Abstract
Carbonic anhydrase (CA) IX is over-expressed in glioblastoma; however, its functions in this context are unknown. Metabolically, glioblastomas are highly glycolytic, leading to a significant lactic acid load. Paradoxically, the intracellular pH is alkaline. We hypothesized that CAIX contributes to the extrusion of hydrogen ions into the extracellular space, thereby moderating intra- and extracellular pH and creating an environment conductive to enhanced invasion. We investigated the role of CAIX as a prognostic marker in patients with glioblastoma and its biological function in vitro. CAIX expression was analyzed in 59 patients with glioblastoma by immunohistochemistry. The expression levels were correlated to overall survival. In vitro, U251 and Ln 18 glioblastoma cells were incubated under hypoxia to induce CAIX expression, and RNA interference (RNAi) was used to examine the function of CAIX on cell attachment, invasion, intracellular energy transfer, and susceptibility to adjuvant treatment. High CAIX expression was identified as an independent factor for poor survival in patients with glioblastoma. In vitro, cell attachment and invasion were strongly reduced after knockdown of CAIX. Finally, the effects of radiation and chemotherapy were strongly augmented after CAIX interference and were accompanied by a higher rate of apoptotic cell death. CAIX is an independent prognostic factor for poor outcome in patients with glioblastoma. Cell attachment, invasion, and survival during adjuvant treatment are significantly influenced by high CAIX expression. These results indicate that inhibition of CAIX is a potential metabolic target for the treatment of patients with glioblastoma.
Keywords: apoptosis, CAIX, hypoxia, invasion, pH
Glioblastoma multiforme is the most frequent primary brain tumor in adults1 and has an exceptionally poor prognosis. Despite optimal treatment consisting of maximal resection followed by radiation and chemotherapy, virtually all tumors recur with a median time to progression of 6.9 months.2 In the search for novel treatment strategies, tumor-specific metabolism has recently become a target of intense investigation.3 Malignant gliomas are biochemically characterized by increased glycolytic metabolism causing high glucose consumption rates and a significant lactic acid load.4 Despite the accumulation of lactic acid, the intracellular pH of malignant gliomas has been found to be alkaline, compared with the normal brain,5 whereas the extracellular compartment is highly acidic.6 In addition to specific genomic alterations7,8 leading to enhanced glycolysis, malignant gliomas display a high degree of intratumoral hypoxia,9,10 which also contributes to activation of glycolysis-related genes through the HIF-1α pathway.11 Of all the genes regulated by hypoxia, carbonic anhydrase IX (CAIX), which catalyzes the reaction CO2 + H2O to HCO3− + H+, shows the strongest transcriptional activation.12 The molecule is composed of 4 domains, the N-terminal proteoglycan domain moderating cell attachment,13 the catalytic domain, a transmembrane region, and a cytoplasmic tail.14 CAIX has the highest catalytic activity among all human α-carbonic anhydrases,15 and the enzymatic domain is exposed to the extracellular space, which may contribute to the intra-extracellular pH gradient observed in malignant gliomas.16 Whereas CAIX is virtually absent in normal brain,17 the molecule has been found to be overexpressed in a number of different brain tumors.18 However, the functions of CAIX in malignant gliomas are unknown. The goal of our study was to determine whether there is a correlation between high CAIX expression and the prognosis in patients with glioblastoma multiforme and to determine whether CAIX contributes to cell attachment, invasion, and susceptibility to chemotherapy and radiation treatment in glioblastoma cells.
Materials and Methods
Patient Population
The study was approved by the University of Regensburg Ethics Committee (protocol 11-101-0179) and conducted in accordance with the ethical standards of the Helsinki Declaration. Patient's written consent was obtained whenever possible; all study results were stored and analyzed in an anonymous fashion. We investigated a cohort of 59 adult (>18 years of age) patients (20 female, 39 male) who were consecutively treated for glioblastoma multiforme at the University of Regensburg Medical Center during 2001–2009 (Table 1). The mean age was 59.5 years (range, 33.6–82.5), and the median Karnofsky score was 90%, (range, 50–100). All patients underwent craniotomy for tumor resection; 32.2% of all patients received a gross total resection; and 63.8% received a subtotal resection, as demonstrated by postoperative MRI scanning obtained within 72 h after surgery. All patients received adjuvant treatment consisting of external beam radiation and chemotherapy with alkylating agents. Follow-up was completed up to March 2011 by reviewing outpatient records and contacting the patient, a family member, or the patient's primary physician. The median overall survival was 18.9 months; tumor progression was the cause of death in all cases. No patient was lost to follow-up.
Table 1.
Characteristics of patients treated for glioblastoma stratified by high and low CAIX expression.
| Characteristic | CAIX high | CAIX low | P |
|---|---|---|---|
| Number of patients | 37 | 22 | |
| Female/Male | 10/27 | 10/12 | .245 |
| Mean age | 59.2 | 59.9 | .835 |
| Median KPS | 90 | 90 | .298 |
| Extent of resection (gross total/subtotal) | 10/27 | 9/13 | .415 |
| Location | .356 | ||
| Frontal | 18 | 8 | |
| Temporal | 11 | 11 | |
| Parietal | 6 | 3 | |
| Occipital | 2 | 0 | |
| Side (left/right) | 13/24 | 10/12 | .612 |
| Median overall survival (mts.) | 15.2 | 34.1 | .001 |
Note: Statistical testing expressed by the P values revealed equal distribution of the parameters between the groups except for median overall survival.
Tissue Processing
All tissue samples were obtained in accordance with the ethical guidelines of the University of Regensburg Medical Center. The tissue samples were collected from surgical specimens and quick-frozen in precooled isopentane, embedded in optimal cutting temperature compound, and stored at −80°C. Histologic diagnosis of the tumor samples was performed by an independent pathologist. A region that was pinpointed by the pathologist as representative for the tumor was chosen for CAIX expression analysis. Cryosections were immunostained using a CAIX-specific antibody (polyclonal rabbit anti-human CAIX, Novus Biologicals; 1:1000) as previously described.18 As a control, sections on the same slide were incubated with nonimmune rabbit IgG at identical protein concentrations. CAIX-positive cells were counted and calculated as percentage of all cells visible in at least 4 different high power fields (200x). The rating of the immunohistochemical staining was performed by 3 observers blinded to the clinical course with use of a 4-grade, semiquantitative scale (1, no cells positive; 2, 1%–10% positive; 3, 10%–30% positive; 4, >30% positive). For the survival studies, a mean rating of >3 was defined as high CAIX expression.
Cell Culture/In Vitro Hypoxia
U251 and Ln 18 glioblastoma cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% FCS, 50 U/mL penicillin, and 50 µg/mL streptomycin in a humidified atmosphere of 5% CO2 and 95% air. To induce CAIX expression, cells were changed into serum-free medium 24 h before the experiment and subsequently incubated for 24 h in a sealed, humidified 5% CO2 module with either 21% oxygen and 25 mM glucose in the culture medium (normoxia) or 0% oxygen (hypoxia) plus 125 mM glucose to further enhance the glycolytic metabolism, as described previously.19
Gene Silencing by RNA Interference
The siRNA sequence (siCAIX) was chosen by searching the coding sequence of CAIX for 2 adenines followed by 16 nucleotides that had a GC content <50% and did not contain >2 thymidines or adenines in a row (Eurofins MWG Operon). The sequence was tested for possible homology to other human genes by searching the National Center for Biotechnology Information database. A scrambled sequence with the identical GC content was used as nonspecific control (NSC). The constructs were transfected into U251 and Ln 18 cells with use of lipofectamine 2000 (Invitrogen) as transfection reagent in serum-free Optimem (Invitrogen) for 4 h at 37 °C. Cells were changed into DMEM with 10% FCS and incubated overnight for recovery.
CAIX Expression Studies
Hypoxic induction of CAIX mRNA expression and the effects of RNA interference were verified by quantitative reverse-transcription polymerase chain reaction (RT-PCR). In brief, total RNA was isolated using an RNeasy mini kit (Qiagen) and quantified. SuperScript II Reverse Transcriptase kit (Invitrogen) was used according to the manufacturer's instructions to obtain single-stranded cDNA. The resulting first-strand cDNA was used as a template for SYBR Green real-time PCR (QuantiFast SYBR Green PCR-Mix; Qiagen) using sequence-specific primers (forward primer: 5′-CCG AGC GAC GCA GCC TTT GA-3′; reverse primer: 5′-GGC TCC AGT CTC GGC TAC CT-3′) and GAPDH as internal standard (forward primer: 5′-GGT CGG TGT GAA CGG ATT TG-3′; reverse primer: 5′-GTG AGC CCC AGC CTT CTC CAT-3′).
Western Blot
Western blotting for CAIX protein expression was performed as described earlier.18 Cells were lysed in a RIPA buffer containing a protease inhibitor cocktail. A 20-µg sample of each protein extract was separated by electrophoresis on a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. After blocking for 1 h in 3% nonfat dry milk, the blot was incubated in the anti-CAIX antibody (Novus Biologicals) at a 1:5000 concentration. Antibody binding was detected by a horseradish peroxidase–linked secondary antibody at a concentration of 1:20 000 for 1 h (Bio Source). Visualization was performed using a chemiluminescence detection system (Pierce SuperSignal). Equal loading was verified by reprobing the blot for β-actin.
Attachment Assay
U251 and Ln 18 cells were seeded at a density of 5 × 103 cells/well in a 96-well dish plate. After allowing the cells to attach for 4 h, the plates were placed on a horizontal shaker for 5 min and washed 3 times with phosphate buffered saline (PBS). Subsequently, 100 µL DMEM supplemented with 10% FCS was added, and the cells were incubated for 4 h. Cells remaining in the 96-well dish plate were quantified using a colorimetric assay (AQ assay; Promega).
Invasion Assay
Invasion chambers (Becton Dickinson Biosciences) containing a matrigel (Trevigen) coated polycarbonate membrane (8 µm pore size) were placed into a 24-well dish plate. After transfection with siCAIX or NSC siRNA, cells were seeded (2 × 105 cells/well) into the invasion chamber and incubated under different metabolic conditions for 48 h. Invasion was quantified by counting the cells remaining on the upper compartment and the cells that invaded through the matrigel layer. Subsequently, a ratio of invading to noninvading cells was calculated.20
Adjuvant Treatment
Influence of CAIX expression on the efficacy of chemotherapy was investigated by temozolomide treatment (MSD) at a concentration of 50 µM over 6 days. Radiation effects were studied by irradiating U251 cells with sublethal doses of 10 Gy with use of a 6-MV linear accelerator (Primus, Siemens).21 Cell toxicity was measured using a colorimetric assay (AQ assay, Promega). Apoptotic cell death was investigated by annexin V labeling (Roche). In brief, 20 µL annexin V solution was mixed with 1 mL of incubation buffer plus 50 µL propidium iodide solution and mixed thoroughly. Subsequently, 120 µL of the reaction mix was added to the cells, which were incubated for 60 min on a horizontal shaker at low speed at room temperature. Annexin V labeling was observed with a fluorescent microscope using the appropriate filters.
ATP Assay
Cells were seeded in serum-free DMEM at a density of 5 × 103 cells/well into a 96-well dish and incubated at different metabolic conditions for 24 h, as described above. ATP levels were measured using a Cell Titer Glo kit (Promega) according to the manufacturer's instructions.
Statistics
Survival time was measured from the date of resection to death of the patient. Survival rate was calculated using the Kaplan-Meier method, and comparisons of survival between the different groups were estimated using the generalized Wilcoxon test for univariate analysis. For multivariate analysis, Cox's proportional hazards model (forward stepwise procedure) was used. In addition to CAIX expression, age, Karnofsky score, and the extent of resection were analyzed as potential prognostic factors. Significance was defined as P < .05 (SPSS, version 17.0; SPSS). Two- and multiple-group comparison was performed by computing Mann-Whitney rank-sum tests and ANOVA on ranks, respectively. Rates and proportion analysis were calculated using the Fisher exact test (SigmaStat, version 3.5; Systat Software).
Results
CAIX Expression in Glioblastoma Specimen and Survival Analysis
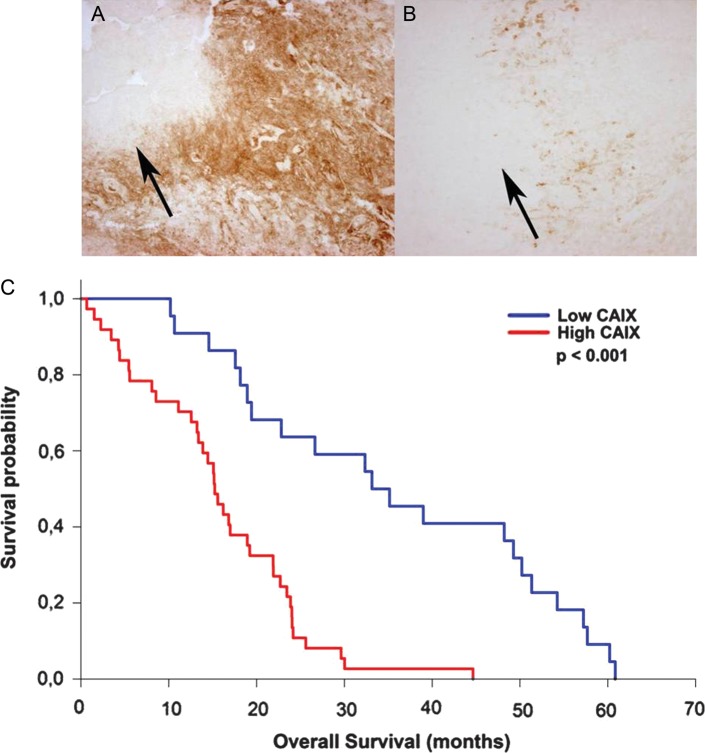
CAIX expression was found in all glioblastoma cases investigated. A subgroup of 22 patients (37.3%) showed low to moderate CAIX expression, whereas 37 patients (62.7%) displayed high to very high CAIX expression levels. Survival analysis revealed that high CAIX expression was associated with significantly worse outcome, with a median survival time of 15.2 months, compared with 34.1 months in patients with low CAIX expression (P < .001, Fig. 1). Multivariate analysis using the Cox's proportional hazards model revealed that high CAIX expression is an independent prognostic factor indicating shorter overall survival (P = .006). In addition to CAIX expression, older age and low Karnofsky score were factors associated with poor prognosis, whereas the extent of resection was not found to be a significant prognostic factor in this patient population.
Fig. 1.
Examples of glioblastomas exhibiting high (A) or low (B) levels of CAIX immunohistochemical staining (100X original magnification). The distribution of CAIX expression was found mostly adjacent to necrotic areas (arrows), the staining was distinctly localized at the cell surface. (C) Kaplan-Meier survival curves of patients with glioblastoma, stratified by high and low CAIX expression. The low CAIX expression group (blue line) has a significantly better overall survival compared to the high CAIX expression group (red line; P < .001).
CAIX Expression, Attachment, Cell Invasion
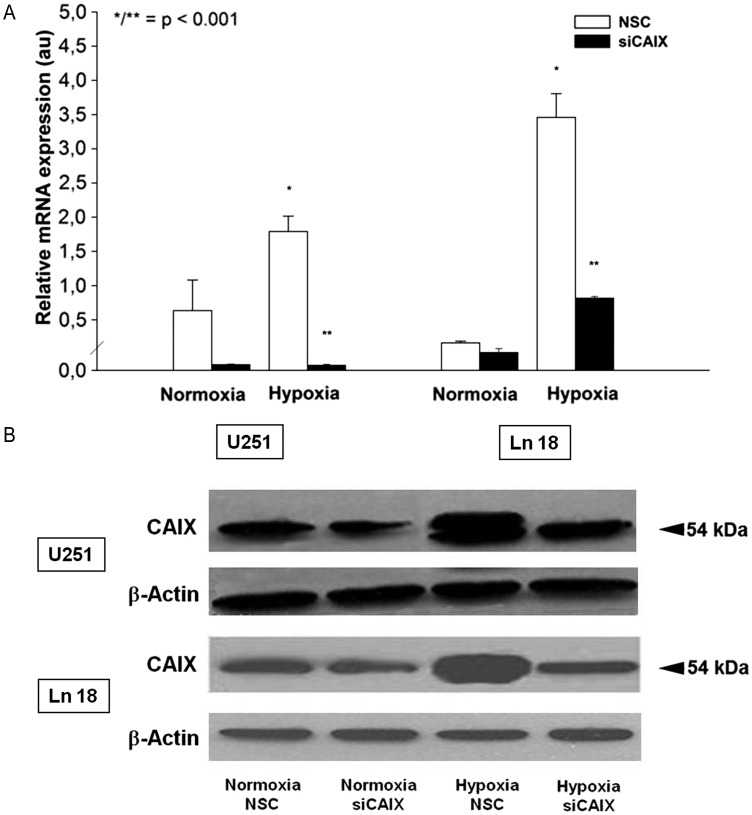
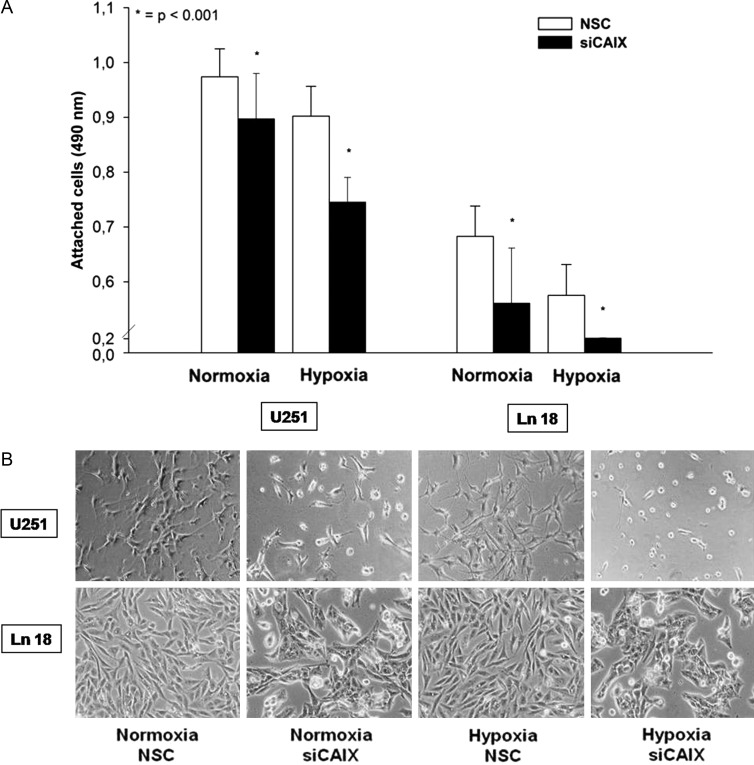
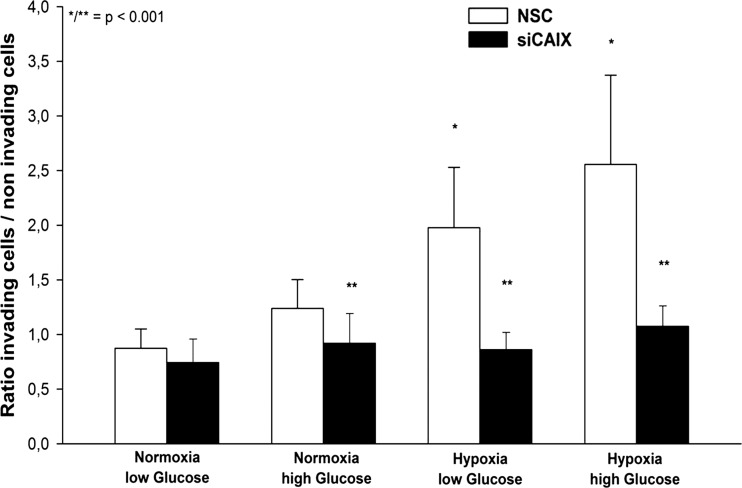
Both U251 and Ln 18 cells showed a low baseline CAIX mRNA expression under normoxic conditions that was further decreased by siRNA transfection; however, this difference was not statistically significant. Hypoxia resulted in a significant increase of CAIX mRNA expression in both cell lines; however, the relative increase of CAIX expression was higher in the Ln 18 cells. Transfection with a CAIX-specific siRNA construct significantly reduced the CAIX expression levels (P < .001, Fig. 2A). Western blotting experiments revealed a detectable CAIX protein expression under normoxic conditions in both cell lines, which was strongly enhanced by hypoxia (Fig. 2B). Of interest, only the hypoxic expression of CAIX showed a double band, which has been observed by other investigators.22,23 In vitro expression studies revealed that CAIX predominantly exists as a dimeric, high-mannose N-linked glycoprotein that is stabilized by intermolecular sulfur bonds.24,25 However, there is no clear explanation for the relative increase of the 54 kDa protein after both hypoxia and desferrioxamine treatment; transfection with siRNA against CAIX resulted in a marked reduction of the CAIX expression. Of interest, the CAIX siRNA-transfected cells displayed a changed morphology, compared with the nonspecific control siRNA (NSC) transfected cells characterized by a small, round cytoplasm, shorter cell processes, and reduced focal adhesion areas (Fig. 3B). Accordingly, CAIX knockdown caused a significant reduction of cell attachment in U251 and Ln 18 cells, compared with the NSC siRNA-transfected cells (P < .001, Fig. 3A), both under normoxic and hypoxic conditions; however, the impact of CAIX knockdown was significantly higher in hypoxic cells. Matrigel invasion assay revealed a significant influence of metabolic conditions on cell invasion. Hypoxia significantly increased matrigel invasion in U251 cells, which was further augmented by high glucose concentration (P < .001, Fig. 4). CAIX knockdown significantly reduced the invasive capacity in all metabolic conditions except for normoxia with low glucose (P < .001).
Fig. 2.
(A) Quantitative RTPCR demonstrates a significantly increased CAIX mRNA expression after hypoxia in U251 and Ln 18 glioblastoma cells compared to normoxia after transfection with nonspecific control siRNA (NSC) (*P < .001, 12 data points per condition). Transfection with sequence specific siRNA targeting CAIX causes a 98% reduction of the CAIX expression under hypoxia (**P < .001). (B) CAIX protein expression tested by Western blot also increases under hypoxia in both cell lines tested and is strongly suppressed by CAIX specific siRNA transfection. Equal loading of the lanes is demonstrated by reprobing the blot with β-Actin. The mRNA and protein samples were harvested at identical time points.
Fig. 3.
(A) Cell attachment is significantly reduced after transfection with CAIX-specific siRNA in U251 and Ln 18 glioblastoma cells compared to the nonspecific control siRNA (NSC) control both under normoxia and 24 h of hypoxia (*P < .001, 32 data points per condition, bar graph shows mean value ± STD). (B) The morphology of U251 and Ln 18 cells changed upon CAIX specific siRNA transfection as shown by phase contrast microscopy. Cells with CAIX knockdown showed a small, round cytoplasm; shorter cell processes; and reduced focal adhesion areas.
Fig. 4.
Cell invasion was strongly enhanced under hypoxic and glycolytic conditions in U251 glioblastoma cells (comparison normoxia vs hypoxia, *P < .001, 32 data points per condition, bar graph shows mean value ± STD). CAIX knockdown caused a significant reduction of the invasive behavior under normoxia with high glucose, hypoxia and glycolysis (comparison nonspecific control siRNA (NSC) vs CAIX knockdown, **P < .001).
Adjuvant Treatment
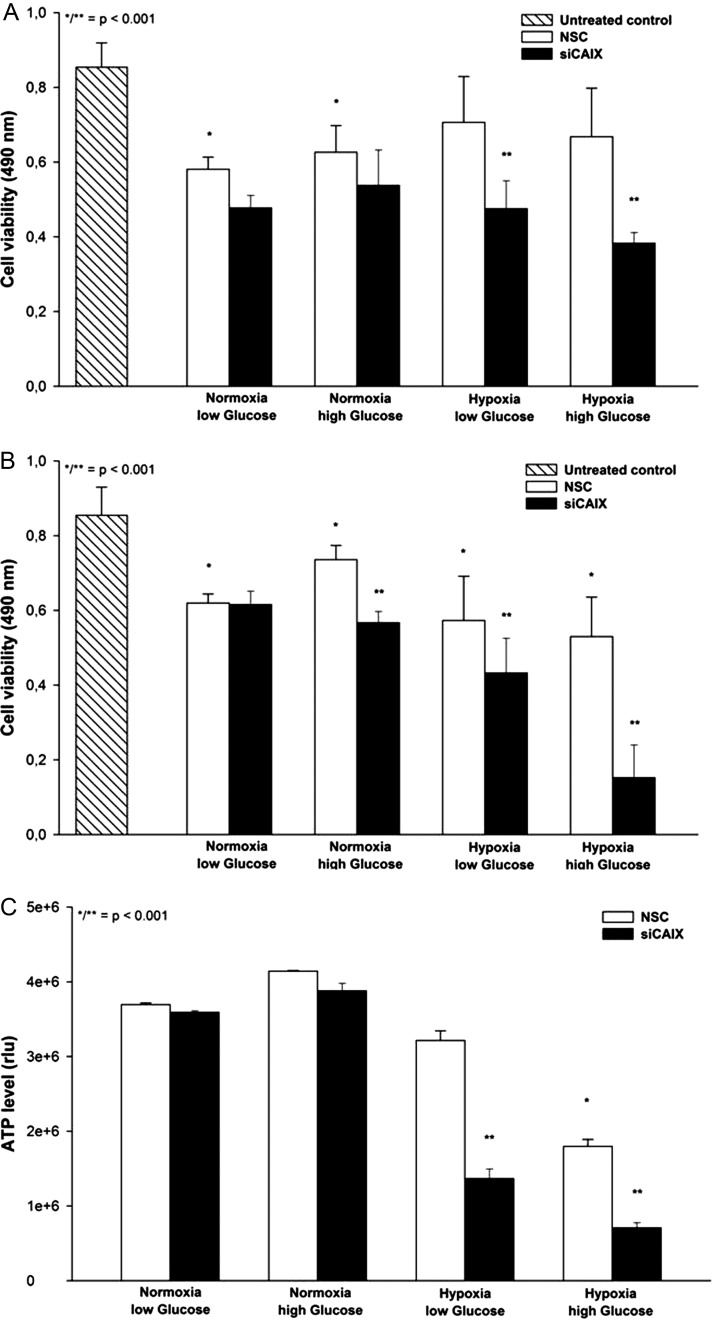
Knockdown of CAIX without any additional treatment caused only moderate cell toxicity (∼10% reduction of cell viablility) in U251 glioblastoma cells under hypoxic and glycolytic conditions (data not shown). In contrast, radiation caused significant toxicity in U251 cells under normoxia, compared with the untreated control; however, this effect was markedly reduced under hypoxic conditions. CAIX knockdown did not induce significant effects under normoxic conditions; however, the cell viability was reduced under hypoxia by 33% in low- and 44% in high-glucose conditions (P < .001, Fig. 5A). Treatment with temozolomide induced significant effects, compared with the untreated control, in all metabolic conditions. CAIX interference augmented the susceptibility to temozolomide significantly in all conditions except for normoxia with low glucose. The strongest effect of CAIX knockdown on cell toxicity was observed under hypoxia with high glucose, with an additional reduction of cell viability by 77% (P < .001, Fig. 5B). This effect was accompanied by a significant reduction of ATP levels under hypoxic conditions with high glucose, which was further decreased after CAIX knockdown by 57% and 61% with low- and high-glucose conditions, respectively (P < .001, Fig. 5C). Finally, annexin V labeling revealed a significantly higher number of apoptotic cells under hypoxia and high glucose after both radiation and temozolomide treatment (Fig. 6A and B).
Fig. 5.
(A) Sublethal radiation caused toxicity under normoxic conditions in U251 glioblastoma cells (comparison to untreated control *P < .001, 24 datapoints per condition, bar graph shows mean value ± STD), which was markedly reduced under hypoxia. CAIX knockdown resulted in a significantly enhanced toxicity in hypoxic and glycolytic cells (comparison nonspecific control siRNA (NSC) vs CAIX knockdown, **P < .001). (B) Susceptibility to chemotherapy with temozolomide (50 µM) was significantly higher after CAIX knockdown in all metabolic conditions except for normoxia with low glucose (**P < .001). Analysis was performed using a colorimetric assay (AQ assay, Promega) and expressed as absorbance at 490 nm (see materials and methods). (C) Glycolysis induced by hypoxia combined with high glucose concentrations caused a significant reduction of ATP levels (comparison normoxia vs glycolysis *P < .001). In addition, CAIX knockdown resulted in a significant decrease of ATP levels, compared with the nonspecific control siRNA (NSC) control (comparison nonspecific control siRNA (NSC) vs CAIX knockdown **P < .001). Analysis was performed using a Cell Titer Glo kit (Promega) and expressed as relative units according to the manufacturer's instructions.
Fig. 6.
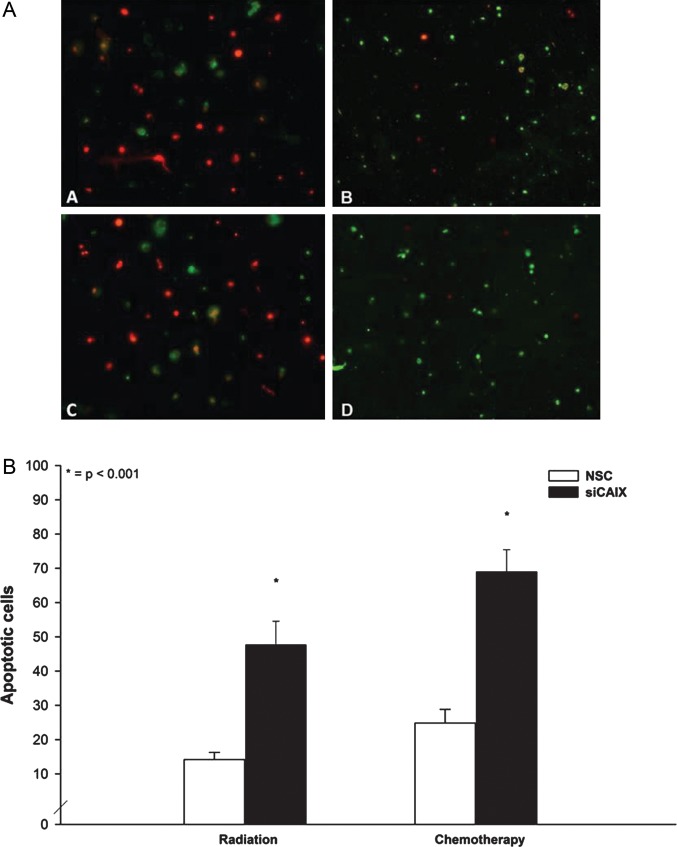
(A) Annexin V labeling (green) revealed an increased number of apoptotic cells upon CAIX knockdown under glycolytic conditions both after radiation (B) and after chemotherapy (D) in U251glioblastoma cells, compared with the nonspecific control siRNA (NSC) controls (A and C). Cells are counterstained with propidium iodide (red) (200X original magnification). B: Quantification revealed a statistically significant higher number of apoptotic cells after CAIX interference under glycolytic conditions both after radiation and temozolomide treatment.
Discussion
CAIX, which is virtually absent in the normal brain,17 has been found to be overexpressed in 100% of the analyzed glioblastoma samples. The CAIX expression was detected exclusively in tumor tissue, whereas in the adjacent normal brain no CAIX expression was found. This tumor-specific expression pattern suggests this molecule as a feasible treatment target. Although CAIX-knockout mice have developed vacuolar degeneration of the brain at older age,26 indicating some regulatory role of CAIX in the brain; treatment with specific CAIX inhibitors did not show any toxicity.27 In addition, we have found a strong association of high CAIX expression and poor overall survival among patients with glioblastoma. In a multivariate analysis of hypoxia-related gene expression in glial tumors of different malignancy grades, Flynn et al.28 found that GLUT-1 and VEGF but neither HIF-1α nor CAIX expression was associated with survival among patients with glioblastoma. Of interest, in this study also, no difference in CAIX expression was detected between low-grade and high-grade gliomas. This is in contrast to a series of investigations showing both a significantly stronger CAIX expression in high-grade gliomas and a clear association between CAIX expression and survival.29–33 The results of these studies indicated that treatment strategies for gliomas targeting CAIX are required.33 A possible explanation for this controversy is the relatively small sample size combined with the multivariate setting in the study of Flynn et al., which may not allow to detect the significant impact of CAIX on patient's survival in this circumstance. Because CAIX gene expression is strongly induced by hypoxia, it can be viewed as an endogenous hypoxia marker that identifies particularly aggressive tumors characterized by fast proliferation and high metabolic activity.34 Alternatively, CAIX may have important functions in the biology of glioblastoma. Because the CAIX gene consists of unique N- and C-terminal domains, it is considered to be a chimeric gene assembled by exon shuffling.35 The extracellular domain of CAIX mediates cells attachment,13 which plays an important role in cancer cell survival.36 Accordingly, we have detected a profound reduction of cell attachment after CAIX knockdown in glioblastoma cells. The highly active catalytic domain of CAIX converts cell-generated carbon dioxide into protons and bicarbonate ions. The juxtamembranous position of the enzyme causes the efflux of protons into the extracellular environment inducing pericellular acidification, which leads to acid-mediated tumor cell invasion.20 Simultaneously, the bicarbonate ions are transported back into the cytosol, possibly by direct interaction with anion exchangers,37 thus providing high pH buffering power.38,39 In our study, CAIX knockdown results in a significantly reduced cell invasion and decreased ATP levels under hypoxic and glycolytic conditions. In addition, the susceptibility of U251 glioblastoma cells to chemotherapy and radiation treatment was strongly enhanced after CAIX interference, which is in accordance to a recent in vivo study.40 In an earlier study, Schmaltz et al. have demonstrated that the induction of apoptotic cell death under hypoxia requires a transient acidification of the intracellular pH.41 The continuous supply of bicarbonate ions by the tumor-associated CAIX may therefore antagonize adjuvant treatment by sustaining an alkaline intracellular environment.38 In fact, we have observed a significantly increased number of apoptotic cells after CAIX knockdown, both after chemotherapy and after radiation treatment under glycolytic conditions.
In conclusion, CAIX was overexpressed in all of the glioblastoma samples in our study group. High CAIX expression is an independent prognostic marker for poor survival among patients with glioblastoma. Selective gene silencing of CAIX in U251 glioblastoma cells resulted in reduced attachment and invasion and enhanced susceptibility to adjuvant treatment. Because new, isoform specific inhibitors of CAIX have been recently synthesized,42 this could provide a new adjuvant treatment form targeting the metabolism of glioblastoma multiforme.
Conflict of interest statement. All authors: No conflicts.
References
- 1.Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009;472:323–342. doi: 10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Wolf A, Agnihotri S, Guha A. Targeting metabolic remodeling in glioblastoma multiforme. Oncotarget. 2010;1(7):552–562. doi: 10.18632/oncotarget.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oudard S, Boitier E, Miccoli L, Rousset S, Dutrillaux B, Poupon MF. Gliomas are driven by glycolysis: putative roles of hexokinase, oxidative phosphorylation and mitochondrial ultrastructure. Anticancer Res. 1997;17(3C):1903–1911. [PubMed] [Google Scholar]
- 5.Hubesch B, Sappey-Marinier D, Roth K, Meyerhoff DJ, Matson GB, Weiner MW. P-31 MR spectroscopy of normal human brain and brain tumors. Radiology. 1990;174(2):401–409. doi: 10.1148/radiology.174.2.2296651. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Martin ML, Herigault G, Remy C, et al. Mapping extracellular pH in rat brain gliomas in vivo by 1H magnetic resonance spectroscopic imaging: comparison with maps of metabolites. Cancer Res. 2001;61(17):6524–6531. [PubMed] [Google Scholar]
- 7.Beckner ME, Gobbel GT, Abounader R, et al. Glycolytic glioma cells with active glycogen synthase are sensitive to PTEN and inhibitors of PI3K and gluconeogenesis. Lab Invest. 2005;85(12):1457–1470. doi: 10.1038/labinvest.3700355. [DOI] [PubMed] [Google Scholar]
- 8.Oudard S, Arvelo F, Miccoli L, et al. High glycolysis in gliomas despite low hexokinase transcription and activity correlated to chromosome 10 loss. Br J Cancer. 1996;74(6):839–845. doi: 10.1038/bjc.1996.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans SM, Judy KD, Dunphy I, et al. Comparative measurements of hypoxia in human brain tumors using needle electrodes and EF5 binding. Cancer Res. 2004;64(5):1886–1892. doi: 10.1158/0008-5472.can-03-2424. [DOI] [PubMed] [Google Scholar]
- 10.Collingridge DR, Piepmeier JM, Rockwell S, Knisely JP. Polarographic measurements of oxygen tension in human glioma and surrounding peritumoural brain tissue. Radiother Oncol. 1999;53(2):127–131. doi: 10.1016/s0167-8140(99)00121-8. [DOI] [PubMed] [Google Scholar]
- 11.Brahimi-Horn MC, Bellot G, Pouyssegur J. Hypoxia and energetic tumour metabolism. Curr Opin Genet Dev. 2011;21(1):67–72. doi: 10.1016/j.gde.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Lal A, Peters H, St Croix B, et al. Transcriptional response to hypoxia in human tumors. J Natl Cancer Inst. 2001;93(17):1337–1343. doi: 10.1093/jnci/93.17.1337. [DOI] [PubMed] [Google Scholar]
- 13.Zavada J, Zavadova Z, Pastorek J, Biesova Z, Jezek J, Velek J. Human tumour-associated cell adhesion protein MN/CA IX: identification of M75 epitope and of the region mediating cell adhesion. Br J Cancer. 2000;82(11):1808–1813. doi: 10.1054/bjoc.2000.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opavsky R, Pastorekova S, Zelnik V, et al. Human MN/CA9 gene, a novel member of the carbonic anhydrase family: structure and exon to protein domain relationships. Genomics. 1996;33(3):480–487. doi: 10.1006/geno.1996.0223. [DOI] [PubMed] [Google Scholar]
- 15.Wingo T, Tu C, Laipis PJ, Silverman DN. The catalytic properties of human carbonic anhydrase IX. Biochem Biophys Res Commun. 2001;288(3):666–669. doi: 10.1006/bbrc.2001.5824. [DOI] [PubMed] [Google Scholar]
- 16.Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26(2):299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov S, Liao SY, Ivanova A, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158(3):905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proescholdt MA, Mayer C, Kubitza M, et al. Expression of hypoxia-inducible carbonic anhydrases in brain tumors. Neuro Oncol. 2005;7(4):465–475. doi: 10.1215/S1152851705000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi Y, Edwards NA, Proescholdt MA, Oldfield EH, Merrill MJ. Regulation and function of aquaporin-1 in glioma cells. Neoplasia. 2007;9(9):777–787. doi: 10.1593/neo.07454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin HJ, Rho SB, Jung DC, Han IO, Oh ES, Kim JY. Carbonic anhydrase IX (CA9) modulates tumor-associated cell migration and invasion. J Cell Sci. 2011;124(Pt 7):1077–1087. doi: 10.1242/jcs.072207. [DOI] [PubMed] [Google Scholar]
- 21.Pohl F, Grosse J, Grimm D, et al. Changes of apoptosis, p53, and bcl-2 by irradiation in poorly differentiated thyroid carcinoma cell lines: a prognostic marker for the prospect of therapeutic success? Thyroid. 2010;20(2):159–166. doi: 10.1089/thy.2008.0345. [DOI] [PubMed] [Google Scholar]
- 22.Robertson N, Potter C, Harris AL. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res. 2004;64(17):6160–6165. doi: 10.1158/0008-5472.CAN-03-2224. [DOI] [PubMed] [Google Scholar]
- 23.Said HM, Staab A, Hagemann C, et al. Distinct patterns of hypoxic expression of carbonic anhydrase IX (CA IX) in human malignant glioma cell lines. J Neurooncol. 2007;81(1):27–38. doi: 10.1007/s11060-006-9205-2. [DOI] [PubMed] [Google Scholar]
- 24.Hilvo M, Baranauskiene L, Salzano AM, et al. Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J Biol Chem. 2008;283(41):27799–27809. doi: 10.1074/jbc.M800938200. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Wang H, Tu C, Shiverick KT, Silverman DN, Frost SC. Role of hypoxia and EGF on expression, activity, localization and phosphorylation of carbonic anhydrase IX in MDA-MB-231 breast cancer cells. Biochim Biophys Acta. 2011;1813(1):159–167. doi: 10.1016/j.bbamcr.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan PW, Parkkila AK, Autio S, et al. Brain phenotype of carbonic anhydrase IX-deficient mice. Transgenic Res. 2012;21(1):163–176. doi: 10.1007/s11248-011-9520-z. [DOI] [PubMed] [Google Scholar]
- 27.Lou Y, McDonald PC, Oloumi A, et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 2011;71(9):3364–3376. doi: 10.1158/0008-5472.CAN-10-4261. [DOI] [PubMed] [Google Scholar]
- 28.Flynn JR, Wang L, Gillespie DL, et al. Hypoxia-regulated protein expression, patient characteristics, and preoperative imaging as predictors of survival in adults with glioblastoma multiforme. Cancer. 2008;113(5):1032–1042. doi: 10.1002/cncr.23678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haapasalo JA, Nordfors KM, Hilvo M, et al. Expression of carbonic anhydrase IX in astrocytic tumors predicts poor prognosis. Clin Cancer Res. 2006;12(2):473–477. doi: 10.1158/1078-0432.CCR-05-0848. [DOI] [PubMed] [Google Scholar]
- 30.Jarvela S, Parkkila S, Bragge H, et al. Carbonic anhydrase IX in oligodendroglial brain tumors. BMC Cancer. 2008;8:1. doi: 10.1186/1471-2407-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korkolopoulou P, Perdiki M, Thymara I, et al. Expression of hypoxia-related tissue factors in astrocytic gliomas. A multivariate survival study with emphasis upon carbonic anhydrase IX. Hum Pathol. 2007;38(4):629–638. doi: 10.1016/j.humpath.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26(2):271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo H, Sohn S, Nam BH, et al. The expressions of carbonic anhydrase 9 and vascular endothelial growth factor in astrocytic tumors predict a poor prognosis. Int J Mol Med. 2010;26(1):3–9. doi: 10.3892/ijmm_00000427. [DOI] [PubMed] [Google Scholar]
- 34.Loncaster JA, Harris AL, Davidson SE, et al. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61(17):6394–6399. [PubMed] [Google Scholar]
- 35.Pastorek J, Pastorekova S, Callebaut I, et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene. 1994;9(10):2877–2888. [PubMed] [Google Scholar]
- 36.Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24(3):425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 37.Svastova E, Hulikova A, Rafajova M, et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004;577(3):439–445. doi: 10.1016/j.febslet.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 38.Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene. 2010;29(50):6509–6521. doi: 10.1038/onc.2010.455. [DOI] [PubMed] [Google Scholar]
- 39.Chiche J, Ilc K, Laferriere J, et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69(1):358–968. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 40.Dubois L, Peeters S, Lieuwes NG, et al. Specific inhibition of carbonic anhydrase IX activity enhances the in vivo therapeutic effect of tumor irradiation. Radiother Oncol. 2011;99(3):424–431. doi: 10.1016/j.radonc.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 41.Schmaltz C, Hardenbergh PH, Wells A, Fisher DE. Regulation of proliferation-survival decisions during tumor cell hypoxia. Mol Cell Biol. 1998;18(5):2845–2854. doi: 10.1128/mcb.18.5.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gluszok S, Frederick R, Foulon C, et al. Design, solid-phase synthesis, and biological evaluation of novel 1,5-diarylpyrrole-3-carboxamides as carbonic anhydrase IX inhibitors. Bioorg Med Chem. 2010;18(21):7392–7401. doi: 10.1016/j.bmc.2010.09.007. [DOI] [PubMed] [Google Scholar]