Abstract
Histological subtyping and grading by malignancy are the cornerstones of the World Health Organization (WHO) classification of tumors of the central nervous system. They shall provide clinicians with guidance as to the course of disease to be expected and the choices of treatment to be made. Nonetheless, patients with histologically identical tumors may have very different outcomes, notably in patients with astrocytic and oligodendroglial gliomas of WHO grades II and III. In gliomas of adulthood, 3 molecular markers have undergone extensive studies in recent years: 1p/19q chromosomal codeletion, O6-methylguanine methyltransferase (MGMT) promoter methylation, and mutations of isocitrate dehydrogenase (IDH) 1 and 2. However, the assessment of these molecular markers has so far not been implemented in clinical routine because of the lack of therapeutic implications. In fact, these markers were considered to be prognostic irrespective of whether patients were receiving radiotherapy (RT), chemotherapy, or both (1p/19q, IDH1/2), or of limited value because testing is too complex and no chemotherapy alternative to temozolomide was available (MGMT). In 2012, this situation has changed: long-term follow-up of the Radiation Therapy Oncology Group 9402 and European Organisation for Research and Treatment of Cancer 26951 trials demonstrated an overall survival benefit from the addition to RT of chemotherapy with procarbazine/CCNU/vincristine confined to patients with anaplastic oligodendroglial tumors with (vs without) 1p/19q codeletion. Furthermore, in elderly glioblastoma patients, the NOA-08 and the Nordic trial of RT alone versus temozolomide alone demonstrated a profound impact of MGMT promoter methylation on outcome by therapy and thus established MGMT as a predictive biomarker in this patient population. These recent results call for the routine implementation of 1p/19q and MGMT testing at least in subpopulations of malignant glioma patients and represent an encouraging step toward the development of personalized therapeutic approaches in neuro-oncology.
Keywords: gliomas, IDH-1, MGMT, 1p/19q, prognosis
The World Health Organization (WHO) classifies tumors of the CNS by histological criteria, assigning them a presumed histogenetic origin and, depending on certain cytological and histological features of anaplasia, grades of I to IV, corresponding to degrees of malignancy defined by the expected clinical course.1 The WHO classification has assumed fundamental clinical relevance, since its histopathological categorization determines how the neuro-oncologist manages an individual patient after surgery, by watchful waiting or with radiotherapy (RT), chemotherapy, or both. Progress in molecular diagnostics has allowed the identification of a number of markers and profiles that identify subtypes of gliomas. Repeatedly these molecular markers have been validated in prospective clinical trials. Molecular marker determination is technically demanding and requires reproducible and validated test procedures. However, molecular tumor characterization becomes more and more necessary in the majority of cases in order to make educated and state-of-the-art individualized treatment decisions. While the currently used 4th edition of the WHO classification, published in 2007, is purposely limited to traditional anatomopathological criteria, any future revision will need to implement also molecular markers for adequate histologicial diagnosis. The current classification lumps together tumor types of identical morphological appearance, while both natural clinical course and different responses to treatment, as well as molecular profiling, indicate distinct entities.2 Further, histopathological diagnoses remain subjective and prone to significant interobserver variation, especially in grades II and III glioma.3
Molecular markers have turned out to be powerful aids for estimating clinical outcomes for certain brain tumors. The potential value of molecular markers thus is to aid in the differential diagnosis of brain tumors that are difficult to distinguish based on histology alone, to estimate outcome within histologically defined tumor entities, and ultimately to predict benefit from specific types of therapy.
Conceptionally, the differentiation between prognostic and predictive factors in the field of gliomas is difficult. Prognostic is meant to signify an effect on outcome that is independent of therapeutic interventions, but the vast majority of patients receive different treatments during the course of their disease. Predictive signifies that a marker allows prediction of benefit specifically from one type of treatment rather than another. The correct understanding of prediction versus prognostication becomes particularly relevant in the discussion of the respective roles of using status of 1p/19q, O6-methylguanine methyltransferase (MGMT), and isocitrate dehydrogenase (IDH) 1/2 to estimate benefit from alkylating agent therapy. Here we discuss the increasing impact and state of the art of these 3 molecular markers for gliomas of adulthood in clinical practice and outline why we believe that testing for these markers should become standard practice based on recent data from large randomized clinical trials (Tables 1 and 2, Fig. 1).
Table 1.
Clinically relevant molecular markers in glioma
| Biological significance | Method of Assessment | Clinical Relevance |
|||
|---|---|---|---|---|---|
| Diffuse Gliomas WHO Grade II | Anaplastic Gliomas WHO Grade III | Glioblastoma WHO Grade IV | |||
| 1p/19q codeletion | Biological role unclear, codeletion of chromosomal arms 1p and 19q linked to oligodendroglial morphology, candidate genes CIC and FUBP1 under evaluation | PCR, FISH | Controversial, probably prognostically favorable | Prognostically favorable in patients receiving RT or chemotherapy or both, predictive for benefit from the addition of PCV to RT | Rare, significance unclear |
| MGMT promoter methylation | DNA repair | Methylation-specific PCR | Controversial | Prognostically favorable in patients receiving RT or chemotherapy or both | Predictive for benefit from alkylating agent chemotherapy |
| IDH1/2 mutations | Link to DNA and histone methylation, energy metabolism, and pro-angiogenic pathways | Immunohistochemistry (IDH1R132H) or sequencing | Prognostically favorable | Prognostically favorable in patients receiving RT or chemotherapy or both | Rare, prognostically favorable, suggestive of secondary glioblastoma |
Table 2.
Frequently asked questions in the molecular neuro-oncology of gliomas in adulthood
| 1p/19q codeletion | |
| Can I use the 1p/19q status for diagnostic purposes? | Sometimes. The presence of the 1p/19q codeletion supports, but the absence of this alteration does not rule out, the diagnosis of an oligodendroglial tumor. |
| Is the 1p/19q status homogeneous within gliomas? | Yes. This is confirmed at least in grades II and III tumors, whereas no data exist for glioblastoma. |
| Can I use the 1p/19q status for prognostic purposes? | Yes. The 1p/19q codeletion is a strong prognosticator in anaplastic glioma patients receiving RT or alkylating agent chemotherapy or both. Its role in low-grade gliomas is less clear but likely to be similar. |
| Can I use the 1p/19q status as a predictive marker for clinical decision making? | Yes. The RTOG 9402 and EORTC 26951 trials suggest that the 1p/19q codeletion is a predictive marker for improved survival for patients treated with PCV in addition to RT vs RT alone. Whether this holds true for TMZ too is not known. |
| MGMT promoter methylation | |
| Can I use the MGMT status for diagnostic purposes? | No. |
| Is the MGMT status homogeneous within gliomas? | Yes. |
| Does the MGMT status change in the course of disease? | No. Most gliomas show the same MGMT status at recurrence. |
| Can I use the MGMT status for prognostic purposes? | Yes. MGMT promoter methylation is positively prognostic in anaplastic glioma patients receiving RT or chemotherapy or both (NOA-04, EORTC 26951). |
| Can I use the MGMT status as a predictive marker for clinical decision making? | Yes. MGMT promoter methylation predicts benefit from alkylating agent chemotherapy in glioblastoma (EORTC 26981) and is particularly useful in elderly glioblastoma patients (NOA-08, Nordic trial). |
| IDH1/2 mutations | |
| Can I use the IDH1/2 status for diagnostic purposes? | Yes. IDH1/2 mutations are common in WHO grades II and III gliomas and can aid in the differential diagnosis vs reactive gliosis and other glioma entities, eg, pilocytic astrocytomas, gangliogliomas, and ependymomas, which typically lack IDH1/2 mutations. |
| Is the IDH1/2 status homogeneous within gliomas? | Yes. This is confirmed at least in WHO grades II and III tumors, whereas no data exist for glioblastoma. |
| Can I use the IDH1/2 status for prognostic purposes? | Yes. IDH1/2 mutations are prognostically favorable, in particular in WHO grades III and IV gliomas. |
| Can I use the IDH1/2 status as a predictive marker for clinical decision making? | No. |
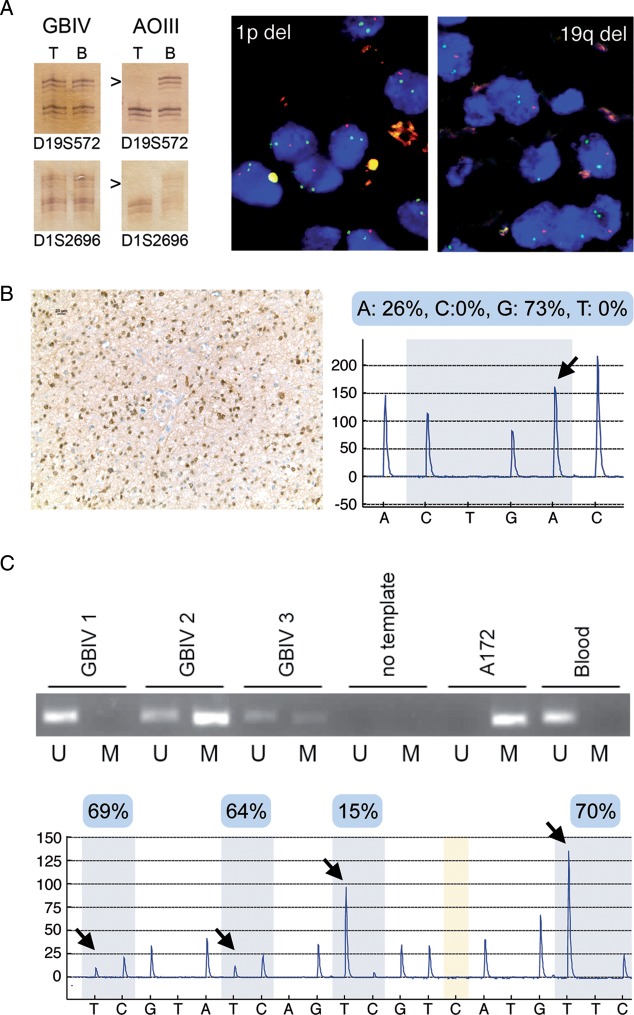
Fig. 1.
Exemplary results obtained by commonly used methods for the assessment of molecular markers in gliomas. (A) 1p/19q codeletion. Left panel: PCR analysis for loss of heterozygosity (LOH) at microsatellite markers on 1p (D1S2696) and 19q (D19S572) in a glioblastoma (GBIV) and in an anaplastic oligodendroglioma (AOIII). Note LOH at both markers (arrowheads) in the tumor DNA (T) as compared to the patient's blood DNA (B) in the AOIII but not in the GBIV. Right panel: Demonstration of 1p and 19q codeletion by FISH in an oligodendroglioma (FISH images were kindly provided by Dr David Capper, Heidelberg). Dual color probe sets detecting loci on 1p23 (red) and 1q25 (green) or 19q13 (red) and 19p13 (green) were used and nuclei were counterstained with 4’,6-diamidino-2-phenylindole (blue). Note that most nuclei show 2 green signals with the reference probes but only 1 red signal with the 1p23 or 19q13 probes. (B) IDH1 mutation. Left panel: diffuse astrocytoma with immunostaining of tumor cells for R132H mutant IDH1 protein, Right panel: pyrogram (reverse sequence) indicating a heterozygous C-to-T point mutation at nucleotide 394 of the IDH1 gene (arrow) resulting in a missense mutation at codon 132 (c.394C>T, R132C) in another case of diffuse astrocytoma. (C) MGMT promoter methylation. Upper panel: Methylation-specific PCR for unmethylated (U) and methylated (M) promoter sequences in 3 GBIVs, a no template control, the glioblastoma cell line A172 with a methylated MGMT promoter, and peripheral blood cells with an unmethylated MGMT promoter. Tumor 1 lacked MGMT promoter methylation, while tumors 2 and 3 had MGMT methylated promoters. Bottom panel: MGMT promoter methylation analysis by pyrosequencing of sodium bisulfite–modified DNA extracted from a GBIV. The mean percentage of methylation at the individual CpG sites (arrows) is noted in the blue boxes on top of the pyrogram.
1p/19q Codeletion
Combined loss of genetic material from chromosomal arms 1p and 19q has long been recognized as a typical molecular signature of oligodendroglial tumors4 and results from an unbalanced translocation that leads to the loss of one hybrid chromosome and thereby loss of heterozygosity.5 The association of this molecular marker with brain tumor formation led to an extensive search for tumor suppressor genes located in these genomic regions, but the first promising candidate genes have only recently been identified by exome sequencing. Most oligodendrogliomas with 1p/19q codeletion indeed carry mutations in the CIC gene, a homolog of the Drosophila gene capicua, located on 19q13.2.6–8 A smaller subset of these tumors carries mutations in the FUBP–1 gene, which encodes the “far upstream element binding protein” on chromosome 1p.6–8 However, the biological role of these mutations remains to be elucidated.
Three randomized clinical trials have demonstrated that anaplastic glioma patients with 1p/19q codeleted tumors live longer when receiving RT or alkylating agent chemotherapy or both.9–11
In trial 9402 of the Radiation Therapy Oncology Group (RTOG), 289 patients with anaplastic oligodendroglioma or anaplastic oligoastrocytoma confirmed at central pathology review were randomized to neoadjuvant procarbazine/CCNU/vincristine (PCV) chemotherapy followed by RT (PCV → RT) versus RT alone. Status of 1p/19q was assessed centrally by fluorescence in situ hybridization (FISH), and 93/201 (46%) tested patients demonstrated combined loss. Although there was no formal crossover design, 80% of the patients randomized initially to RT alone received chemotherapy at progression. An initial analysis after a minimum follow-up of 3 years showed a median progression-free survival (PFS) of 2.6 years for PCV → RT compared with 1.7 years for RT alone (P = .004); however, median overall survival (OS) was similar: 4.9 years with PCV → RT versus 4.7 years with RT alone (P = .26). The absence of a survival benefit and the occurrence of severe (grade 3 or 4) toxicity in 65% of the PCV-treated patients were felt to outweigh the moderate gain in PFS. Median OS was longer in cases of 1p/19q codeleted tumors than in cases of tumors lacking this aberration (>7 y vs 2.8 y, P < .001). However, there was no significant effect of type of treatment on survival by 1p/19q status. In patients with 1p/19q codeleted tumors, median OS was not reached with PCV → RT and was reached at 6.6 years with RT alone (P = .28). For the other patients, median OS was reached at 2.7 versus 2.8 years with versus without PCV (P = .33).9 Yet, at extended follow-up in 2012, these conclusions needed to be revoked.12 In patients with tumors lacking 1p/19q codeletions, median OS was still similar at 2.6 years for PCV → RT versus 2.7 years for RT alone (hazard ratio [HR], 0.85; 95% confidence interval [CI], 0.58–1.23; P = .39). However, 126 patients with 1p/19q codeleted tumors had median OS of 14.7 years with PCV → RT versus 7.3 years with RT alone (P = .03), translating into an HR of 0.59 (95% CI, 0.37–0.95; P = .03). This difference in OS was observed despite reoperation rates that were similar in both arms (43% with PCV → RT vs 54% with RT alone) and salvage chemotherapy that was more frequently administered in the RT-alone arm (57% vs 81%).12
The European Organisation for Research and Treatment of Cancer (EORTC) 26951 trial, which was similarly designed to the RTOG trial and somewhat larger, was initially reported in 2006 and updated at the 2012 plenary session of the American Society of Clinical Oncology. A total of 368 patients with locally diagnosed anaplastic oligodendroglioma or anaplastic oligoastrocytoma were randomized to RT or RT followed by PCV (RT → PCV). A subgroup of 78 patients (21%) demonstrated 1p/19q codeletions as assessed by FISH. Again, the addition of PCV after RT increased PFS (23 vs 13.2 months, P = .0018), but median OS at a median follow-up of 60 months was similar (40.3 vs 30.6 months, P = .23). There was also no specific effect of treatment when split by 1p/19q status: for patients with 1p/19q codeleted tumors, median OS was not reached with either RT → PCV or RT alone, while for patients whose tumors had a partial or no deletion, median survival times were 25.2 and 21.4 months for RT → PCV and RT alone, respectively.10 The 2012 update demonstrates a survival advantage for early adjuvant PCV chemotherapy in patients with codeleted tumors. With now long-term median follow-up of >10 years, median survival has still not been reached in the subgroup of 42 patients with codeleted tumors and initially receiving RT → PCV, compared with a median survival of 9.3 years for the 38 patients who initially received RT alone, and frequently received chemotherapy only at progression (P = .059). Also in concordance with the RTOG results, median OS for patients without codeleted tumors was still similar, with 25 months for RT → PCV versus 21 months for RT alone (P = .19).13
The German Neuro-Oncology Group trial NOA-04 randomized 318 patients with anaplastic astrocytoma, anaplastic oligodendroglioma, and mixed anaplastic oligoastrocytoma, after biopsy or resection, 2:1:1 to receive RT, PCV, or temozolomide (TMZ), all as single modality treatment. At unacceptable toxicity or progression, patients randomized to RT were further randomized to PCV or TMZ, whereas patients randomized to chemotherapy were to receive RT. Histology was centrally confirmed before randomization, and 23% (74 patients) had 1p/19q codeletion by FISH. The primary endpoint was treatment failure, defined as death, progression after RT and one line of chemotherapy, or ineligibility for salvage at first relapse, with PFS and OS as secondary endpoints. At first analysis after a maximum follow-up of 54 months, when 43% of patients had reached the primary endpoint of treatment failure, RT and chemotherapy induced (i) similar PFS, (ii) similar time to treatment failure, and (iii) similar OS across all histologies. The 1p/19q codeletion was again a favorable prognostic factor, translating into a risk reduction of approximately 50%, which was independent of treatment arm.11 The current long-term data of the RTOG 9402 and EORTC 26951 trials suggest that the follow-up of NOA-04 is still too short to allow an estimate of a predictive value of 1p/19q status for benefit from RT alone versus chemotherapy alone. Moreover, crossover at progression will impact the survival endpoint in NOA-04.
In glioblastomas, 1p/19q codeletions are rare and of unknown biological significance.14 Patients with WHO grade II gliomas do not always receive RT or chemotherapy after the first surgical intervention. Such patients treated with surgery alone allow estimation of whether the 1p/19q codeletion specifically mediates increased responsiveness to RT or alkylating agents or whether these tumors take a less aggressive course even in the absence of genotoxic treatment. Small retrospective studies indeed indicate no difference in time to reintervention in patients with WHO grade II gliomas managed by surgery alone by 1p/19q status,15,16 lending support to the hypothesis of specific therapy sensitivity conferred by 1p/19q codeletion. However, 2 French series reported slower growth rates in 1p/19q codeleted low-grade gliomas.17,18 Future studies need to determine the mechanistic roles of the CIC or FUBP1 genes that seem to be targeted by mutation and 1p/19q codeletion in association with increased sensitivity to RT and alkylating agent chemotherapy in 1p/19q codeleted oligodendrogliomas.
Accordingly, 1p/19q codeletion predicts longer OS when glioma patients receive RT or chemotherapy alone or their combination, but the long-term follow-up of 2 randomized trials strongly suggests a survival benefit of initial combined modality treatment using PCV plus RT over RT alone in patients with 1p/19q codeleted tumors.12,13 These observations have important implications for ongoing clinical trial activities and for clinical practice. Based on the data from RTOG 9402, the CODEL trial (NCT 00887146) has been suspended because RT alone is no longer considered appropriate for patients with 1p/19q codeleted anaplastic oligodendroglial tumors. Whether the neuro-oncology community will accept the old PCV regimen added to RT as a new standard of care in patients with these tumors is also uncertain at present.
MGMT Promoter Methylation
MGMT is a DNA repair protein that removes the alkylation of the O6 position of guanine, the most cytotoxic lesion induced by alkylating agent chemotherapy. An association of MGMT expression or activity and the benefit from alkylating agent chemotherapy in glioma patients was reported 15 years ago.19,20 The molecular modification associated with loss of MGMT expression is aberrant methylation of the MGMT promoter region, leading to gene silencing and consequently reduced proficiency to repair DNA damage induced by alkylating agent chemotherapy. In the pivotal trial of TMZ for newly diagnosed glioblastoma,21 MGMT promoter methylation was strongly associated with the extent of benefit from the addition of TMZ in the experimental arm but had only minor prognostic impact for PFS in patients receiving initial RT alone,22 suggestive of a predictive effect. It is now widely accepted that MGMT status can be reliably tested using a standardized methylation-specific PCR, while validation of clinical use for other assays is lacking.23 MGMT status is homogeneous within individual tumors and, for glioblastomas, is retained in recurrent tumors.24,25
The strong prognostic role of MGMT promoter methylation was confirmed prospectively in the RTOG 0525/EORTC/North Central Cancer Treatment Group Intergroup Study using a centralized quantitative methylation-specific PCR assay. The study compared 3 weeks on, 1 week off adjuvant dose-intensified TMZ with the standard TMZ regimen: OS was 23.2 months in patients with MGMT promoter-methylated tumors versus 16 months in patients unmethylated tumors.26
A predictive, as opposed to a prognostic, role of MGMT status can be assessed particularly well in patients receiving either RT or chemotherapy alone versus the combination. Such single modality treatments are often administered to elderly patients where the combined modality may be less active or less well tolerated or both. In an unselected sample of 233 glioblastoma patients aged ≥70 years,27 median PFS was 4.8 months and median OS was 7.7 months. For the whole cohort, PFS was 5.2 versus 4.7 months and OS was 8.4 versus 6.4 months in patients with versus without MGMT promoter methylation. Patients with MGMT promoter-methylated tumors had longer PFS when receiving RT plus chemotherapy or chemotherapy alone compared with patients receiving RT alone. Patients with MGMT promoter-unmethylated tumors appeared to derive no survival benefit from chemotherapy, regardless of whether given at diagnosis together with RT or as a salvage treatment.27 A similar conclusion was reached in a French study of elderly glioblastoma patients.28 The conclusion that MGMT promoter methylation may indeed be a useful predictive biomarker to stratify elderly glioblastoma patients for RT versus alkylating agent chemotherapy has now been established in 2 prospective randomized trials, the NOA-08 trial29 and the Nordic trial.30
NOA-08 randomized patients with glioblastoma (n = 331) or anaplastic astrocytoma (n = 40) aged 66 or more to RT alone or one week on one week off dose-intensified TMZ alone and sought to demonstrate non-inferiority of TMZ compared with RT. Median PFS and OS did not differ between arms. MGMT promoter methylation was associated with prolonged OS of 11.9 versus 8.2 months. More importantly in the context of molecular signatures, patients with MGMT promoter methylation had longer PFS when treated with TMZ (8.4 vs 4.6 months), whereas patients without MGMT promoter methylation had longer PFS when receiving RT (4.6 vs 3.3 months).
In the Nordic trial, newly diagnosed glioblastoma patients ≥60 years of age were randomized to standard RT (60 Gy) versus hypofractionated RT (34 Gy over 2 wk) versus TMZ (200 mg/m2 days 1–5 every 28 days for 6 cycles). OS with standard RT was inferior to that with TMZ and hypofractionated RT. MGMT promoter methylation was associated with significantly better OS in TMZ-treated patients (9.7 vs 6.8 months; 95% CI, 8.0–11.4 vs 5.9–7.7; HR, 0.56; 95% CI, 0.34–0.93; P = .03) but not in RT patients (8.2 vs 7.0 months; 95% CI, 6.6–9.9 vs 5.7–8.3; HR, 0.97; 0.69–1.38; P = .88). Accordingly, these consistent trial results are practice changing, and elderly glioblastoma patients eligible for either RT or TMZ should undergo MGMT testing prior to clinical decision making.
Furthermore, MGMT promoter methylation was associated with potential benefit from the integrin antagonist cilengitide in patients of newly diagnosed glioblastoma.31 If this is confirmed in the ongoing phase III registration trial (CENTRIC, NCT 00689221), MGMT status determination will have direct implication in the choice of treatment for all patients and will be required routinely.
Interestingly, this powerful predictive value of MGMT status in glioblastoma was not observed in 2 randomized trials in anaplastic glioma patients. NOA-04 reported a strong positive prognostic effect of MGMT promoter methylation for PFS in patients receiving either RT or alkylating agent chemotherapy, with no evidence for preferential activity of chemotherapy in MGMT promoter-methylated patients.11 Similarly, the EORTC 26951 trial observed the same extent of PFS advantage in patients with tumors with MGMT promoter methylation receiving either RT alone or RT → PCV.32 This observation, which at first glance is paradoxical, may be explained by the fact that despite similar clinical and radiological presentations, WHO grades III and IV glioma are distinct entities with different pathogeneses and prevailing tumorigenic pathways. It is tempting to conclude that this difference may be due to the different biology of IDH1/2 mutant tumors, in particular its association with the cytosine–phosphatidyl–guanine (CpG) island methylator phenotype (CIMP), which dominates grade III anaplastic tumors versus IDH1/2 wild-type tumors, which dominate grade IV primary glioblastomas.33–36 In fact, CIMP-positive grade II and III gliomas almost invariably harbor a methylated MGMT promoter, while CIMP-negative gliomas regardless of tumor grade display an MGMT methylation frequency of approximately 50%.37
IDH1/2 Mutations
Mutations of IDH1 and 2 genesare common and characteristic molecular lesions in grades II and III gliomas.38–41 The IDH1 gene encodes cytosolic IDH1, whereas the IDH2 gene encodes mitochondrial IDH2. The development of an antibody recognizing the mutant IDH1R132H protein, the most common IDH1 mutant in gliomas, facilitated the rapid implementation of IDH1 assessment within routine diagnostic neuropathology.42 IDH mutations cluster at codon 132 of IDH1 and codon 172 of the IDH2 gene, suggesting that they confer a gain of function to the mutant enzymes. Mutant IDH proteins exhibit altered substrate specificity resulting in increased production of D-2-hydroxyglutarate, which acts as an oncometabolite, instead of α-ketoglutarate, which is produced by wild-type IDH enzymes.35,43 In contrast to other molecular markers in gliomas, D-2-hydroxyglutarate may be used as a biomarker also for monitoring the natural course of disease and response to therapy. While D-2-hydroxyglutarate levels may be too low in the serum of IDH1/2 mutant glioma patients to be of diagnostic value,44 it may become possible in the future to monitor local, tumor-associated mutant IDH1/2 activity using MR spectroscopy for the detection of D-2-hydroxyglutarate.45
Serial stereotactic biopsies revealed a homogeneous pattern of IDH1 status within gliomas of WHO grades II and III, even within and outside of anaplastic foci.46 In most glioma entities, patients with IDH mutant tumors show longer OS than those with IDH wild-type tumors; in fact, OS in patients with WHO grade IV glioblastoma with IDH mutation is longer than that of patients with grade III anaplastic astrocytoma without IDH mutation.33 The prognostic impact of IDH1 mutations in diffuse astrocytoma remains controversial because at least one series with long follow-up reported a less benign postsurgical course of disease in IDH mutant tumors until interventions with RT or chemotherapy were made, commonly for progressive disease.47 However, IDH1 status has not been shown to predict preferential benefit from a specific type of treatment, eg, RT versus chemotherapy.
In general, current analyses focus on the interrelations of various molecular markers—for instance, nearly all 1p/19q codeleted tumors also show IDH1/2 mutations among low-grade and anaplastic gliomas. In that regard, there is also speculation that the predictive value of MGMT status for benefit from alkylating agent chemotherapy may be restricted to patients with IDH wild-type tumors rather than being dependent on WHO grade.36 Altogether, current data suggest that IDH1/2 mutations can be used to distinguish a separate class or lineage of gliomas and that IDH1/2 status may become the cornerstone to separate various subtypes of glial tumors in the near future.
Outlook
Molecular markers have already shaped the design and conduct of several investigator-initiated and industry clinical trials in the field of gliomas, and information from such trials has been invaluable to demonstrate the potential impact of routine molecular testing. To date, a set of 3 molecular markers—1p/19q codeletion, MGMT promoter methylation, and IDH1/2 mutation—have gained importance in routine clinical decision making. The use of these markers will improve patient outcome and reduce cost and toxicity from ineffective treatments. More advances are to be expected: testing for the epidermal growth factor receptor (EGFR) vIII mutation may be implemented if randomized trials demonstrate activity of EGFR vIII–targeted vaccination; furthermore, pending demonstration of activity and approval, there will be an urgent need to define biomarkers allowing selection among the increasing repertoire of anti-angiogenic agents for glioblastoma. In contrast, the potentially more powerful high-throughput analyses, which have led to a reclassification, eg, of glioblastomas based on RNA expression profiles and DNA methylation patterns,48–51 have not yet had the awaited clinical impact because they have so far not allowed derivation of useful information for clinical decision making. Yet, we anticipate that such molecular signatures, possibly complemented by targeted sequencing of a panel of glioma-associated genes using next-generation sequencing technologies, will ultimately be made widely available and be increasingly used in neuro-oncology.
Conflict of interest statement: M.W. has received research grants from MSD, Merck Serono, and Roche and honoraria for lectures or for serving on advisory boards from Magforce, MSD, Merck Serono, and Roche. R.S. has served on speaker's' bureaus and/or advisory boards for Merck & Co/MSD and Roche and received indirect research support from Merck KGaA Darmstadt (EMD Serono) as the principal investigator for clinical trials with cilengitide. M.E.H. is an adviser to MDxHealth and EMD Serono, has served on advisory boards for Merck KGaA, and has received a research grant from AstraZeneca. M.v.d.B has received honoraria from MSD, Merck Serono, and Roche and has received research grants from Roche. J.C.T. has received honoraria for lectures or for serving on advisory boards from MSD, Merck Serono, Medac, and Roche. M. S. has received honoraria for serving on advisory boards from Merck Serono and Roche. W.W. has received research support from MSD, Eli Lilly, and Apogenix and honoraria for lectures or for serving on advisory boards from Magforce, MSD, and Roche. G. R. has received honoraria for serving on advisory boards from Merck Serono.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hegi ME, Janzer RC, Lambiv WL, et al. Presence of an oligodendroglioma-like component in newly diagnosed glioblastoma identifies a pathogenetically heterogeneous subgroup and lacks prognostic value: central pathology review of the EORTC_26981/NCIC_CE.3 trial. Acta Neuropathol. 2012;123:841–852. doi: 10.1007/s00401-011-0938-4. [DOI] [PubMed] [Google Scholar]
- 3.Van den Bent M. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician's perspective. Acta Neuropathol. 2010;120:297–304. doi: 10.1007/s00401-010-0725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reifenberger J, Reifenberger G, Liu L, et al. Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol. 1994;145:1175–1190. [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkins RB, Blair H, Ballman KV, et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66:9852–9861. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- 6.Bettegowda C, Agrawal N, Jiao Y, et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011;333:1453–1455. doi: 10.1126/science.1210557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yip S, Butterfield YS, Morozova O, et al. Concurrent CIC mutations, IDH mutations, and 1p/19q loss distinguish oligodendrogliomas from other cancers. J Pathol. 2012;226:7–16. doi: 10.1002/path.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahm F, Koelsche C, Meyer J, et al. CIC and FUBP1 mutations in oligodendrogliomas, oligoastrocytomas and astrocytomas. Acta Neuropathol. 2012;123:853–860. doi: 10.1007/s00401-012-0993-5. [DOI] [PubMed] [Google Scholar]
- 9.Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 10.Van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715–2722. doi: 10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 11.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. J Clin Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 12.Cairncross JG, Wang M, Shaw EG, et al. Chemotherapy plus radiotherapy (CT-RT) versus RT alone for patients with anaplastic oligodendroglioma: long-term results of the RTOG 9402 phase III study. [Abstract] J Clin Oncol. 2012;30:2008b. [Google Scholar]
- 13.Van den Bent MJ, Hoang-Xuan K, Brandes AA, et al. Long-term follow-up results of EORTC 26951: A randomized phase III study on adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors (AOD) [Abstract] J Clin Oncol. 2012;30:2. [Google Scholar]
- 14.Weller M, Felsberg J, Hartmann C, et al. for the German Glioma Network. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma. A prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27:5743–5750. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 15.Weller M, Berger H, Hartmann C, et al. for the German Glioma Network. Combined 1p/19q loss in oligodendroglial tumors: predictive or prognostic biomarker? Clin Cancer Res. 2007;13:6933–6937. doi: 10.1158/1078-0432.CCR-07-0573. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann C, Hentschel B, Tatagiba M, et al. for the German Glioma Network. Molecular markers in low-grade gliomas: predictive or prognostic? Clin Cancer Res. 2011;17:4588–4599. doi: 10.1158/1078-0432.CCR-10-3194. [DOI] [PubMed] [Google Scholar]
- 17.Ricard D, Kaloshi G, Amiel-Benouaich A, et al. Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann Neurol. 2007;61:484–490. doi: 10.1002/ana.21125. [DOI] [PubMed] [Google Scholar]
- 18.Gozé C, Bezzina C, Gozé E, et al. 1P19Q loss but not IDH1 mutations influences WHO grade II gliomas spontaneous growth. J Neurooncol. 2012;108:69–75. doi: 10.1007/s11060-012-0831-6. [DOI] [PubMed] [Google Scholar]
- 19.Friedman HS, McLendon RE, Kerby T, et al. DNA mismatch repair and O6-alkylguanine-DNA alkyltransferase analysis and response to Temodal in newly diagnosed malignant glioma. J Clin Oncol. 1998;16:3851–3857. doi: 10.1200/JCO.1998.16.12.3851. [DOI] [PubMed] [Google Scholar]
- 20.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 21.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for patients with newly diagnosed glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 22.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and response to temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 23.Weller M, Stupp R, Reifenberger G, et al. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nature Rev Neurol. 2010;6:39–51. doi: 10.1038/nrneurol.2009.197. [DOI] [PubMed] [Google Scholar]
- 24.Grasbon-Frodl EM, Kreth FW, Ruiter M, et al. Intratumoral homogeneity of MGMT promoter hypermethylation as demonstrated in serial stereotactic specimens from anaplastic astrocytomas and glioblastomas. Int J Cancer. 2007;121:2458–2464. doi: 10.1002/ijc.23020. [DOI] [PubMed] [Google Scholar]
- 25.Felsberg J, Thon N, Eigenbrod S, et al. Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int J Cancer. 2011;129:659–670. doi: 10.1002/ijc.26083. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert MR, Wang M, Aldape KD, et al. RTOG 0525: A randomized phase III trial comparing standard adjuvant temozolomide (TMZ) with a dose-dense (dd) schedule in newly diagnosed glioblastoma (GBM) [Abstract] J Clin Oncol. 2011;29:2006. [Google Scholar]
- 27.Reifenberger G, Hentschel B, Felsberg J, et al. Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer. 2012;131:1342–1350. doi: 10.1002/ijc.27385. [DOI] [PubMed] [Google Scholar]
- 28.Gállego Pérez-Larraya J, Ducray F, Chinot O, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29:3050–3055. doi: 10.1200/JCO.2011.34.8086. [DOI] [PubMed] [Google Scholar]
- 29.Wick W, Platten M, Meisner C, et al. for the NOA-08 Study Group of the Neuro-oncology Working Group (NOA) of the German Cancer Society. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13:707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 30.Malmström A, Grønberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy for patients aged over 60 years with glioblastoma: the Nordic randomized phase 3 trial. Lancet Oncol. 2012 doi: 10.1016/S1470-2045(12)70265-6. in press. [DOI] [PubMed] [Google Scholar]
- 31.Stupp R, Hegi ME, Neyns B, et al. Phase I//IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28:2712–2718. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 32.Van den Bent MJ, Dubbink HJ, Sanson M, et al. MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: a report from EORTC Brain Tumor Group Study 26951. J Clin Oncol. 2009;27:5881–5886. doi: 10.1200/JCO.2009.24.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astroctytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavourable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120:707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 34.van den Bent MJ, Gravendeel LA, Gorlia T, et al. A hypermethylated phenotype in anaplastic oligodendroglial brain tumors is a better predictor of survival than MGMT methylation in anaplastic oligodendroglioma: a report from EORTC study 26951. Clin Cancer Res. 2011;17:7148–7155. doi: 10.1158/1078-0432.CCR-11-1274. [DOI] [PubMed] [Google Scholar]
- 35.Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wick W, Meisner C, Hentschel B, et al. Prognostic or predictive value of MGMT promoter methylation in malignant gliomas: depends on IDH1 mutations. [Abstract] Neuro-Oncology. 2012 doi: 10.1212/WNL.0b013e3182a95680. [DOI] [PubMed] [Google Scholar]
- 37.Bady P, Sciuscio D, Diserens AC, et al. MGMT methylation analysis of glioblastoma on the Infinium methylation BeadChip identifies two distinct CpG regions associated with gene silencing and outcome, yielding a prediction model for comparisons across datasets, tumor grades, and CIMP-status. Acta Neuropathol. 2012 doi: 10.1007/s00401-012-1016-2. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balss J, Meyer J, Mueller W, et al. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 39.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27:4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 41.Dubbink HJ, Taal W, van Marion R, et al. IDH1 mutations in low-grade astrocytomas predict survival but not response to temozolomide. Neurology. 2009;73:1792–1795. doi: 10.1212/WNL.0b013e3181c34ace. [DOI] [PubMed] [Google Scholar]
- 42.Capper D, Weissert S, Balss J, et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010;20:245–254. doi: 10.1111/j.1750-3639.2009.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capper D, Simon M, Langhans CD, et al. 2-hydroxyglutarate concentration in serum from patients with gliomas does not correlate with IDH1/2 mutation status or tumor size. Int J Cancer. 2012;131:766–768. doi: 10.1002/ijc.26425. [DOI] [PubMed] [Google Scholar]
- 45.Choi C, Ganji SK, DeBerardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in subjects with IDH-mutated gliomas. Nature Med. 2012;18:624–629. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunz M, Thon N, Eigenbrod S, et al. Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro-Oncology. 2011;13:307–316. doi: 10.1093/neuonc/noq196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thon N, Eigenbrod S, Kreth S, et al. IDH1 mutations in grade II astrocytomas are associated with unfavorable progression-free survival and prolonged postrecurrence survival. Cancer. 2012;118:452–460. doi: 10.1002/cncr.26298. [DOI] [PubMed] [Google Scholar]
- 48.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 49.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17:510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sasaki M, Knobbe CB, Munger JC, et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012 doi: 10.1038/nature11323. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]