Abstract
A phase II study of bevacizumab (BVZ) plus irinotecan (CPT-11) was conducted in cases of pediatric recurrent ependymoma (EPN) to estimate sustained objective response rate and progression-free survival (PFS). Eligible patients received 2 doses of single-agent BVZ intravenously (10 mg/kg) 2 weeks apart and then BVZ + CPT-11 every 2 weeks until progressive disease, unacceptable toxicity, or a maximum of 2 years of therapy. Correlative studies included diffusion-weighted and T1 dynamic contrast enhanced permeability imaging and tumor immunohistochemistry for vascular endothelial growth factor (VEGF)–A and –B, hypoxia inducible factor–2α, VEGF receptor (R)–2, and carbonic anhydrase (CA)–9. Thirteen evaluable patients received a median of 3 courses (range, 2–12) of BVZ + CPT-11. No sustained response was observed in any patient. Median time to progression in 10 patients was 2.2 months (range, 1.9–6.3). Two patients had stable disease for 10 months and 12 months, respectively. Six-month PFS was 25.7% (SE = 11.1%). Grades I–III toxicities related to BVZ treatment included fatigue in 4 patients, systemic hypertension in 2, epistaxis in 1, headache in 1, and avascular necrosis of bone in 1. Although there was a decrease in the mean diffusion ratio following 2 doses of BVZ, it did not correlate with PFS. BVZ + CPT-11 was well tolerated but had minimal efficacy in cases of recurrent EPN.
Keywords: bevacizumab, CPT-11, efficacy, ependymoma, recurrent
Ependymomas (EPNs) are glial-based tumors that arise from the lining of the ventricles of the entire CNS.1 These tumors constitute approximately8%–10% of all primary CNS tumors in children.2 Complete surgical resection followed by radiotherapy remains the best therapeutic option in cases of newly diagnosed disease.1,3 However, cases of recurrent disease continue to have poor outcomes despite available salvage therapies.4,5 It is possible that targeting key molecular signaling pathways that drive tumor progression in cases of recurrent disease might allow disease stabilization and prolong survival.6 Tumor angiogenesis is one likely target owing to its role in tumor growth, infiltration, and metastasis.7 Vascular endothelial growth factor (VEGF) is a powerful endothelial mitogen and one of the most potent stimulators of angiogenesis.8 Owing to its central role in tumor angiogenesis, especially in brain tumors,9,10 VEGF inhibition has been utilized in multiple recent clinical trials, with or without chemotherapy.11,12 Like other primary brain tumors, EPN overexpress VEGF, which has been correlated with poor survival.13 VEGF inhibition might therefore provide tumor control in cases of recurrent EPNs. In a single-arm phase II study from Duke University Medical Center in cases of recurrent malignant glioma, the use of the humanized monoclonal anti-VEGF antibody bevacizumab (BVZ; Avastin, GenenTech) concomitantly with irinotecan (CPT-11; Camptosar, Pfizer) resulted in an objective response rate of 63% and 6-month progression-free survival (PFS) of 38%.14 Based on these encouraging results, the Pediatric Brain Tumor Consortium (PBTC) initiated a phase II study of this combination in cases of pediatric recurrent brain tumors (including malignant glioma, brain stem glioma, EPN, medulloblastoma, and low-grade glioma) to assess the efficacy and toxicity of this regimen in this patient population.15 We now report on our findings of efficacy in patients with recurrent EPN.
Materials and Methods
Study Objectives
The primary objective of the study was to estimate the rate of sustained (≥8 wk) objective response to BVZ + CPT-11 in cases of pediatric recurrent EPN over 4 courses of therapy. Secondary objectives included estimation of treatment-related toxicities and PFS, changes in perfusion/diffusion on MRI during treatment, and expression of (1) VEGF-A and -B, (2) hypoxia inducible factor (HIF)–2α, (3) VEGF receptor (R)–2, and (4) carbonic anhydrase (CA)–9 by tumor immunohistochemistry (IHC) correlated with tumor response and PFS.16
Eligibility Criteria
Inclusion criteria
Patients younger than21 years of age with recurrent or progressive histologically confirmed EPN and measurable disease were eligible for this study. Subjects were required to have a Karnofsky/Lansky score of ≥50, to have had ≤2 chemotherapy regimens prior to enrollment, and to be ≥3 weeks post myelosuppressive chemo- or biologic therapy, ≥6 weeks post major surgical resection, and ≥3 months post local radiotherapy. Required evidence of adequate organ function included an absolute neutrophil count of ≥1500/µL (unsupported), platelets ≥100 000/µL (unsupported), hemoglobin ≥8 g/dL, serum creatinine no greater than the institutional upper limit of normal (ULN), blood urea nitrogen (BUN) ≤25 mg/dL, bilirubin ≤1.5 × ULN, and serum glutamic oxaloacetic transaminase and serum glutamic pyruvic transaminase ≤3 × ULN. Eligibility also required absence of active systemic illness, stable neurologic function and corticosteroid dose (if any), and an agreement to use a medically acceptable form of birth control (in those of child-bearing or fathering potential).
Exclusion criteria
Patients were excluded from the study if they had evidence of symptomatic intracerebral hemorrhage (grade >II) on neuroimaging studies obtained within 2 weeks prior to enrollment, had prior exposure to BVZ or CPT-11, were on anticoagulation or other investigational agents, or could not be available for follow-up. Patients were also excluded if they had uncontrolled hypertension, bleeding diathesis, severe proteinuria, nonhealing wounds or bone fractures, or history of stroke or had had major surgical procedures within 6 weeks or gastrointestinal perforation within 6 months prior to registration. The institutional review board of each PBTC institution approved the protocol before initial patient enrollment; continuing approval was maintained throughout the study. Patients or their legal guardians gave written informed consent, and assent was obtained as appropriate at the time of enrollment.
Treatment Plan and Dose Modifications
Patients first received 2 doses of BVZ at 10 mg/kg (i.v.) 2 weeks apart. Perfusion, permeability, and diffusion imaging were done prior to and following administration of BVZ alone to assess the effects of this VEGF inhibitor on these parameters. The first dose of CPT-11 was given within 3 days after the second dose of BVZ. The starting dose of CPT-11 was 125 mg/m2 (i.v.) for patients not on enzyme-inducing anticonvulsant drugs (EIACDs), which was increased in subsequent courses to 150 mg/m2 if tolerated; for patients on EIACDs, a starting dose of 250 mg/m2 was increased in 25-mg/m2 increments every 2 weeks to a maximum of 350 mg/m2, as tolerated. The dose of CPT-11 was adjusted as previously reported based on hematologic and nonhematologic toxicities in subsequent courses.17 Patients who could not receive CPT-11 owing to toxicity could continue treatment with BVZ in the absence of severe thrombocytopenia. BVZ was withheld for related grade ≥III toxicities and restarted when toxicity had resolved to grade <II without dose modification; CPT-11 was to be withheld in such instances. Patients who went off treatment and could not restart BVZ within 4 weeks were taken off the study. Patients could receive therapy for a maximum of 2 years in the absence of unacceptable toxicity or progressive disease.
Required Observations Prior to, During, and Off Study
Clinical examination (including neurologic function and blood pressure) was performed at baseline and every 2 weeks. Laboratory tests (complete blood count with differential, BUN, creatinine, liver function, and urine for protein) were obtained at baseline and every 2–4 weeks during therapy. An MRI brain scan (±spine) was obtained every 8 weeks during the first 6 months and every 12 weeks thereafter. MR perfusion and diffusion scans were done at baseline, within 24–48 hours of the second dose of BVZ (week 3, prior to the first dose of CPT-11), then every 8 weeks for the first 6 months and every 12 weeks through the first year of therapy, and at disease progression or cessation of therapy. Owing to concerns over the effects of BVZ on the epiphyseal plate, growing children were required to have radiographs of the knee at baseline and at periodic intervals thereafter. Patients with any abnormality of the epiphyseal growth plate were required to have MRI scans of the knee for confirmation.
Evaluation of Response
Patients were evaluable for response assessment if they had received the first course of treatment, unless there was clear evidence of progression during the first course. Evaluative categories were complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) using Macdonald criteria.18 Patients with PD ≤50% were allowed to remain on study as long as there were no clinical symptoms or signs or evidence of new site of disease.
Evaluation of Permeability, Perfusion, and Diffusion Parameters
An MR perfusion region of interest (ROI) was placed in the solid part of the target tumor lesion with the highest cerebral blood volume (CBV) and divided by an ROI from the frontal white matter and the ratio value recorded. Regarding diffusion images, a ROI (3 mm in diameter) within the solid part of the tumor (determined from the T1, T2 fluid attenuated inversion recovery (FLAIR), T2, and postgadolinium T1 sequences) from the apparent coefficient diffusion map was divided by the value from an ROI obtained in the frontal lobe white matter. Of note, the tumor ROI determined in diffusion analysis corresponded to the ROI generated in perfusion analysis. MR permeability imaging was performed with 3D axial T1 dynamic contrast enhanced sequences with kinetic modeling to yield permeability (Kps) and CBV measurements. ROIs were placed within the areas of highest permeability on the Kps maps, and Kps values and fractional CBV values were recorded. While ROI measurements may be difficult for small lesions (1–2 mm in diameter), the reproducibility of these measurements for larger tumors is robust owing to the same operator performing all ROI measurements.
Immunohistochemistry
In consenting patients, paraffin-embedded tumor sections were obtained from already available tumor tissue and underwent IHC staining for 4 protein markers (VEGF, VEGFR-2, HIF-2, and CA-9) scored by a neuropathologist (R.E.M.), who was unaware of clinical and image analysis.16
Study Design and Statistical Analysis
The primary objective of the study was to determine the true objective response (CR + PR) to BVZ + CPT-11 in cases of pediatric recurrent EPN as observed during the first 4 courses of treatment. Simon's minimax 2-stage design with 10% type I and II error rates was used to assess a ≤5% objective response rate, which was deemed unacceptable vs a ≥25% objective response rate, which was considered desirable. Thirteen patients were enrolled during the first stage, and ≥1 objective response was required to enroll an additional 7 patients in the second stage. The treatment regimen would be deemed ineffective if <2 responses were observed in 20 patients. The study plan also specified models for exploring correlations between MR perfusion/diffusion imaging and fluorodeoxyglucose-PET uptake and responses in the same tumor site. In the absence of any objective response, Cox proportional hazards models were used to explore relationships between PFS and functional changes in tumor as measured by MR perfusion/diffusion imaging (diffusion ratio, CBV-maximum [3Dmax], Kps-3Dmax) and between PFS and IHC scores. Kaplan–Meier estimates of distributions of PFS were obtained based on all eligible patients who received ≥1 dose of BVZ. PFS was measured from the date of initial treatment to the earliest date of disease progression, second malignancy, or death for patients who failed and to the date of last contact for patients who remained at risk for failure.
Results
Between February 2008 and December 2009, 15 patients were enrolled on this stratum. One patient was found to be ineligible owing to invalid informed consent. One patient (#11, Table 2) was inevaluable owing to receiving more than the protocol-specified dose of the study drug.
Table 2.
Clinical characteristics and outcome of 14 eligible patients with recurrent ependymoma treated with BVZ + CPT-11
| Patient No. | Age/Sex | Tumor Grade | No. of Prior Recurrences/Site of Recurrence/Size (cm) | Prior Therapy (interval post-XRT, y)/interval from last therapy to enrollment (mo) | No. of Courses | Outcome |
|---|---|---|---|---|---|---|
| 1 | 4.2/F | III | 1/lateral ventricle (1.2 × 0.8) | Surgery/focal XRT (2.4)/29 | 2 | Local PD |
| 2 | 5.3/M | II | 3/Infrantentorial (NOS) (2.3 × 3.2) | Surgery, CTX, CDDP, VCR, VP-16, CSI + focal XRT, oral VP-16 (1.1)/1 | 2 | Local PD |
| 3 | 9.7/M | II | 1/IV ventricle (3.7 × 1.5) | Surgery/focal XRT (1.6)/19 | 2 | Local PD |
| 4 | 9.5/M | II | 3/Infratentorial (NOS) | Surgery, CTX, VCR, focal XRT (8.1)/1 | 3 | Local PD |
| 5 | 9.4/M | II | 2/Spinal cord (2.8 × 2.2) | Surgery, Focal XRT (6.9)/83 | 4 | Local PD |
| 6 | 4.8/F | II | 1/Cerebellum (2.1 × 1.4) | Surgery, focal XRT (2.3)/27 | 2 | Local PD |
| 7 | 12.4/M | II | 4/Infratentorial (NOS) (1.4 × 1.0) | Surgery, focal XRT x4 (6.0)/72 | 2 | Local PD |
| 8 | 8.9/M | III | 3/IV ventricle (0.5 × 0.5) | Surgery, focal XRT, CTX, VCR, CDDP, VP-16; oral VP-16, 13- CR, celocoxib, TMZ (1.6)/6 | 2 | Local PD |
| 9 | 7.1/F | III | 1/IV ventricle (0.9 × 0.9) | Surgery, focal XRT (1.4)/17 | 4 | Local PD |
| 10 | 16.7/M | II | 2/Cerebellum (2.2 × 2.0) | Surgery, focal XRT (1.5)/18 | 12 | SD/off Rx for toxicity |
| 11 | 14.7/F* | III | 5/Parietal Lobe (1.3 × .1.2) | Surgery, focal XRT, enzastaurin, pannitumab (NA)/0.6 | 2 | PD/spinal cord |
| 12 | 7.7/F | II | 5/Cerebellum (3.2 × 3) | Surgery, HDC, VCR, carboplatin, VP-16, TMZ, celocoxib, focal XRT (3.2)/0.3 | 7 | Local PD |
| 13 | 13/M | II | 3/Infratentorial (NOS) (2.9 × 2.2) | Surgery, VCR, carboplatin, CTX, focal XRT, AZD2171 (3.1)/0.8 | 10 | SD/off Rx for toxicity |
| 14 | 3/F | II | 0/Frontal lobe (1.9 × 0.8) | Surgery, focal XRT (0.8)/10 | 4 | SD/off Rx for refusal to follow-up |
Abbreviations: XRT, radiotherapy; CR, cis–retinoic acid CTX, chemotherapy; CDDP, cisplatin; VCR, vincristine; VP-16, etoposide; CSI, craniospinal irradiation; TMZ, temozolomide; HDC, high-dose chemotherapy; NOS, not otherwise specified. * The patient was not evaluable owing to receiving incorrect doses of the study drug.
Patient Characteristics
The median age at enrollment for eligible patients was 9.7 years (range, 3–19.5) (Table 1). Four of 14 patients (28%) had grade III EPN. The median number of prior recurrences was 2.5 (range, 0–5) (Table 2); all patients had received radiation therapy ± chemotherapy prior to entry. The median time from prior radiotherapy to protocol enrollment was 2.3 years (range, 0.8–8.1). The median performance status was 90 (range, 70–100). The median number of courses of BVZ + CPT-11 received was 3 (range, 2–12) (Table 2).
Table 1.
Clinical characteristics of patients with recurrent ependymoma
| Patient Characteristics | |
|---|---|
| N | 15 |
| n eligible | 14 |
| n evaluable | 13 |
| Median age at enrollment, y (range) | 9.7 (3–19.5) |
| Histology | Grade II = 10, grade III = 4 |
| n on EIACD | 2 |
| Median no. of courses (range) | 3 (2–12) |
| Objective responses (CR + PR) | 0 |
| n cases stable disease for >12 weeks | 2 |
| n cases progressive disease | 10 |
| Median time to progression (range) | 2.2 mo (1.9–6.3) |
| 6-mo PFS (±SE) | 25.7% (±11.1%) |
Toxicity
The common grades I–III toxicities related to BVZ and CPT-11 are listed in Table 3. The most common toxicity related to BVZ treatment was grades I–III fatigue in 4 patients. One patient had moderate hypertension. One patient (#10, Table 2) had grade I avascular necrosis of bilateral distal femoral shafts and proximal tibiae. This patient had previously had a prolonged course of steroids to control neurologic symptoms from his brain tumor, but an x-ray of the left knee obtained (but not required per protocol) prior to starting protocol therapy was normal. After 12 courses of treatment, an x-ray of the left knee showed increased sclerosis of the lateral femoral condyle. MRI scan of both knees showed changes consistent with medullary infarcts (avascular necrosis) of the distal metadiaphysis. This patient was taken off protocol therapy for this toxicity. Another patient (#13, Table 2), with an EPN of the fourth ventricle and a preexisting arachnoid cyst of the craniocervical junction, developed severe headaches (grade III) after 10 courses of treatment in the presence of stable tumor but worsening arachnoid cyst. He was taken off the study because he required surgery for the arachnoid cyst, and the BVZ was possibly contributing to his headaches. Grades II–III neutropenia (n = 5) and elevated transaminases were associated with CPT-11 (Table 3).
Table 3.
Adverse events from toxicities related to bevacizumab or irinotecan
| Toxicity | Grade | Bevacizumab, n | Irinotecan, n |
|---|---|---|---|
| Fatigue | I–III | 4 | |
| Epistaxis | I | 1 | |
| Hypertension | I–III | 1 | |
| Avascular necrosis of bilateral distal femurs and proximal tibiae | IV | 1 | |
| Neutropenia | II–III | 5 | |
| Thrombocytopenia | I–II | 0 | |
| Diarrhea | I–III | 12 | |
| Elevated transaminases | I–III | 3 |
Responses and PFS
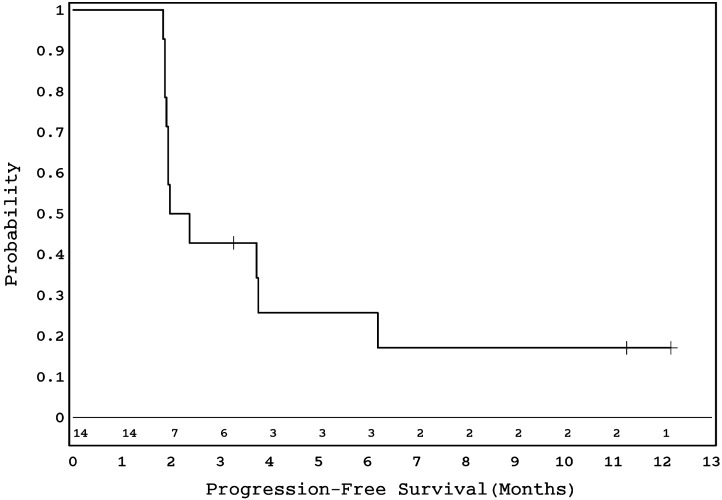
No sustained objective responses were observed in 13 evaluable patients (Tables 1 and 2). One patient who had SD at 4 months (#14, Table 2) came off the study at parental request. Two patients had SD lasting 12 months (#10 in Table 2 was taken off treatment owing to avascular necrosis) and 10 months (#13 in Table 2 was taken off treatment owing to persistent headaches), respectively. Ten patients suffered PD at the primary site at a median time of 2.2 months (range, 1.9–6.3) from beginning protocol therapy. The 6-month PFS for the entire cohort was 25.7% (SE = 11.1%) (Fig. 1).
Fig. 1.
Kaplan–Meier survival curve showing PFS in 14 eligible patients with recurrent ependymoma.
Changes in Diffusion Ratio, CBV-3Dmax, Kps-3Dmax, and Volume FLAIR Following 2 Doses of BVZ and During Treatment with BVZ+CPT-11 and Correlation with PFS
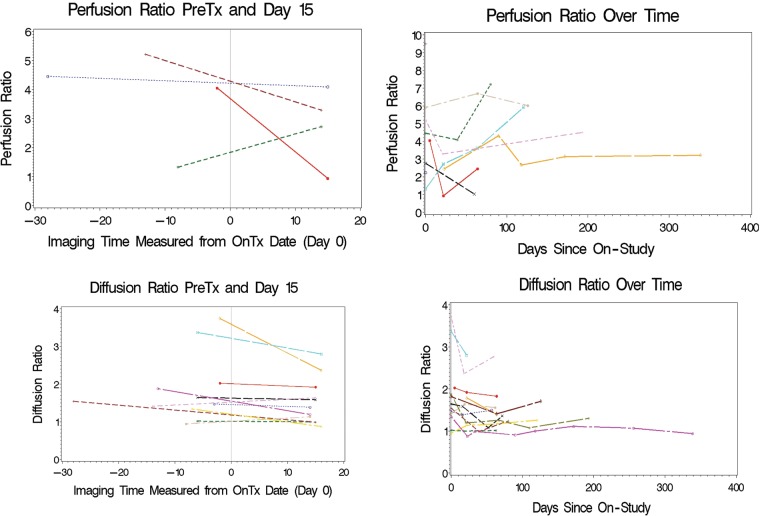
Perfusion- and diffusion-weighted images were obtained at baseline and following 2 doses of BVZ in 4 and 12 patients, respectively. There was a decrease in median diffusion ratio between pretreatment and day 15 scans (P = .054) (Fig. 2). However, there was no correlation between this change and PFS. Nine patients had diffusion-weighted imaging available at baseline and at the end of course 2. No significant change was observed in the median diffusion ratio between these 2 time points (P = .164). Similar analyses were not possible using perfusion parameters (owing to the small number of patients with paired scans) but are represented graphically in Fig. 2. An example of changes in neuroimaging in 1 patient is represented in Fig. 3 (#13, Table 2).
Fig. 2.
Change in perfusion ratio and diffusion ratio following 2 doses of BVZ (left panels upper and lower, respectively) and over the entire course of treatment (right panels upper and lower, respectively).
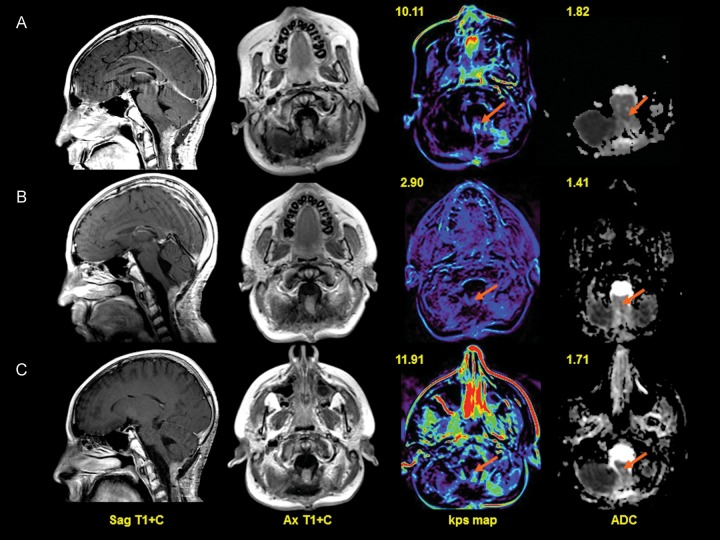
Fig. 3.
Row A. Sagittal (first column) and axial T1 images (second column) with gadolinium at baseline demonstrate enhancing tumor at the cervicomedullary junction involving the medulla and upper cervical spinal cord. There is increased T1 permeability value (third column, arrow) within the tumor (Kps = 10.11/100 cm3) and mild restricted tumor diffusion (fourth column, arrow) on the apparent diffusion coefficient (ADC) map. Row B. At 2 months posttherapy, there is minimal decrease in the enhancing mass on sagittal and axial T1 images at the cervicomedullary junction. T1 permeability maps (third column, arrow) demonstrate decreased permeability within the lesion (Kps = 2.90 mL/100 cm3) and reduced diffusion values (fourth column, arrow) within the tumor. Row C. At 4 months after therapy, there is stable enhancing tumor at the craniocervical junction on sagittal and axial T1 images (second column). There is an increased permeability (third column, arrow) value within the tumor (Kps = 11.91 mL/100 cm3) and increased tumor diffusion values (fourth column, arrow).
Expression of VEGF, HIF-2α, VEGFR-2, and CA-9 by Tumor IHC Correlated with PFS
IHC analysis was performed in available tumor samples from 7 of 13 patients (grade II, n = 3; grade III, n = 4). All these samples were from tissue obtained from either diagnosis or prior recurrences. Median expressions for VEGF, HIF-2α, VEGFR-2, and CA-9 were 40% (range, 5%–70%), 0.01% (0–10%), 15% (5%–80%), and 20% (0%–60%), respectively. There was no correlation between level of IHC expression and PFS.
Discussion
BVZ, a humanized monoclonal antibody, was developed as a specific inhibitor of all VEGF-A isoforms and has been FDA approved in combination with chemotherapy for certain adult cancers, including recurrent glioblastoma multiforme (GBM).19–21 Inhibition of VEGF results in a decrease in vascular permeability and interstitial fluid pressure, a more orderly blood flow owing to pruning of unnecessary blood vessels, a decrease in the number of tumor-initiating cells owing to disruption of their perivascular niche, and an increase in chemotherapy drug delivery into the tumor.22,23 The clinical trial reported here is the first efficacy study in pediatric cases of recurrent EPN. Our results indicate that this combination was not effective in producing objective responses. Also, the observed rate of disease stabilization (6-month PFS = 27.7%) does not appreciably improve upon the suboptimal responses and outcome observed with standard salvage regimens used for recurrent EPN.4,5 This outcome is similar to what we have previously reported in children with recurrent malignant glioma and diffuse intrinsic brain stem glioma enrolled in the same study.15 In striking contrast, cases of adult recurrent or newly diagnosed malignant glioma (driven predominantly by VEGF-mediated angiogenesis) treated with single-agent BVZ or BVZ + CPT-11 have consistently shown evidence of sustained tumor shrinkage and improvement in overall survival.11,24
The reasons for treatment failure in our study are unclear. The biologic heterogeneity of EPN is well known, and responses to various biologic agents might be different according to recently described specific molecular categories.25 Although VEGF and VEGFR-2 expression were observed in a proportion of our patients, especially those with anaplastic tumors, it is possible that VEGF is not the sole mediator of angiogenesis in these multiply relapsed tumors and that other angiogenic pathways (eg, fibroblast growth factor or placenta growth factor) sustain tumor growth in these cases, as has been observed in other solid tumors.26 Such innate refractoriness to anti-angiogenic therapy does not require a genetic mutation to induce it and occurs despite inhibition of the intended angiogenic target.27 In the face of angiogenic inhibition, the tumor derives its blood supply through elaboration of additional pro-angiogenic factors, increases pericyte coverage of tumor endothelial cells, infiltrates deeper into the surrounding tissue, and co-opts normal vasculature.27 While the latter phenomenon has been known to cause diffuse, infiltrative, non-enhancing tumor spread that has been noted in about 30% of adult GBM patients who receive BVZ therapy,28 only 1 of our patients was noted to have metastatic disease on treatment (Table 2).
Toxicity was fairly predictable and manageable in this study. With half the patients receiving not more than 3 cycles, it is likely that the majority were spared from significant side effects of BVZ and/or CPT-11. In particular, there were no patients with CNS ischemia or hemorrhage. One patient went off the study owing to avascular necrosis of the diametaphysis of the distal femoral shafts and upper tibial metaphyses following a year of treatment. Avascular necrosis of the mandible has been associated with BVZ-containing regimens in cases of adult cancers of the prostate and breast and occasionally malignant glioma.29,30 It is likely that avascular necrosis of the weight-bearing bones developed from microvessel injury following BVZ treatment in our patient, in the context of previous prolonged steroid exposure, and was aggravated by minor trauma and the stress of weight bearing.
Because BVZ-induced VEGF inhibition is expected to decrease blood flow, CBV and Kps were measured before and after treatment. Also, diffusion imaging parameters were obtained as a measure of change in cellular content of the tumor following anti-VEGF treatment to be used as a predictor of tumor response or PFS.31,32 Similar to what has been observed in adult cases of solid tumors following anti-VEGF therapy,33,34 there was some evidence for decrease in the median tumor diffusion ratio following 2 doses of single-agent BVZ compared with baseline owing to reduction in edema consequent to decrease in vascular permeability (Fig. 2). However, diffusion or perfusion ratio values did not change significantly during subsequent treatment (Fig. 2), indicating that compensatory angiogenic factors might have contributed to maintenance of tumor blood flow and interstitial edema that could not be further overcome with continuing BVZ therapy. However, our correlative neuroimaging data should be interpreted cautiously owing to the small sample size. While interobserver variability did not exist in our study owing to measurements made by a single operator during central review, the interpretation of perfusion changes following anti-angiogenic therapy in small tumors can be difficult, especially if the tumor contrast enhancement is heterogeneous. This study also sought to assess the prognostic value of baseline IHC expression of certain angiogenic markers in available paraffin-embedded tumor tissue.16 In a recent Duke study of adult cases of recurrent malignant glioma treated with BVZ + CPT-11, higher VEGF expression correlated well with responses to the drug combination but was not associated with a survival benefit, and overexpression of CA-9 predicted for a worse outcome.16 In our current study, although both VEGFR-2 and CA-9 expression appear higher in anaplastic EPN compared with grade II tumors, the small sample size, variable times of collection of these tissue samples, and absence of objective responses to BVZ + CPT-11 precluded meaningful evaluation of associations between these parameters. The prognostic value of such markers needs to be further explored in larger studies using anti-angiogenic therapy.
In summary, BVZ + CPT-11 did not demonstrate efficacy in cases of pediatric recurrent EPN. Direct anti-angiogenic therapies like BVZ work best in the context of early tumors that have a need for a high vascular supply. Following multiple recurrences, tumors have multiple oncogenic mutations and are resistant to hypoxia and less dependent on angiogenesis for survival. Since anti-angiogenic therapy is likely to work better in the setting of minimal tumor burden and earlier in the disease course of such patients, it might be worthwhile using this combination at the time of initial diagnosis. Other strategies also need to be explored based on the identification of unique signaling pathways that drive the relentless progression of these tumors.35,36
Funding
This work was supported by a Pediatric Brain Tumor Consortium grant U01CA81457, a National Center for Research Resources grant M01RR00188, and the American Lebanese Syrian Associated Charities.
Conflict of interest statement. None declared.
Acknowledgments
Presented in part at the Society for Neuro-Oncology, Montreal, Canada, November 2010.
References
- 1.Mclendon R, Ng HK, Gururangan S, et al. Ependymoma, NOS, in PubCan2010, International Agency for Research on Cancer (IARC) Lyon, France: World Health Organization (WHO); [Google Scholar]
- 2.Bouffet E, Tabori U, Huang A, et al. Ependymoma: lessons from the past, prospects for the future. Childs Nerv Syst. 2009;25(11):1383–1384. doi: 10.1007/s00381-009-0915-6. author reply 1385. [DOI] [PubMed] [Google Scholar]
- 3.Merchant TE. Current management of childhood ependymoma. Oncology (Williston Park) 2002;16(5):629–642, 644. discussion 645–6, 648. [PubMed] [Google Scholar]
- 4.Merchant TE, Boop FA, Kun LE, et al. A retrospective study of surgery and reirradiation for recurrent ependymoma. Int J Radiat Oncol Biol Phys. 2008;71(1):87–97. doi: 10.1016/j.ijrobp.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 5.Bouffet E, Capra M, Bartels U. Salvage chemotherapy for metastatic and recurrent ependymoma of childhood. Childs Nerv Syst. 2009;25(10):1293–1301. doi: 10.1007/s00381-009-0883-x. [DOI] [PubMed] [Google Scholar]
- 6.Johnson R, Wright KD, Gilbertson RJ. Molecular profiling of pediatric brain tumors: insight into biology and treatment. Curr Oncol Rep. 2009;11(1):68–72. doi: 10.1007/s11912-009-0011-9. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 8.Karkkainen MJ, Petrova TV. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19(49):5598–5605. doi: 10.1038/sj.onc.1203855. [DOI] [PubMed] [Google Scholar]
- 9.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333(2):328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 11.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 12.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 13.Korshunov A, Golanov A, Timirgaz V. Immunohistochemical markers for prognosis of ependymal neoplasms. J Neurooncol. 2002;58(3):255–270. doi: 10.1023/a:1016222202230. [DOI] [PubMed] [Google Scholar]
- 14.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 15.Gururangan S, Chi SN, Young Poussaint T, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28(18):3069–3075. doi: 10.1200/JCO.2009.26.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26(2):271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman HS, Petros WP, Friedman AH, et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17(5):1516–1525. doi: 10.1200/JCO.1999.17.5.1516. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald DR, Cascino TL, Schold SC, Jr., et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 19.Cohen MH, Gootenberg J, Keegan P, et al. FDA drug approval summary: bevacizumab plus FOLFOX4 as second-line treatment of colorectal cancer. Oncologist. 2007;12(3):356–361. doi: 10.1634/theoncologist.12-3-356. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MH, Shen YL, Keegan P, et al. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 21.Summers J, Cohen MH, Keegan P, et al. FDA drug approval summary: bevacizumab plus interferon for advanced renal cell carcinoma. Oncologist. 2010;15(1):104–111. doi: 10.1634/theoncologist.2009-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8(4):309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korshunov A, Witt H, Hielscher T, et al. Molecular staging of intracranial ependymoma in children and adults. J Clin Oncol. 2010;28(19):3182–3190. doi: 10.1200/JCO.2009.27.3359. [DOI] [PubMed] [Google Scholar]
- 26.Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14(20):6371–6375. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- 27.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91(3):329–336. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 29.Estilo CL, Fornier M, Farooki A, et al. Osteonecrosis of the jaw related to bevacizumab. J Clin Oncol. 2008;26(24):4037–4038. doi: 10.1200/JCO.2007.15.5424. [DOI] [PubMed] [Google Scholar]
- 30.Disel U, Besen AA, Ozyilkan O, et al. A case report of bevacizumab-related osteonecrosis of the jaw: Old problem, new culprit. Oral Oncol. 2012;48:e2–3. doi: 10.1016/j.oraloncology.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 31.Hamstra DA, Galban CJ, Meyer CR, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol. 2008;26(20):3387–3394. doi: 10.1200/JCO.2007.15.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pope WB, Lai A, Mehta R, et al. Apparent diffusion coefficient histogram analysis stratifies progression-free survival in newly diagnosed bevacizumab-treated glioblastoma. AJNR Am J Neuroradiol. 2011;32(5):882–889. doi: 10.3174/ajnr.A2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;27(18):3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkinson JM, Shelat AA, Carcaboso AM, et al. An integrated in vitro and in vivo high-throughput screen identifies treatment leads for ependymoma. Cancer Cell. 2011;20(3):384–399. doi: 10.1016/j.ccr.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson RA, Wright KD, Poppleton H, et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466(7306):632–636. doi: 10.1038/nature09173. [DOI] [PMC free article] [PubMed] [Google Scholar]