Abstract
PDGFRA is a critical gene in glioma biology. Similar to EGFR, PDGFRA has been shown to be overexpressed, amplified, mutated, or truncated in gliomas, particularly glioblastomas. In addition, PDGFRA has been recently shown to be rearranged in glioblastoma. However, the frequency, cooccurrence, and clinical value of PDGFRA abnormalities in diffuse gliomas remain unclear. We investigated PDGFRA abnormalities and their clinical impact on 619 primary diffuse gliomas, including 167 grade II, 168 grade III, and 284 grade IV gliomas, with use of BAC-aCGH and validated our findings by quantitative polymerase chain reaction (PCR). We studied PDGFRA expression using reverse-transcription quantitative PCR in 84 gliomas and 12 non-tumor samples. In 138 samples, we also screened PDGFRA point mutations in exons 5, 7, 8, 9, 10, 11, and 23; presence of KDR-PDGFRA fusion gene; and PDGFRA truncation. PDGFRA was amplified and gained in 5.2% and 1.9% of samples, respectively. In addition PDGFRA was point-mutated, rearranged, and truncated in 2.9%, 0%, and 0.7% of cases, respectively. PDGFRA point mutations were observed exclusively in grade IV gliomas and in 12.5% of PDGFRA-amplified tumors. High-level PDGFRA amplification was associated with PDGFRA overexpression, high malignancy grade, and older patient age. Of interest, high-level PDGFRA amplification has an independent negative prognostic value for progression-free survival and overall survival among patients with grade III tumors. PDGFRA is altered through various genetic mechanisms in a subset of high-grade gliomas in patients who might be ideal candidates for PDGFRA inhibitor treatment, and PDGFRA gene amplification could be used as a prognostic biomarker in anaplastic gliomas.
Keywords: amplification, glioma; mutation; PDGFRA, prognosis
Gliomas are the most common primary brain tumors in adults.1 The WHO classifies diffuse gliomas based on the proliferating cell type (ie, astrocytoma, oligodendroglioma, or oligoastrocytoma) and the grade of malignancy (ie, from II to IV).2
Although significant progress has been made in the treatment of patients with glioma, this disease remains incurable. Over the past several years, important advancements in the understanding of molecular gliomagenesis have been accomplished, leading to new therapeutic perspectives.3 Indeed, multiple growth factor receptors with tyrosine kinase activity have been shown to participate in glioma tumorigenesis and are currently targetable by innovative drugs (eg, EGFR, PDGFRA, or VEGFR).4
Platelet-derived growth factor receptor A (PDGFRA) is the second most frequently mutated tyrosine kinase receptor, following EGFR, in glioblastomas (GBMs).4,5 PDGFRA is a transmembrane receptor with 5 immunoglobulin-like repeats in its extracellular domain and a tyrosine kinase (TK) in its intracellular domain. The binding of a ligand to the receptor activates pivotal downstream signal transduction pathways that promote oncogenesis, including MAP kinase, PI3K/AKT, JAK/STAT, and PLC-PKC.6
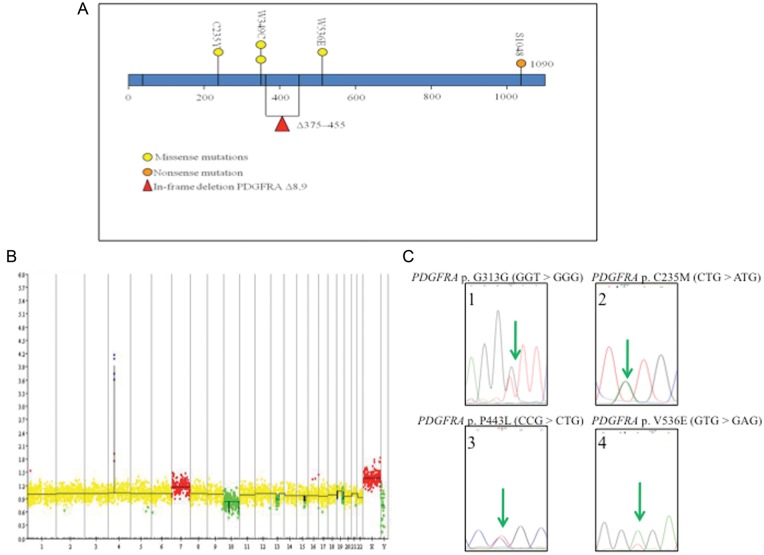
PDGFRA plays an important role in the normal development of the CNS by regulating normal glial cell proliferation and oligodendrocyte differentiation.7 PDGFRA has also been implicated in several cancers, including CNS malignancies.8 Indeed, several PDGFRA abnormalities have been detected in gliomas (eg, amplification, overexpression, in-frame deletion, point mutation, and rearrangement)4,5,9–15 (Fig. 1A).
Fig. 1.
Previously reported PDGFRA mutations in gliomas. (A) Representation of the previously described PDGFRA mutations according to the different protein domains. Missense mutations,5 nonsense mutations,16 and in-frame deletion.13–15 (B) BAC-aCGH of a GBM exhibiting PDGFRA amplification (green circle). (C) Chromatograms of PDGFRA point-mutations reported in glioma.
The clinical significance, prevalence, and co-occurrence of these PDGFRA abnormalities in the various glioma subcategories have not yet been determined. Indeed, these mutations have been studied mainly in GBM.4,13,14,16 This led us to conduct the present study to assess PDGFRA status in diffuse gliomas, because these abnormalities are currently candidate targets for innovative molecular therapies in personalized medicine.
Materials and Methods
Patients
The following inclusion criteria were used for patients and tumors in the present study: age ≥18 years at pathological diagnosis, histological diagnosis of diffuse glioma, primary tumor with no history of brain tumor, detailed clinical information at diagnosis and during follow-up, availability of paired blood and tumor samples, consent form for molecular analysis provided by the patient, and available PDGFRA gene copy number status determined via BAC-array based comparative genomic hybridization (BAC-aCGH).
On the basis of the aforementioned inclusion criteria, 619 patients were enrolled in the present study: (1) 167 WHO grade II gliomas (88 oligodendrogliomas, 59 oligoastrocytomas, and 20 astrocytomas); (2) 168 WHO grade III gliomas (27 astrocytomas, 70 oligodendrogliomas, and 71 oligoastrocytomas); and (3) 284 WHO grade IV gliomas (200 classic GBMs and 84 GBMs with an oligodendroglial component [GBMO]).
DNA Extraction and BAC-aCGH
DNA extraction was performed using the DNeasy Mini kit (Qiagen) according to the manufacturer's recommendations. DNA concentration and quality were determined spectrophotometrically (NanoDrop).
BAC-aCGH experiments were conducted as previously described.17 Fluorescence levels (cyanine-5–labeled tumor DNA/cyanine-3–labeled control DNA) were measured and processed using GLAD pipeline to determine the genomic status of each BAC (amplification, gain, balanced, hemizygous deletion, or homozygous deletion). Extreme cyanine 5/cyanine 3 ratios of ≥3 and ≤0.6 were considered to be suggestive of amplifications and homozygous deletions, respectively (Fig. 1B).
Validation of PDGFRA Copy Number Status Using Quantitative Polymerase Chain Reaction (qPCR)
PDGFRA-amplified tumors detected on BAC-aCGH were further confirmed using real-time qPCR analysis. The PDGFRA primers amplified a genomic fragment overlapping PDGFRA intron 15 and exon 15 (TaqMan PDGFRA Copy Number Assays [FAM Dye], Assay ID Hs02749151_cn; Applied Biosystems). The reference primers amplified a genomic fragment from RNase P (TaqMan RNase P Detection Reagents [HEX Dye], no. 4316831; Applied Biosystems).
RNA Extraction and PDGFRA Expression Using Reverse Transcription (RT) and qPCR
Total RNAs were isolated using the RNeasy Lipid Tissue Minikit (Qiagen) according to the manufacturer's recommendations. The RNA concentration was determined using spectrophotometer (NanoDrop). RNA was reverse transcribed using the SuperScript system (Invitrogen) according to the manufacturer's protocol. RNA quality was assessed using Agilent RNA Nano 6000 LabChip kits and an Agilent 2100 Bioanalyzer (minimum RNA integrity number of 7).
RT-qPCR was performed using a Universal Probe Library (Roche Applied Science).18 The primers are reported in Table 1. cDNA was synthesized from 1 µg of total RNA and used for qPCR. Thermal cycling protocol was 10 min at 95°C, denaturation for 10 s at 95°C, annealing for 30 s at 60°C, and extension for 1 s at 72°C for 45 cycles, followed by 10 s at 40°C of cooling using the LightCycler 480 real-time PCR system. Data were expressed as ΔCt values [ΔCt = Ct of the target gene (PDGFRA) – Ct of the control gene (PPIA)]. The 2-ΔΔCt equation was used to determine the fold expression changes relative to nontumor tissues.
Table 1.
Primers used for qPCR testing PDGFRA mRNA expression level
| Gene | Forward or Reverse | Sequence | UPL probe |
|---|---|---|---|
| PDGFRA | F | AGGTGGTTGACCTTCAATGG | #80 |
| R | TTTGATTTCTTCCAGCATTGTG | ||
| PPIA | F | CCTAAAGCATACGGGTCCTG | #47 |
| R | TTTCACTTTGCCAAACACCA |
Abbreviations: qPCR, quantitative PCR; PDGFRA, platelet-derived growth factor receptor alpha; PPIA, peptidylprolyl isomerase A (cyclophilin A); F, forward; R, reverse; UPL, Universal Probe Library.
PDGFRA mutations
PDGFRA-amplified tumors were screened for point mutations in exons 5, 7, 8, 9, 10, 11, and 23, as described previously.19 The primers are reported in Table 2.
Table 2.
Primers used for PDGFRA Sanger sequencing
| Exon | F or R | Sequence |
|---|---|---|
| 5 | F | TGTAAAACGACGGCCAGTCCACTGCTGAGGAATGCGGT |
| R | CAGGAAACAGCTATGACCTCCCAGAGGTGGGAAGCTAAGG | |
| 7 | F | TGTAAAACGACGGCCAGTGGCAAAGGCATCACAATGCTG |
| R | CAGGAAACAGCTATGACCAGGGATGTGATTTCAAGCATCTCTTA | |
| 8 | F | TGTAAAACGACGGCCAGTGTCAGGCCGTGGCTGAACTG |
| R | CAGGAAACAGCTATGACCAAAGTGCCAGGCTTTCCTTGG | |
| 9 | F | TGTAAAACGACGGCCAGTTGCTGCTAACCATGTGGGTCTG |
| R | CAGGAAACAGCTATGACCGAGCCCTGGACCTTCTTCTCACA | |
| 10 | F | TGTAAAACGACGGCCAGTGAGAAGGGAGGGCTCCAGGC |
| R | CAGGAAACAGCTATGACCTTTGGCGAAAGTCACACGGC | |
| 11 | F | TGTAAAACGACGGCCAGTAGGCAGCCCTCACACTTCCC |
| R | CAGGAAACAGCTATGACCCAGCTGCATCGGGTCCACAT | |
| 23 | F | TGTAAAACGACGGCCAGTTCCACGTGGCCTACCACAGC |
| R | CAGGAAACAGCTATGACCCACACCACTGAGATGCTACTGAGGC |
Abbreviations: PDGFRA, platelet-derived growth factor receptor alpha; F, forward; R, reverse.
Screening for PDGFRAΔ8,9 Mutants and for KDR-PDGFRA Fusions (KP Gene)
RNA amplified from PDGFRA was reverse transcribed to search for truncated genes that lacked exons 8 and 9. The same samples were screened for the KP fusion gene. All primers and PCR conditions were identical to those described previously.13,14 In brief, PCR amplification was performed using FastStart Taq DNA polymerase (Roche) in a thermocycling program consisting of 3 min at 94°C, followed by 30 cycles of denaturation for 45 s at 94°C, annealing for 30 s at 55°C, and extension for 90 s at 72°C, followed by a 10 min final extension. To validate these findings, the obtained cDNA was sequenced.
IDH1 and IDH2 Mutational Status
IDH1 and IDH2 mutations in the hotspot codons R132 and R172, respectively, were assessed by bidirectional cycle sequencing of PCR-amplified primers, as previously described.20
Chromosome Arm 1p/19q Codeletion Was Detected Using BAC-aCGH and Validated Using Microsatellite Analysis
Chromosome arms 1p/19q status was assessed using BAC-aCGH and validated by microsatellites polymorphisms analysis using the following markers, as described previously: D1S450, D1S2667, D1S234, D1S2890, D1S2841, D19S425, D19S219, D19S412, and D19S418.21
Statistical analysis
For univariate analysis, χ2 or Fisher's exact, and Mann-Whitney U tests were used to compare categorical and continuous variables, respectively. PDGFRA expression level was compared among nontumor tissues, nonamplified tumors, and PDGFRA-amplified tumors with use of the nonparametric Kruskal-Wallis test. The Spearman's rank order correlation test was used to analyze the relationship between PDGFRA amplification and other alterations (truncation, point mutation, and expression). A Spearman's ρ value of −1 indicated perfect inverse/negative correlation, and a value of 1 indicated perfect/positive correlation. The agreement between BAC-aCGH and microsatellite analysis of 1p19q codeletion detection and between BAC-aCGH and qPCR for PDGFRA amplification was assessed using Fleiss κ statistics. A κ value of <0 indicated poor agreement, whereas a κ value of 0.81–1.00 indicated almost perfect agreement.22
Overall survival (OS) and progression-free survival (PFS) were evaluated from diagnosis to death and to the first clinical and/or radiological documentation of progression, respectively. All patients without evidence of relapse at the time of the last clinical visit were censored. OS and PFS were estimated using Kaplan-Meier methodology and compared using the log-rank test. A Cox proportional hazards model was used for the multivariate analysis. Two-sided P values <.05 were interpreted as statistically significant. Analyses were performed in R, version 2.13.1 (http://www.R-project.org).
Results
Patient and Tumor Characteristics
From 2005 through 2009, 619 diffuse gliomas were characterized using BAC-aCGH (from 345 men and 274 women; sex ratio, 1.3). The median age of the study population was 49.5 years (interquartile range, 37.2–61.2 years). The clinical characteristics of patients with PDGFRA-amplified and nonamplified diffuse gliomas are shown in Table 3. All patients provided their written consent for molecular analysis.
Table 3.
Patients and tumors characteristics
| Characteristics | PDGFRA Amplified (%) | PDGFRA Non-amplified (%) | P |
|---|---|---|---|
| No. of tumors (619) | 32 (5.2) | 587 (94.8) | |
| Age at diagnosis | |||
| Median (years) | 58.8 | 48.7 | 0.0002 |
| Interquartile range | 51.2–68.6 | 37–60.9 | |
| Gender | |||
| Female (274) | 16 (5.8) | 258 (94.2) | NS |
| Male (345) | 16 (4.6) | 329 (95.4) | |
| Tumor WHO grade | |||
| Grade II (167) | 0 | 167 (100) | 8.032e-06 |
| Grade III (168) | 6 (3.6) | 162 (96.4) | |
| Grade IV (284) | 26 (9.1) | 258 (90.9) | |
| Tumor phenotype | |||
| Astrocytoma (248) | 18 (7.3) | 230 (92.7) | NS |
| Oligoastrocytoma (130) | 3 (2.3) | 127 (97.7) | |
| Oligodendroglioma (including GBMO) (241) | 11 (4.6) | 230 (95.4) | |
Abbreviations: NS, not significant; WHO, World Health Organization; GBMO, glioblastoma with an oligodendroglial component.
PDGFRA High-Level Amplification and Gain
PDGFRA high-level amplification and gain were evaluated using BAC-aCGH validated by qPCR (κ = 0.89; 95% confidence interval [CI], 0.69–1.00); these events were detected in 32 (5.2%) of 619 and 12 (1.9%) of 619 cases, respectively (Fig. 1B).
Correlation with Tumor Pathology
Both PDGFRA high-level amplification and gain were detected in 0 (0%) of 167 WHO grade II, 6 (3.6%) of 168 WHO grade III, and 26 (9.15%) of 284 WHO grade IV gliomas (Table 3). In addition, PDGFRA gain was detected in 1 (0.6%) of 167 WHO grade II, 2 (1.1%) of 168 WHO grade III, and 9 (3.1%) of 284 WHO grade IV gliomas.
PDGFRA high-level amplification was not associated with tumor phenotype (18 of 248, 3 of 127, and 11 of 230 of astrocytic, oligodendrocytic, and oligoastrocytic gliomas, respectively; P = 0.1) (Table 3), even when GBMO were analyzed as oligodendrocytic tumors. PDGFRA high-level amplification was strongly associated with a high grade of malignancy (P = 8.032e-06) (Table 3). Likewise, PDGFRA gain was also associated with a high grade of malignancy (P = 6.793e-06).
Correlation with Clinical Characteristics and Outcome of Patients
Patients with PDGFRA-amplified tumors were significantly older (59.8 years vs 48.7 years; P = .0002). No statistically significant association with patient sex was observed (Table 3).
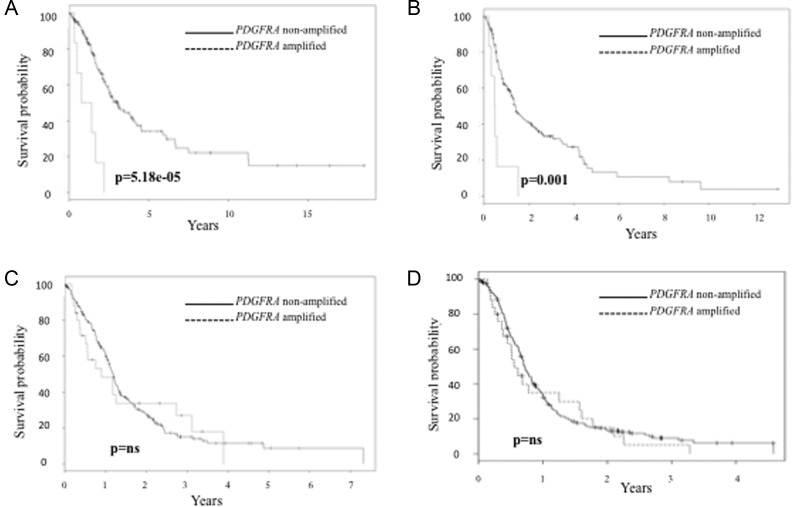
In WHO grade III gliomas, PDGFRA amplification had a negative impact on OS and PFS (P = 5.18e-05, Fig. 2A; P = .001, Fig. 2B) and had an independent prognostic value in multivariate analysis that included chromosome arm 1p/19q status and IDH mutation (Tables 4 and 5). Both IDH mutation and 1p/19q codeletion were associated with longer PFS and OS in univariate and multivariate analysis (Tables 4 and 5). The agreement between BAC-aCGH and microsatellite analyses was very good (κ = 0.91; 95% CI, 0.78–1.00). Chromosome arms 1p/19q codeletion was observed in 22% of WHO grade III tumors.
Fig. 2.
Prognostic value of PDGFRA abnormalities in gliomas (continuous line indicates PDGFRA- non amplified tumors and broken line indicates PDGFRA amplified tumors). (Panel A). OS in PDGFRA amplified vs nonamplified WHO III gliomas. (Panel B). PFS in PDGFRA amplified vs nonamplified tumors in WHO grade III gliomas. (Panel C). OS in PDGFRA amplified vs nonamplified WHO IV gliomas. Panel d. PFS in PDGFRA amplified vs nonamplified tumors in WHO grade IV gliomas.
Table 4.
Univariate and multivariate analysis of progression free survival in WHO III tumors
| Variable | n | Unvariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| median, years (95%CI) | P value | HR (95%CI) | P value | |||
| Sex | Women | 77 | 3.8 (2.4–5.2) | NS | ||
| Men | 91 | 2.2 (1.5–2.9) | ||||
| Age | <45 | 83 | 1.7 (0.8–2.6) | .006 | 1 | NS |
| ≥45 | 85 | 3 (2–4.1) | 1.6 (0.9–2.6) | |||
| KPS | ≥70 | 9 | 1.3 (0.2–2.5) | .04 | 1 | NS |
| <70 | 150 | 0.8 (0.2–1.3) | 1.8 (0.8–4) | |||
| Surgery | Biopsy | 60 | 2.1 (1.5–2.7) | 4e-04 | 1 | NS |
| Resection | 100 | 3.8 (2.7–4.9) | 1.4 (0.8–2.3) | |||
| Phenotype | Astrocytoma | 27 | 1.4 (1.1–1.7) | NS | ||
| Oligodendroglioma | 70 | 1.3 (0.8–1.8) | ||||
| Oligoastrocytoma | 71 | 1.3 (0.9–1.8) | ||||
| Treatment | RT alone | 40 | 1.2 (0.4–2.6) | NS | ||
| All other regimens | 128 | 1.0 (0.5–2.1) | ||||
| PDGFRA amplification | No | 162 | 1.4 (1.1–1.7) | .001 | 1 | .004 |
| Yes | 6 | 1.3 (1.1–1.5) | 4.1 (1.5–10.7) | |||
| IDH mutation | No | 66 | 0.7 (0.5–1) | 1e-09 | 1 | .001 |
| Yes | 90 | 3 (1.7–4.4) | 0.3 (0.2–0.7) | |||
| 1p19q codeletion | No | 131 | 0.9 (0.6–1.2) | 3.6e-05 | 1 | .035 |
| Yes | 37 | 3.5 (2–4.9) | 0.5 (0.2–0.9) | |||
Abbreviations: KPS, Karnofsky Performance Status; PDGFRA, platelet-derived growth factor receptor alpha; IDH 1 and 2, isocitrate dehydrogenase 1 and 2; Oligodendroglial includes oligodendroglioma and oligoastrocytoma.
Table 5.
Univariate and multivariate analysis of overall survival in WHO III tumors
| Variable | n | Unvariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| median, years (95%CI) | P value | HR (95%CI) | P value | |||
| Sex | Women | 77 | 2.3 (1.4–3.1) | NS | ||
| Men | 91 | 1.5 (0.8–2.9) | ||||
| Age | <45 | 83 | 2.2 (1.2–3.3) | .0004 | 1 | .004 |
| ≥45 | 85 | 1.6 (0.9–2.9) | 2.4 (1.3–4.3) | |||
| KPS | ≥70 | 9 | 3 (2–4.1) | .0064 | 1 | .01 |
| <70 | 150 | 1.5 (0.8–1.7) | 2.9 (1.3–7) | |||
| Surgery | Biopsy | 60 | 2 (1.5–2.6) | .0004 | 1 | NS |
| Resection | 100 | 3.8 (2.7–4.8) | 1.09 (0.6–1.9) | |||
| Phenotype | Astrocytoma | 27 | 2.5 (1.7–3.4) | NS | ||
| Oligodendroglioma | 70 | 3.1 (1.4–4.8) | ||||
| Oligoastrocytoma | 71 | 2.5 (2.2–2.9) | ||||
| Treatment | RT alone | 40 | 2.7 (1.7–4.6) | NS | ||
| All other regimens | 128 | 1.6 (0.8–2.8) | ||||
| PDGFRA amplification | No | 162 | 3.0 (2.2–3.8) | 5.18e-05 | 1 | .0003 |
| Yes | 6 | 0.6 (0.1–1.9) | 5.9 (2.2–15.6) | |||
| IDH mutation | No | 66 | 1.5 (1.2–1.8) | 1.1e-12 | 1 | .004 |
| Yes | 90 | 6.6 (2.9–10.5) | 0.4 (0.2–0.7) | |||
| 1p19q codeletion | No | 131 | 2.2 (1.8–2.5) | 2.68e-07 | 1 | .002 |
| Yes | 37 | 11.2 (2.45–20) | 0.2 (0.09–0.59) | |||
Abbreviations: PDGFRA, platelet-derived growth factor receptor alpha; IDH 1 and 2, isocitrate dehydrogenase 1 and 2; Oligodendroglial includes oligodendroglioma and oligoastrocytoma
In WHO grade IV tumors, PDGFRA amplification does not have any prognostic significance in terms of OS and PFS (Fig. 2C and D).
PDGFRA Mutations
Four of the 32 PDGFRA-amplified tumors (12.5%) exhibited somatic point mutations in PDGFRA. All tumors with point mutations were WHO grade IV. One silent mutation, a G > T transversion at codon G313 (GGT > GGG) (Fig. 1C, Panel 1), was detected. The other 3 were missense mutations: 1 at codon C235M (CTG > ATG) (Fig. 1C, Panel 2), 1 at codon P443L (CCG > CTG) (Fig. 1C, Panel 3), and 1 at codon V536E (GTG > GAG) (Fig. 1C, Panel 4). Each point mutation occurred in different samples, and all have been reported previously.5 Of interest, among WHO grade IV tumors, PDGFRA mutation was observed more frequently in GBMO, although this difference in frequency did not reach statistical significance (75% vs 24%; P = 0.07).
Screening for PDGFRA Truncation and KDR-PDGFRA Fusion in PDGFRA-Amplified Samples
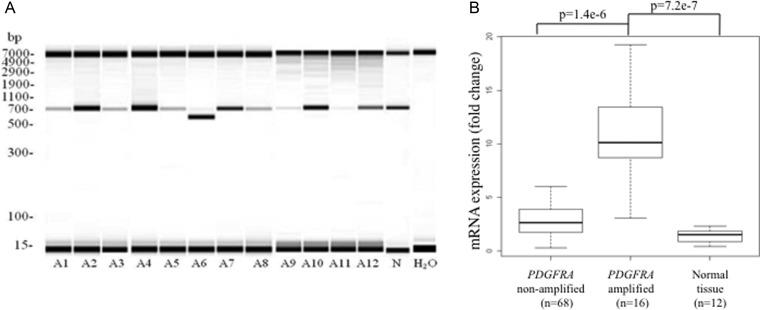
PDGFRA truncation (PDGFRAΔ8,9) was observed in 1 of the 12 PDGFRA-amplified tumors (Fig. 3A) and validated by direct sequencing. None of our PDGFRA-amplified tumors contained the fusion gene KDR-PDGFRA.14
Fig. 3.
(A) Reverse-Transcription PCR from total RNA searching for truncated PDGFRA transcript (PDGFRAΔ8,9). The low weight molecular band (ie, 642 base pairs) and the high weight molecular band (885 base pairs) indicate PDGFRA wild-type and truncated PDGFRA transcripts (ie, PDGFRAΔ8,9) respectively. Each line represents a distinct PDGFRA-amplified glioblastoma. PDGFRAΔ8,9 truncation was detected in 1/12 PDGFRA-amplified glioblastoma (line A6). Line “N” indicates a glioblastoma with normal copy number of PDGFRA. H20 indicates water as a negative control of the experiment. (B) mRNA expression of PDGFRA according to PDGFRA copy number status (amplified, nonamplified and normal brain tissue).
PDGFRA mRNA Expression Analysis
The expression level of PDGFRA mRNA in 16 PDGFRA-amplified tumors (all WHO grade IV) was compared with that in 68 nonamplified tumors (including 20 WHO grade IV, 26 WHO grade III, and 22 WHO grade II) and 12 nontumor brain tissues (epilepsy samples). Of interest, tumors harboring PDGFRA amplification exhibited an increased level of PDGFRA mRNA expression compared with their nonamplified counterparts and with nontumor brain tissue (P = 1.4e-6 and P = 1.5e-7, respectively) (Fig. 3B).
PDGFRA Molecular Abnormalities in PDGFRA Nonamplified Gliomas
We studied a cohort of 106 PDGFRA non-amplified glial tumors, including 32 low-grade gliomas (10 astrocytomas, 12 oligodendrogliomas, and 10 oligoastrocytomas); 32 anaplastic gliomas (6 astrocytomas, 18 oligodendrogliomas, and 8 oligoastrocytomas); and 42 GBMs. None of them was PDGFRA-point-mutated, PDGFRA-truncated, or KDR-PDGFRA-rearranged.
Correlation Between PDGFRA Amplification and PDGFRA Mutation, Rearrangement, IDH Mutation, and 1p/19q Codeletion
PDGFRA amplification was strongly associated with the PDGFRA expression level (Fig. 3B). In addition, an inverse correlation was observed between mutation and truncation. PDGFRA amplification was about twice more frequent in the mutated samples than in the truncated samples.
PDGFRA amplification and IDH mutation were inversely correlated; 12.9% of PDGFRA-amplified tumors were IDH mutated, compared with 2.6% of nonamplified tumors (P = 0.001). In addition, tumors featuring PDGFRA locus gain were less frequently IDH mutated, compared with tumors with normal PDGFRA copy number status (16.7% vs 43.2%; P = .08). Moreover, the presence of 1p/19q codeletion and PDGFRA amplification were mutually exclusive in WHO grade III tumors (0% of 1p/19q codeleted tumors were PDGFRA-amplified vs 4.6% of non-codeleted; P = .03).
Discussion
PDGFRA is a critical gene in neurogenesis and gliomagenesis. To our knowledge, this study represents the largest cohort of diffuse gliomas and the most comprehensive analysis of the prevalence, the clinicopathological significance, and the co-occurrence of the PDGFRA abnormalities most frequently reported in gliomas. Of interest, PDGFRA high-level amplification has an independent negative prognostic value in grade III tumors in terms of PFS and OS.
In our study, PDGFRA amplification was restricted to high-grade gliomas and was not observed in diffuse low-grade gliomas. A previous study has described PDGFRA amplification in low-grade gliomas (this study found amplification in 6 of 20 low-grade gliomas, but 83% of amplified tumors were astrocytomas).9 Our series included 20 WHO grade II astrocytomas, none of which were PDGFRA amplified. These conflicting results require further investigation to specify the frequency of PDGFRA amplification in low-grade gliomas (Table 6).
Table 6.
Frequency and prognostic significance of PDGFRA alterations in gliomas
| PDGFRA alteration | n | Histology and grade |
Prognostic impact | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AI | AII | OII | MII | AIII | OIII | MIII | GBM | ||||
| Point Mutation | 91 | 1/91 | 4 | ||||||||
| 116 | 4/116 | NP | 5 | ||||||||
| 86† | NP | 9 | |||||||||
| Rearrangement (truncation) | 212 | 6/212 (6/15*) | NP | 14 | |||||||
| Rearrangement (fusion KP) | 212 | 1/212 | NP | ||||||||
| Amplification | 206 | 23/206 | NP | 4 | |||||||
| 170 | 44/170 | NP | 5 | ||||||||
| 57 | 0/1 | 5/10 | 1/10 | 0/2 | 2/13 | 1/2 | 3/19 | No association with survival | 9 | ||
| 87 | 0/1 | 0/11** | 0/12** | 15/63 | NP | 14 | |||||
| 47 | 9/47 | No association with survival | 24 | ||||||||
| 78 | 1/35 | 0/19 | 0/9 | 5/15 | Poorer prognosis in univariate analysis in the overall population (WHOII and WHOIII gliomas) | 25 | |||||
| 390 | 33/390 | No association with survival | 26 | ||||||||
Abbreviations: PDGFRA. platelet-derived growth factor receptor alpha; †histology and grade was not described, 4 silent mutations out of 86 gliomas.
*PDGFRA amplified samples; **phenotype is not specified; KP. KDR-PDGFRA fusion gene; NP. not performed; A. astrocytoma; O. oligodendroglioma; M. mixed glioma or oligoastrocytoma; GBM. glioblastoma multiforme; I. WHO grade I; II. WHO grade II; III. WHO grade III; IV. WHO grade IV.
Previous studies have reported that PDGFRA amplification occurs in 8.5%–26% of GBMs.4,5,9,14 This wide range might be attributed to distinct definitions of gene amplification. Taken together, the published data and our results suggest that PDGFRA high-level amplification is observed in approximately 10% of GBMs (Table 6).4,14
PDGFRA amplification is strongly correlated with deleterious effects in WHO III gliomas in terms of PFS and OS. These results are consistent with our previous study, which focused on anaplastic oligodendrogliomas.23 This prognostic value of PDGFRA amplification is independent of age, KPS, type of surgery, IDH mutation, and 1p/19q codeletion. In our series of WHO grade III, tumor phenotype (ie, astrocytoma, oligoastrocytoma, and oligodendroglioma) does not impact significantly patients' survival although a trend is observed. The prognostic value of phenotype in anaplastic oligodendroglial tumors is debated, and some discrepancies might be at least partially explained by some variability in neuropathologic classification.24 Similarly, intra- and interobserver variability in pathologic diagnosis might induce some variability in the prevalence of 1p/19q codeletion in anaplastic oligodendroglial tumors. Our results are at the lower limit of the range of the prevalence reported in the literature.25,26
Techniques used for 1p/19q codeletion testing in anaplastic oligodendroglial tumors might also explain variability in the prevalence of 1p/19q codeletion. Indeed, techniques testing a large number of genomic loci simultaneously (eg, comparative genomic hybridization or multiplex ligation-dependent probe amplification), compared with the techniques investigating a limited number of loci (eg, FISH or microsatellites analysis), allow a better distinction of whole chromosome arms 1p/19q codeletion vs partial 1p/19q codeletion.27,28 The former alteration corresponding to a reciprocal unbalanced translocation t(1;19)(q10;p10) is actually the favorable prognostic biomarker in anaplastic oligodendroglial tumors.29,30 Finally, the design of studies (eg, retrospective), because of biases issues, might also explain some variability in biomarkers assessment in oligodendroglial tumors.
No prognostic value for PDGFRA amplification was detected in WHO grade IV gliomas. Indeed, the prognostic significance of PDGFRA amplification has been debated in the literature (Table 6). Previous studies did not associate PDGFRA amplification with worse prognosis in any histological entity.31 However, Pupitti et al. showed that PDGFRA amplification was associated with worse prognosis in a univariate analysis in gliomas.32 Conversely, Nobusawa et al. studied 390 GBMs and did not find an association between the amplification of PDGFRA and survival.33 As is the case for EGFR amplification, PDGFRA amplification seems not to have a prognostic value in GBM (Table 6).34
The prognostic value of PDGFRA mutations in gliomas has not been established previously (Table 6).9,31,35 Similarly, the KP-fusion gene has been described only recently,14 and there are no studies analyzing the clinical significance of these mutations. Unfortunately, we did not identify any sample with a KP-fusion gene; this seems to be a rare phenomenon in glioma.
Our series focused on PDGFRA-amplified gliomas because PDGFRA truncation and rearrangement has been described as unique to this group.14 Whether the percentage of PDGFRA mutations in nonamplified tumors is similar to that in amplified tumors remains unknown, with the exception of PDGFRA deletion, 8,9 which has been detected exclusively in PDGFRA amplified gliomas.14 In a series of 106 PDGFRA nonamplified gliomas, we did not find any PDGFRA mutation or rearrangement. In our series, the percentage of gliomas with truncations is much lower than that previously reported (8.3% vs 40%).14
We found a statistically significant association between PDGFRA amplification and the overexpression of PDGFRA mRNA (P = 0.045). In previous studies, the correlation between PDGFRA expression and copy number status was evaluated via immunohistochemistry.9,36–38 These studies found that PDGFRA was overexpressed in approximately 50% of malignant gliomas.9,36–38 In addition, the prognostic value of PDGFRA expression remains hotly debated.9,36–38
PDGFRA is a critical gene in glioma and particularly in GBM. It is amplified in approximately 10% of GBMs, but PDGFRA amplification has poor independent prognostic value in anaplastic gliomas. Taken together, our data and those reported in previous studies highlight PDGFRA alterations as a potential prognostic biomarker and a therapeutic target. Indeed, multiple PDGFR inhibitors are under development in academic and industrial laboratories.39,40 To date, some have been tested in humans with limited success, but the patients in these trials were not selected on the basis of PDGFRA status.41,42 PDGFRA truncation appears as a rare phenomenon in diffuse gliomas. Indeed, it is observed in 8%–40% of PDGFRA-amplified GBMs, which means in approximately 0.8%–4% of all GBMs. These results do not support the development of a vaccine therapy against PDGFRAΔ,8,9 similar to that developed for EGFRvIII observed in ∼20% of all GBM. However, further studies are warranted to specify the exact prevalence of this abnormality in various normal tissues, diffuse gliomas, and other cancer types.43,44
Funding
This work was supported by Obra Social la Caixa (to A.A.). This work is part of the national program Cartes d'Identité des Tumeurs (http://cit.ligue-cancer.net/), which is funded and developed by the Ligue Nationale Contre le Cancer.
Acknowledgments
Institut Hospitalier Universitaire de Neurosciences Translationnelles de Paris.
Conflict of interest statement. None declared.
References
- 1.Central Brain Tumor Registry of the United States. Available at http://www.CBTRUS.org . Accessed 12 December, 2011. [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 7.Richardson WD, Pringle N, Mosley MJ, Westermark B, Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988;53(2):309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- 8.Shih AH, Holland EC. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006;232(2):139–147. doi: 10.1016/j.canlet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Martinho O, Longatto-Filho A, Lambros MBK, et al. Expression, mutation and copy number analysis of platelet-derived growth factor receptor A (PDGFRA) and its ligand PDGFA in gliomas. Br J Cancer. 2009;101(6):973–982. doi: 10.1038/sj.bjc.6605225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermanson M, Funa K, Hartman M, et al. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52(11):3213–3219. [PubMed] [Google Scholar]
- 11.Nistér M, Libermann TA, Betsholtz C, et al. Expression of messenger RNAs for platelet-derived growth factor and transforming growth factor-alpha and their receptors in human malignant glioma cell lines. Cancer Res. 1988;48(14):3910–3918. [PubMed] [Google Scholar]
- 12.Di Rocco F, Carroll RS, Zhang J, Black PM. Platelet-derived growth factor and its receptor expression in human oligodendrogliomas. Neurosurgery. 1998;42(2):341–346. doi: 10.1097/00006123-199802000-00080. [DOI] [PubMed] [Google Scholar]
- 13.Kumabe T, Sohma Y, Kayama T, Yoshimoto T, Yamamoto T. Amplification of alpha-platelet-derived growth factor receptor gene lacking an exon coding for a portion of the extracellular region in a primary brain tumor of glial origin. Oncogene. 1992;7(4):627–633. [PubMed] [Google Scholar]
- 14.Ozawa T, Brennan CW, Wang L, et al. PDGFRA gene rearrangements are frequent genetic events in PDGFRA-amplified glioblastomas. Genes Dev. 2010;24(19):2205–2218. doi: 10.1101/gad.1972310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke ID, Dirks PB. A human brain tumor-derived PDGFR-alpha deletion mutant is transforming. Oncogene. 2003;22(5):722–733. doi: 10.1038/sj.onc.1206160. [DOI] [PubMed] [Google Scholar]
- 16.Rand V, Huang J, Stockwell T, et al. Sequence survey of receptor tyrosine kinases reveals mutations in glioblastomas. Proc Natl Acad Sci USA. 2005;102(40):14344–14349. doi: 10.1073/pnas.0507200102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idbaih A, Marie Y, Lucchesi C, et al. BAC array CGH distinguishes mutually exclusive alterations that define clinicogenetic subtypes of gliomas. Int J Cancer. 2008;122(8):1778–1786. doi: 10.1002/ijc.23270. [DOI] [PubMed] [Google Scholar]
- 18.Mouritzen P, Noerholm M, Nielsen PS, et al. ProbeLibrary: A new method for faster design and execution of quantitative real-time PCR. Nature Methods. 2005;2:313–316. [Google Scholar]
- 19.Sihto H, Sarlomo-Rikala M, Tynninen O, et al. KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol. 2005;23(1):49–57. doi: 10.1200/JCO.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 20.Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75(17):1560–1566. doi: 10.1212/WNL.0b013e3181f96282. [DOI] [PubMed] [Google Scholar]
- 21.Hoang-Xuan K, Capelle L, Kujas M, et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22(15):3133–3138. doi: 10.1200/JCO.2004.10.169. [DOI] [PubMed] [Google Scholar]
- 22.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 23.Idbaih A, Crinière E, Marie Y, et al. Gene amplification is a poor prognostic factor in anaplastic oligodendrogliomas. Neuro Oncol. 2008;10(4):540–547. doi: 10.1215/15228517-2008-022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician's perspective. Acta Neuropathol. 2010;120(3):297–304. doi: 10.1007/s00401-010-0725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24(18):2715–2722. doi: 10.1200/JCO.2005.04.6078. [DOI] [PubMed] [Google Scholar]
- 26.Cairncross G, Berkey B, Shaw E, Jenkins R, Scheithauer B, et al. Intergroup Radiation Therapy Oncology Group Trial 9402. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24(18):2707–2714. doi: 10.1200/JCO.2005.04.3414. [DOI] [PubMed] [Google Scholar]
- 27.Idbaih A, Marie Y, Pierron G, et al. Two types of chromosome 1p losses with opposite significance in gliomas. Ann Neurol. 2005;58(3):483–487. doi: 10.1002/ana.20607. [DOI] [PubMed] [Google Scholar]
- 28.Jeuken J, Cornelissen S, Boots-Sprenger S, Gijsen S, Wesseling P. Multiplex ligation-dependent probe amplification: a diagnostic tool for simultaneous identification of different genetic markers in glial tumors. J Mol Diagn. 2006;8(4):433–443. doi: 10.2353/jmoldx.2006.060012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffin CA, Burger P, Morsberger L, et al. Identification of der(1;19)(q10;p10) in five oligodendrogliomas suggests mechanism of concurrent 1p and 19q loss. J Neuropathol Exp Neurol. 2006;65(10):988–994. doi: 10.1097/01.jnen.0000235122.98052.8f. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins RB, Blair H, Ballman KV, et al. A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res. 2006;66(20):9852–9861. doi: 10.1158/0008-5472.CAN-06-1796. [DOI] [PubMed] [Google Scholar]
- 31.Joensuu H, Puputti M, Sihto H, Tynninen O, Nupponen NN. Amplification of genes encoding KIT, PDGFRalpha and VEGFR2 receptor tyrosine kinases is frequent in glioblastoma multiforme. J Pathol. 2005;207(2):224–231. doi: 10.1002/path.1823. [DOI] [PubMed] [Google Scholar]
- 32.Puputti M, Tynninen O, Sihto H, et al. Amplification of KIT, PDGFRA, VEGFR2, and EGFR in gliomas. Mol Cancer Res. 2006;4(12):927–934. doi: 10.1158/1541-7786.MCR-06-0085. [DOI] [PubMed] [Google Scholar]
- 33.Nobusawa S, Stawski R, Kim Y-H, Nakazato Y, Ohgaki H. Amplification of the PDGFRA, KIT and KDR genes in glioblastoma: a population-based study. Neuropathology. 2011;31(6):583–588. doi: 10.1111/j.1440-1789.2011.01204.x. [DOI] [PubMed] [Google Scholar]
- 34.Quan AL, Barnett GH, Lee SY, et al. Epidermal growth factor receptor amplification does not have prognostic significance in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2005;63(3):695–703. doi: 10.1016/j.ijrobp.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 35.Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99–08. Clin Cancer Res. 2006;12(16):4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 36.Varela M, Ranuncolo SM, Morand A, et al. EGF-R and PDGF-R, but not bcl-2, overexpression predict overall survival in patients with low-grade astrocytomas. J Surg Oncol. 2004;86(1):34–40. doi: 10.1002/jso.20036. [DOI] [PubMed] [Google Scholar]
- 37.Ribom D, Andrae J, Frielingsdorf M, Hartman M, Nistér M, Smits A. Prognostic value of platelet derived growth factor alpha receptor expression in grade 2 astrocytomas and oligoastrocytomas. J Neurol Neurosurg Psychiatr. 2002;72(6):782–787. doi: 10.1136/jnnp.72.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulsson J, Lindh MB, Jarvius M, et al. Prognostic but not predictive role of platelet-derived growth factor receptors in patients with recurrent glioblastoma. Int J Cancer. 2011;128(8):1981–1988. doi: 10.1002/ijc.25528. [DOI] [PubMed] [Google Scholar]
- 39.Haberler C, Gelpi E, Marosi C, et al. Immunohistochemical analysis of platelet-derived growth factor receptor-alpha, -beta, c-kit, c-abl, and arg proteins in glioblastoma: possible implications for patient selection for imatinib mesylate therapy. J Neurooncol. 2006;76(2):105–109. doi: 10.1007/s11060-005-4570-9. [DOI] [PubMed] [Google Scholar]
- 40.Bareford MD, Park MA, Yacoub A, et al. Sorafenib enhances pemetrexed cytotoxicity through an autophagy-dependent mechanism in cancer cells. Cancer Res. 2011;71(14):4955–4967. doi: 10.1158/0008-5472.CAN-11-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neyns B, Sadones J, Chaskis C, et al. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol. 2011;103(3):491–501. doi: 10.1007/s11060-010-0402-7. [DOI] [PubMed] [Google Scholar]
- 42.Iwamoto FM, Lamborn KR, Robins HI, et al. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06–02) Neuro Oncol. 2010;12(8):855–861. doi: 10.1093/neuonc/noq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moscatello DK, Ramirez G, Wong AJ. A naturally occurring mutant human epidermal growth factor receptor as a target for peptide vaccine immunotherapy of tumors. Cancer Res. 1997;57(8):1419–1424. [PubMed] [Google Scholar]
- 44.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]