurosecretory apparati capable of inducing vasodilatation, as triggered by antidromic nerve impulses (“neurogenic inflammation” [2]). When the complement of such nerve fibers contained within individual nerve fascicles is excited intraneurally, the area of resulting neurogenic vasodilatation matches precisely a sensory fascicular territory of skin [14] (Fig. 10). This is a relatively delayed phenomenon. However, the expected independent vasoconstrictor effect of activating sympathetic vasoconstrictor fibers does not follow a fascicular dermatome. It elicits diffuse vasoconstriction of a broad area, for example, the whole hand. This effect likely reflects a segmental somatosympathetic vasoconstrictor response induced by painful input during intraneural stimulation.
Fig. 10.
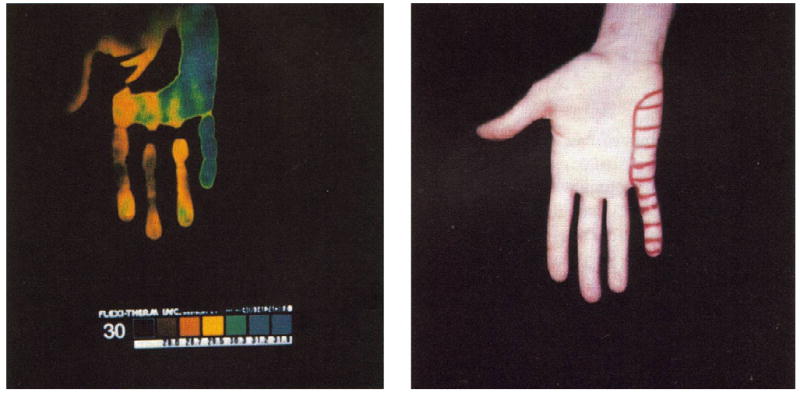
Left panel shows liquid crystal thermogram of the palm of the hand portrayed on the right. The baseline thermogram before stimulation displayed a uniformly yellow color. Following prolonged painful intrafascicular stimulation, regional warming developed (blue). The projected field of sensations evoked during painful intraneural stimulation are marked in the skin of the hand.
A fascinating interaction between antidromic vasodilatation and orthodromic vasoconstriction phenomena occurs during sustained painful intraneural microstimulation [16]. The sequence is as follows: there is a first stage of diffuse sympathetic mediated vasoconstriction during the painful stimulation. There is a second stage, that develops after discontinuing repetitive painful stimulation, during which a progressive vasodilatation very clearly confined to a sensory fascicular territory of skin becomes established. This reflects neurogenic inflammation due to neurosecretion of vasoactive substances from C nociceptors [1]. During a third stage involving re-initiation of the painful intraneural microstimulation, the vasodilatation becomes rectified and the whole segment again becomes vasoconstricted. Clearly, sympathetic mediated vasoconstriction overwhelms nociceptor-mediated antidromic vasodilatation. We have wondered whether fostering sympathetic mediated vasoconstriction and cooling might help relieve passively the heat dependent pain in patients with “erythralgia” [6] or ABC syndrome [12]. Indeed, this is occasionally observed in ABC patients. In them, interjection of sympathetic outflow not only blanches the rubor, but neutralizes the spontaneous pain and the mechanical and heat hyperalgesias. Such pain suppression likely results from the rectifying effect of cooling, upon the temperature-dependent hyperexcitable anomaly of nociceptor membranes. Thus, for circumstantial reasons, sympathetic activity may subdue antidromic vasodilatation and may even abolish pain evoked pathologically from nociceptors sensitized to heat. This, of course, is the opposite of the controverted “sympathetically maintained pain” state. Often, patients expressing an overt ABC syndrome are misconstrued clinically as harboring RSD/CRPS/SMP. However, their pain and heat hyperalgesia are predictably worsened by sympathetic blocks that warm up their “erythralgic” skin.
The case of Mr. R.C. is an experiment of nature in the realm of neuropathic pain, neurogenic vasodilatation, and misdiagnosis of potential contributions by nociceptor afferents versus sympathetic efferentes to clinical “autonomic signs” and “sympathetically mediated pains.” The patient, age 50, fell off a bicycle, twisted his back, and developed low back and radicular pain projected to the Left L5 root, associated with sensory dysfunction (static mechanical hyperalgesia) over the lateral aspect of the leg, dorsum of the foot and mostly the big toe, plus weakness of ankle and big toe dorsiflexion. There was a striking reddish discoloration and warming of the skin in the lateral aspect of the symptomatic leg, and the dorsum of the foot and big toe. MRI of the lower spine revealed an intraforaminal L4–5 disc herniation. A few days post lumbar laminectomy, discectomy and spinal fusion with instrumentation, the symptoms and signs worsened severely. Re-operation revealed a grossly compressed Left L5 root that relaxed after disengaging the fixating pedicular screws. One consultant diagnosed CRPS I (despite presence of a clearly documented nerve root injury) and implied sympathetic nerve fiber irritation (despite absence of sympathetic outflow into nerve roots distal to L2 segment in humans). Part of the diagnostic hypothesis was inspired by the presence of hyperalgesia/allodynia, plus the objective vasomotor signs (despite the presence of hyperthermia rather than hypothermia as expected from sympathetic irritation). Lumbar sympathetic blocks failed to relieve symptoms or signs (no formal record of outcome of skin temperature or hyperalgesia is available). Careful reassessment of the anatomical distribution of the “erythralgia” (diagrams, physician’s reports, photographs) demonstrated that the vasomotor signs reflected vasodilatation strictly confined to the L5 dermatome, as per the standard dermatome charts of Henry Head. It may be remembered that Head’s classic zoster-inspired human dermatomes closely matched the otherwise inspired “dermatomes in man” [4]. It is pertinent to the present case to remember that one method used by Foerster in his study took advantage of the “vasodilatation method” triggered by electrical stimulation of exposed nerve roots. All this has nothing to do with the sympathetic system. This was a striking case of C nociceptor-mediated antidromic vasodilatation due to irritation of unmyelinated fibers in the L5 root.
Fig. 6.
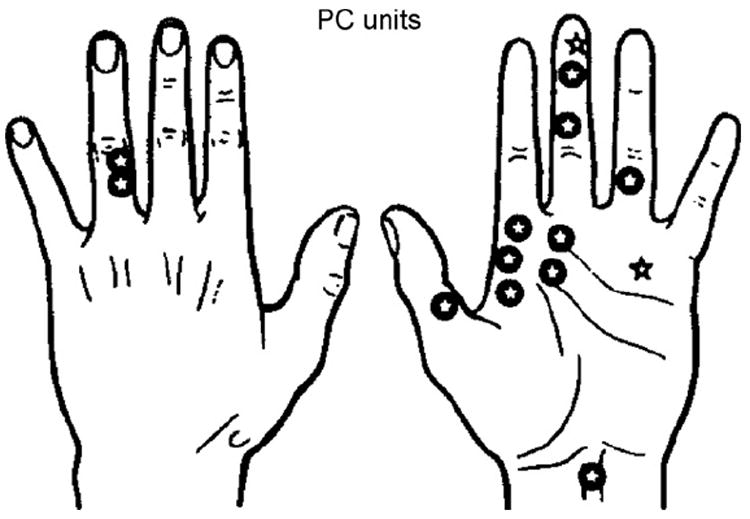
Location of receptive fields of 14 PC units. INMS-evoked sensations from 12 units (✪) and no sensation from 2 units (✰).
Fig. 7.
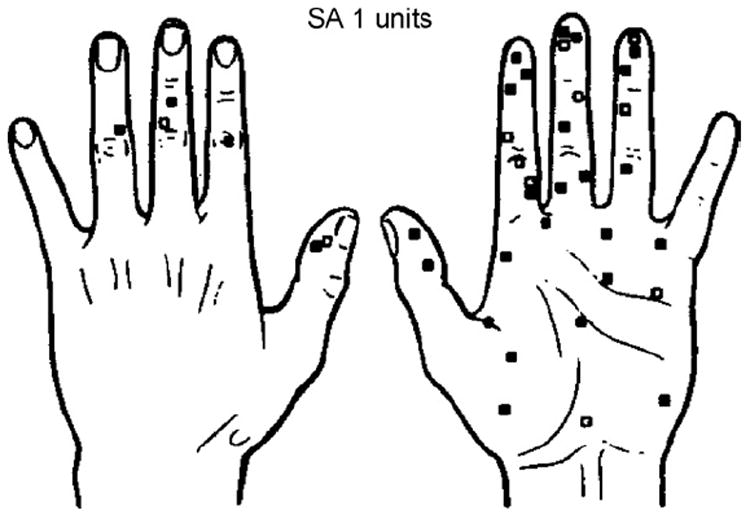
Location of receptive fields of 39 SA I units. INMS evoked a sensation from 28 units (■) and no sensation from 11 units (□).
References
- 1.Brain SD, Escott KJ. Calcitonin gene-related peptide. Agents Actions (Suppl) 1994;41:C262–C263. [Google Scholar]
- 2.Chahl LA, Szolcsányi J, Lembeck F. Antidromic vasodilatation and neurogenic inflammation. Satellite symposium of the 29th International Congress of Physiological Sciences; Newcastle, Australia. 1983. [Google Scholar]
- 3.Cline MA, Ochoa J, Torebjörk HE. Chronic hyperalgesia and skin warming caused by sensitized C nociceptors. Brain. 1989;112:621–647. doi: 10.1093/brain/112.3.621. [DOI] [PubMed] [Google Scholar]
- 4.Foerster O. The dermatomes in man. Brain. 1933;56:1–39. [Google Scholar]
- 5.Hallin RG, Wu G. Novel information on peripheral tactile mechanisms in man acquired with concentric needle electrode microneurography. Behavioral Brain Research. 2002;135(1–2):11–18. doi: 10.1016/s0166-4328(02)00149-3. [DOI] [PubMed] [Google Scholar]
- 6.Lewis T. Pain. Macmillan; London: 1942. pp. 1–191. [Google Scholar]
- 7.Marchettini P, Simone DA, Caputi G, Ochoa JL. Pain from excitation of identified muscle nociceptors in humans. Brain Research. 1996;740:109–116. doi: 10.1016/s0006-8993(96)00851-7. [DOI] [PubMed] [Google Scholar]
- 8.Melzack R, Wall PD. On the nature of cutaneous sensory mechanisms. Brain. 1962;85:331–356. doi: 10.1093/brain/85.2.331. [DOI] [PubMed] [Google Scholar]
- 9.Ochoa J, Torebjörk HE. Paresthesiae from ectopic impulse generation in human sensory nerves. Brain. 1980;103:835–853. doi: 10.1093/brain/103.4.835. [DOI] [PubMed] [Google Scholar]
- 10.Ochoa J, Torebjörk HE. Sensations evoked by intraneural microstimulation of single mechano-receptor units innervating the human hand. Journal of Physiology. 1983;342:633–654. doi: 10.1113/jphysiol.1983.sp014873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochoa J, Torebjörk HE, Culp W. Determinants of subjective attributes of normal cutaneous sensation and of paresthesiae from ectopic nerve impulse generation. In: Von Euler, Franzén O, Lindblom U, Ottoson D, editors. Somatosensory Mechanisms, Wenner-Gren Center International Symposium. Vol. 41. 1984. pp. 379–387. [Google Scholar]
- 12.Ochoa J. The newly recognized painful ABC syndrome: thermographic aspects. Thermology. 1986;2:65–107. [Google Scholar]
- 13.Ochoa J, Cline MA, Dotson R, Marchettini P. Effects of Injury on Trigeminal and Spinal Somatosensory Systems. Alan R. Liss Publishers; New York: 1987. Pain and paresthesias provoked mechanically in human cervical root entrapment (sign of Spurling). Single sensory unit antidromic recording of ectopic, bursting, propagated nerve impulse activity; pp. 389–397. [Google Scholar]
- 14.Ochoa J, Comstock WJ, Marchettini P, Nizamuddin G. Intrafascicular nerve stimulation elicits regional skin warming that matches the projected field of evoked pain. In: Schmidt RF, Schaible HG, Vahle-Hinz C, editors. Fine Afferent Nerve Fibers and Pain. Vol. 44. VCH Verlagsgesellschaft; Weinheim: 1987. pp. 475–479. [Google Scholar]
- 15.Ochoa J, Torebjörk HE. Sensations evoked by selective intraneural microstimulation of identified C nociceptor fibres in human skin nerves. Journal of Physiology. 1989;415:583–599. doi: 10.1113/jphysiol.1989.sp017737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochoa J, Yarnitsky D, Marchettini P, Dotson R, Cline M. Interactions between sympathetic vasoconstrictor outflow and C nociceptor-induced antidromic vasodilatation. Pain. 1993;54:191–196. doi: 10.1016/0304-3959(93)90208-7. [DOI] [PubMed] [Google Scholar]
- 17.Schady W, Torebjörk HE, Ochoa J. Peripheral projections of nerve fibers in the human median nerve. Brain Research. 1983;277:249–261. doi: 10.1016/0006-8993(83)90932-0. [DOI] [PubMed] [Google Scholar]
- 18.Schady W, Torebjörk HE, Ochoa J. Cerebral localisation function from the input of single mechanoreceptive units in man. Acta Physiologica Scandinavica. 1983;119:277–285. doi: 10.1111/j.1748-1716.1983.tb07338.x. [DOI] [PubMed] [Google Scholar]
- 19.Simone DA, Marchettini P, Caputi G, Ochoa JL. Identification of muscle afferents subserving sensation of deep pain in humans. Journal of Neurophysiology. 1994;72:883–889. doi: 10.1152/jn.1994.72.2.883. [DOI] [PubMed] [Google Scholar]
- 20.Torebjörk HE, Ochoa J. Specific sensations evoked by activity in single identified sensory units in man. Acta Physiologica Scandinavica. 1980;110:445–447. doi: 10.1111/j.1748-1716.1980.tb06695.x. [DOI] [PubMed] [Google Scholar]
- 21.Torebjörk HE, Vallbo AB, Ochoa J. Intraneural microstimulation in humans. Its relation to specificity of tactile sensations. Brain. 1987;110:1502–1529. doi: 10.1093/brain/110.6.1509. [DOI] [PubMed] [Google Scholar]
- 22.Torebjörk HE, Ochoa J. New method to identify nociceptor units innervating glabrous skin of the human hand. Experimental Brain Research. 1990;81:509–514. doi: 10.1007/BF02423499. [DOI] [PubMed] [Google Scholar]
- 23.Vallbo AB. Sensations evoked from the glabrous skin of the human hand by electrical stimulation of unitary mechanosensitive afferents. Branch Research. 1981;215:359–363. doi: 10.1016/0006-8993(81)90517-5. [DOI] [PubMed] [Google Scholar]
- 24.Wall PD. The hyperpathic syndrome: a challenge to specificity theory. In: Zotterman Y, editor. Somatosensory mechanisms. Wenner-Gren International Symposium. Vol. 27. Pergamon Press; Oxford: 1984. pp. 489–502. [Google Scholar]
- 25.Wall PD, McMahon SB. Microneuronography and its relations to perceived sensation: a critical review. Pain. 1985;21:209–229. doi: 10.1016/0304-3959(85)90086-7. [DOI] [PubMed] [Google Scholar]


