Summary
The regulation of tolerance to self-proteins and the suppression of T-cell responses have in part been attributed to the activity of CD25+CD4+ T regulatory (Treg) cells. Further, Treg cells can inhibit the antitumor effectiveness of adoptive immunotherapy and active immunization approaches in preclinical models. In an effort to selectively eliminate Treg cells from human peripheral blood mononuclear cell to potentially bolster antitumor responses, we have evaluated the Treg-cell depleting capacity of the CD25-directed immunotoxin, RFT5-SMPT-dgA. In preclinical studies, incubation of human peripheral blood mononuclear cell with RFT5-SMPT-dgA mediated a partial reduction in the levels of CD25+, Foxp3- expressing CD4+ T cells in vitro. Administration of RFT5-SMPT-dgA to 6 patients with metastatic melanoma induced a transient but robust reduction in the number of CD25high CD4+ T cells in vivo (a 97.5% mean reduction at nadir; from 69.4±12.4 cells/μL to 1.7±0.3 cells/μL). The reduction in FOXP3+ CD4+ T-cell number was less comprehensive (a 71.3% mean reduction at nadir; from 66.6±16.5 cells/μL to 14.2±3.9 cells/μL). This resulted in the selective persistence of a stable number of CD25low/neg FOXP3+ CD4+ T cells in vivo. No objective antitumor responses were seen in any patient. Our results indicate that the CD25-directed, RFT5-SMPT-dgA immunotoxin can mediate a transient, partial reduction in Treg-cell frequency and number in vitro and in vivo and suggest that comprehensive eradication of human Treg cells in vivo may require the ability to target and eliminate FOXP3+ CD4+ T cells expressing both high and low levels of CD25.
Keywords: human, immunotoxin, RFT5-SMPT-dgA, CD25, regulatory T cell, depletion
Self/tumor antigens, such as cancer/testis antigens, differentiation antigens and overexpressed self-tissue proteins, are common targets of naturally occurring tumor infiltrating lymphocytes (TILs) and immunotherapeutic strategies. As such, the ability to shift the immune balance from self-tolerance toward anti–self-recognition holds substantial promise for the bolstering of antitumor responses in vivo. Immunosuppressive cellular elements such as T regulatory (Treg) cells, which regulate responses to self-proteins, may thus represent a prohibitive barrier to effective tumor treatment. In human metastatic melanoma, tumor-reactive TIL isolated from excised, progressive lesions regularly recognize self/tumor antigens including the melanocyte differentiation antigens MART-1 and gp100. Moreover, functional circulating Treg cells and tumor infiltrating, tumor antigen-specific Treg cells from patients with metastatic melanoma have been described.1,2 Additionally, Treg cells from metastatic melanoma reportedly inhibit the function of the infiltrating T-cell population.3
In humans, naturally developing Treg cells comprise about 5% to 10% of the peripheral CD4+ T-cell compartment and seem to preserve homeostatic peripheral self-tolerance via suppression of self-antigen–reactive T cells.4 Their role as regulators of self-tolerance is evident in autoimmune individuals with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX), a recessive and frequently fatal genetic disorder resulting from a lack of functional Treg cells.5 Thus, the ability to selectively eliminate Treg cells may strengthen preexistent, self/tumor antigen-specific TIL responses in vivo. However, the ability to comprehensively eliminate human Treg cells in vivo has been limited by the lack of an exclusive cell surface marker for identification and depletion of the entire Treg-cell population. Treg cells constitutively express the high affinity α-chain of the IL-2 receptor (CD25), glucocorticoid- induced tumor necrosis factor receptor (GITR), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and Forkhead box P3 (Foxp3),6 a transcription factor required for Treg-cell development and function.7 In the absence of clinically-approved strategies for targeted depletion of FOXP3-expressing cells in vivo,8 clinical efforts to selectively deplete Treg cells have relied heavily on targeting Treg-cell associated surface molecules.
In murine models, administration of a CD25-specific antibody can effectively deplete Treg cells to promote autoimmune induction9 and improve antitumor responses in vivo.10–15 The impact of clinical-grade, CD25-depleting reagents on circulating Treg-cell number in vivo and tumor immunity in humans has been largely limited to in vitro study.16 A clinical-grade CD25-specific antibody (anti-Tac, daclizumab) exists yet possesses a long half-life in vivo which may affect Treg and activated effector T cells alike. Anti-Tac is known to block T-cell activation to reduce immune disorders17 but its Treg-cell depleting capacity in vivo is not well established.18 Clinical reagents with short half-lives in vivo, such as immunotoxins (ITs), which combine the targeting specificity of a monoclonal antibody with a cellular toxin, have been used to selectively eliminate cell subpopulations in vivo.19 RFT5-SMPT-dgA is an IT comprised of the IL-2Rα-specific murine IgG1 antibody RFT5 linked to deglycosylated ricin A chain (dgA) via the sterically hindered heterobifunctional disulfide linker SMPT [4-succinimidyl-oxycarbonyl-α-methyl-a-(2-pyridyldithio)-toluene]. RFT5-SMPT-dgA exhibited potent antitumor activity in severe combined immunodeficiency mice xenografted with L540 cells which express CD2520 and has been administered in a phase 1 to 2 clinical trial in patients with Hodgkin lymphoma (HL),21,22 a disease characterized by constitutive CD25 expression on malignant cells.23 In the current study, RFT5-SMPT-dgA was evaluated for the ability to selectively eliminate CD25+ Treg cells from human peripheral blood mononuclear cells (PBMCs) in vitro and in vivo.
MATERIALS AND METHODS
Patient Samples and Culture Media
Patient PBMCs were isolated by Ficoll-Hypaque separation after obtaining an informed consent and were cryopreserved in heat-inactivated human AB serum (HSA, Gemini Bioproducts, Woodland, CA) with 10% dimethyl sulfoxide and stored at −180°C until the time of study. Serum samples were collected and stored at 4°C until study. Complete media (CM) consisted of RPMI 1640 (Invitrogen Corp, Carlsbad, CA) supplemented with 2mM glutamine (Biofluids, Rockville, MD), 25mM HEPES buffer (Biofluids), 100 U/mL penicillin (Bio-fluids), 100 μg/mL streptomycin (Biofluids), 50 μM 2-mercaptoethanol (Invitrogen), and 10% heat-inactivated fetal bovine sera (FBS, Gemini Bioproducts).
Treatment Regimen
All patients in this study had metastatic melanoma and were entered on an institutional review board-approved protocol in the Surgery Branch of the National Cancer Institute. Informed consent was obtained from all subjects. Starting on day 0, patients received 3 mg/m2 of RFT5-SMPT-dgA intravenously every other day for a total of 3 doses. Four to five weeks after the last dose of RFT5-SMPT-dgA, patients were evaluated for tumor response and toxicity. This constituted one treatment course.
Patient Eligibility
Patients who were 18 years of age or greater, with measurable metastatic melanoma which was not responsive to standard therapy, who met standard laboratory safety criteria, and who did not have concomitant major medical illnesses or require steroid therapy were eligible for enrollment. Patients had clinical Eastern Cooperative Oncology Group performance status of 0 or 1 or 2. Eligibility criteria required serum creatinine levels ≤1.6 mg/dL and bilirubin ≤2.0 mg/dL. Serum eligibility criteria included serum albumin >2.5 g/dL, aspartate aminotransferase/alanine aminotransferase <2.5 times normal, and serum human antimouse antibody (HAMA) levels ≤1 μg/mL. Blood eligibility criteria included white blood cell ≥3000/mm3, and platelets ≥90,000/mm3. Excluded from the protocol were patients who previously received RFT5-SMPT-dgA on another trial or monoclonal antibody therapy within 12 weeks of enrollment, had prior radiotherapy, had a resting left ventricular ejection fraction of <45%, or extensive lung disease and patients with autoimmune disease, immunodeficiency, HIV infection, or other concurrent malignancies.
IT
The RFT5-SMPT-dgA IT was prepared as described previously24 by linking the IL-2Rα–specific murine IgG1 antibody RFT5 to deglycosylated ricin A chain (dgA; Inland Laboratories, Austin, TX) via the sterically hindered heterobifunctional disulfide linker SMPT [4-succinimidyl-oxycarbonyl-α-methyl-a-(2-pyridyldithio)- toluene; Pierce Endogen, Rockford, IL]. Vials of drug stored at −80°C were thawed and filtered through a 0.22 μm filter. RFT5-SMPT-dgA was formulated as a sterile solution at 0.5 mg/mL in 0.85% NaCl USP, containing 5mM lysine.
Antibodies and Flow Cytometry
Fluorescein isothiocyanate-conjugated antihuman CD4 and CD8 antibodies and antigen-presenting cell–-conjugated CD3 antibody were all obtained from BD Biosciences (San Jose, CA). For CD25 detection, PE-conjugated CD25 antibody (4E3 clone; Miltenyi Biotech, Auburn, CA) was used. CD25high expression was defined by comparison to isotype antibody staining. FOXP3 (clone PCH101) and control rat IgG2a antibodies were obtained from eBiosciences. Thawed PBMCs were resuspended in fluorescence-activated cell sorting buffer consisting of phosphate-buffered saline with 2% FBS (Gemini Bioproducts) at 107 cells/mL and blocked with 10%normal mouse Ig (Caltag Labs, Burlingame, CA) for 10 minutes on ice. Cells (106) in 100 μL were stained with fluorochrome-conjugated monoclonal antibody at 4°C for 40 minutes in the dark. In some cases, cells were briefly stained with propidium iodide for nonviable cell exclusion and analyzed in a FACSCalibur (BD Biosciences). FOXP3 staining was performed according to manufacturer’s instructions (eBiosciences).
In Vitro Sensitization
The 10-day in vitro sensitization was carried out as previously described.25 Briefly, after 48-hour culture in CM with or without RFT5-SMPT-dgA, PBMCs were washed and plated at 3 × 106/well in 24-well plates with 1 μM soluble peptide [Flu58–66 GILGFVFTL or gp100280–288(288 V) YLEPGPVTV] for 10 days. Cells were harvested, washed, and plated in 96-well plates with T2 cells alone or pulsed with either peptide at 1 μM. After 24 hours the supernatant from each well was harvested and interferon-γ was measured using enzyme-linked immunosorbent assay according to the manufacturer’s instructions (Pierce Endogen).
Real-time Polymerase Chain Reaction
Levels of mRNA Foxp3 were analyzed by quantitative real-time polymerase chain reaction using the TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA) as described previously.26
Lymphocyte Separation
CD4+ cells were separated from whole PBMC by magnetic bead selection using negative isolation (Dynal Biotech; Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. CD4+ cells were further purified into CD25− and CD25+ fractions using the Dynal Treg kit according to the manufacturer’s instructions. Separations were performed in phosphate-buffered saline with 0.1% bovine serum albumin.
Proliferation Assay
PBMCs treated with 0 or 100 ng/mL RFT5-SMPT-dgA for 48 hours were plated in 96-well plates coated with CD3 antibody (OKT3; 1 μg/mL) at a cell concentration of 50 × 103 PBMCs per well. On days 2 and 4 of cell culture, 1 μCi [3H]-thymidine incorporation was added per well and further cultured for 18 hours before harvesting for measurement on days 3 and 5. Plates were harvested onto nylon filters using the Betaplate system and radioactivity quantified using a Betaplate counter. Results are expressed as the mean counts per minute of 24 cultures ±SEM per condition.
HAMA and Human Antiricin Chain Antibody Detection
HAMA and human antiricin chain antibody (HARA) were measured as described previously.27
RESULTS
Impact of RFT5-SMPT-dgA on CD25+CD4+ T Cells In Vitro
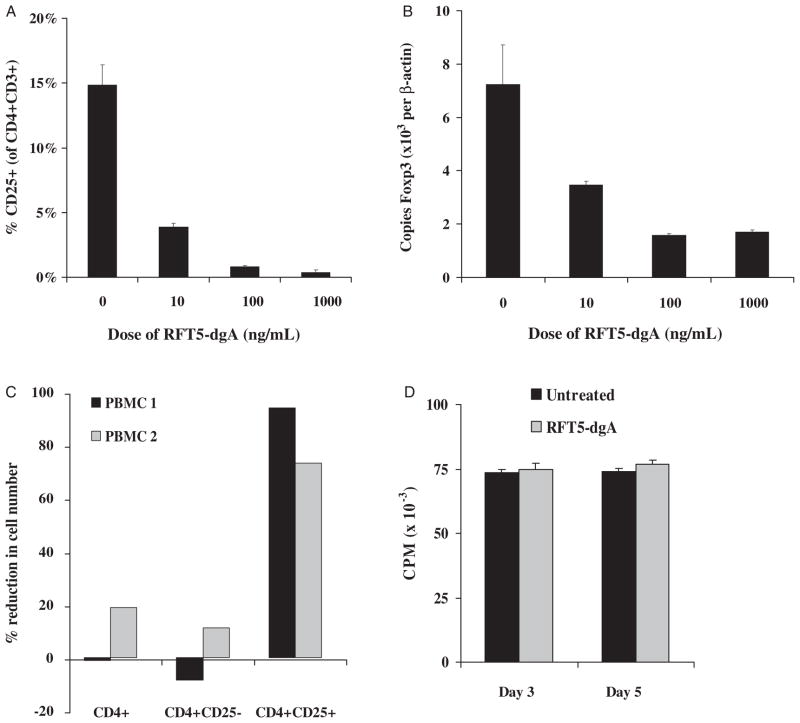
Resting PBMCs were incubated with doses of RFT5-SMPT-dgA ranging from 0 to 1000 ng/mL final concentration in vitro for 48 hours and assessed for CD25 and Foxp3 expression by CD4+ T cells in 2 independent experiments. At high concentrations, the percentage of CD3+ CD4+ lymphocytes expressing CD25 decreased from 14.9±1.5% to 0.4±0.2%, for a 97.6% mean reduction (Fig. 1A). This paralleled a decrease in Foxp3 expression from 7.2±1.5 to 1.7±0.1 copies per 103 β-actin copies as quantified by real-time quantitative polymerase chain reaction, representing a 77.4% mean reduction (Fig. 1B). Sensitivity to RFT5-SMPT-dgA was detectable at 10 ng/mL, but a maximum impact was observed near 100 ng/mL. Serial harvesting of PBMC at 12, 24, 48, and 72 hours after a single administration of IT at 100 ng/mL suggested maximum reduction in CD25 and Foxp3 expression by CD4+ T cells occurred beginning 48 hour after exposure in vitro (data not shown).
FIGURE 1.
Varying doses of RFT5-SMPT-dgA were incubated with resting human PBMC for 48 hours and the percent of residual CD25+ CD4+ cells (A) and the number of Foxp3 mRNA copies per 104 copies of β-actin mRNA (B) evaluated. A dose-related reduction in these 2 surrogate markers of human Treg cells was seen. This experiment was representative of 3 independent dose titrations performed. C, Whole CD4+, CD4+ CD25−, or CD4+ CD25+ cell subsets were purified from resting human PBMC after 48-hour incubation in CM with or without RFT5-SMPT-dgA (100 ng/mL) and cell yield determined in 2 independent experiments. Percent reduction was calculated as cell number of the IT-treated PBMC subset relative to the cell count of the untreated PMBC subset. D, PBMCs were cultured for 48 hours in CM containing RFT5-SMPT-dgA (100 ng/mL) or CM alone (untreated), washed, stimulated with plate-bound anti-CD3 antibody and measured for [3H]-thymidine incorporation on days 3 and 5 of cell culture. Results are expressed as the mean counts per minute of 24 independent well cultures ± SEM per condition.
To quantify the impact of RFT5-SMPT-dgA on resting Treg cells, large numbers of freshly isolated PBMCs were treated with or without IT. After 48-hour incubation, PBMCs were mechanically sorted into CD4+ fractions by negative isolation and then into CD4+CD25− and CD4+CD25+ fractions and counted (Fig. 1C). In 2 independent experiments performed on separate patient PBMC samples (containing 1.5 × 109 and 3.0 × 108 cells, respectively), the impact of RFT5-SMPTdgA on the absolute number of CD25+CD4+ cells was profound, producing a 94.1% and 73.3% reduction compared with untreated controls (from 5.1 × 106 to 0.3 × 106 and from 0.15 × 106 to 0.04 × 106, respectively). In both experiments, the impact upon the absolute CD4+ count (+1.3% and −18.8%) and the absolute CD4+CD25− count (+8.5% and −11.1%, respectively) was minimal, suggesting a preferential cytotoxicity of RFT5-SMPT-dgA directed against cells expressing CD25. Together, these data demonstrate the capacity of the CD25-directed IT, RFT5-SMPT-dgA, to mediate a partial elimination of human regulatory T cells in vitro.
To determine the impact of RFT5-SMPT-dgA treatment on the surviving non-CD25+ T-cell population, we evaluated their proliferation and reactivity in vitro. PBMC harvested after a 48-hour culture in CM alone or containing 100 ng/mL RFT5-SMPT-dgA were stimulated with plate-bound anti-CD3 antibody and measured for [3H]-thymidine incorporation on days 3 and 5 of cell culture (Fig. 1D). No difference in proliferation was detected at either time point suggesting that the surviving non-CD25+ cells were unharmed by treatment. To test the ability of IT-surviving effector cell precursors to respond to peptide stimulation, RFT5-SMPT-dgA treated and untreated PBMCs were sensitized in vitro with relevant (Flu58–66), irrelevant [gp100280–288 (288 V)], or no soluble peptide for 10 days. The cells were then washed, cultured overnight with T2 cells alone or pulsed with each peptide and assayed for interferon-γ secretion (Table 1). In 2 independent experiments, both the untreated and RFT5-SMPT-dgA-treated PBMC responded only to relevant Flu58–66 peptide stimulation, however, the magnitude of response was greater in the treated group (4177 vs. 2517 pg/mL, Table 1; 4720 vs. 799 pg/mL, data not shown). Thus, the response to antigen stimulation among RFT5-SMPT-dgA-surviving cells was equivalent, if not better than, that of the untreated cells suggesting that the IT pretreatment had no deleterious effects on the remaining non-CD25+ CD4+ and CD8+ cells.
TABLE 1.
Effector Cell Precursors are not Negatively Affected by RFT5-SMPT-dgA Pretreatment In Vitro
| Stimulation | Preconditioning
|
|||||
|---|---|---|---|---|---|---|
| CM Alone
|
CM+RFT5-dgA
|
|||||
| None | g280 | Flu | None | g280 | Flu | |
| Flu/T2 | 112 | 74 | 2517 | 46 | 54 | 4177 |
| g280/T2 | 670 | 607 | 602 | 664 | 579 | 553 |
| T2 alone | 44 | 31 | 118 | 50 | 59 | 99 |
| None | 39 | 49 | 39 | 50 | 50 | 45 |
Patient PBMC were incubated with or without RFT5-SMPT-dgA at 100 ng/mL for 48 h, washed and cultured for 10 d at 3 × 106 cells/well in CM containing IL-2 (50CU/mL) alone (none) or with 1 μM of soluble peptide (Flu: Flu58–66 GILGFVFTL; g280: gp100280).
As RFT5-SMPT-dgA could mediate a partial reduction in CD25+ Foxp3-expressing CD4+ T-cell numbers in vitro without impairing the function of the remaining lymphocytes, a phase 2 prospective trial was developed to determine whether objective clinical responses could be obtained in patients with metastatic melanoma after administration of RFT5-SMPT-dgA, with secondary objectives to determine whether administration of RFT5-SMPT-dgA could mediate changes in circulating CD25+ CD4+ Treg-cell levels and evaluate the toxicity profile of patients treated.
Patient Characteristics and Treatment
Six patients with progressive metastatic melanoma (5M/1F) were enrolled to receive RFT5-SMPT-dgA therapy; 5 patients had visceral metastases. Patients age ranged from 39 to 64 years old (mean of 51.5±4.2). All patients had received prior surgery, chemotherapy, and immunotherapy. The dosing regimen consisted of RFT5-SMPT-dgA (3 mg/m2) administered intravenously every other day for 3 total doses (9 mg/m2 total) with patient evaluation occurring 4 to 5 weeks after the last dose of IT. All patients received a full course of RFT5-SMPT-dgA therapy, except 1 patient who was withdrawn from the protocol after experiencing abdominal pain and hypotension shortly after administration of the first IT dose.
Treatment-related Toxicities, Antibody Responses, and Clinical Outcome
As established in a previous phase 1 trial in patients with HL, RFT5-SMPT-dgA has a maximum-tolerated dose (MTD) of 15 mg/m2 per course with moderate toxicities including vascular leak syndrome, dyspnea, fatigue, and malaise.22 Although RFT5-SMPT-dgA has been administered to patients with hematologic cancers, the impact of RFT5-SMPT-dgA on patients with metastatic melanoma, who have comparatively smaller numbers of circulating CD25+ lymphocytes, is unknown. A sub-MTD of 9 mg/m2 per course was therefore chosen based upon the findings that grade III or IV toxicities were seldom observed among patients treated at doses of 5 to 10 mg/m2 and the suggestion that Treg cells might be sensitive to RFT5-SMPT-dgA at lower doses.21,28 Patients with metastatic melanoma receiving IT at 9 mg/m2 per course experienced a similar panel of toxicities (Table 2). Mild to moderate side effects were observed in the 6 patients receiving RFT5-SMPT-dgA, including 4 cases of grade II hypoalbuminemia and 3 cases of grade II hypocalcemia. Individual cases of grade I/II fatigue, fever, dyspnea, abdominal pain, leukopenia, and altered transaminase levels were also observed. Few patients experienced grade III events but individual cases of fatigue, transient VLS, hypoalbuminemia, and a line placement-related infection did develop (Table 2).
TABLE 2.
Toxicity Events After RFT5-SMPT-dgA Treatment
| Toxicity | Grade
|
|||
|---|---|---|---|---|
| I | II | III | IV | |
| Fatigue | 1 | 1 | ||
| Fever | 1 | |||
| Line infection | 1 | |||
| Abdominal pain | 1 | 1 | ||
| Dyspnea | 1 | |||
| Back pain | 1 | 1 | ||
| Joint pain | 1 | |||
| aVLS | 1 | |||
| Hypoalbuminemia | 4 | 1 | ||
| Hypocalcemia | 3 | |||
| Hypophosphatemia | 1 | |||
| Hyperkalemia | 1 | |||
| Hemoglobin | 1 | |||
| Leukopenia | 1 | |||
| Neutropenia | 1 | |||
| SGPT ALT | 1 | |||
| SOT AST | 1 | |||
| Total | 4 | 18 | 4 | 0 |
aVLS indicates acute vascular leakage syndrome; SGPT ALT, serum glutamate pyruvate transaminase or alanine transaminase; SOT AST, serum glutamic oxaloacetic transaminase or aspartate transaminase.
As an eligibility criterion, all enrolled patients were negative for HAMA response before treatment. To determine whether immune responses had been elicited against RFT5-SMPT-dgA after treatment, serum from treated patients was assayed for HAMA and HARA responses (Table 3). After RFT5-SMPT-DGA therapy, HAMA responses were detectable in assessed serum samples from 2/4 patients. In the testing of nearly 1000 patients from multiple trials, HARA were seldom detected (<1.3%) and were therefore not tested before treatment. After IT treatment, 2/4 patients, the same 2 patients who developed HAMA, also developed HARA responses. Of note, patient 1 developed a profound HARA response of >5 mg/mL, which was persistent more than 2 months after the start of treatment. HARA were not detected in a retrospective analysis of serum from this patient obtained before treatment. The development of HAMA and HARA responses in these patients is consistent with the HAMA and HARA induced in 69% of HL patients receiving RFT5-SMPT-dgA at the MTD.22 No patient receiving RFT5-SMPT-dgA therapy experienced an objective cancer regression (0/5) or overt autoimmune disease, however, patient 1 has stable disease 14 months after the start of therapy.
TABLE 3.
HAMA/HARA Responses in Patients Receiving Immunotoxin Therapy
| Treated Patient | Day (Relative to First Dose) | HAMA (ng/mL) | HARA (ng/mL) |
|---|---|---|---|
| 1 | Pre | 0 | 0 |
| 28 | NT | 5200678 | |
| 35 | 3931 | 4002919 | |
| 69 | 13076 | 500470 | |
| 2 | Pre | 0 | NT |
| 35 | 0 | 0 | |
| 3* | Pre | 0 | NT |
| 4 | Pre | 0 | NT |
| 33 | 0 | 0 | |
| 5 | Pre | 0 | NT |
| 27 | 1718 | 148154 | |
| 6 | Pre | 0 | NT |
| Post | NT | NT |
Values represent serum antibody concentrations in ng/mL.
Antibody concentrations >1 μg/mL are bolded.
Patient treatment was terminated due to toxicities.
NT indicates samples not tested; Pre, pretreatment.
IT-mediated Reduction in CD25+ FOXP3+ CD4+ T Cells In Vivo
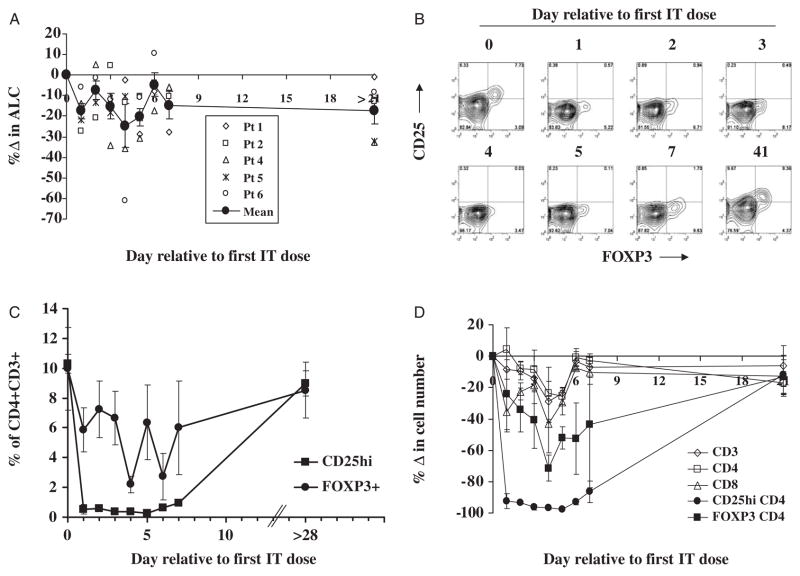
At the start of RFT5-SMPT-dgA therapy, the mean patient lymphocyte cell count was 1614±191 cells/μL (Fig. 2A). After administration of the first dose of IT, a minor, transient reduction in mean lymphocyte count was observed on day 1 (a 17.5% reduction to 1346±194 cells/μL; P≤0.009, 2-tailed paired t test for means) and again on day 5 postinfusion (a 20.4% reduction to 1278±151 cells/μL; P≤0.009). Cell counts were similar to pretreatment levels at the intervening days. One month after the first dose of RFT5-SMPT-dgA, the mean cell count was restored to pretreatment levels.
FIGURE 2.
A, The percent change in absolute lymphocyte count (ALC) was calculated relative to pretreatment ALC values on day 0. The mean percent change in ALC ± SEM is shown in filled circles. B, Representative dot plots show the expression of CD25 (y-axis) and FOXP3 (x-axis) on CD4+ CD3+-gated T cells before and on the indicated day after the start of IT-therapy in PBMC from patient 4. C, The mean frequency of CD4+ CD3+-gated T cells from all treated patients that expressed high levels of CD25 or FOXP3 ± SEM was longitudinally measured. D, The absolute number of T cells (CD3+) that were CD4+, CD8+, CD25high, or FOXP3+ was calculated and the percent change relative to pretreatment cell numbers determined.
To determine the impact of RFT5-SMPT-dgA on CD25+ Treg T cells in vivo, circulating CD4+ T cells from patients receiving the entire IT dosing regimen were measured for expression of CD25. Representative kinetic analysis in Figure 2B shows that CD25 expression on CD4 T cells from the peripheral blood of patient 4 was substantially reduced for the first week after initial IT administration and was restored to pretreatment levels after 1 month. The mean frequency of CD25 expressing CD4+ T cells from all patients was significantly reduced beginning on day 1 after the start of therapy from 10.3±1.4% to a 0.7±0.3% (P<0.0001; Fig. 2C). This reduction was maintained for the entire week after the first IT infusion. At 1 month after the first IT dose, the mean frequency of CD4 T cells expressing CD25 (9.0%±1.9%) was similar to pretreatment levels. Similarly, the mean frequency of CD25+ CD8+ T cells from all treated patients was reduced from 3.8%±1.2% to nearly 1% for the entire week after the first IT-dose and restored to normal levels after 1 month (4.3%±3.2%; data not shown).
To better determine the impact of RFT5-SMPT-dgA on Treg cells in vivo, CD4+ T cells were measured for expression of FOXP3 protein, a surrogate marker of Treg cells whose intracellular expression cannot be directly affected by potential blocking effects of RFT5-SMPT-dgA (Figs. 2B, C). Before IT administration, the mean percentage of CD4+ CD3+ cells expressing FOXP3 protein from all patients was 10.0%±2.8%. Figure 2B shows that FOXP3 expression was primarily restricted to CD4+ T cells expressing high levels of CD25, although FOXP3 was also detected in a subset of CD4+ T cells expressing lower levels of CD25. RFT5-SMPT-dgA administration induced a reduction in mean FOXP3+ CD4+ T-cell frequency that was significant from 3 to 7 days after the first IT dose (P≤0.05) with a nadir of 2.2±1.3% FOXP3+ CD4+ T cells occurring on day 4 (Fig. 2C). Thus, the mean frequency of FOXP3+ CD4+ T cells was reduced by up to 78% after RFT5-SMPT-dgA administration. One month after the first dose of IT, the mean frequency of FOXP3+ CD4+ T cells (8.5%±1.9%) was restored to pretreatment levels. These results show that the reduction in circulating FOXP3+ CD4 T-cell frequency mediated by RFT5-SMPT-dgA infusion was significant, albeit transient and incomplete.
To quantify to impact of RFT5-SMPT-dgA-therapy on circulating lymphocyte numbers, CD25+ or FOXP3+ CD4+ T-cell counts were enumerated and compared with total T-cell and CD8+ T-cell numbers (Fig. 2D). Compared with pretreatment numbers (1264±145 cells/μL), total CD3+ T-cell counts were not significantly reduced after IT-administration, with the exception of day 5 of therapy (951±87 cells/μL; P≤0.003). A similar effect was observed for CD4+ CD3+ T cells, dropping from 789±118 cells/μL to 566±57 cells/μL on day 5 (P≤0.01). Despite the fact that few circulating CD8+ T cells expressed CD25 before treatment (3.8%±1.2%; data not shown), CD8+ T-cell counts, which began at 320±104 cells/μL, were significantly reduced on days 1, 2, 5, and 6 post-IT infusion with a maximum significant reduction of 29.5% on day 5 (P<0.02). The mean frequency of CD25+ CD8+ T cells from all treated patients was reduced to nearly 1% for the entire week after the first IT-dose and restored to normal levels after 1 month (4.3%±3.2%). The greatest overall effect of IT-administration, however, was observed on CD25+ CD4+ T-cell counts which were significantly reduced from 69±12 cells/μL before therapy, for the entire 7 days after the first RFT5-SMPT-dgA dose (P≤0.05), with a nadir of 1.7±0.3 cells/μL on day 5 for a 97.5% mean reduction. Albeit to a lesser degree, FOXP3+ CD4+ T-cell counts were similarly reduced after RFT5-SMPT-dgA infusion with statistically significant reductions occurring 4 and 5 days after the first dose of IT (from 66±16 cells/μL to 14±4 cells/μL and 36±14 cells/μL, respectively; P≤0.004), accounting for a 71.3% mean reduction at nadir on day 4.
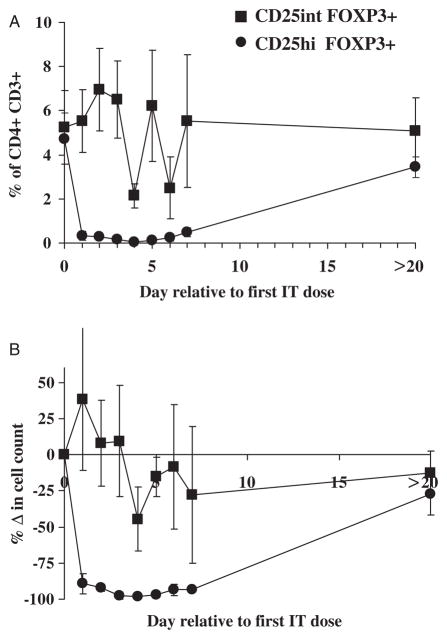
Selective Survival of CD25int FOXP3+ CD4 T Cells In Vivo
Although CD25 is highly expressed on the surface of the majority of FOXP3+ CD4 T cells in vivo, a smaller number of FOXP3+ CD4+ T cells do not express high levels of CD25 (CD25int, Fig. 2B). Accordingly, these latter cells may elude the cytotoxic effects of the CD25-directed IT in vivo and thereby account for the inability of RFT5-SMPT-dgA to mediate comprehensive elimination of FOXP3+ CD4 T cells in vivo. To determine the impact of CD25 expression on the susceptibility of FOXP3+ T cells to the cytotoxic effects of RFT5-SMPT-dgA, the frequency and number of FOXP3+ CD4 T cells expressing high or intermediate/low levels of CD25 was measured. Before treatment, the overall frequency of CD4 T cells that were FOXP3+ and expressed either high or intermediate/low levels of CD25 was similar (4.7±1.2% were CD25high; 5.2±1.7% were CD25int; Fig. 3A). CD25high FOXP3+ CD4 T-cell frequencies were significantly reduced for the first 6 days after first IT dose to 0.4%±1.7% on day 4 for a 98.7% reduction (P<0.02; Fig. 3B). Alternatively, CD25int FOXP3+ CD4 T cells were not significantly reduced at any time point after IT administration, indicating their continued survival. It is possible that our inability to detect CD25high FOXP3+ cells after IT therapy results in part from blocking of CD25 by residual bound RFT5-SMPT-dgA. However, use of another CD25 detecting antibody specific for an alternative CD25 epitope yielded nearly identical results, suggesting that the CD25 high population had in fact been eliminated (data not shown). Isolation and assessment of the CD25int FOXP3+ CD4 T-cell population for in vitro suppressor function was technically inhibited by the inability to selectively enrich live FOXP3-expressing cells, coupled with the lack of high CD25 expression by CD4 T cells after IT administration. Nevertheless, our data show that the limitation of this CD25-directed IT therapy for comprehensive elimination of FOXP3+ Treg cells in vivo seems to reflect the innate inability of RFT5-SMPT-dgA to selectively target and destroy FOXP3+ T cells that do not express high levels of CD25.
FIGURE 3.
FOXP3+ CD4+ T cells expressing intermediate levels of CD25 persist after CD25-directed IT therapy. A, The frequency of FOXP3+CD4+CD3+-gated T cells expressing either intermediate (squares) or high (circles) levels of CD25 after administration of RFT5-SMPT-dgA therapy was calculated. B, The mean change in the absolute number of FOXP3+CD4+ CD3+ T cells was calculated relative to levels measured before the initiation of IT therapy.
DISCUSSION
CD25high CD4+ Treg cells represent a good therapeutic target in the treatment of human cancers because these cells exhibit immune-suppressive function and are prevalent in the peripheral blood and tumor microenvironment of these individuals.29 Indeed, Treg cells reportedly contribute to the growth of human ovarian carcinomas in vivo by suppressing tumor-specific T-cell immunity and are associated with reduced survival of patients with ovarian cancer.30 The noted association between objective tumor regression and autoimmunity in patients receiving nonspecific immunotherapeutic agents such as IL-231 and anti-CTLA4 antibody,32,33 and after lymphodepleting preconditioning and autologous transfer of melanoma-reactive T cells31,32,34 suggests that immune responses directed against self-proteins may induce cancer regression and supports the use of self-tolerance–modulating agents for cancer therapy. In mouse studies, immune-modulation via elimination of CD25+CD4+ Treg cells can markedly improve cancer immunotherapy. 13,35 For example, cotransfer of CD25-depleted CD4+ T helper cells with tumor/self-reactive CD8+ T cells and vaccination into CD4+ T-cell deficient recipient mice resulted in regression of established tumor and concomitant autoimmunity, whereas cotransfer of CD25+ Treg cells abolished this effect.35 These findings suggest that Treg cells may account, in part, for the poor clinical response rates reported in cancer patients receiving conventional immunotherapy and that elimination of Treg cells via the selective depletion of CD25-expressing cells in vivo may improve cancer therapy.
As recombinant ITs can deliver a cytotoxic signal targeted by the specificity of a high affinity monoclonal antibody,36 we evaluated whether a CD25-directed IT could serve as a clinical agent to target and eliminate CD25+ Treg cells in vivo. In preclinical testing, incubation of human PBMC in vitro with 1000 ng/mL of RFT5-SMPT-dgA induced a 98% reduction in CD25+ CD4 T-cell frequency with a concomitant 77% reduction in Foxp3 copy number. The capacity of RFT5-SMPT-dgA to reduce Treg cells in vitro was similar to that noted in our previous evaluation of another CD25-directed IT, LMB-2.16 There was no detectable negative impact on the CD25-negative T-cell population surviving RFT5-SMPT-dgA incubation. In fact, the reactivity of in vitro expanded Flu-specific T cells from IT-treated PBMC was increased relative to the untreated PBMC control in the 2 PBMC samples tested. On the basis of its ability to reduce Treg cells in vitro while preserving the function of the remaining cell population, RFT5-SMPT-dgA was selected for clinical evaluation.
In a previous phase 1/2 trial to determine whether administration of RFT5-SMPT-dgA could reduce the risk of graft-versus-host disease after human leukocyte antigen-matched, unrelated marrow transplantation by targeting allo-activated T cells expressing CD25, acute graft-versus-host disease was exacerbated in patients who received small doses of RFT5-SMPT-dgA (1 to 3 mg/m2) suggesting a susceptibility of CD25+ Treg cells to the toxic effects of the IT.28 The impact of IT administration on CD25+ Treg-cell number and frequency in vivo was, however, not evaluated. In the current trial, patients with metastatic melanoma received a total of 9 mg/m2 of RFT5-SMPT-dgA; less than the 15 mg/m2 MTD established in patients with HL.22 Treatment-related toxicities were largely mild to moderate and consistent with previously reported results22 with few grade III toxicities observed suggesting that patients with melanoma are not at an increased risk for toxicities compared with patients with hematologic malignancies who harbor comparatively large numbers of circulating CD25+ cells. Two of four evaluated patients developed positive HAMA and HARA responses. Importantly, administration of RFT5-SMPT-dgA induced a transient but robust 97.5% mean reduction in the number of CD25high CD4+ T cells in vivo at nadir that was maintained for at least 1 week. The reduction in FOXP3+ CD4+ T-cell number was less comprehensive (a 71.3% mean reduction at nadir; from 66.6±16.5 cells/μL to 14.2±3.9 cells/μL) resulting in the selective persistence of a relatively stable number of CD25int/low FOXP3+ CD4+ T cells in vivo. Whether RFT5-SMPT-dgA administration mediated the elimination of activated self/tumor antigen-specific T cells in vivo is unknown. Despite the transient reduction in Treg-cell numbers, no patient experienced an objective clinical response or autoimmunity suggesting a more comprehensive and/or durative elimination may be necessary to alter the balance in self-tolerance in vivo. Although our trial was designed to treat up to 41 patients to determine whether the RFT5-SMPT-dgA can produce a response rate targeted to be 20%, we chose to terminate the trial after laboratory data showed an incomplete elimination of Treg cells in all 6 treated patients. Eligibility for retreatment required disease stabilization or a clinical response without the development of positive HAMA or HARA responses. Because no patient fulfilled this criterion, the impact of multiple course RFT5-SMPTdgA administration on Treg cells reduction or cancer regression was not evaluated. Further clinical investigation will be required to resolve whether the level of Treg-cell depletion achieved in these 6 heavily pretreated patients is sufficient to induce cancer regression in a subset of patients with melanoma or other cancers.
IT-mediated elimination of Treg cells was not complete on the basis of the evaluation of the surrogate Treg-cell marker, FOXP3. Our data and that of others37 show that although CD25 is highly expressed on the surface of many FOXP3+ CD4 T cells in vivo, a second population of human FOXP3+ CD4+ T cells expresses low to intermediate levels of CD25 and may thereby be resistant to the eliminatory effects of CD25-directed clinical agents. Accordingly, we found that although the frequency of FOXP3+ CD4 T cells that were CD25high or CD25int/low was similar before treatment, only CD25high FOXP3+ CD4 T-cell frequencies were significantly reduced after IT dosing. The mean frequency of CD25int/low FOXP3+ CD4 T cells was not significantly reduced at any time point. Our data indicate that the inability of RFT5-SMPT-dgA and other high affinity IL-2 receptor targeted agents to mediate comprehensive elimination of FOXP3+ CD4 T cells in vivo may result from an intrinsic inability to selectively target and destroy FOXP3+ T cells that do not express high levels of CD25. This may also help explain the observed disconnect between level of CD25 and Foxp3 reduction seen after treatment of human PBMC with the CD25-directed IT LMB-2 in vitro.38 The limitation of CD25-directed approaches for the total elimination of Treg cells in vivo suggests that alternative strategies which target a combination of Treg-cell–associated surface molecules or directly target and eliminate FOXP3+ cells8 in vivo may be more effective. Elucidating the cell surface phenotype of the surviving CD25int/low FOXP3+ CD4 T cells in vivo may thus be important in developing successful combinatory Treg-cell depleting clinical approaches.
In previous clinical trials designed to selectively eliminate Treg cells in vivo through targeting of the IL-2 receptor, comprehensive elimination of Treg cells has also remained elusive and the impact of Treg-cell depletion on cancer treatment still remains unknown. Selective depletion of functional CD25+ Treg cells from large-scale patient cell samples has been performed ex vivo under clinical-grade conditions for use in adoptive immunotherapy. 26 However, in vivo transfer of autologous CD25-depleted mononuclear populations to lymphopenic patients in combination with high-dose IL-2 was not sufficient to mediate prolonged reduction of Treg cells, and contrarily resulted in a transient elevation in Treg-cell numbers in vivo.39 Candidate Treg-cell depleting agents, which are easily administered to patients, have been more commonly used in clinical studies. Indeed, administration of anti–CTLA-4 antibody is effective in inducing cancer regression and autoimmunity in treated patients,32,33 although the antitumor effects of CTLA-4 blockade seem related to increased T-cell activation rather than inhibition or depletion of Treg cells which express CTLA-4.40 Alternatively, an incomplete, transient reduction in Treg cells in vivo was seen after administration of LMB-2 IT to 8 patients with melanoma, however, LMB-2 therapy did not augment immune responses to cancer vaccination and no patient responses were observed.41 In another approach, DAB389IL-2 (denileukin diftitox, ONTAK), a cytotoxin comprised of the interleukin-2 cytokine enzymatically linked to the active portion of the diphtheria toxin,42 has been clinically used to direct the toxic effects of diphtheria to malignant cells of hematologic origin expressing CD25.43–48 In the treatment of 13 patients with metastatic melanoma with DAB389IL-2, we found no significant impact on CD25+ Treg cells or cancer regression.49 This was consistent with the inability of DAB389IL-2 to eliminate CD4+CD25+ Treg cells in vitro. In some patients, the frequency of CD25+CD4+ cells actually increased as did normalized levels of Foxp3 expression. In 2 other clinical studies, DAB389IL-2 mediated an incomplete reduction in Treg cells in vivo that was reportedly capable of augmenting the potency of cancer vaccines.50,51 The impact of these reductions on clinical response was not evaluated. The mechanisms which account for the inability of DAB389IL-2 to comprehensibly eliminate Treg cells in vivo are not known but may be attributable to the ability of DAB389IL-2 to provide homeostatic IL-2 signals to Treg cells,52,53 a low binding affinity of IL-2 for its receptor relative to antibodies, or the selective survival of Treg cells expressing the low affinity IL-2 receptor complex37 as suggested by the current study.
The ability to surmount peripheral self-tolerance may hold significant promise to bolster in vivo antitumor responses in patients with cancer. Overcoming the immunoregulatory influences of Treg cells in vivo will require clinical-grade reagents and novel approaches to specifically target and comprehensively eliminate or abrogate the function of Treg cells in vivo.
Acknowledgments
Supported (in part) by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
The authors thank the research nurses and clinical care team at the Surgery Branch, National Cancer Institute. They also thank Drew Ivey, UTSWMC, for coordinating the shipment of samples and Kelly Mapes and Ayesha Ahmed for carrying out the HAMA/HARA assays.
Footnotes
Financial Disclosure: The authors have declared there are no financial conflicts of interest related to this work.
References
- 1.Javia LR, Rosenberg SA. CD4+CD25+ suppressor lymphocytes in the circulation of patients immunized against melanoma antigens. J Immunother. 2003;26:85–93. doi: 10.1097/00002371-200301000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 3.Viguier M, Lemaitre F, Verola O, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 5.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 7.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 8.Nair S, Boczkowski D, Fassnacht M, et al. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67:371–380. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]
- 9.McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002;168:5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 10.Golgher D, Jones E, Powrie F, et al. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32:3267–3275. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Onizuka S, Tawara I, Shimizu J, et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 12.Prasad SJ, Farrand KJ, Matthews SA, et al. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J Immunol. 2005;174:90–98. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 14.Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka H, Tanaka J, Kjaergaard J, et al. Depletion of CD4+ CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J Immunother (1997) 2002;25:207–217. doi: 10.1097/00002371-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Attia P, Powell DJ, Jr, Maker AV, et al. Selective elimination of human regulatory T lymphocytes in vitro with the recombinant immunotoxin LMB-2. J Immunother. 2006;29:208–214. doi: 10.1097/01.cji.0000187959.45803.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Junghans RP, Waldmann TA, Landolfi NF, et al. Anti-Tac-H, a humanized antibody to the interleukin 2 receptor with new features for immunotherapy in malignant and immune disorders. Cancer Res. 1990;50:1495–1502. [PubMed] [Google Scholar]
- 18.Kreijveld E, Koenen HJ, Klasen IS, et al. Following anti-CD25 treatment, a functional CD4+CD25+ regulatory T-cell pool is present in renal transplant recipients. Am J Transplant. 2007;7:249–255. doi: 10.1111/j.1600-6143.2006.01604.x. [DOI] [PubMed] [Google Scholar]
- 19.Kreitman RJ. Recombinant immunotoxins for the treatment of haematological malignancies. Expert Opin Biol Ther. 2004;4:1115–1128. doi: 10.1517/14712598.4.7.1115. [DOI] [PubMed] [Google Scholar]
- 20.Winkler U, Gottstein C, Schon G, et al. Successful treatment of disseminated human Hodgkin’s disease in SCID mice with deglycosylated ricin A-chain immunotoxins. Blood. 1994;83:466–475. [PubMed] [Google Scholar]
- 21.Engert A, Diehl V, Schnell R, et al. A phase-I study of an anti-CD25 ricin A-chain immunotoxin (RFT5-SMPT-dgA) in patients with refractory Hodgkin’s lymphoma. Blood. 1997;89:403–410. [PubMed] [Google Scholar]
- 22.Schnell R, Vitetta E, Schindler J, et al. Treatment of refractory Hodgkin’s lymphoma patients with an anti-CD25 ricin A-chain immunotoxin. Leukemia. 2000;14:129–135. doi: 10.1038/sj.leu.2401626. [DOI] [PubMed] [Google Scholar]
- 23.Strauchen JA, Breakstone BA. IL-2 receptor expression in human lymphoid lesions. Immunohistochemical study of 166 cases. Am J Pathol. 1987;126:506–512. [PMC free article] [PubMed] [Google Scholar]
- 24.Thorpe PE, Wallace PM, Knowles PP, et al. Improved antitumor effects of immunotoxins prepared with deglycosylated ricin A-chain and hindered disulfide linkages. Cancer Res. 1988;48:6396–6403. [PubMed] [Google Scholar]
- 25.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Impact of cytokine administration on the generation of antitumor reactivity in patients with metastatic melanoma receiving a peptide vaccine. J Immunol. 1999;163:1690–1695. [PMC free article] [PubMed] [Google Scholar]
- 26.Powell DJ, Jr, Parker LL, Rosenberg SA. Large-scale depletion of CD25+ regulatory T cells from patient leukapheresis samples. J Immunother. 2005;28:403–411. doi: 10.1097/01.cji.0000170363.22585.5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitetta ES, Stone M, Amlot P, et al. Phase I immunotoxin trial in patients with B-cell lymphoma. Cancer Res. 1991;51:4052–4058. [PubMed] [Google Scholar]
- 28.Martin PJ, Pei J, Gooley T, et al. Evaluation of a CD25-specific immunotoxin for prevention of graft-versus-host disease after unrelated marrow transplantation. Biol Blood Marrow Transplant. 2004;10:552–560. doi: 10.1016/j.bbmt.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 30.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 31.Phan GQ, Attia P, Steinberg SM, et al. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19:3477–3482. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
- 32.Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreitman RJ. Recombinant toxins for the treatment of cancer. Curr Opin Mol Ther. 2003;5:44–51. [PubMed] [Google Scholar]
- 37.Gavin MA, Torgerson TR, Houston E, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Attia P, Maker AV, Haworth LR, et al. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powell DJ, Jr, de Vries CR, Allen T, et al. Inability to mediate prolonged reduction of regulatory T Cells after transfer of autologous CD25-depleted PBMC and interleukin-2 after lympho-depleting chemotherapy. J Immunother (1997) 2007;30:438–447. doi: 10.1097/CJI.0b013e3180600ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175:7746–7754. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powell DJ, Jr, Silva AF, Merino MJ, et al. Administration of a CD25-directed Immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J Immunol. 2007;179:4919–4928. doi: 10.4049/jimmunol.179.7.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waters CA, Schimke PA, Snider CE, et al. Interleukin 2 receptor-targeted cytotoxicity. Receptor binding requirements for entry of a diphtheria toxin-related interleukin 2 fusion protein into cells. Eur J Immunol. 1990;20:785–791. doi: 10.1002/eji.1830200412. [DOI] [PubMed] [Google Scholar]
- 43.Dang NH, Hagemeister FB, Pro B, et al. Phase II study of denileukin diftitox for relapsed/refractory B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22:4095–4102. doi: 10.1200/JCO.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 44.Duvic M, Cather J, Maize J, et al. DAB389IL2 diphtheria fusion toxin produces clinical responses in tumor stage cutaneous T cell lymphoma. Am J Hematol. 1998;58:87–90. doi: 10.1002/(sici)1096-8652(199805)58:1<87::aid-ajh18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 45.Foss FM. DAB(389)IL-2 (ONTAK): a novel fusion toxin therapy for lymphoma. Clin Lymphoma. 2000;1:110–116. doi: 10.3816/clm.2000.n.009. [DOI] [PubMed] [Google Scholar]
- 46.LeMaistre CF, Saleh MN, Kuzel TM, et al. Phase I trial of a ligand fusion-protein (DAB389IL-2) in lymphomas expressing the receptor for interleukin-2. Blood. 1998;91:399–405. [PubMed] [Google Scholar]
- 47.Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 48.Paschal BR. Remission of follicular non-Hodgkin’s lymphoma with denileukin diftitox (ONTAK) after progression during rituximab, CHOP and fludarabine therapy. Leuk Lymphoma. 2003;44:731–733. doi: 10.1080/1042819031000063854. [DOI] [PubMed] [Google Scholar]
- 49.Attia P, Maker AV, Haworth LR, et al. Inability of a fusion protein of IL-2 and diphtheria toxin (Denilukin Diftitox, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28:582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahnke K, Schonfeld K, Fondel S, et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007;120:2723–2733. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- 52.Walz G, Zanker B, Murphy JR, et al. A kinetic analysis of the effects of interleukin-2 diphtheria toxin fusion protein upon activated T cells. Transplantation. 1990;49:198–201. doi: 10.1097/00007890-199001000-00044. [DOI] [PubMed] [Google Scholar]
- 53.Walz G, Zanker B, Brand K, et al. Sequential effects of interleukin 2-diphtheria toxin fusion protein on T-cell activation. Proc Natl Acad Sci USA. 1989;86:9485–9488. doi: 10.1073/pnas.86.23.9485. [DOI] [PMC free article] [PubMed] [Google Scholar]