Abstract
Malignant gliomas are associated with high morbidity and mortality because they are highly invasive into surrounding brain tissue, making complete surgical resection impossible. Osteopontin is abundantly expressed in the brain and is involved in cell adhesion, migration, and invasion. The aim of the present study was to investigate the mechanisms of glioma cell migration. Migration and invasion activity were determined by transwell and wound-healing assays. Gene and protein expressions were analyzed by reverse transcription–PCR, real time–PCR, and Western blotting. Nrf2-DNA binding activity was determined by electrophoretic mobility shift assay. Establishment of migration-prone sublines were performed to select highly migratory glioma. An intracranial xenograft mouse model was used for the in vivo study. Application of recombinant human osteopontin enhanced the migration of glioma cells. Expression of heme oxygenase (HO)–1 mRNA and protein also increased in response to osteopontin stimulation. Osteopontin-induced increase in cell migration was antagonized by HO-1 inhibitor or HO-1 small interfering (si)RNA. Osteopontin-mediated HO-1 expression was reduced by treatment with MEK/ERK and phosphatidylinositol 3-kinase/Akt inhibitors, as well as siRNA against Nrf2. Furthermore, osteopontin stimulated Nrf2 accumulation in the nucleus and increased Nrf2-DNA binding activity. In migration-prone sublines, cells with greater migration ability had higher osteopontin and HO-1 expression, and zinc protoporphyrin IX treatment could effectively reduce the enhanced migration ability. In an intracranial xenograft mouse model, transplantation of migration-prone subline cells exhibited higher cell migration than parental tumor cells. These results indicate that osteopontin activates Nrf2 signaling, resulting in enhanced HO-1 expression and cell migration in glioma cells.
Keywords: glioma, HO-1, migration, Nrf2, osteopontin
Most primary brain tumors are derived from glial cells and are collectively referred to as gliomas.1 Malignant gliomas, the most common malignant tumor of the brain in adults, are particularly invasive into surrounding brain tissue.2,3 Migration capacity is an essential prerequisite for invasion and precedes malignant tumor formation.4 This pathologic characteristic of metastasis or invasion into the surrounding brain tissue renders complete surgical resection impossible and induces resistance to conventional radiotherapy and chemotherapy,5,6 which makes successful treatment very difficult. The better strategies of treatment will ultimately require understanding of the molecular mechanisms of malignant progression of human glioma as well as identifying and specifically targeting the critical signaling effectors.7,8
Osteopontin, an adhesive glycoprotein containing the arginyl–glycyl–aspartic acid (RGD) motif, acts as a multifunctional cytokine that has been linked to a variety of pathophysiologic cell functions.9–11 Osteopontin has been implicated in cell adhesion and migration functions and is one of the extracellular matrix molecules present in bone, brain, kidney, and placenta.9,12 In the CNS, focal ischemic injury induces osteopontin expression, which acts as a chemoattractant that recruits glial cells to assist the glial scar formation following ischemic injury.10,13 Importantly, osteopontin also expresses in human glioma and is correlated with malignancy.14–16 Moreover, previous reports have shown that hypoxia,17 12-O-tetradecanoylphorbol-13-acetate,18 and hyaluronic acid19 stimulate osteopontin expression and induce cell migration in glioma cells.
Heme oxygenase (HO; also referred to as heat shock protein [HSP]32) is a rate-limiting enzyme that catalyzes the conversion of heme to carbon monoxide, biliverdin, and ferrous iron. HO-1 is induced by various stress-related cellular stimuli, such as oxidative stress and hypoxia-ischemia.20 HO-1 and its products in the brain are important for maintaining cellular homeostasis and have antioxidant, anti-inflammatory, and anti-apoptotic effects.21,22 However, whether HO-1 expression in tumor cells regulates cell migration and metastasis is still controversial. Studies have found that HO-1 inhibits breast cancer invasion via suppressing the expression of matrix metalloproteinase–9.23,24 However, a growing body of evidence indicates that HO-1 activation might play a role in carcinogenesis and could potentially influence tumor growth and metastasis.25 Several studies have demonstrated that HO-1 overexpression is implicated in tumorigenesis, growth, and resistance to chemo- and radiotherapy in several types of malignancies.25–28 Recent reports have shown that HO-1 plays a complex role in the stimulation of angiogenesis29 and metastasis.30 Importantly, it has also been reported that HO-1 expression is correlated with macrophage infiltration31 and neurogenic factor expression32 in glioma cells.
Osteopontin plays an important role in tumor metastasis. However, little information is available on osteopontin's effect on the regulation of HO-1 expression and related molecular mechanisms. The present study shows that osteopontin might be capable of increasing HO-1 expression and subsequently regulating glioma cell migration. Moreover, signaling pathways of extracellular-signal-regulated kinase (ERK), Akt, and Nrf-2 may be involved in the increase of HO-1 expression and cell migration by osteopontin.
Materials and Methods
Materials
Osteopontin, LY294002, and wortmannin were obtained from Sigma-Aldrich. Primary antibodies against phosphatidylinositol 3-kinase (PI3K) (p85), β-actin, Akt, and phospho-Akt (Ser473) were purchased from Santa Cruz Biotechnology. On-Target smart pool Nrf2 small interfering (si)RNA and control nontargeting pool siRNA were purchased from Dharmacon. Akt kinase and ERK activity assay kits were purchased from Cell Signaling and Neuroscience. Akt inhibitor (1L-6-hydroxymethyl-chiro-inositol 2(R)-2-O-methyl-3-O-octadecylcarbonate) was purchased from Chemicon. Antibody specific for osteopontin was purchased from R&D Systems.
Cell Cultures
C6 and U251 cells were purchased from the American Type Culture Collection. C6 cells were maintained with F12 medium (Invitrogen), while U251 cells were maintained in 75-cm2 flasks with Dulbecco's modified Eagle's medium (DMEM; Gibco BRL/ Invitrogen). All cells were cultured in medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco BRL/Invitrogen), 100 U/mL penicillin, and 100 mg/mL streptomycin at 37°C and incubated in a humidified atmosphere consisting of 5% CO2 and 95% air.
Transfection
U251 cells were transiently transfected with Nrf2 or control siRNA by Lipofectamine (LF)2000 (Invitrogen) for 24 h. Plasmid DNA and LF2000 were premixed in Opti–Minimal Essential Medium (Invitrogen) for 20 min and then applied to the cells. An equal volume of medium containing 20% FBS was added 4–6 h later. After transfection for 24 h, LF2000-containing medium was replaced with fresh serum-free medium.
Reverse Transcription–PCR and Quantitative Real Time–PCR
Total RNA was extracted from cells using a Trizol reagent (Invitrogen), and the reverse transcription (RT) reaction was performed using 1 μg of total RNA converted into cDNA using the Promega RT kit and amplified using the following oligonucleotide primers:
HO-1: 5′-CACGCCTACACCCGCTACCT-3′ and
5′-TCTGTCACCCTGTGCTTGAC-3′;
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH): 5′-AGGGCTGCTTTTAACTCTGGT-3′ and
5′-CCCCACTTGATTTTGGAGGGA-3′.
Each PCR cycle was carried out for 30 s at 95°C, for 30 s at 55°C, and for 1 min at 68°C. PCR products were then separated electrophoretically in a 2% agarose gel and stained with ethidium bromide. The band intensity was quantified with a densitometric scanner and presented as the relative level of GAPDH.
Quantitative real-time PCR using Sybr Green I Master Mix was performed with a model 7900 Sequence Detector (Applied Biosystems). After pre-incubation at 95°C for 10 min, PCR was performed for 40 cycles of 95°C for 10 s and 60°C for 1 min. The threshold was set above the nontemplate control background and within the linear phase of target gene amplification for calculating the cycle number at which the transcript was detected (denoted as CT).
Western Blot Analysis
Whole-cell lysis extracts were prepared as per our previous report.33 In brief, cells were lysed with radioimmunoprecipitation assay buffer for 30 min on ice. The supernatants were collected by centrifugation at 13 000 g for 30 min and stored at -20°C.
Nuclear extracts were prepared as described previously.34 Cells were suspended in buffer A for 10 min on ice. The lysates were separated into cytosolic and nuclear fractions by centrifugation at 12 000 g for 10 min. The supernatants containing cytosolic proteins were collected. A pellet containing nuclear fraction was resuspended in buffer C for 30 min on ice. The supernatants containing nuclear proteins were collected by centrifugation at 13 000 g for 20 min and stored at -80°C.
Protein samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Millipore) blocked with 5% nonfat milk in phosphate buffered saline (PBS) and probed overnight with primary antibody at 4°C. After undergoing PBS washes, the membranes were incubated with secondary antibody. Blots were visualized by enhanced chemiluminescence using Kodak X-Omat LS film. The blots were subsequently stripped by incubation in stripping buffer and reprobed a loading control antibody.
Protein Kinase Assays
ERK and Akt protein kinase assays were performed according to the manufacturer's protocols. Equal amounts of protein were incubated with recombinant Elk and GSK3β fusion protein-agarose for 16–18 h at 4°C with gentle rotation, respectively. The beads were washed 3 times with wash buffer and kept on ice. The kinase reactions were performed by incubating immunoprecipitated beads with 50 μL of kinase buffer contained within 200 μM adenosine triphosphate at 30°C for 30 min. To assess ERK or Akt activities, the reaction mixtures were analyzed by SDS-PAGE using specific antibody against Elk or GSK3α/β phosphorylation, respectively.
Migration Assay and Invasion Assay
In vitro migration and invasion assays were performed using Costar transwell inserts (pore size, 8 μm) in 24-well plates as described previously.33,34 For invasion assay, filters were precoated with 25 μL matrigel basement membrane matrix (BD Biosciences). Before performing the migration assay, cells were pretreated for 30 min with different concentrations of inhibitors or transfected with various dominant-negative (DN) mutants for 24 h. Approximately 1 × 104 cells in 200 μL of serum-free medium were placed in the upper chamber, and 500 μL of the same medium containing osteopontin was placed in the lower chamber. The plates were incubated for 24 h at 37°C in 5% CO2, then the cells were stained with 0.05% crystal violet and 2% methanol in PBS for 15 min. Nonmigratory cells on the upper surface of the filters were removed by wiping with a cotton swab and were washed with PBS. The cell number of 3 fields per well was counted under a microscope at 100× magnification and expressed as the “fold of control.” Images of migratory cells were observed and acquired at 24 h with a digital camera and light microscope.
Electrophoretic Mobility Shift Assay
Electrophoretic mobility shift assay (EMSA) was performed using the Panomics gel shift kit according to the manufacturer's protocol. Nuclear extract (2 μg) from U251 glioma cells was incubated with poly d(I-C) at room temperature for 5 min and then incubated with biotin-labeled probes, followed by incubation at room temperature for 30 min. After electrophoresis on an 8% polyacrylamide gel, the samples on the gel were transferred onto a presoaked Immobilon-Nyt membrane (Millipore). The membrane was cross-linked in an oven for 3 min, developed by adding the blocking buffer and streptavidin–horseradish peroxidase conjugate, and then subjected to Western blot analysis.
Wound-Healing Assay
Cancer cells were treated with osteopontin or vehicle for 24 h. A cell-free gap of 500 mm was created after removing the Ibidi Culture-Insert. The cells that migrated into the wound area were observed and acquired at 0 and 24 h with a digital camera and light microscope.
Establishment of Migration-prone Sublines
Subpopulations from glioma cells were selected according to their differential migration ability34; the cell culture insert system was used as described earlier. After 24 h of migration, cells that penetrated through pores and migrated to the underside of the filters were trypsinized and harvested for a second-round selection. The original cells that did not pass through membrane pores were designated as P0. After 10 rounds of selection, the migration-prone subline was designated as P10.
In Vivo Intracranial Xenograft Studies
Cells were freshly prepared and adjusted to 1 × 108 cells/mL before implantation. An intracranial xenograft was performed according to protocols approved by the China Medical University Animal Care and Use Committee. Briefly, nude mice were anesthetized and placed in a stereotactic frame, and the skulls were exposed by incision. In each skull, a hole was made 0.6 mm anterior, 1.8 mm to the right of the bregma, and cells (5 μL) were injected using a 10-μL Hamilton syringe with a 26S-gage needle mounted in a stereotactic holder. The syringe was lowered to a depth of 3 mm, and the cells were injected at a rate of 10 μL/min. After intracranial implantation, a 5-min waiting period was observed before slowly withdrawing the syringe to prevent any reflux. The skull was then cleaned and the incision was sutured. Tumors were allowed to grow, and animals were sacrificed on day 28.
Under deep anesthesia with trichloroacetaldehyde, each mouse was transcardially perfused with a saline solution (0.9% NaCl) and then fixed with 4% paraformaldehyde dissolved in 0.1 M PBS. The brains were removed from the skulls, postfixed overnight in buffered 4% paraformaldehyde at 4°C, stored in a 30% sucrose solution at 4°C until they sank, and were frozen sectioned on a sliding microtome in 10-µm-thick coronal sections. To determine the brain volume, brain sections were stained with hematoxylin.
Statistical Analysis
Statistical analysis was performed using Graphpad Prism 4.01 software. Values are means ± SEM. Statistical analysis of the difference between 2 samples was performed using Student's ttest.
Results
Osteopontin Increases Cell Migration and Invasion in Glioma Cells
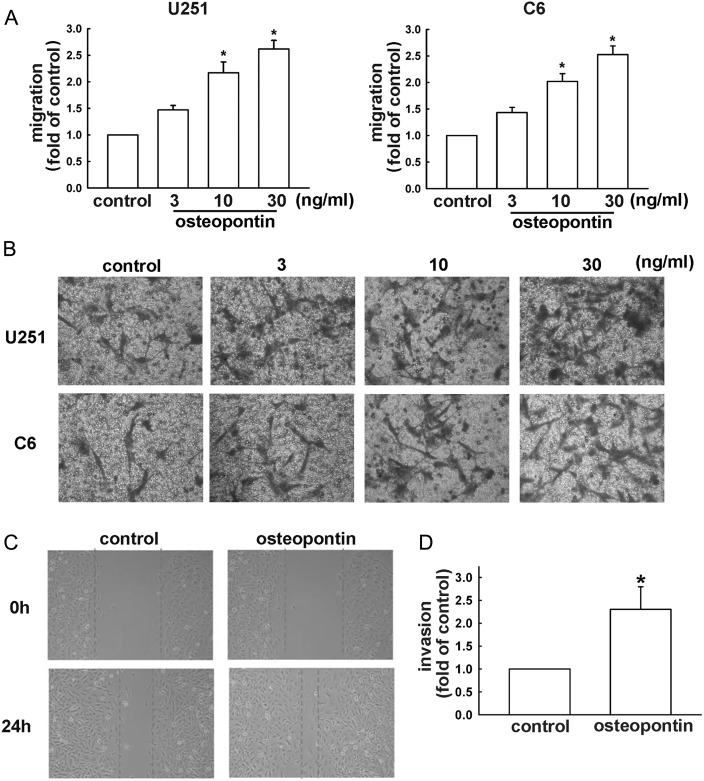
It has been shown that osteopontin plays an important role in glioblastoma motility.16 Osteopontin-regulated glioma cell migration was examined using the transwell assay with correction of proliferation effects.33,35 As shown in Fig. 1A, osteopontin enhanced migration of both mouse and human malignant glioma cells (C6 and U251 cells, respectively) in a concentration-dependent manner. Pictures of migrating cells are shown in Fig. 1B. To confirm that osteopontin is indeed responsible for cell migration in human glioma cells, the specific osteopontin antibody was used. On the other hand, osteopontin also increased wound-healing activity in human U251 glioma cells (Fig. 1C). Furthermore, osteopontin increased the chemoinvasive ability of U251 cells through matrigel basement membrane matrix (Fig. 1D).
Fig. 1.
Osteopontin induces the migration activity of glioma cells. By using a cell culture insert system, in vitro migration activities were examined. (A) After incubating cells with various concentrations of osteopontin for 24 h, we found that osteopontin induced migration activity in U251 and C6 cells. Results are expressed as means ± SEM of 3 independent experiments. (B) Cells were treated with various concentrations of osteopontin for 24 h. The migrated cells were visualized by phase-contrast imaging. (C) Cells were seeded on the migration insert for 24 h and treated with or without osteopontin (10 ng/mL) for another 24 h. The migrated cells were determined by wound-healing assay and visualized by phase-contrast imaging. (D) Treatment with osteopontin (10 ng/mL) enhanced invasion of U251 cells. Results are expressed as means ± SEM of 3 independent experiments. *P < .05 compared with control group.
Osteopontin-Directed Glioma Cell Migration Involves HO-1 Expression
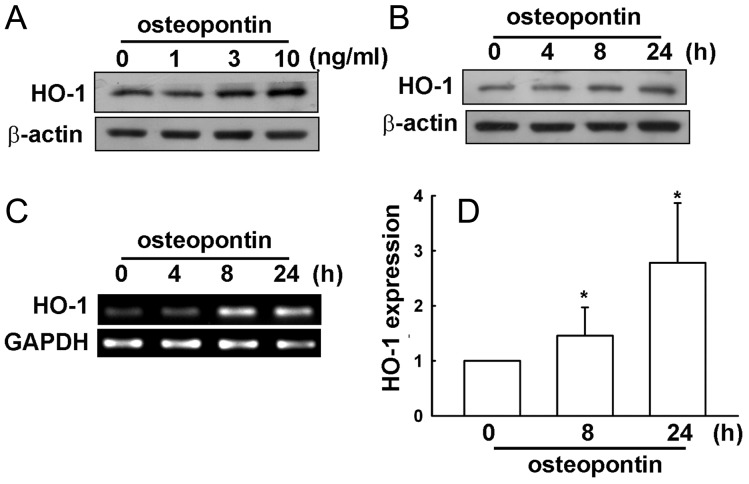
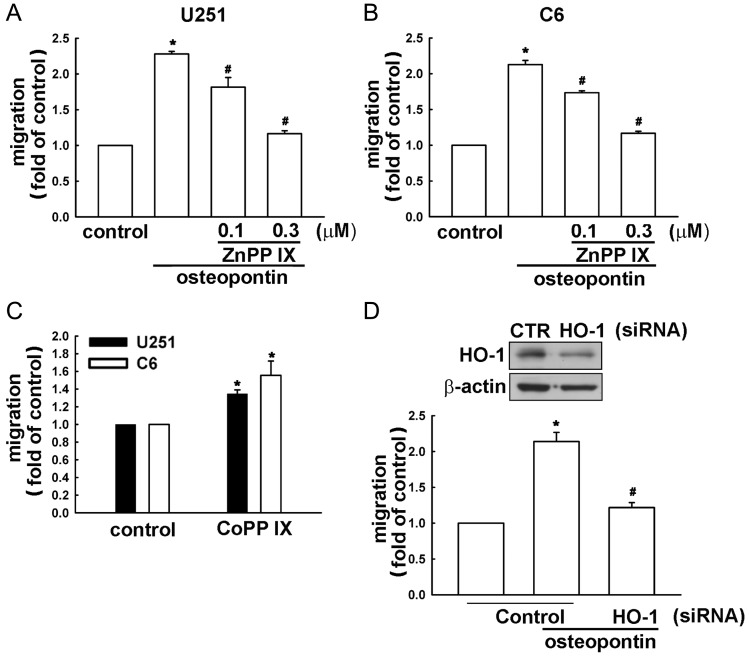
Several studies have demonstrated that HO-1 overexpression is implicated in tumorigenesis, growth, and resistance to chemo- and radiotherapy in several types of malignancies.25–28 However, whether HO-1 affects tumor cell migration and metastasis in glioma cells is still controversial. We hypothesized that HO-1 may be involved in osteopontin-directed migration of glioma cells. Following osteopontin stimulation, HO-1 protein and mRNA expression were assessed by Western blot, RT-PCR, and real time–PCR analysis. As shown in Fig. 2A and B, osteopontin increased HO-1 protein expression in concentration- and time-dependent manners. Treatment of osteopontin also increased HO-1 mRNA expression, which was determined by RT-PCR (Fig. 2C), and quantification, determined by real time–PCR (Fig. 2D). Pretreatment of cells with ZnPPIX, a pharmacologic inhibitor of HO-1, markedly inhibited osteopontin-induced cell migration in U251 and C6 cells (Fig. 3A and B). Moreover, treatment with CoPPIX, an activator of HO-1, also mildly increased glioma cell migration (Fig. 3C). Furthermore, transfection of cells with HO-1 siRNA for 24 h inhibited HO-1 protein expression (upper panel, Fig. 3D) and reduced osteopontin-induced glioma cell migration (lower panel, Fig. 3D). These results suggest that osteopontin-induced glioma cell migration may occur via HO-1 upregulation.
Fig. 2.
Osteopontin increases HO-1 upregulation in U251 glioma cells. Cells were stimulated with osteopontin with various concentrations (1, 3, or 10 ng/mL for 24 h; A) or with a concentration of 10 ng/mL for indicated time periods (4, 8, and 24 h; B). After cell lysate extracts were collected, HO-1 protein levels were determined by using Western blot analysis. (C) Cells were treated with osteopontin (10 ng/mL), and HO-1 mRNA expression was analyzed by RT-PCR. (D) mRNA expression was quantitated by real time–PCR. Results are expressed as means ± SEM of 3 independent experiments. *P < .05 compared with control group.
Fig. 3.
Osteopontin-directed glioma cell migration involves HO-1 expression. (A and B) Cells were treated with ZnppIX (0.1 or 0.3 μM) for 30 min, followed by stimulation with osteopontin (10 ng/mL). (C) Cells were stimulated with CoPPIX for 24 h. (D, lower panel) Cells were pretransfected with control or HO-1 siRNA for 24 h, followed by stimulation with osteopontin (10 ng/mL) for another 24 h. In vitro migration activities were examined after osteopontin treatment for 24 h. Results are expressed as means ± SEM of 3 independent experiments. *P < .05 compared with the control group; #P < .05 compared with the osteopontin treatment group. Cells were pretransfected with control or HO-1 siRNA for 24 h, and protein expression was determined by Western blot (D, upper panel).
Akt and ERK Signaling Pathways Are Involved in Osteopontin-Mediated HO-1 Upregulation and Glioma Cell Migration
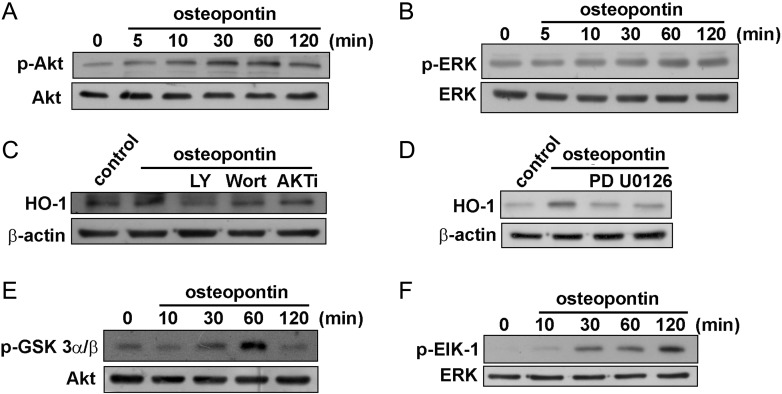
It has been reported that osteopontin increases the phosphorylation of Akt and ERK.36–38 Recently, we also showed that HO-1 expression increased through activation of Akt and ERK.39 Therefore, we examined the role of Akt and ERK in HO-1 upregulation. Stimulation of osteopontin increased Akt (Fig. 4A) and ERK (Fig. 4B) phosphorylation in a time-dependent manner. Moreover, cells were pretreated with PI3K inhibitors and Akt inhibitor or MEK and ERK inhibitors for 30 min, followed by incubation with osteopontin. As shown in Fig. 4C and D, osteopontin-induced HO-1 expression was inhibited by treatment with the specific PI3K/Akt or MEK/ERK inhibitors. Furthermore, using GSK3α/β-agarose fusion protein as the Akt substrate, an increase in Akt activity at 30–60 min was observed in osteopontin-treated glioma cells (Fig. 4E). Using Elk-1–agarose fusion protein as the ERK substrate, an increase in ERK activity at 30–120 min was observed in osteopontin-treated glioma cells (Fig. 4F). These results indicate that the Akt and ERK pathways are involved in osteopontin-induced HO-1 upregulation in glioma cells.
Fig. 4.
Involvement of PI3K/Akt in osteopontin-induced HO-1 expression in U251 glioma cells. Cells were incubated with osteopontin for indicated time periods. The phosphorylation of Akt (A) and ERK (B) were determined by Western blot analysis. (C and D) Cells were pre-incubated with LY294002, wortmannin, Akt inhibitor (AKT i), or PD98059 or U0126 for 30 min followed by stimulation with osteopontin for 24 h, and HO-1 protein expression was analyzed by Western blot. (E and F) Cells were incubated with osteopontin for indicated time periods, and cell lysates were then immunoprecipitated with GSK3- or Elk-1–fusion protein agarose beads. One set of immunoprecipitates was subjected to 10% SDS-PAGE and analyzed by immunoblotting with the anti-phospho-GSK3α/β antibody or anti-phospho-Elk-1 antibody. Equal amounts of the immunoprecipitated kinase complex presented in each kinase assay were confirmed by immunoblotting of Akt or ERK. Results are expressed as 4 independent experiments.
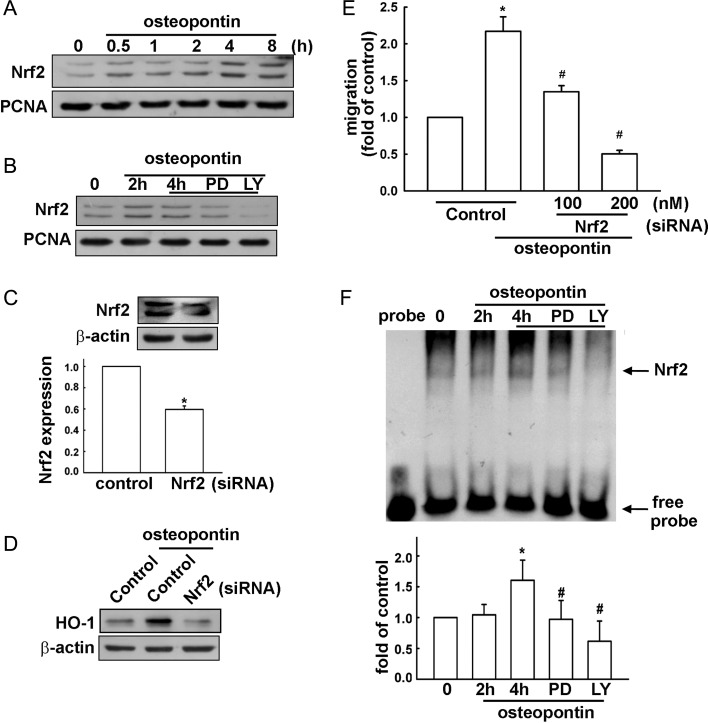
Involvement of Nrf2 in Osteopontin-Induced Cell Migration in Human Glioma Cells
It has been reported that the stress-response element/Nrf2 transcription factor pathway is important for HO-1 expression.40–42 We therefore examined whether Nrf2 signaling is involved in osteopontin-induced HO-1 expression. First, Nrf2 activation was assessed with the accumulation of Nrf2 in nucleus. Treatment of osteopontin resulted in an accumulation of Nrf2 in a time-dependent manner in the nuclei of U251 cells (Fig. 5A). In addition, treatment with ERK and Akt inhibitors reduced osteopontin-induced accumulation of Nrf2 in the nuclei for 4 h (Fig. 5B and Supplementary Fig. S1A). To investigate whether osteopontin-induced HO-1 expression was mediated through Nrf2 activation, siRNA against Nrf2 was used. As shown in Fig. 5C, transfection with Nrf2 siRNA for 24 h reduced Nrf2 expression in U251 glioma cells. Moreover, transfection with Nrf2 siRNA effectively reduced osteopontin-induced HO-1 expression (Fig. 5D) and reduced osteopontin-enhanced glioma cell migration in a dose-dependent manner (Fig. 5E). Furthermore, stimulation of cells with osteopontin for 2 h or 4 h increased the DNA binding activity of Nrf2 in nuclear extracts (Fig. 5F). In addition, there was no detectable DNA binding complex without loading nuclear protein. Treatment with an ERK or Akt inhibitor significantly reduced osteopontin-increased DNA binding activity of Nrf2 (Fig. 5F and Supplementary Fig. S1B). These results suggest that the stimulatory effect of osteopontin is mediated through Nrf2 activation and increases HO-1 expression and then glioma cell migration.
Fig. 5.
Osteopontin-induced HO-1 upregulation involves Nrf2 activation in U251 glioma cells. (A) Cells were treated with osteopontin for the indicated time periods, and the level of nuclear Nrf2 expression was determined by immunoblotting with Nrf2-specific antibody. (B) Cells were treated with ERK or Akt inhibitors for 30 min followed by stimulation with osteopontin for 4 h, and the level of nuclear Nrf2 expression was determined by immunoblotting with Nrf2 specific antibody. Cells were transfected with control or Nrf2 siRNA for 24 h, and Nrf2 (C) and HO-1 protein expressions (D) were determined by Western blot; osteropontin-induced cell migration was measured in the transwell assay (E). Results are expressed as means ± SEM of 3 independent experiments. *P < .05 compared with the control group; #P < .05 compared with the osteopontin treatment group. (F) Nuclear extracts were collected from cells treated with osteopontin for the indicated time periods (2 h or 4 h), and the binding of Nrf2 to the Nrf2-DNA binding element was examined by EMSA analysis. Lane 1 was loaded without nuclear extracts (probe). Cells were pretreated with PD98059 or LY294002, were treated with osteopontin for 4 h, and were then analyzed by EMSA. The quantitative data are shown in the lower panel. *P < .05 compared with the control group; #P < .05 compared with the osteopontin treatment for 4 h. Note that osteopontin increases the binding of Nrf2 to the Nrf2-DNA binding element, and treatment with PD98059 and LY294002 reduced osteopontin-increased DNA binding activity of Nrf2. Results are expressed as means ± SEM of 3 independent experiments. *P < .05 compared with the control group; #P < .05 compared with the osteopontin treatment for the 4 h group.
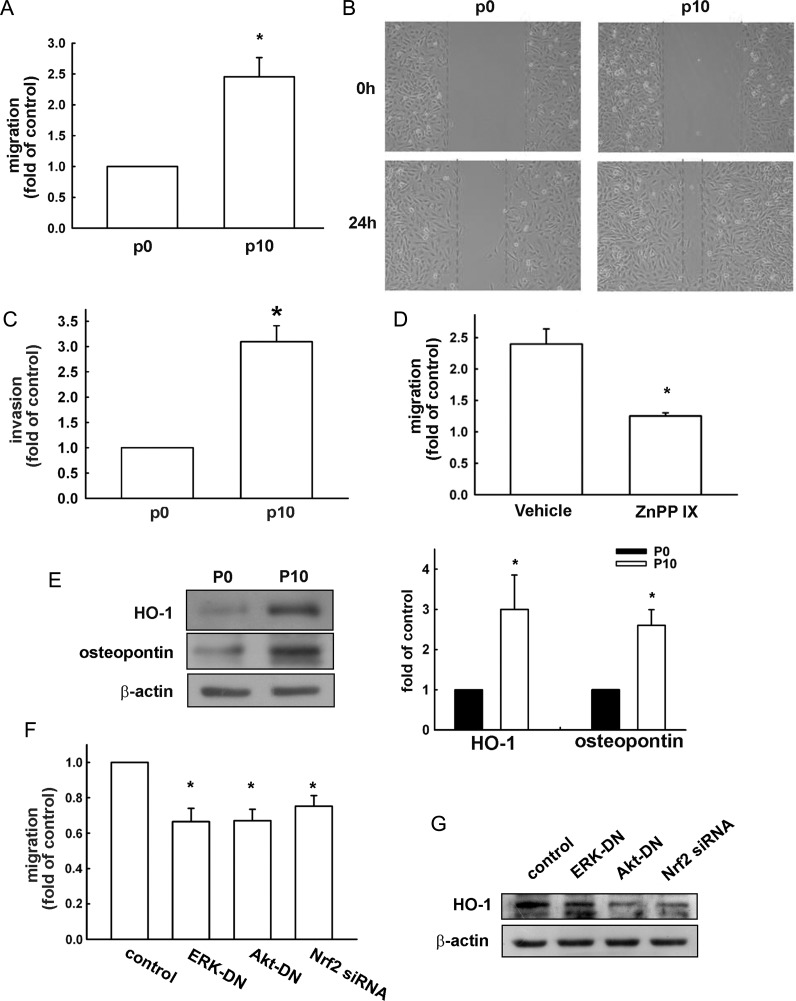
Increased Osteopontin Expression in Migration-prone Cells
We selected U251 sublines with higher cell mobility, as described in the Materials and Methods. The migration-prone subline P10 had higher cell mobility and migrated more easily through the cell culture insert basement membrane matrix than did the original U251 cells designated as P0 (the difference was approximately 2.4-fold; Fig. 6A). Wound-healing activity was also increased in the migration-prone subline P10 (Fig. 6B). Furthermore, the chemoinvasive ability of P10 U251 cells through Matrigel basement membrane matrix was enhanced (Fig. 6C). Pretreatment of ZnPPIX for 24 h also effectively reduced the enhancement of cell migration (Fig. 6D). Surprisingly, we found that P10 had markedly increased expression of HO-1 and osteopontin protein levels (Fig. 6E). Transfection with ERK-DN, Akt-DN, or Nrf-2 siRNA reduced osteopontin-enhanced cell migration (Fig. 6F) and HO-1 expression (Fig. 6G). These results suggest that ERK, Akt, Nrf-2 activation, and HO-1 upregulation are involved in glioma cell migration.
Fig. 6.
Upregulation of osteopontin and HO-1 expression in migration-prone cells. (A) After 10 rounds of selection of U251 cells by a cell culture insert system, the migration-prone subline (P10) exhibited higher migration ability than the original U251 cells. Results are expressed as means ± SEM of 3 independent experiments. *P < .05 compared with the original group (P0). (B) The original U251 cells (P0) and the migration-prone subline (P10) were seeded for 24 h, and cell migration was determined by wound-healing assay and visualized by phase-contrast imaging. (C) The migration-prone subline (P10) exhibited higher invasion ability than the original U251 cells. Results are expressed as means ± SEM of 3 independent experiments. *P < .05 compared with the control group. (D) Treatment with ZnPPIX (0.3 μM) for 24 h reduced the migration ability of the migration-prone subline (P10) compared with the vehicle treatment. Results are expressed as means ± SEM of 3 independent experiments. *P < .05 compared with the control group (vehicle treatment). (E) The cell lysates of P10 and the original U251 glioma cells (P0) were collected after 24 h of culturing, and osteopontin and HO-1 protein levels were determined using Western blot analysis. Note that P10 expressed higher osteopontin and HO-1 protein levels. The quantitative results are shown in the right panel. Results are expressed as means ± SEM of 3 independent experiments. *P < .05 compared with the P0 group. P10 cells were pretransfected with ERK-DN, Akt-DN, or Nrf-2 siRNA for 24 h, cell migration activity was determined by a cell culture insert system (F), and HO-1 expression was examined by Western blot (G).
Higher Osteopontin and HO-1 Expression of Human Glioma Cells Correlated with Cell Invasiveness In Vivo
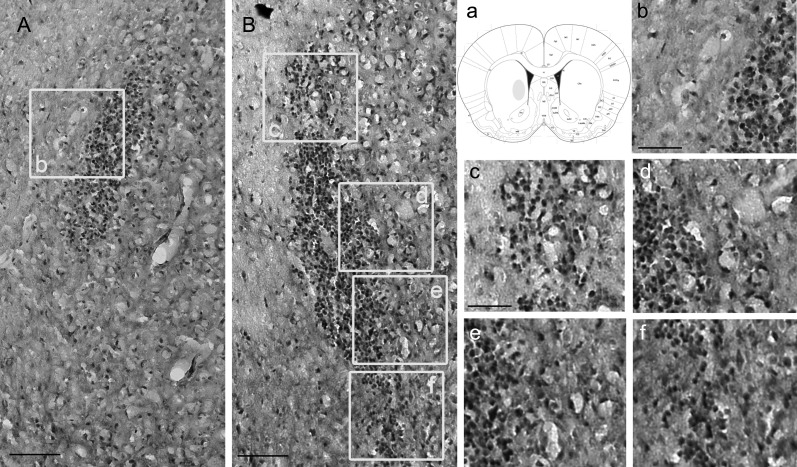
To more adequately evaluate the migratory activity of the higher expression of osteopontin and HO-1 in P10 cells in mouse brain tissue, we performed an intracranial glioma xenograft experiment. Normally, P0 cells inoculated in the brains of nude mice grow as a noninvasive solid tumor mass (ball-like),43,44 form a sharper cranial margin, and are less invasive (Fig. 7A, b). We implanted the migration-prone subline P10 into the brains of nude mice and followed the mice over time. After about4 weeks, the mice were euthanized and the brains removed for evaluation. Brain striatally injected normal U251 cells P0 (Fig. 7A) and the migration-prone subline P10 (Fig. 7B) were shown, demonstrating dissemination of migration-prone subline P10 glioma into the normal brain. Metastatic P10 U251 cells grew orthotopically in mouse brain tissue with a diffuse tumor boundary (Fig. 7B, c–f) and fingerlike protrusions (Fig. 7B, d), indicating infiltrative growth and tumor spread. In contrast to P10 tumors, P0 U251 tumors maintained a distinct border with the brain parenchyma, with little localized invasion and a smaller tumor size (Fig. 7A, b). These results confirmed the in vitro experiments suggesting that migration-prone subline P10 glioma cells had more enhanced migratory activity than did normal U251 P0 cells.
Fig. 7.
High migration activity of human glioma cells correlated with cell invasiveness in vivo. After 10 rounds of selection of U251 cells by a cell culture insert system, the migration-prone subline (P10; B) and the original group (P0; A) were implanted into nude mice intracranially at the site indicated as a; mice were killed 28 days after implantation, and representative sections were stained with hematoxylin. Scale bar = 200 μm in A, B. Higher magnification of the migration-prone subline P0 are shown in b. Higher magnification of the migration-prone subline P10 is shown in c, d, e, and f (n = 3 mice in each group). Scale bar = 100 μm in b–f.
Discussion
Glioblastoma is the most common primary brain tumor in adults and the second most common tumor in children. Glioblastoma is associated with high morbidity and mortality because its pathologic characteristic is highly invasive into surrounding brain tissue, making complete surgical resection impossible. Current therapies fail to prevent glioma invasion into normal contiguous brain tissue, leading to poor prognosis after surgery and/or radiation therapy. The elucidation of the molecular biology of cancer cells in the past decade has identified alterations in various signaling pathways in glioblastoma. Currently, this information is being exploited to develop potential therapeutic targets. Osteopontin is an adhesive glycoprotein implicated in cell adhesion and migration functions45,46 and may play a role in controlling the inflammation associated with neuronal damage and cell death.10,13,47 It has been reported that osteopontin, the cell attachment protein, is expressed in normal adult brain and has the potential to promote glioma cell invasion.48 A recent report also showed that osteopontin in glioblastoma not only induces cancer cell migration but also is associated with infiltration of leucocytes.49 Therefore, osteopontin could be a useful target in examining the molecular mechanism in glioblastoma.
HO-1 participates in maintaining cellular homeostasis and plays an important protective role by reducing oxidative injury, attenuating the inflammatory response, inhibiting cell apoptosis, and regulating cell proliferation.50–52 HO-1 has recently been reported as an important molecule in tumor angiogenesis and metastasis. However, the role of HO-1 in glioma cell migration is unclear. HO-1 can be induced in melanoma in response to anticancer drugs, augment tumor cell metastasis, and decrease survival of tumor-bearing mice.53 Overexpression or activation of HO-1 potentiates angiogenesis and cell metastasis. On the other hand, inhibition of HO activity inhibits the occurrence of metastasis.30,54 Furthermore, pharmacologic inhibitors of HO-1 have anticarcinogenic effects in colon cancer and sarcoma.55 However, mechanisms underlying modulation of HO-1 in glioma cell migration remain unknown. In the present study, we found that osteopontin significantly increased cell migration in human glioma cells. We further demonstrated that the enhancement effect of osteopontin may be attributed to its expression levels of HO-1. This premise is supported by results of inhibition or knockdown of HO-1 in glioma cells decreasing osteopontin-induced cell migration. In addition, using HO-1 activator mildly increased cell migration activity in glioma cells. Importantly, we selected sublines that showed higher migration ability. Our results suggest that migration-prone sublines expressing higher levels of osteopontin and HO-1 have higher migration activity. The more prominent expression of osteopontin in migration-prone cells further indicates that osteopontin may be involved in autocrine or paracrine functions that enhance migration and invasion. Our results also confirmed a previous report that the expression level of osteopontin in glioma cells parallels cell invasion ability.16 Nrf2 is a major transcription factor that regulates expression of antioxidant defense genes through binding to antioxidant response elements in the promoter region of antioxidant genes, such as HO-1.56–59 Importantly, a recent report has shown high correlations between tumor metastasis and Nrf2/HO-1 expression in gallbladder cancer.60 Our results showed that Nrf2 activation is essential for osteopontin-stimulated HO-1 expression, based on the fact that Nrf2 siRNA inhibits the enhancement of osteopontin-induced migration. Furthermore, osteopontin stimulated Nrf2 accumulation in nucleus and increased Nrf2-DNA binding activity. These results suggest that Nrf2 activation is required for osteopontin-induced HO-1 expression and cell migration in human glioma.
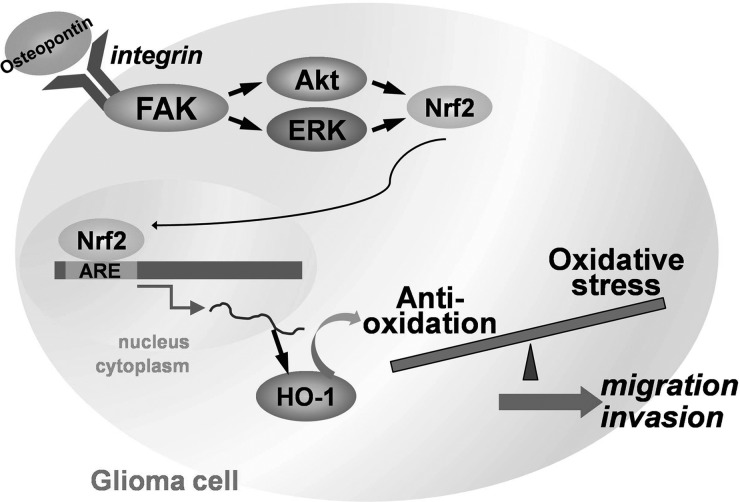
In conclusion, we present here a novel mechanism of osteopontin-directed migration and HO-1 upregulation in human glioma cells by activation of Akt, ERK, and Nrf2-dependent pathways (Fig. 8). Our results also indicate that HO-1 can be a novel therapeutic target.
Fig. 8.
Schematic diagram of the signaling pathways involved in osteopontin-induced inflammatory mediator expression in human glioma cells. Treatment of cells with osteopontin might bind with osteopontin receptor to activate ERK and Akt signaling pathways, which leads to HO-1 expression and increases the cell migration of human glioma.
Funding
This work was supported by grants from the National Science Council (NSC 101-2320-B-039-048-MY2), China Medical University (CMU100-S-11), and Taichung Tzu Chi General Hospital (TTCRD-10008).
Supplementary Material
Acknowledgments
The authors thank S. H. Ko and Y. R. Chen for technical support.
Conflict of interest statement: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molina JR, Hayashi Y, Stephens C, Georgescu MM. Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia. 2010;12(6):453–463. doi: 10.1593/neo.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang Z, Cheng L, Guryanova OA, Wu Q, Bao S. Cancer stem cells in glioblastoma—molecular signaling and therapeutic targeting. Protein Cell. 2010;1(7):638–655. doi: 10.1007/s13238-010-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor LP. Diagnosis, treatment, and prognosis of glioma: five new things. Neurology. 2010;75(18 Suppl 1):S28–32. doi: 10.1212/WNL.0b013e3181fb3661. [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Koul D, Shen R, Bergh S, et al. Inhibition of Akt survival pathway by a small-molecule inhibitor in human glioblastoma. Mol Cancer Ther. 2006;5(3):637–644. doi: 10.1158/1535-7163.MCT-05-0453. [DOI] [PubMed] [Google Scholar]
- 7.Kuo TC, Yang JS, Lin MW, et al. Emodin has cytotoxic and protective effects in rat C6 glioma cells: roles of Mdr1a and nuclear factor kappaB in cell survival. J Pharmacol Exp Ther. 2009;330(3):736–744. doi: 10.1124/jpet.109.153007. [DOI] [PubMed] [Google Scholar]
- 8.Chu PM, Chen LH, Chen MT, et al. Targeting autophagy enhances BO-1051-induced apoptosis in human malignant glioma cells. Cancer Chemother Pharmacol. 2012;69(3):621–633. doi: 10.1007/s00280-011-1747-0. [DOI] [PubMed] [Google Scholar]
- 9.El-Tanani MK. Role of osteopontin in cellular signaling and metastatic phenotype. Front Biosci. 2008;13:4276–4284. doi: 10.2741/3004. [DOI] [PubMed] [Google Scholar]
- 10.Choi JS, Kim HY, Cha JH, Choi JY, Lee MY. Transient microglial and prolonged astroglial upregulation of osteopontin following transient forebrain ischemia in rats. Brain Res. 2007;1151:195–202. doi: 10.1016/j.brainres.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Gross TS, King KA, Rabaia NA, Pathare P, Srinivasan S. Upregulation of osteopontin by osteocytes deprived of mechanical loading or oxygen. J Bone Miner Res. 2005;20(2):250–256. doi: 10.1359/JBMR.041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16(2):79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Wung JK, Perry G, Kowalski A, et al. Increased expression of the remodeling- and tumorigenic-associated factor osteopontin in pyramidal neurons of the Alzheimer's disease brain. Curr Alzheimer Res. 2007;4(1):67–72. doi: 10.2174/156720507779939869. [DOI] [PubMed] [Google Scholar]
- 14.Yin B, Li KH, An T, Chen T, Peng XZ. Nectin-like molecule 1 inhibits the migration and invasion of U251 glioma cells by regulating the expression of an extracellular matrix protein osteopontin. Chin Med Sci J. 2010;25(2):100–104. doi: 10.1016/s1001-9294(10)60030-2. [DOI] [PubMed] [Google Scholar]
- 15.Yan W, Qian C, Zhao P, et al. Expression pattern of osteopontin splice variants and its functions on cell apoptosis and invasion in glioma cells. Neuro Oncol. 2010;12(8):765–775. doi: 10.1093/neuonc/noq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jan HJ, Lee CC, Shih YL, et al. Osteopontin regulates human glioma cell invasiveness and tumor growth in mice. Neuro Oncol. 2010;12(1):58–70. doi: 10.1093/neuonc/nop013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Said HM, Hagemann C, Staab A, et al. Expression patterns of the hypoxia-related genes osteopontin, CA9, erythropoietin, VEGF and HIF-1alpha in human glioma in vitro and in vivo. Radiother Oncol. 2007;83(3):398–405. doi: 10.1016/j.radonc.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Tucker MA, Chang PL, Prince CW, Gillespie GY, Mapstone TB. TPA-mediated regulation of osteopontin in human malignant glioma cells. Anticancer Res. 1998;18(2A):807–812. [PubMed] [Google Scholar]
- 19.Kim MS, Park MJ, Moon EJ, et al. Hyaluronic acid induces osteopontin via the phosphatidylinositol 3-kinase/Akt pathway to enhance the motility of human glioma cells. Cancer Res. 2005;65(3):686–691. [PubMed] [Google Scholar]
- 20.Cuadrado A, Rojo AI. Heme oxygenase-1 as a therapeutic target in neurodegenerative diseases and brain infections. Curr Pharm Des. 2008;14(5):429–442. doi: 10.2174/138161208783597407. [DOI] [PubMed] [Google Scholar]
- 21.Chung HT, Choi BM, Kwon YG, Kim YM. Interactive relations between nitric oxide (NO) and carbon monoxide (CO): heme oxygenase-1/CO pathway is a key modulator in NO-mediated antiapoptosis and anti-inflammation. Methods Enzymol. 2008;441:329–338. doi: 10.1016/S0076-6879(08)01218-4. [DOI] [PubMed] [Google Scholar]
- 22.Wu BJ, Kathir K, Witting PK, et al. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med. 2006;203(4):1117–1127. doi: 10.1084/jem.20052321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Hu F, Guo S, et al. BMP-6 inhibits MMP-9 expression by regulating heme oxygenase-1 in MCF-7 breast cancer cells. J Cancer Res Clin Oncol. 2011;137(6):985–995. doi: 10.1007/s00432-010-0963-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin CW, Shen SC, Hou WC, Yang LY, Chen YC. Heme oxygenase-1 inhibits breast cancer invasion via suppressing the expression of matrix metalloproteinase-9. Mol Cancer Ther. 2008;7(5):1195–1206. doi: 10.1158/1535-7163.MCT-07-2199. [DOI] [PubMed] [Google Scholar]
- 25.Jozkowicz A, Was H, Dulak J. Heme oxygenase-1 in tumors: is it a false friend? Antioxid Redox Signal. 2007;9(12):2099–2117. doi: 10.1089/ars.2007.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prawan A, Kundu JK, Surh YJ. Molecular basis of heme oxygenase-1 induction: implications for chemoprevention and chemoprotection. Antioxid Redox Signal. 2005;7(11–12):1688–1703. doi: 10.1089/ars.2005.7.1688. [DOI] [PubMed] [Google Scholar]
- 27.Berberat PO, Dambrauskas Z, Gulbinas A, et al. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin Cancer Res. 2005;11(10):3790–3798. doi: 10.1158/1078-0432.CCR-04-2159. [DOI] [PubMed] [Google Scholar]
- 28.Dulak J, Loboda A, Zagorska A, Jozkowicz A. Complex role of heme oxygenase-1 in angiogenesis. Antioxid Redox Signal. 2004;6(5):858–866. doi: 10.1089/ars.2004.6.858. [DOI] [PubMed] [Google Scholar]
- 29.Miyake M, Fujimoto K, Anai S, et al. Heme oxygenase-1 promotes angiogenesis in urothelial carcinoma of the urinary bladder. Oncol Rep. 2011;25(3):653–660. doi: 10.3892/or.2010.1125. [DOI] [PubMed] [Google Scholar]
- 30.Liu PL, Tsai JR, Charles AL, et al. Resveratrol inhibits human lung adenocarcinoma cell metastasis by suppressing heme oxygenase 1-mediated nuclear factor-kappaB pathway and subsequently downregulating expression of matrix metalloproteinases. Mol Nutr Food Res. 2010;54(Suppl 2):S196–204. doi: 10.1002/mnfr.200900550. [DOI] [PubMed] [Google Scholar]
- 31.Nishie A, Ono M, Shono T, et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5(5):1107–1113. [PubMed] [Google Scholar]
- 32.Morita K, Lee MS, Her S. Possible relation of hemin-induced HO-1 expression to the upregulation of VEGF and BDNF mRNA levels in rat C6 glioma cells. J Mol Neurosci. 2009;38(1):31–40. doi: 10.1007/s12031-008-9156-5. [DOI] [PubMed] [Google Scholar]
- 33.Chen JH, Huang SM, Chen CC, et al. Ghrelin induces cell migration through GHS-R, CaMKII, AMPK, and NF-kappaB signaling pathway in glioma cells. J Cell Biochem. 2011;112(10):2931–2941. doi: 10.1002/jcb.23209. [DOI] [PubMed] [Google Scholar]
- 34.Lu DY, Leung YM, Cheung CW, Chen YR, Wong KL. Glial cell line-derived neurotrophic factor induces cell migration and matrix metalloproteinase-13 expression in glioma cells. Biochem Pharmacol. 2010;80(8):1201–1209. doi: 10.1016/j.bcp.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 35.Yeh WL, Lu DY, Liou HC, Fu WM. A forward loop between glioma and microglia: Glioma-derived extracellular matrix-activated microglia secrete IL-18 to enhance the migration of glioma cells. J Cell Physiol. 2012;227(2):558–568. doi: 10.1002/jcp.22746. [DOI] [PubMed] [Google Scholar]
- 36.Zhang R, Pan X, Huang Z, Weber GF, Zhang G. Osteopontin enhances the expression and activity of MMP-2 via the SDF-1/CXCR4 axis in hepatocellular carcinoma cell lines. PLoS One. 2011;6(8):e23831. doi: 10.1371/journal.pone.0023831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed M, Kundu GC. Osteopontin selectively regulates p70S6K/mTOR phosphorylation leading to NF-kappaB dependent AP-1-mediated ICAM-1 expression in breast cancer cells. Mol Cancer. 2010;9:101. doi: 10.1186/1476-4598-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson BW, Bonsal L, Chellaiah MA. Regulation of Erk1/2 activation by osteopontin in PC3 human prostate cancer cells. Mol Cancer. 2010;9:260. doi: 10.1186/1476-4598-9-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu DY, Tsao YY, Leung YM, Su KP. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology. 2010;35(11):2238–2248. doi: 10.1038/npp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vargas MR, Pehar M, Cassina P, et al. Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear factor erythroid 2-related factor 2 (Nrf2) in spinal cord astrocytes: consequences for motor neuron survival. J Biol Chem. 2005;280(27):25571–25579. doi: 10.1074/jbc.M501920200. [DOI] [PubMed] [Google Scholar]
- 41.Joung EJ, Li MH, Lee HG, et al. Capsaicin induces heme oxygenase-1 expression in HepG2 cells via activation of PI3K-Nrf2 signaling: NAD(P)H:quinone oxidoreductase as a potential target. Antioxid Redox Signal. 2007;9(12):2087–2098. doi: 10.1089/ars.2007.1827. [DOI] [PubMed] [Google Scholar]
- 42.Makabe S, Takahashi Y, Watanabe H, Murakami M, Ohba T, Ito H. Fluvastatin protects vascular smooth muscle cells against oxidative stress through the Nrf2-dependent antioxidant pathway. Atherosclerosis. 2010;213(2):377–384. doi: 10.1016/j.atherosclerosis.2010.07.059. [DOI] [PubMed] [Google Scholar]
- 43.Lal S, Lacroix M, Tofilon P, Fuller GN, Sawaya R, Lang FF. An implantable guide-screw system for brain tumor studies in small animals. J Neurosurg. 2000;92(2):326–333. doi: 10.3171/jns.2000.92.2.0326. [DOI] [PubMed] [Google Scholar]
- 44.Piao Y, Lu L, de Groot J. AMPA receptors promote perivascular glioma invasion via beta1 integrin-dependent adhesion to the extracellular matrix. Neuro Oncol. 2009;11(3):260–273. doi: 10.1215/15228517-2008-094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaumann A, Petrow P, Mentzel T, et al. Osteopontin expression in primary sarcomas of the pulmonary artery. Virchows Arch. 2001;439(5):668–674. doi: 10.1007/s004280100452. [DOI] [PubMed] [Google Scholar]
- 46.Wu CY, Wu MS, Chiang EP, et al. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56(6):782–789. doi: 10.1136/gut.2006.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ichikawa H, Itota T, Nishitani Y, Torii Y, Inoue K, Sugimoto T. Osteopontin-immunoreactive primary sensory neurons in the rat spinal and trigeminal nervous systems. Brain Res. 2000;863(1–2):276–281. doi: 10.1016/s0006-8993(00)02126-0. [DOI] [PubMed] [Google Scholar]
- 48.Ding Q, Stewart J, Jr., Prince CW, et al. Promotion of malignant astrocytoma cell migration by osteopontin expressed in the normal brain: differences in integrin signaling during cell adhesion to osteopontin versus vitronectin. Cancer Res. 2002;62(18):5336–5343. doi: 10.1100/tsw.2002.247. [DOI] [PubMed] [Google Scholar]
- 49.Atai NA, Bansal M, Lo C, et al. Osteopontin is up-regulated and associated with neutrophil and macrophage infiltration in glioblastoma. Immunology. 2011;132(1):39–48. doi: 10.1111/j.1365-2567.2010.03335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsu HY, Chu LC, Hua KF, Chao LK. Heme oxygenase-1 mediates the anti-inflammatory effect of Curcumin within LPS-stimulated human monocytes. J Cell Physiol. 2008;215(3):603–612. doi: 10.1002/jcp.21206. [DOI] [PubMed] [Google Scholar]
- 51.Cheng PY, Lee YM, Shih NL, Chen YC, Yen MH. Heme oxygenase-1 contributes to the cytoprotection of alpha-lipoic acid via activation of p44/42 mitogen-activated protein kinase in vascular smooth muscle cells. Free Radic Biol Med. 2006;40(8):1313–1322. doi: 10.1016/j.freeradbiomed.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 52.Huang YN, Wu CH, Lin TC, Wang JY. Methamphetamine induces heme oxygenase-1 expression in cortical neurons and glia to prevent its toxicity. Toxicol Appl Pharmacol. 2009;240(3):315–326. doi: 10.1016/j.taap.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 53.Was H, Cichon T, Smolarczyk R, et al. Overexpression of heme oxygenase-1 in murine melanoma: increased proliferation and viability of tumor cells, decreased survival of mice. Am J Pathol. 2006;169(6):2181–2198. doi: 10.2353/ajpath.2006.051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sunamura M, Duda DG, Ghattas MH, et al. Heme oxygenase-1 accelerates tumor angiogenesis of human pancreatic cancer. Angiogenesis. 2003;6(1):15–24. doi: 10.1023/a:1025803600840. [DOI] [PubMed] [Google Scholar]
- 55.Fang J, Sawa T, Akaike T, et al. In vivo antitumor activity of pegylated zinc protoporphyrin: targeted inhibition of heme oxygenase in solid tumor. Cancer Res. 2003;63(13):3567–3574. [PubMed] [Google Scholar]
- 56.Wagner AE, Boesch-Saadatmandi C, Breckwoldt D, et al. Ascorbic acid partly antagonizes resveratrol mediated heme oxygenase-1 but not paraoxonase-1 induction in cultured hepatocytes—role of the redox-regulated transcription factor Nrf2. BMC Complement Altern Med. 2011;11:1. doi: 10.1186/1472-6882-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shin JW, Ohnishi K, Murakami A, et al. Zerumbone induces heme oxygenase-1 expression in mouse skin and cultured murine epidermal cells through activation of Nrf2. Cancer Prev Res. 2011;4(6):860–870. doi: 10.1158/1940-6207.CAPR-10-0354. [DOI] [PubMed] [Google Scholar]
- 58.Seo WY, Goh AR, Ju SM, et al. Celastrol induces expression of heme oxygenase-1 through ROS/Nrf2/ARE signaling in the HaCaT cells. Biochem Biophys Res Commun. 2011;407(3):535–540. doi: 10.1016/j.bbrc.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 59.Quesada A, Ogi J, Schultz J, Handforth A. C-terminal mechano-growth factor induces heme oxygenase-1-mediated neuroprotection of SH-SY5Y cells via the protein kinase cepsilon/Nrf2 pathway. J Neurosci Res. 2011;89(3):394–405. doi: 10.1002/jnr.22543. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Zhang M, Zhang L, et al. Correlation of Nrf2, HO-1, and MRP3 in gallbladder cancer and their relationships to clinicopathologic features and survival. J Surg Res. 2010;164(1):e99–e105. doi: 10.1016/j.jss.2010.05.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.