GTGs are highly conserved membrane proteins found in plants, animals, and fungi. Arabidopsis thaliana GTG1 is found in Golgi bodies and endoplasmic reticulum. GTG knockout mutants show defects in fertility, hypocotyl and root growth, and responses to light and sugars but respond normally to abscisic acid. These results show that Arabidopsis GTGs are fundamental to plant growth and development.
Abstract
G protein–coupled receptor-type G proteins (GTGs) are highly conserved membrane proteins in plants, animals, and fungi that have eight to nine predicted transmembrane domains. They have been classified as G protein–coupled receptor-type G proteins that function as abscisic acid (ABA) receptors in Arabidopsis thaliana. We cloned Arabidopsis GTG1 and GTG2 and isolated new T-DNA insertion alleles of GTG1 and GTG2 in both Wassilewskija and Columbia backgrounds. These gtg1 gtg2 double mutants show defects in fertility, hypocotyl and root growth, and responses to light and sugars. Histological studies of shoot tissue reveal cellular distortions that are particularly evident in the epidermal layer. Stable expression of GTG1pro:GTG1-GFP (for green fluorescent protein) in Arabidopsis and transient expression in tobacco (Nicotiana tabacum) indicate that GTG1 is localized primarily to Golgi bodies and to the endoplasmic reticulum. Microarray analysis comparing gene expression profiles in the wild type and double mutant revealed differences in expression of genes important for cell wall function, hormone response, and amino acid metabolism. The double mutants isolated here respond normally to ABA in seed germination assays, root growth inhibition, and gene expression analysis. These results are inconsistent with their proposed role as ABA receptors but demonstrate that GTGs are fundamentally important for plant growth and development.
INTRODUCTION
Membrane proteins have important physiological functions in all organisms, with fundamental roles including signaling, transport, and bioenergetics. The recently described G protein–coupled receptor-type G protein/Golgi pH regulator (GTG/GPHR) membrane protein family is highly conserved in eukaryotes (Maeda et al., 2008; Pandey et al., 2009). GPHR of hamster (Cricetulus griseus) exhibits voltage-dependent anion channel activity and is believed to be critical in the control of Golgi acidification, with mutant cell lines showing altered Golgi function (Maeda et al., 2008). The human (Homo sapiens) Hs GPHR protein was initially annotated as a putative orphan G protein–coupled receptor (GPR89), although no function has been assigned to it. In Arabidopsis thaliana, there are two members of this family, GTG1 and GTG2, which were recently described as novel types of G protein–coupled GTPases that function as receptors for the plant hormone abscisic acid (ABA) (Pandey et al., 2009). ABA regulates diverse processes in plant growth and development and functions in stress responses (Cutler et al., 2010). A wide range of proteins have been implicated as ABA receptors, including Flowering time control protein A (FCA), Mg-chelatase H subunit (CHLH), G-protein coupled receptor2 (GCR2), and most recently the GTGs and the Pyrabactin resistant (PYR)/PYR-like (PYL)/Regulatory component of ABA receptor (RCAR) family of START domain proteins (Klingler et al., 2010).
Several observations have suggested that a G protein–coupled receptor (GPCR) might participate in ABA signal transduction in plants (Wang et al., 2001; Pandey et al., 2006, 2009). GPCRs and heterotrimeric G proteins consisting of α, β, and γ subunits function together in G protein signaling (Temple and Jones, 2007). Typical GPCRs are seven-transmembrane-domain receptors that are activated by ligands and interact with Gα subunits. Classically, upon signal perception, GPCRs change conformation, allowing the Gα subunit to exchange GDP for GTP. Once activated, the heterotrimeric complex dissociates into GTP-Gα and a Gβγ dimer, and these two independent elements can interact with downstream signaling effectors. The intrinsic GTPase activity of Gα leads to reassociation of the trimer to the inactive state until the next signaling event (Assmann, 2002). In humans, there are 23 Gα, five Gβ, and 12 Gγ subunits and hundreds of predicted GPCRs. By contrast, there appears to be a much smaller repertoire of G proteins in plants with Arabidopsis having one Gα (GPA1), one Gβ (AGB1), three Gγ subunits (AGG1, AGG2, and AGG3) and one regulator of G protein signaling. There are also three extra large G proteins that are involved in the regulation of root growth (Ding et al., 2008). Mutations in these G protein components can lead to altered phenotypes involving a wide range of processes, including hormone regulation of seed germination and seedling growth, stomatal regulation, plant defense responses, and root, rosette leaf, flower, and silique development (Temple and Jones, 2007; Trusov et al., 2008).
Several dozen genes have been predicted to function as GPCRs in plants based on topology predictions (Moriyama et al., 2006) and interaction with GPA1 using the split-ubiquitin system in yeast (Gookin et al., 2008). However, these may not be GPCRs in the classic sense as Arabidopsis GPA1 is not constitutively GTP bound and may not require GPCR-mediated guanine-nucleotide exchange to accomplish signal transduction (Johnston et al., 2007a). Only a few of the putative plant GPCRs have been functionally characterized. GCR1 and GCR2 have been shown to physically bind GPA1 in Arabidopsis; however, GCR1 is not thought to function in direct ABA perception (Cutler et al., 2010). GCR2 was originally suggested to function as an ABA receptor, but its role as a GPCR involved in ABA signaling is still in doubt (Gao et al., 2007; Johnston et al., 2007b; Liu et al., 2007; Guo et al., 2008; Risk et al., 2009; Cutler et al., 2010).
More recently, Arabidopsis GTG1 and GTG2 have been identified and described as GPCR-type G proteins that have been shown not only to interact with GPA1 in vitro but also to exhibit specific GTP binding and intrinsic GTPase activity (Pandey et al., 2009). The evidence for a role of Arabidopsis GTGs in ABA perception came both from binding studies reporting specific, saturable ABA binding to purified recombinant GTG proteins and from genetic studies that indicated that a gtg1 gtg2 mutant was hyposensitive to ABA in several classic responses, such as seed germination, cotyledon greening, and primary root growth. In addition, expression of ABA-responsive genes was markedly reduced in the gtg1 gtg2 mutant (Pandey et al., 2009).
We independently cloned GTG1 and GTG2 from Arabidopsis and isolated new gtg1 gtg2 double mutants. We show that the GTGs contribute to fertility, plant growth, and responses to light and sugars, but our molecular and genetic data using these mutants indicate that they respond normally in classic ABA responses.
RESULTS
Primary Structure of GTG1 and GTG2 and Phylogenetic Analysis of the GTG/GPHR Family
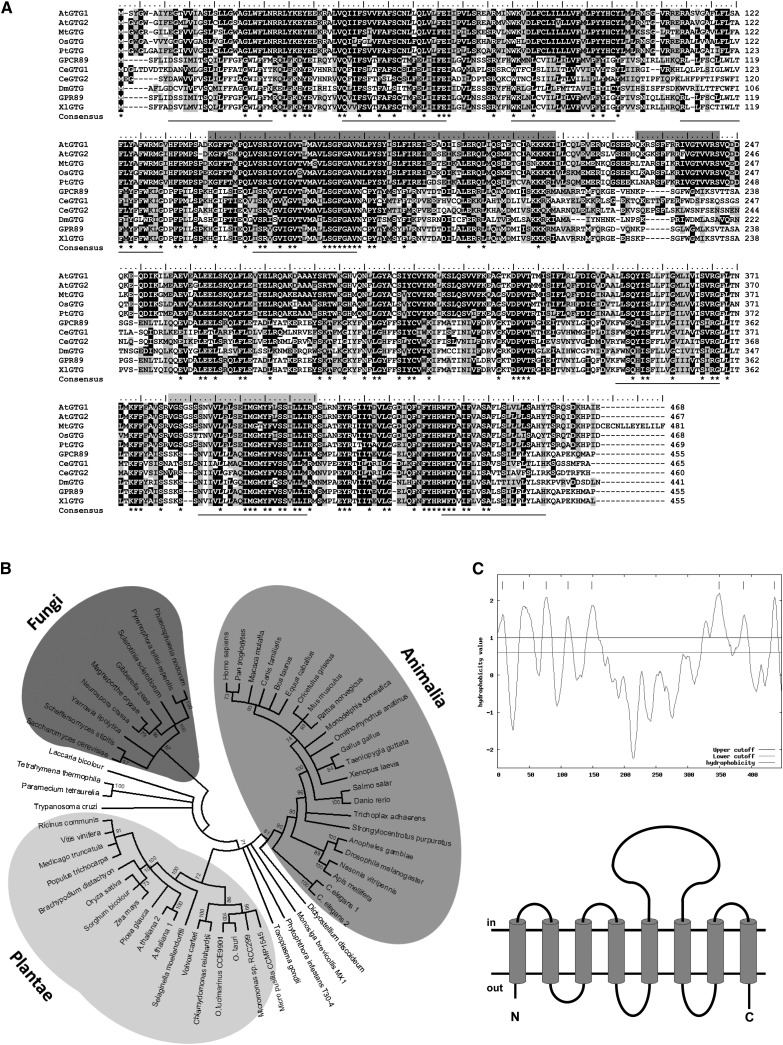
GTG1 and GTG2 cDNA and genomic sequences were independently cloned from Arabidopsis Columbia (Col-8). cDNA sequences are identical to those predicted in The Arabidopsis Information Resource (http://www.Arabidopsis.org). GTG1 and GTG2 are 468– and 467–amino acid proteins, respectively, with a predicted molecular mass of 53.5 kD. They are 90% identical at the amino acid level and are part of a conserved family of proteins found in plants, animals, and fungi (Figure 1A; see Supplemental Table 1 online).
Figure 1.
GTG/GPHR Is a Conserved Family of Predicted Membrane Proteins Found in Plants, Animals, and Fungi.
(A) Sequence alignment of GTG/GPHR proteins from a range of plants and animals. At GTG1 and At GTG2 (Arabidopsis), Mt GTG (Medicago truncatula), Os GTG (Oryza sativa), Pt GTG (Populus trichocarpa), GPCR89 (H. sapiens), Ce GTG1 and Ce GTG2 (Caenorhabditis elegans), Dm GTG (Drosophila melanogaster), GPR89 (Mus musculus), and Xl GTG (Xenopus laevis). The protein sequences show a minimum of 39% sequence identity, with identical amino acids shown in black; asterisks indicate completely conserved amino acids. Membrane domains are indicated by underlining. Dark-gray shading above sequences indicates potentially important domains: a 70–amino acid sequence domain referred to as the DUF3735 family (Pfam), which is found in eukaryotes and has a conserved LSG sequence motif; a region in GTG1 and GTG2 with similarity to the degenerate Ras GTPase-activating protein domain. Light-gray shading indicates a protein kinase ATP binding region signature.
(B) Phylogenetic analysis of predicted full-length GTG/GPHR proteins from a range of eukaryotic organisms. A nonrooted, bootstrap consensus tree in circle formation is shown. The sequences and alignment used to generate the tree are available as Supplemental Data Set 1 online. The GenBank accession numbers of the proteins used are shown in Supplemental Table 2 online.
(C) Hydropathy plot and topology model of deduced GTG1 amino acid sequence. A 10–amino acid interval size was used for this analysis (positive values are hydrophobic) (http://gcat.davidson.edu/DGPB/kd/kyte-doolittle.htm; Kyte and Doolittle, 1982). Predicted protein structure was generated using the ConPred_v2 transmembrane domain prediction software (available at http://bioinfo.si.hirosaki-u.ac.jp/∼ConPred2).
From phylogenetic analysis, the plant, animal, and fungal GTG/GPHR proteins group separately according to their kingdom: Plantae, Fungi, and Animalia (Figure 1B; see Supplemental Table 2 and Supplemental Data Set 1 online). Several sequences from organisms that are classified into different kingdoms are somewhat distinct from these and each other. These include GTG/GPHRs from Dictyostelium discoideum (Amoebozoa), Monosiga brevicollis (Opisthokonta), Trypanosoma cruzi (Excavata), Phytophthora infestans, Toxoplasma gondii, Tetrahymena thermophila, and Paramecium tetraurelia (Chromalveolata). The algal GTGs separate from those of the land plants. Alignment of the predicted protein sequences of Arabidopsis GTG1 and GTG2 with those of different plant and animal GTGs is shown in Figure 1A. The plant sequences show a high level of conservation and share regions of similarity with animal GTGs throughout the protein. Overall, GTG1 and GTG2 are 42 and 43% identical, respectively, at the amino acid level to hamster GPHR, which has recently been proposed as an anion channel critical for Golgi acidification and function (Maeda et al., 2008). There is less conservation with fungal sequences from Neurospora crassa and Saccharomyces cerevisiae, but again regions of similarity are seen throughout the proteins (see Supplemental Figure 1 and Supplemental Table 1 online).
GTG1 and GTG2 are predicted to consist of eight to 10 transmembrane domains with a large cytoplasmic domain between the 5th and 6th membrane-spanning helices (Figure 1C; see Supplemental Table 3 online). Overlapping the 5th membrane domain is a highly conserved region spanning ∼70 amino acids (amino acids 140 to 210) with a single completely conserved G residue that may be functionally important (Figure 1A). Proteins with this conserved region are annotated as members of the DUF3735 family (domain of unknown function 3735), which has only been identified in eukaryotes and contains the conserved amino acid motif LSG (Welcome Trust Sanger Institute; http://pfam.sanger.ac.uk/family). Additionally, Pandey et al. (2009) reported the presence of a Ras GTPase-activating protein domain (amino acids 227 to 243) and an ATP/GTP binding region signature (amino acids 380 to 410). These are annotated in Figure 1A, but it should be noted that the nucleotide binding site overlaps a predicted transmembrane domain and may therefore not be solvent exposed. The nucleotide coding sequences of GTG1 and GTG2 are over 80% conserved; GTG1 and GTG2 consist of 13 exons that are the same size, except for exon four of GTG1, which has an additional three nucleotides. These exons are separated by intron/exon splice points that are conserved between the two genes. These data suggest the two genes are probably the result of a relatively recent gene duplication event that is likely to have occurred after the divergence of Arabidopsis from other plants and may explain why Arabidopsis has two functional genes unlike most other plants, which have one.
Isolation of T-DNA Insertion Mutants
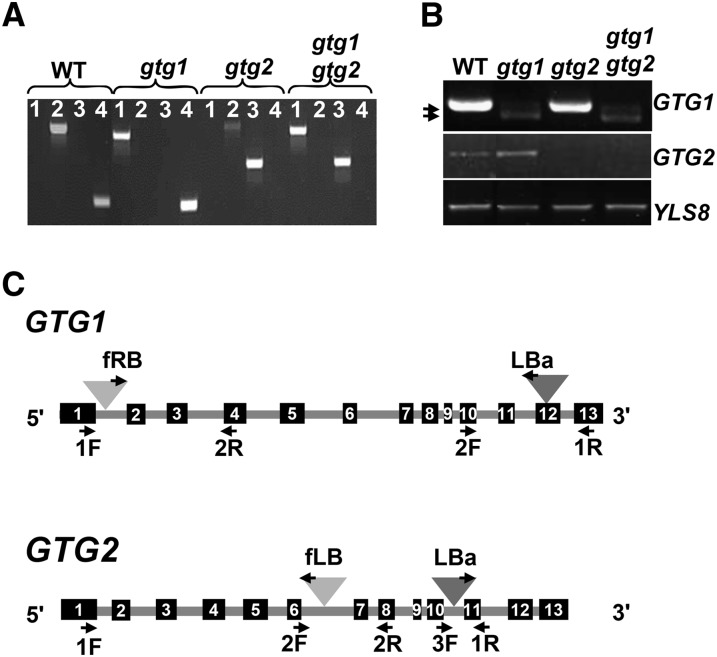
We isolated SALK and FLAG T-DNA insertion mutants in Col and Wassilewskija (Ws) backgrounds, respectively, for both GTG1 and GTG2. These have been designated gtg1-2, gtg1-3, gtg2-2, and gtg2-3 and are new mutant alleles compared with those previously isolated (Pandey et al., 2009). Using these single mutants, we generated the double mutants gtg1-2 gtg2-2 and gtg1-3 gtg2-3, hereafter referred to as Col gtg1 gtg2 and Ws gtg1 gtg2, respectively. Wild-type and mutant alleles were genotyped by PCR using gene-specific diagnostic primers flanking the insertion site (see Supplemental Table 4 online). The positions of the T-DNA inserts are shown in Figure 2C and were confirmed by sequencing. PCR using wild-type genomic DNA as a template generated products of the correct size for GTG1 and GTG2, but no equivalent products were observed for the mutants (Figure 2A; see Supplemental Figure 2A online).
Figure 2.
Isolation of T-DNA Insertional Mutants for GTG1 and GTG2.
(A) Genotyping mutant lines in Col background by PCR on genomic DNA. Genomic DNA from the wild type (WT), gtg1-2, gtg2-2, and gtg1-2 gtg2-2 were used with primers pairs (1) GTG1 2F plus T-DNA LBa primers, which detect T-DNA in GTG1; (2) GTG1 2F plus GTG1 1R, which are gene-specific primers amplifying GTG1; (3) GTG2 1R plus T-DNA LBa, which detect T-DNA in GTG2; and (4) GTG2 3F and GTG2 1R, which are gene-specific primers amplifying GTG2.
(B) Gene expression in mutant lines (Col background) analyzed by RT-PCR. Expression of full-length transcripts for GTG1 (1407 bp) and GTG2 (1404 bp) was determined using primer pairs GTG1 F plus GTG1 Stop R and GTG2 P2 F plus GTG2 Stop R, respectively. Control gene YLS8 was amplified using primers YLS8 1F and YLS8 2R (607-bp product for cDNA). Products using GTG2-specific primers were generated with wild-type (center panel, lane 1) and gtg1-2 mutant (center panel, lane 2) DNA. GTG1-specific primers products were generated with wild-type (top panel, lane 1) and gtg2-2 mutant plants (top panel, lane 3) DNA. Two very faint products, indicated by the arrows, were observed using the GTG1 primers in gtg1-2 (top panel, lane 2) and gtg1-2 gtg2-2 plants (top panel, lane 4).
(C) GTG1 and GTG2 genomic organization with T-DNA insertion positions. Dark-gray triangles, position of T-DNA insertion in gtg1-2 and gtg2-2 (Col); light-gray triangles, position of T-DNA insertion in gtg1-3 and gtg2-3 (Ws). Primers used in the genotyping are indicated. Black boxes indicate exons 1 to 13; gray line indicates introns. All primers are listed in Supplemental Table 4 online.
RT-PCR expression analysis with gene-specific primers resulted in full-length 1.4-kb products for GTG1 and GTG2 from wild-type samples, but no full-length product could be amplified from gtg1-3, gtg2-2, and gtg2-3 homozygous mutant samples, indicating that these are null mutants (Figure 2B; see Supplemental Figure 2B online). However, for gtg1-2 and the Col gtg1 gtg2 double mutants, RT-PCR using GTG1-specific primers produced two faint products slightly <1.4 kb (Figure 2B). Sequencing showed these to have resulted from T-DNA excision. In the case of the smaller 1276-bp product, a region 671 bases from the end of the 11th intron through to the start of the 13th exon of GTG1 has been spliced out, whereas the larger 1385-bp product results from the loss of 22 nucleotides that span the T-DNA insertion in the 12th exon. If translated, the 1276- and 1385-bp cDNAs would generate truncated proteins of 387 and 405 amino acids, respectively. Although the loop domain remains intact in the two possible proteins, both lack the last two predicted membrane domains and therefore may not be functional.
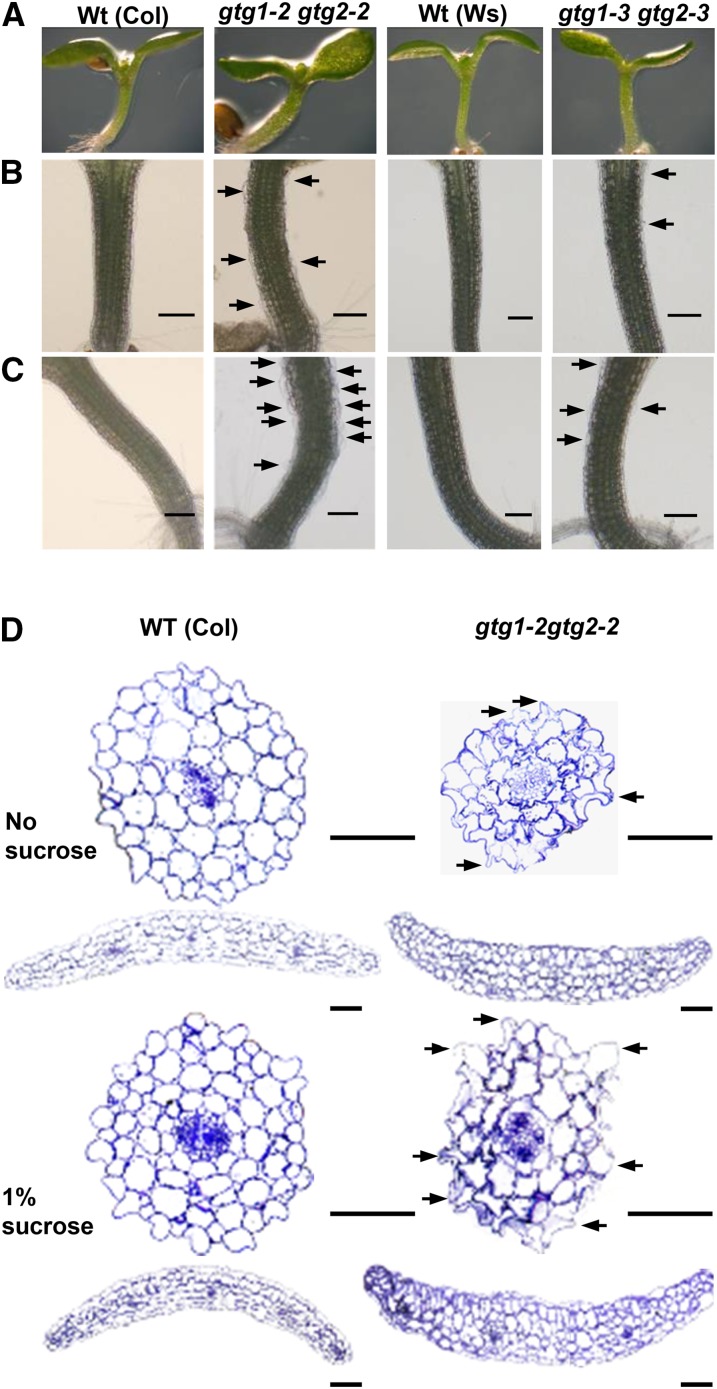
gtg1 gtg2 Mutants Show Defects in Seedling Growth
When grown on standard agar plates, the single mutants appeared similar to the wild type (data not shown); however, the gtg1 gtg2 mutants showed an altered morphology. The hypocotyls were slightly thicker, and large distended cells were often seen at the hypocotyl surface (Figures 3A and 3B). This phenotype was exacerbated in the presence of Suc (Figure 3C). In transverse sections of the hypocotyl, there was a severe disruption of cellular shape in the double mutants, and irregularly shaped cells were particularly evident in the epidermal layer (Figure 3D; see Supplemental Figure 3 online). A disrupted epidermal layer was also seen in cotyledon sections from the double mutants (Figure 3D; see Supplemental Figure 3 online).
Figure 3.
gtg1 gtg2 Mutants Exhibit Cellular Distortion.
Wild type (Wt) and double mutant seedlings grown in the light for 6 d.
(A) Images of wild-type and double mutant shoots grown on 0.5× MS showing distortion in hypocotyl and cotyledons.
(B) Light microscopy of hypocotyls of seedlings grown on 0.5× MS showing distended epidermal cells in the double mutants as indicated by black arrows. Bars = 200 μm.
(C) Light microscopy of hypocotyls of seedlings grown on 0.5× MS plus 1% Suc showing increased distortion of cells in double mutants. Bars = 200 μm.
(D) Transverse sections through hypocotyls and cotyledons of 6-d-old seedlings grown on 0.5× MS plus or minus 1% Suc. Bar = 200 μm.
To investigate the response to light, the insertional mutants were grown in the dark and under low white light (10 μmol m−2 s−1) in the absence and presence of Suc. The mutants showed no significant difference in hypocotyl length from the wild type when grown in the dark but, under low white light, the double mutants were shorter than their corresponding wild type, significantly in the case of the Col gtg1-2 gtg2-2 (Figure 4A). When Suc was included in the medium, the double mutants in both backgrounds were significantly shorter than the wild type under low white light (Figure 4B).
Figure 4.
gtg1 gtg2 Mutants Exhibit a Shorter Hypocotyl in the Light.
Seedlings were grown in the absence (A) and presence (B) of 1% Suc under D and low WL conditions (10 μmol m−2 s−1). Data represent mean ± se (n = three independent biological repeats, each containing five replicate plates with 25 seedlings per plate). *P ≤ 0.05, Student’s t test. WT, wild type.
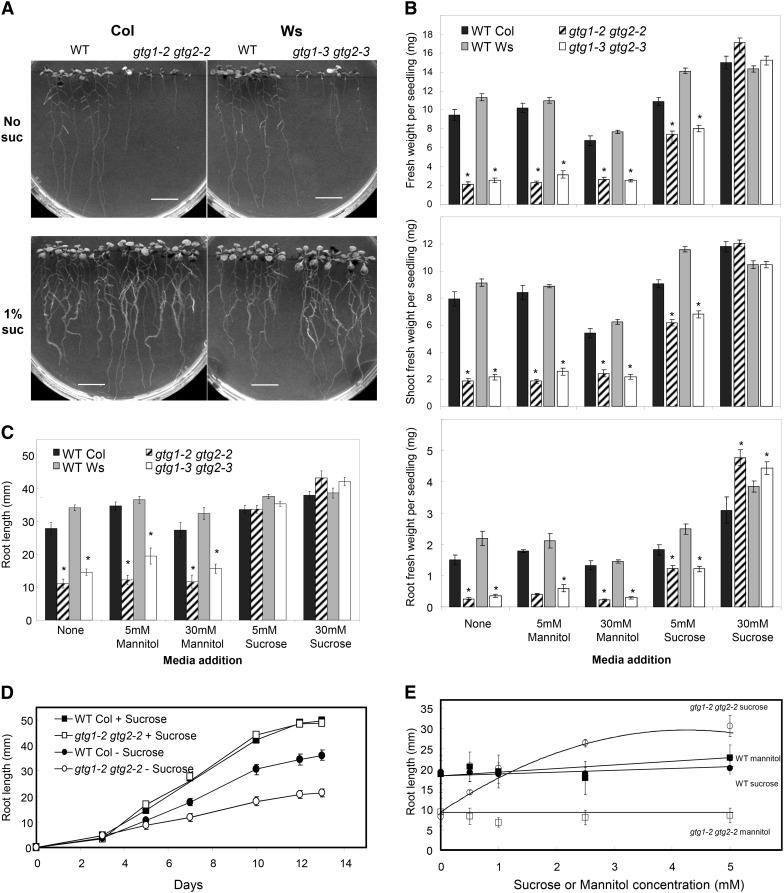
In addition to a short hypocotyl phenotype, gtg1 gtg2 mutants were generally smaller than wild-type seedlings (Figure 5A) with reduced shoot and root biomass (Figure 5B) and a shorter primary root length (Figures 5A and 5C). Suc restored this defect, and in its presence, growth of the double mutant was similar to or exceeded that of the wild type (Figures 5A to 5C). This is not simply an osmotic effect as mannitol had little effect on the growth of the gtg1 gtg2 mutants (Figure 5B). Time-course measurements showed that, in the absence of Suc, the gtg1 gtg2 mutants germinated at the same time as the wild type and root growth was similar until day five, when growth rate was reduced in the mutant compared with wild type (Figure 5D). In the presence of Suc, no marked difference in root growth rate was observed in the gtg1 gtg2 mutants compared with the wild type (Figure 5D): 5 mM Suc could partially rescue the biomass defect on half-strength Murashige and Skoog (MS) and fully rescue the root length defect, and even 1 mM Suc could rescue the root length defect (Figure 5E).
Figure 5.
Response of gtg1 gtg2 Mutants to Suc.
(A) Root growth of gtg1 gtg2 mutants is inhibited in the absence of Suc. Representative plates are shown for the wild type (WT) and gtg1 gtg2 mutants on 0.5× MS containing 0 or 1% Suc.
(B) and (C) Response of mutants to growth on Suc and mannitol. Seedling ([B], top), shoot ([B], middle), and root ([B], bottom) fresh weight and root length (C) are shown for seedlings grown on Suc or mannitol.
(D) Time course for root extension with and without 1% Suc.
(E) Concentration curve for root extension of the wild type and gtg1 gtg2 mutants with Suc or mannitol. Data are from representative experiments and represent the mean weights or root lengths ± se determined from six replicate plates per treatment each containing six seedlings per genotype. *P ≤ 0.05, Student’s t test.
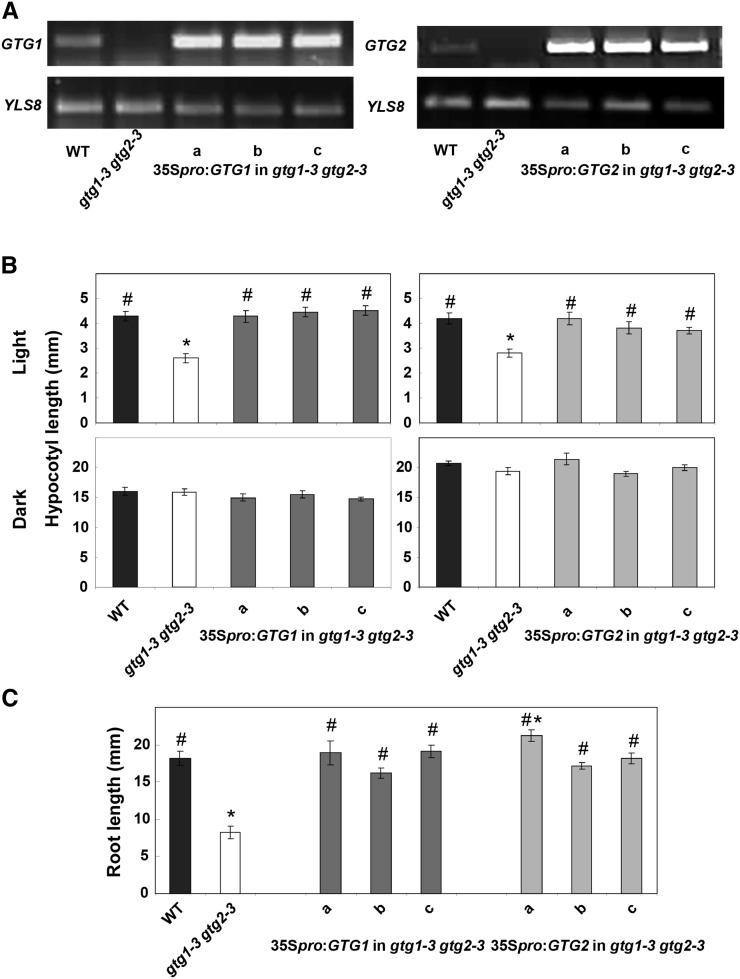
Expression of GTG1 or GTG2 Complements the gtg1-3 gtg2-3 Mutant
To confirm that these phenotypes resulted from the loss of GTG proteins, we transformed the Ws gtg1 gtg2 mutant with either 35Spro:GTG1 or 35Spro:GTG2. Three independent T3 homozygous lines were characterized for each gene. Expression analysis by RT-PCR showed that these lines all expressed the relevant gene (Figure 6). In all cases, both GTG1 and GTG2 fully rescued the short hypocotyl and root phenotypes of Ws gtg1 gtg2 mutant seedlings (Figures 6B and 6C).
Figure 6.
GTG1 or GTG2 Expression Rescues the gtg1-3 gtg 2-3 Mutant.
(A) RT-PCR showing expression of GTG1 and GTG2. Expression of GTG1 (1407 bp) and GTG2 (1404 bp) was determined using primer pairs GTG1 F plus GTG1 Stop R and GTG2 P2 F plus GTG2 Stop R, respectively, in the wild type (WT), gtg double mutants, and three independent 35Spro:GTG expressing lines (a to c). The control gene YLS8 was amplified using primers YLS8 1F and YLS8 2R (607-bp product).
(B) Hypocotyl length is restored in gtg double mutants expressing either GTG1 or GTG2. Hypocotyl length was determined after 6 d of growth on 0.5× MS in low light (10 μmol m−2 s−1) or in darkness. Data represent mean of three experiments ± se each containing four plates with 25 seedlings per genotype.
(C) Root length is restored in gtg double mutants expressing either GTG1 or GTG2. Root length was determined after 10 d for seedlings grown on 0.5× MS. Data represent mean of three experiments ± se, each containing five plates, each with six seedlings per genotype. For (B) and (C), * = significantly different from the wild type, # = significantly different from gtg1-3 gtg2-3; P ≤ 0.05, Student’s t test.
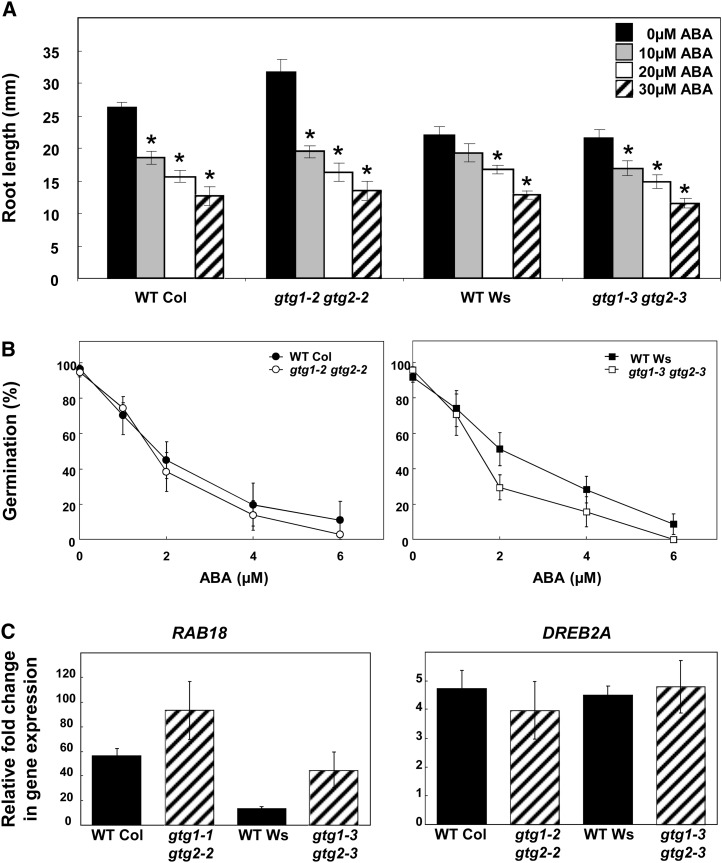
gtg1 gtg2 Double Mutants Are Responsive to ABA
GTG1 and GTG2 were recently reported to be GTG proteins functioning in Arabidopsis as ABA receptors (Pandey et al., 2009). In that study, the double mutant gtg1-1 gtg2-1 showed hyposensitivity to ABA. Therefore, we tested several characteristic ABA responses in the mutant alleles we isolated. Both the gtg1-2 gtg2-2 and gtg1-3 gtg2-3 double mutants showed a similar inhibition of root extension by ABA compared with their respective wild types (Figure 7A). There was also no significant difference in the response to ABA for seed germination in double mutants compared with the wild type (Figure 7B). Finally, we tested the expression of the ABA-induced genes Responsive to ABA18 (RAB 18) and Dre-Binding Protein2A (DREB2A) by real-time RT-PCR using 7-d-old seedlings treated for 4 h with 50 μm ABA or a mock ethanol treatment (negative control). RAB18 and DREB2A both showed significant induction by ABA in wild-type seedlings, but, in contrast with the previous study (Pandey et al., 2009), no loss of induction was seen in either gtg1-2 gtg2-2 or gtg1-3 gtg2-3 mutants (Figure 7C), with RAB18 showing a stronger induction by ABA in the mutants.
Figure 7.
gtg1 gtg2 Double Mutants Are ABA Responsive.
(A) Root growth is inhibited by ABA in the wild type (WT) and gtg double mutants. Data shown represent the mean ± se of three experiments, each with four plates per treatment containing six seedlings per genotype. *Significantly different from value at 0 μM ABA, P ≤ 0.05 Student’s t test.
(B) Germination is inhibited by ABA in the wild type and gtg double mutants. For each ABA concentration, the percentage of germination is presented. Data represent mean ± se of three experiments, each with three plates containing 25 seeds per genotype.
(C) ABA-responsive genes are induced in the wild type and gtg double mutants. Induction of RAB18 and DREB2A expression by 50 μM ABA in the wild type and gtg double mutants using real-time PCR with Actin2 used for normalization. All data are means of four independent biological replicates ± se.
Distortion of Segregation Ratios during Isolation of Double Mutants
When generating the gtg1 gtg2 double mutants, we observed a distortion in the segregation ratios. Crossing of the single gtg1 and gtg2 mutants resulted in gtg1(+/−) gtg2(+/−) double heterozygotes, which were self-fertilized to generate the double homozygous mutant. However, the resulting progeny did not segregate according to the expected Mendelian segregation ratio of 1 in 16 double mutants with no double mutants observed either in the Col or Ws backgrounds. This was confirmed by PCR genotyping of the progeny of 42 Ws gtg1-3(+/−) gtg2-3(+/−) double heterozygous plants. To increase the chances of isolating a double homozygous gtg1-3 gtg2-3 mutant, progeny from 28 gtg1-3(−/−) gtg2-3(+/−) and 52 gtg1-3(+/−) gtg2-3(−/−) parental plants were planted. Although an average of 20 double mutants from a total of 80 seeds (ratio of 1 in 4) would be expected, we only identified three double homozygous plants from the gtg1-3(+/−) gtg2-3(−/−) parental line, a result that was significantly different from predicted (χ2 test, P ≤ 0.05). Similar results were obtained for the Col gtg1-2(+/−) gtg2-2 (−/−) mutant with one double homozygous plant obtained out of 24 progeny tested.
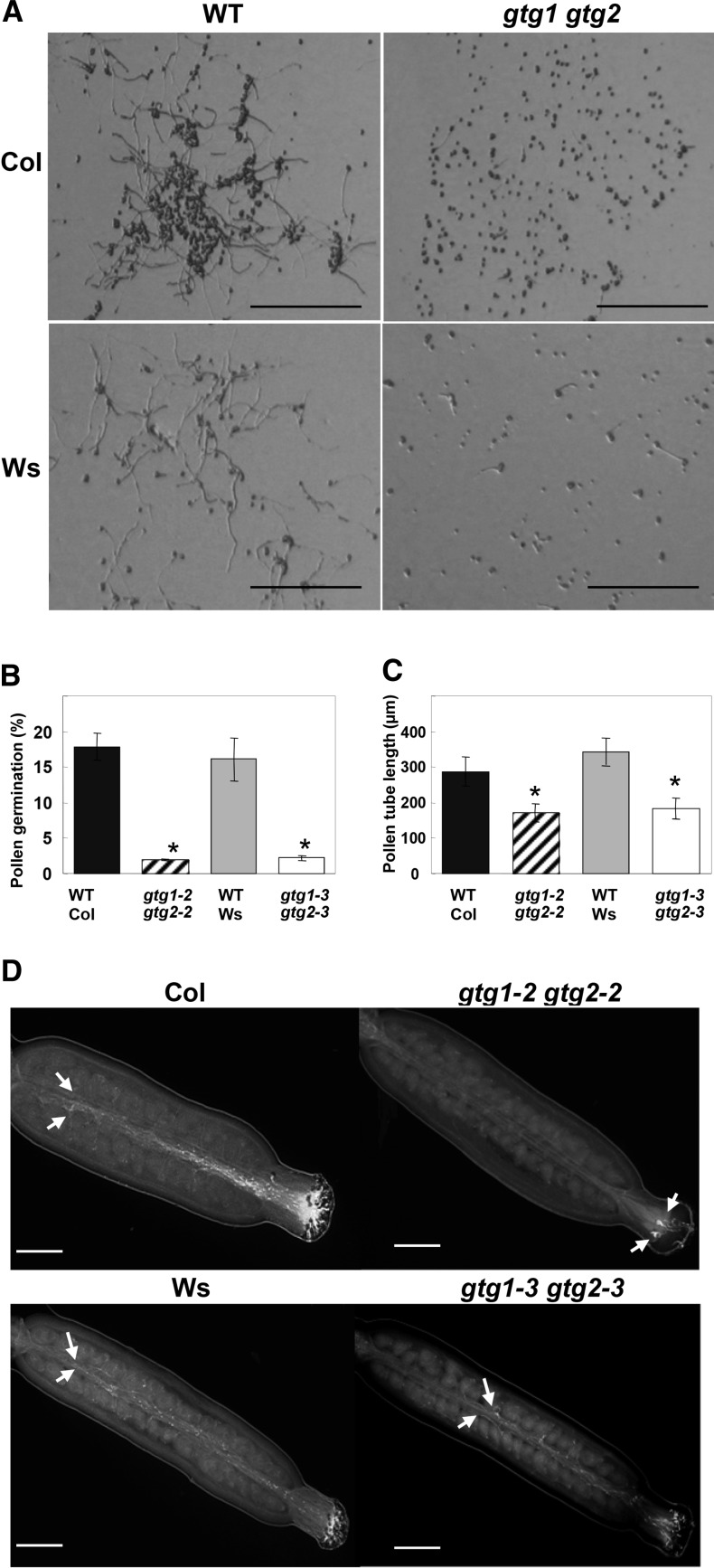
gtg1 gtg2 Mutants Have Defective Pollen
To test whether the distorted segregation ratios could be due to possible defects in gtg1 gtg2 double mutant pollen, a series of in vitro and in vivo pollen germination and pollen tube growth assays were performed (Figure 8). In the in vitro assay where pollen was allowed to germinate on agarose, the double mutants in both backgrounds showed reduced pollen germination and pollen tube extension growth (Figures 8A to 8C). When self-fertilizing stigma were observed, pollen tube growth was less evident in the double mutants than the wild type (Figure 8D). The majority of double mutant pollen tubes penetrated no further than half way along the pistil, whereas the pollen tubes penetrated the entire length of the wild-type stigma (Figure 8D). A semi–in vivo assay in which wild-type and double mutant pollen grains were allowed to germinate and grow through a wild-type stigma showed that wild-type pollen tubes extended the length of the stigma, while mutant pollen tubes showed less growth over the time period examined. This was particularly evident for the Col background (see Supplemental Table 5 online). No obvious defects were observed in the overall flower structure, and anther growth appeared similar in double mutants and their respective wild types.
Figure 8.
gtg1 gtg2 Pollen Have a Reduced Germination Rate and Pollen Tube Growth.
(A) In vitro pollen tube growth for the wild type (WT) and gtg double mutants. Representative images are taken for the wild type and gtg double mutants after 2 h. Bars = 200 μm.
(B) Pollen germination. Data are from a representative experiment of pollen grown in vitro and show the mean ± se from three plants measuring ∼200 pollen from each.*Significantly different from the wild type; P ≤ 0.05, Student’s t test.
(C) Pollen tube length in the wild type and double mutants. Data are from a representative experiment of pollen grown in vitro for 16 h and show the mean ± se of 40 to 60 pollen tubes for germinated pollen.*P ≤ 0.05, Student’s t test.
(D) In vivo pollen growth assay for the wild type and gtg double mutants. Arrows indicate end of extension of pollen tubes, and bar = 200 μm.
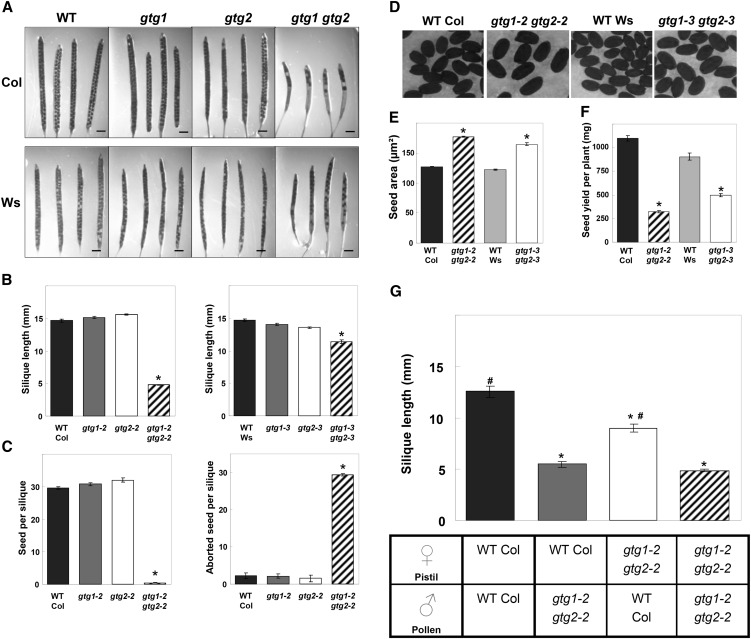
Silique Length and Seed Yield Are Reduced in gtg1 gtg2 Mutants
When the wild type, gtg single, and gtg double mutants were grown on soil under standard conditions, there was a reduced silique length in the double (but not the single) mutants, and this was most marked in the Col gtg1 gtg2 mutant (Figures 9A and 9B). Seed number per silique was also severely reduced in the double mutants with a greater proportion of undeveloped or aborted ovules per silique (Figure 9C), and again this was most pronounced in the Col gtg1 gtg2 mutant. This led to an overall reduction in seed yield per plant (Figure 9F). Although seed number was reduced, double mutant seed was considerably larger than the wild type (Figures 9D and 9E). We tested whether the silique length could be rescued by fertilizing Col gtg1 gtg2 mutants with wild-type pollen. Col gtg1 gtg2 mutant plants fertilized with their own pollen had short siliques, whereas when fertilized with wild-type pollen, silique length was significantly increased but not to wild-type length (Figure 9G). When Col gtg1 gtg2 pollen was used to fertilize wild-type plants, the siliques remained short (Figure 9G).
Figure 9.
Silique and Seed Phenotypes in gtg Mutants.
(A) The gtg double mutants have shorter siliques with fewer seed. WT, the wild type. Bars = 1 mm.
(B) Silique length for the wild type and gtg mutants. Col (left panel) and Ws (right panel). Data represent mean ± se determined for 10 siliques from the primary inflorescences of six plants. *Significantly different from the wild type; P ≤ 0.05, Student’s t test.
(C) Seed number and undeveloped seed per silique. Col (left panel) and Ws (right panel). Data represent mean ± se determined for five siliques from six to eight plants. *Significantly different from the wild type; P ≤ 0.05, Student’s t test.
(D) and (E) Seed size is greater in gtg double mutants than the wild type. Data represent mean ± se for 250 seed (50 seed from five independent plants). *Significantly different from the wild type; P ≤ 0.05, Student’s t test.
(F) Seed yield per plant. Data represent mean ± se from four to six independent plants. *Significantly different from the wild type; P ≤ 0.05, Student’s t test.
(G) Fertilization of gtg1-2 gtg2-2 stigmas with wild-type pollen restores silique length. Reciprocal crosses were made between wild-type and gtg1-2 gtg2-2 plants. For each cross, five maternal plants were used with five to eight stigmas from each. Each stigma was crossed using pollen from at least three different flowers, each from individual plants. Data represent mean ± se from at least 25 siliques for each cross. *Significantly different from wild type × wild type; # = significantly different from gtg1-2 gtg2-2 × gtg1-2 gtg2-2; P ≤ 0.05, Student’s t test.
GTG1 and GTG2 Show Similar Expression Patterns in Both Seedlings and Mature Plants
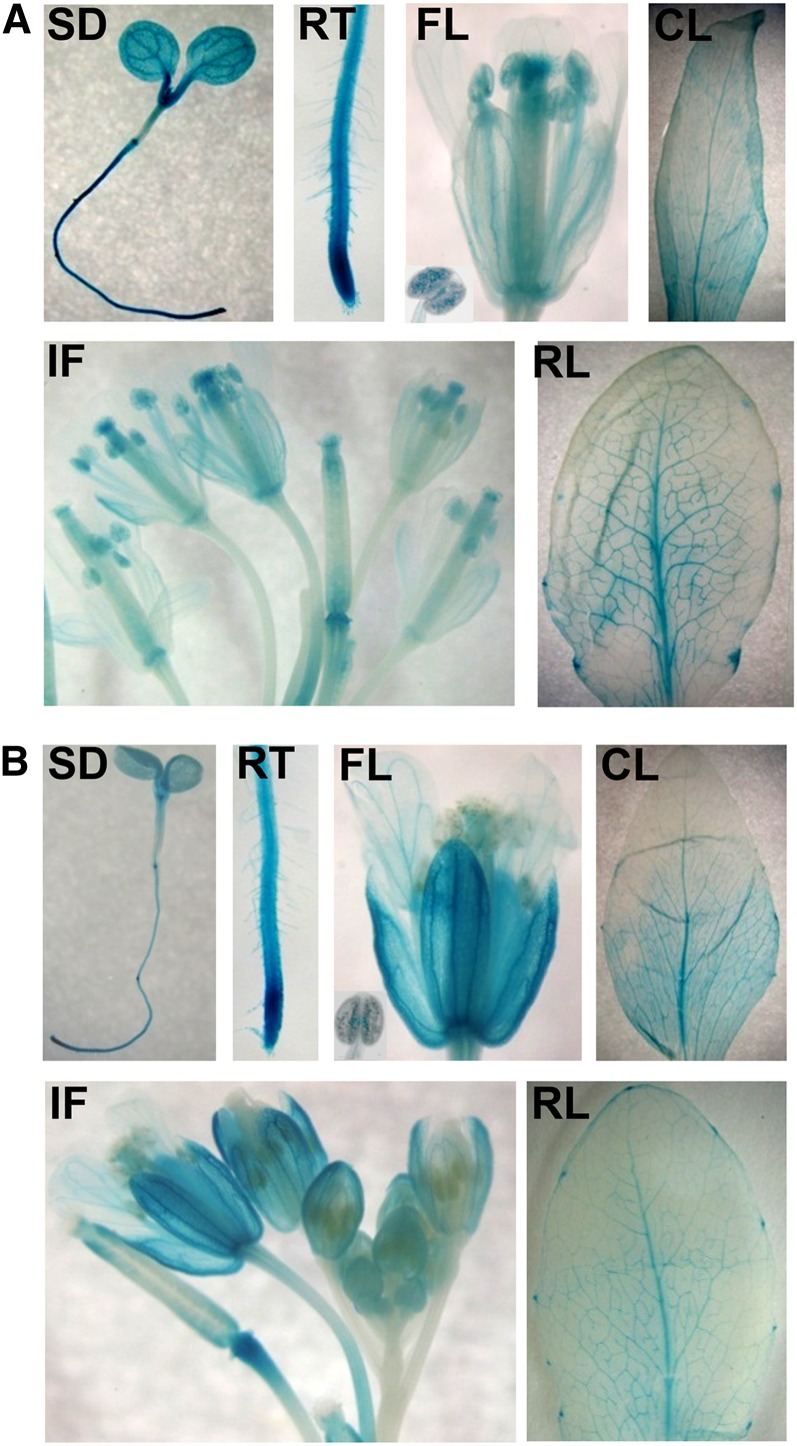
The expression patterns of GTG1 and GTG2 were determined using Arabidopsis plants expressing either GTGpro:GUS (for β-glucuronidase) or GTGpro:GTG-GUS fusions. As both construct types showed very similar expression patterns, the data are shown only for the GTGpro:GTG-GUS–expressing lines. GTG1 and GTG2 are expressed throughout the plant with a similar pattern (Figure 10). Both genes are expressed in the cotyledons, hypocotyl, and root with very strong expression observed in the root tip (Figure 10). High expression was observed in the vascular system in the root, hypocotyl, and cotyledon. In mature plants, expression occurred throughout, but was again particularly strong in the vascular system of mature leaves as well as the hydathodes. The flowers showed expression throughout, with the highest expression levels at the base of the carpel as well as in the stigma, stamen, and pollen.
Figure 10.
GUS Reporter Constructs Reveal a Broad Expression Pattern for GTG1 and GTG2.
(A) Expression of GTG1 genomic GTG1:GTG1-GUS reporter.
(B) Expression of GTG2 genomic GTG2:GTG2-GUS reporter.
CL, cauline leaf; FL, flower and insert photograph of anther showing GUS-stained pollen; IF, inflorescence; RL, rosette leaf; RT, root tip; SD, seedling.
Cellular Localization of GTG Proteins
Transient expression of 35Spro:GTG1-GFP (for green fluorescent protein) and 35Spro:GTG2-GFP in Arabidopsis protoplasts showed localization close to the periphery of the cells as had been reported previously (Pandey et al., 2009). However, we also frequently observed fluorescence within the interior of the protoplast (see Supplemental Figure 4 online). To analyze localization further, we generated Arabidopsis lines transformed with the genomic construct of GTG1 carrying a C-terminal GFP tag (GTG1pro:GTG1-GFP). Overall tissue-specific expression in the seedling was similar to that seen using the GUS-tagged constructs with a high level in the root tip region and also in the tip of developing lateral roots (Figure 11A, a to g). Closer examination of GTG1pro:GTG1-GFP localization within roots showed GFP expression predominantly within Golgi bodies of nonelongated cells within the cell division zone of the root tip (Figure 11A, h and i). As cells began to elongate, Golgi localization was not so apparent, and in fully elongated root cells, there was a more diffuse pattern (Figure 11A, j). Time-lapse studies confirmed that GTG1-GFP was localized to Golgi bodies because of their dynamic characteristics (see Supplemental Movie 1 online). To confirm endomembrane localization, 35Spro:GTG1-GFP was transiently expressed in tobacco (Nicotiana tabacum) leaf cells. GFP fluorescence was located in the endoplasmic reticulum (ER) and Golgi bodies (Figure 11B, a), and Golgi body localization was confirmed by coexpression with the Golgi marker sialyl transferase using 35Spro:ST-mRFP (Figure 11B, b; see Supplemental Movie 2 online).
Figure 11.
Localization of GTG1-GFP in Arabidopsis and Tobacco.
(A) Stable expression of GTG1pro:GTG1-GFP in Arabidopsis. Images a to d show GTG1-GFP expression in root tip, 4′,6-diamidino-2-phenylindole (DAPI)–stained nuclei, combined image, and the transmission light image, respectively. Bars in a to d = 100 μm. GTG1-GFP expression was observed in e, primary root tip, nuclei stained with DAPI; f, primary root tip, nuclei stained with DAPI, combined with transmission light image; g, lateral root primordium. h and i, GTG1-GFP expression in unexpanded root cells showing Golgi localization; j, expanded root file cells showing more diffuse localization. Bars in e to j = 10 μm.
(B) Transient expression of 35Spro:GTG1-GFP in tobacco leaf cells showing ER and Golgi localization. a, expression of GTG1-GFP in ER and Golgi (chloroplasts appear red); b, colocalization of GTG1-GFP (green) with the Golgi marker ST-mRFP (red) shown in yellow. ST-mRFP also marks the apoplastic space (thick red lines) and chloroplasts appear blue. Bars = 20 μm.
Microarray Analysis of the gtg1-2 gtg2-2 Mutant
To provide a better understanding of the impact of the loss of GTG proteins and, therefore, their role in the cell, we performed a microarray analysis on 6-d-old white-light-grown seedlings using the Arabidopsis ATH1 Genome Array through the Nottingham Arabidopsis Stock Centre (NASC) transcriptomics facility. The expression of 295 genes showed significantly altered expression (twofold or greater) in the Col gtg1 gtg2 double mutant compared with the wild type. Increased expression was observed for 194 genes, while 101 had reduced expression in the double mutant. The full list of genes showing twofold regulation together with P values is given in Supplemental Data Set 2 online.
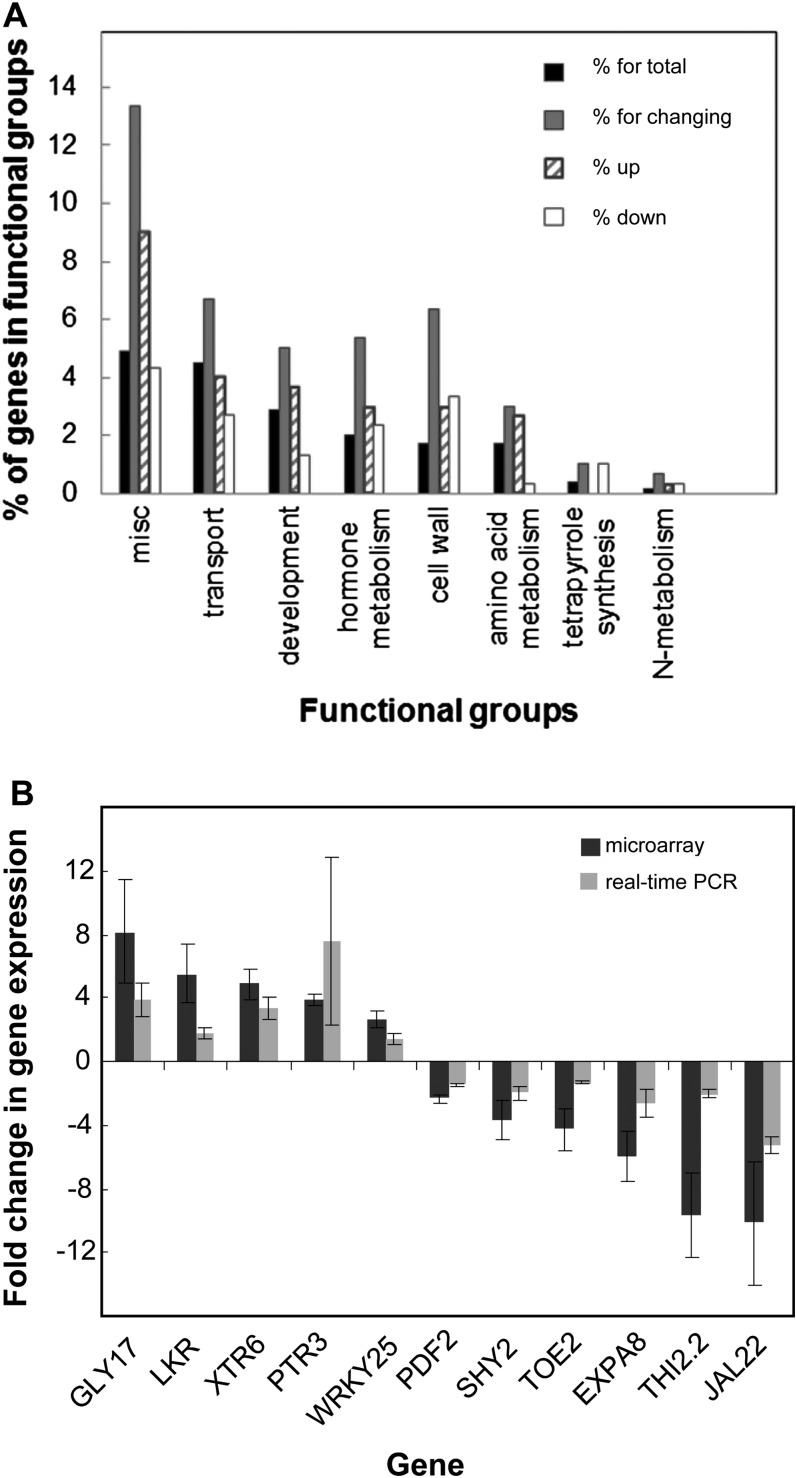
MapMan software (http://mapman.gabipd.org/web/guest/home) was used to examine the functional groups to which these genes belong. Of the 295 genes, 240 belonged to functional groups (see Supplemental Figure 5 online). For a limited number of these functional groups, genes changing in the mutant were enriched compared with the expected number of genes for which we detected expression at the seedling stage. These groups are shown in Figure 12A, and the individual genes with functional groups are given in Supplemental Data Set 3 online. The genes in these groups are important in cell wall formation and modification; resource recycling, including lipid and amino acid metabolism and transport of amino acids, peptides, and sugars; and genes characterized as hormone regulated. In the latter category, there were 16 genes, seven of which are auxin regulated with five showing reduced expression in the double mutant. In addition, four genes, Auxin Response Factor11, Auxin Resistant1, Auxin Resistant3, and Short Hypocotyl2 (SHY2)/Indole-3-acetic acid inducible (IAA3), that, although not designated in this category, are considered to be important in auxin responses, also showed altered expression in Col gtg1 gtg2. Interestingly, MapMan identified only one gene in the hormone functional group that may be involved in ABA regulation: ABF3/DPBF5 (for Abscisic Acid Responsive Elements Binding Factor3), a bZIP transcription factor shown to bind to and activate the DREB2A promoter in an ABRE-dependent manner (Kim et al., 2011). This gene was downregulated in the Col gtg1 gtg2 double mutant.
Figure 12.
Microarray Analysis and Real-Time PCR Reveal Altered Gene Expression in the gtg1 gtg2 Double Mutants Compared with the Wild Type.
(A) Functional groups with a significantly higher percentage of genes changing in the mutant. The number of genes in each functional group is expressed as a percentage. Black bars: percentage assigned for total genes expressed (mapped 9148 genes from 9009). Gray bars: percentage assigned for the genes showing a significant change (twofold or greater) in the mutant (P ≤ 0.1) and includes up- and downregulated genes (mapped 300 from 295 genes); the upregulated genes (striped bars) and downregulated genes (white bars) are indicated separately. Functional groups are those designated by MapMan software (http://mapman.gabipd.org/web/guest/home).
(B) Comparison of expression changes analyzed by microarray and real-time PCR. Microarray expression (black bars) and real-time PCR expression (gray bars) comparing 11 genes in 6-d-old gtg1-2 gtg2-2 and wild-type Col seedlings grown on 0.5× MS. YLS8 was used for data normalization for real-time PCR. Data shown are fold changes for up- and downregulated genes (mean ± se of three or four independent biological replicates).
The reliability of the microarray data was analyzed by selecting 11 genes for further analysis using real-time PCR. A variety of genes was selected that showed a range of expression changes in Col gtg1 gtg2 compared with the wild type in the microarray analysis. As shown in Figure 12B, the real-time PCR results were generally consistent with those obtained from the microarray experiments. SHY2/IAA3 was confirmed as being downregulated, and strong inhibition was also seen for jacalin-related lectin22 (JAL22), one member of a family of genes important in recognition of cell surface carbohydrates and the mobilization and recycling of carbohydrates. Other downregulated genes included Protodermal Factor2 (PDF2), a homeobox gene important in shoot epidermal cell differentiation (Abe et al., 2003). Upregulated genes included the peptide transporter Peptide Transporter Protein3 (PTR3), genes involved in amino acid and carbohydrate metabolism, Saccharopine Dehydrogenase (LKR) and Glyoxalase I family protein17 (GLY 17), Xyloglucan Endotransglycosylase6 (XTR6), a xyloglucan endotransglycosylase likely to be involved in cell wall synthesis, and WRKY DNA-binding protein25 (WRKY25), a jasmonate- and salicylic acid–regulated transcription factor involved in plant defense responses (Zheng et al., 2007).
Given the relative prevalence of auxin-responsive genes identified in the microarray analysis, we investigated the effect of mutations in GTGs on auxin distribution and responses. To test whether auxin distribution was altered in the mutants, the Col gtg1 gtg2 mutant and wild type were transformed with the auxin reporter construct IAA2pro:GUS (Marchant et al., 2002). However, no marked difference in the distribution of staining was observed in the root (see Supplemental Figure 6 online), suggesting that auxin distribution is unaffected in Col gtg1 gtg2. Next, we examined hypocotyl and root growth in wild-type and mutant seedlings treated with indole-3-acetic acid (IAA). For roots, this was done in the absence of Suc, a condition in which the mutants are shorter than the wild type. As reported previously, hypocotyl extension and primary root extension are both inhibited by IAA in wild-type seedlings (Stowe-Evans et al., 1998; Collett et al., 2000; Rahman et al., 2007). In both Col and Ws backgrounds, the gtg1 gtg2 double mutants also showed inhibition of hypocotyl and root elongation in response to IAA (see Supplemental Figure 5 online), indicating that the gtg1 gtg2 mutants can still respond to auxin.
DISCUSSION
In this study, we have identified double mutants in the closely-related genes GTG1 and GTG2 in both Ws and Col backgrounds. Both double mutants showed a reduction in hypocotyl growth (Figure 4), which was associated with altered cell shape and a thicker more uneven hypocotyl (Figure 3), as well as an inhibition of root growth that could be rescued by Suc (Figure 5). Mature plants also showed severe fertility defects with inhibition of pollen tube growth, short siliques, and a reduced seed yield (Figures 8 and 9). The Ws gtg1 gtg2 mutant is a null mutant, whereas the Col mutant has a very low level of two truncated transcripts, which if translated could lead to small amounts of a truncated protein lacking the last two transmembrane domains (Figure 2). It is not known whether these would be functional, but the mutant phenotype was generally stronger in the Col background than in Ws, suggesting that these truncated transcripts do not contribute significantly. Single mutants showed wild-type responses, indicating that GTG1 and GTG2 function redundantly; their high sequence similarity and apparent recent gene duplication are consistent with this (Figure 1).
Are GTG Proteins Required for ABA Signaling?
Arabidopsis GTG proteins were identified and annotated as putative GPCRs based on an in silico analysis (Pandey et al., 2009). They suggested that GTGs function as plasma membrane–localized ABA receptors and reported that a gtg1 gtg2 double mutant (Ws background) exhibited reduced ABA sensitivity in classic ABA responses. This included reduced sensitivity to ABA inhibition of germination and root growth, as well as the loss of ABA-induced gene expression (Pandey et al., 2009). However, the mutants isolated in this study showed no loss of response to ABA (Figure 7). In addition, detailed morphological analysis of ABA-deficient seedlings, such as aba1 (Barrero et al., 2005), has not revealed evidence of distended hypocotyl cells and other characteristic features observed for gtg double mutants in our study. Although ABA-deficient seedlings are typically reduced in hypocotyl and root growth, this reduced stature is maintained in more mature plants (Finkelstein et al., 2002). We observed no obvious differences under standard conditions in stature between wild-type and gtg double mutant plants even though GTG1 and GTG2 are expressed throughout development.
The original proposal that GTG proteins function as ABA receptors came from experiments demonstrating ABA binding (Pandey et al., 2009), but concerns have been raised about the ABA binding assays used (Risk et al., 2009). The GTG1 and GTG2 proteins were reported to bind ABA with Kd values of 35.8 and 41 nM, respectively (Pandey et al., 2009), but this could mean that saturation would occur under normal physiological conditions (McCourt and Creelman, 2008; Risk et al., 2009). The low stoichiometry of binding (0.01 mol ABA/mol protein), suggested to be due to suboptimal conditions of protein purification, solubilization, and renaturation (Pandey et al., 2009), means that the binding affinities should be interpreted with caution (Risk et al., 2009).
GTG proteins are actually one of many recent ABA receptor candidates. The FCA, GCR2, and CHLH proteins have all been proposed as ABA receptors based in part on their ability to bind ABA (Razem et al., 2006; Shen et al., 2006; Liu et al., 2007). FCA, the first proposed ABA receptor, was subsequently shown to be unable to bind ABA (Risk et al., 2008), and the original publication has also since been retracted (Razem et al., 2008). Some controversy also surrounds the role of GCR2, which has also been shown to lack ABA binding (Risk et al., 2009), while various gcr2 mutant combinations all fail to show loss of ABA sensitivity (Gao et al., 2007; Guo et al., 2008). The role of CHLH as an ABA receptor has also been questioned with similar concerns about the ABA binding assays used (Risk et al., 2009) and the absence of any deficiency in ABA signaling in the barley (Hordeum vulgare) CHLH mutant xanF (Müller and Hansson, 2009). Recently, evidence has been presented that suggests that although CHLH does affect ABA signaling in stomatal guard cells, it is not an ABA receptor (Tsuzuki et al., 2011). In contrast with the proteins described above, the evidence that the PYR/PYL/RCAR family of START domain proteins function as ABA receptors is compelling. In work from a number of laboratories, it has been shown that PYR/PYL/RCAR proteins interact with protein phosphatase 2C to bind ABA in the cytoplasm (Ma et al., 2009; Park et al., 2009; Santiago et al., 2009; Nishimura et al., 2010).
It has long been suspected that there may be both intracellular and extracellular receptors for ABA (Cutler et al., 2010), and it has been proposed that the GTGs are plasma membrane ABA receptors (Pandey et al., 2009). Our results from Arabidopsis stable expression lines show that GTG1 is localized within the cell in the Golgi, while transient expression in tobacco shows expression in the ER and Golgi with an overlap with the Golgi marker sialyl transferase (Figure 11; see Supplemental Figure 7 online). We did observe GTG1 and GTG2 at the protoplast periphery in transient assays as also seen by Pandey et al. (2009), but we also found them intracellularly. An internal localization is consistent with that observed with the ortholog GPHR from Chinese hamster (Cricetulus griseus), which was found to colocalize to the Golgi as well as the ER (Maeda et al., 2008).
GTG Proteins as GPCRs
The GTG proteins are predicted to have eight or nine membrane spanning regions (Figure 1) and were interpreted by Pandey et al. (2009) as having GPCR-like topology. They also show sequence homology to a human orphan receptor, GPR89. However, neither the GTGs nor GPR89 are predicted to have the classic GPCR topology of seven-membrane-spanning α-helical segments separated by alternating intracellular and extracellular loop regions (Moriyama et al., 2006; Rosenbaum et al., 2009). Also, there is no functional evidence that Hs GPR89 functions as a GPCR. Indeed, GPHR from Chinese hamster, the only mammalian homolog of the GTGs characterized to date, exhibits voltage-dependent anion channel activity, believed to be critical in the control of Golgi acidification and as a consequence in a range of Golgi functions, including trafficking (Maeda et al., 2008). The Golgi/ER localization observed for Arabidopsis GTGs would also argue against them functioning as classic GPCRs. Another feature of GPCRs is that they interact with heterotrimeric G proteins (Temple and Jones, 2007). The Arabidopsis Gα subunit GPA1 interacted with GTG1 and GTG2 in split ubiquitin and coimmunoprecipitation assays (Pandey et al., 2009). The plant GTG proteins are proposed to contain a conserved ATP/GTP binding region, and the Arabidopsis GTG proteins were shown to specifically bind and hydrolyze GTP (Pandey et al., 2009). However, this may not be a general property of this family as Hs GPHR did not exhibit GTP binding or GTPase activity (Pandey et al., 2009). It should also be noted that the proposed ATP/GTP binding region overlaps with a predicted membrane spanning domain and may not be functional.
While the role of GPA1 in GTG protein function awaits further confirmation since the split-ubiquitin system and coimmunoprecipitation can both give rise to spurious results (Risk et al., 2009), can the phenotype of the gtg double mutants be fully or partially accounted for by changes in Gα function? One significant aspect of the growth phenotype of the gtg1 gtg2 double mutant is that inhibition of hypocotyl growth was light dependent with no difference seen in the dark. Interestingly, overexpression of Gα also results in a light-dependent inhibition of hypocotyl elongation, although this was not associated with a noticeable alteration in tissue structure (Okamoto et al., 2001; Jones et al., 2003). Gα mutants also display moderately shorter hypocotyls, but in this case, the phenotype is observed in both light and dark conditions (Ullah et al., 2001; Trusov et al., 2008). Overexpression of Gα also inhibits seedling root growth, while gpa1 mutants showed a wild-type root growth response in the absence of additional treatments (Ullah et al., 2003; Okamoto et al., 2009). Finally, overexpression of Gα has also been shown to result in reduced silique size, while Gα mutants were not strongly affected (Ullah et al., 2003). From these observations, we might speculate that aspects of the gtg1 gtg2 phenotype might result from increased Gα activity from the absence of the interaction with GTGs. If GTGs do function as major Gα-interacting proteins, then the phenotype with regard to the role of Gα might be expected to be somewhat analogous to Gβ mutants (increased Gα and decreased Gβ). Interestingly, Gβ mutants do have shorter hypocotyls with increased girth as well as shorter siliques (Ullah et al., 2003). While the role of G protein signaling in the phenotype of gtg1 gtg2 mutants clearly requires further analysis, the similarity in the processes regulated does suggest there may be a close association between the two.
The Role of GTGs in Arabidopsis
It has been noted that GTGs appear to affect a wide range of processes, all of which show some overlap with regulation by G proteins. The molecular basis of the gtg1 gtg2 phenotype is still unclear, but our data provide some important clues. In keeping with the mutant phenotype, the GTGs are widely expressed in different organs and tissues. Our results using genomic GUS fusions are similar to those shown previously with promoter GUS constructs (Pandey et al., 2009) with strong expression in roots, particularly at the root tip and shoots as well as in flowers with high expression in the anthers, pollen, and the stigma.
A common feature of all aspects of the phenotype is reduced growth, and our microarray data are consistent with this phenotype. A major category of genes affected were those involved in cell wall synthesis and modification, suggesting that the gtg1 gtg2 mutants may have cell wall defects. This is certainly consistent with the observed distortions of the hypocotyl and cotyledon cells (particularly epidermal cells), which seems to be related to irregular cellular expansion. The Golgi plays a major role in cell wall synthesis, and the localization of GTGs to the Golgi and ER suggests that if Golgi function is impaired in gtg1 gtg2 mutants this could result in altered cell wall synthesis and subsequent growth defects. However, it should be remembered that growth can occur normally under certain conditions; for example, hypocotyl growth in the dark or root growth in the presence of Suc and a signaling role for the GTGs cannot yet be excluded. Interestingly, in relation to a connection with G protein signaling (see discussion above), a recent study has revealed a novel role for G proteins in regulating cell wall modifications (Klopffleisch et al., 2011). There are precedents for defects in Golgi function resulting in reduced growth through altered signaling. The det3 mutant lacking V-ATPase activity in the trans-Golgi network has reduced hypocotyl growth due to an oxylipin-dependent process (Brüx et al., 2008), while RAB geranylgeranyl transferase deficiency leading to reduced vesicle trafficking also results in reduced growth (Hála et al., 2010). In both cases, however, seedlings displayed a constitutive photomorphogenic phenotype in the dark, which was not observed for gtg double mutants.
The rescue of root growth on Suc (but not mannitol) may be related to possible roles in sugar signaling or transport, and it is interesting in this regard that the hypocotyl phenotype is exacerbated on Suc both in terms of length and cell distortion. Previous studies have identified two genetically distinct pathways controlling hypocotyl elongation in the dark and the light and the procuste1 mutant of Arabidopsis has reduced hypocotyl growth in the dark but elongates normally in the light (Desnos et al., 1996), the opposite of the gtg1 gtg2 phenotype. The physiological basis of this genetic difference may lie in the observation that basipetal auxin transport is required only for elongation in light-grown seedlings (Jensen et al., 1998). We originally hypothesized that the observed effect of the gtg1 gtg2 mutations might be caused by disruption of auxin transport. This hypothesis was supported by the microarray data, which demonstrated changes in a number of auxin-regulated genes. However, we were unable to rescue the hypocotyl or root defects with auxin and the mutants responded normally to these treatments. There also didn’t appear to be any disruption to the localization of auxin, based on the expression of the IAA2pro:GUS reporter. It is likely that the changes in gene expression observed here reflect the central role played by auxin in plant growth (Jaillais and Chory, 2010).
One of the most significant phenotypes we observed was the effect of the gtg1 gtg2 mutants on fertility. In vitro assays showed that pollen germination is reduced in gtg1 gtg2 mutant pollen as is pollen tube elongation, and this was also apparent in vivo. The reduced pollen tube growth and the distorted segregation ratios for the original isolation of the double mutant suggest that the fertility defects are related to pollen functionality. The observed defects in pollen tube extension and, hence, their ability to fertilize ovules in the double mutant could also explain the defects seen in silique size as there is a strong correlation between seed number and silique length in Arabidopsis (Cox and Swain, 2006). The partial rescue of silique length when gtg1 gtg2 mutants were fertilized with wild-type pollen is consistent with these results and the primary fertility defect residing in the pollen.
In conclusion, we demonstrated that Arabidopsis GTG proteins are fundamental to growth and development of seedlings and also have a critical role in pollen tube growth and plant fertility. In contrast with a previous report, the mutants isolated here respond normally to ABA. Given the high conservation of the GTG family of proteins across eukaryotes (animals, fungi, and plants), it might be expected that they perform a fundamental and common cellular function. In this respect, another role put forward for this family is as voltage-dependent anion channels, which are important in the control of acidification and Golgi functions (Maeda et al., 2008). Certainly the localization data we have shown for Arabidopsis GTG1, together with some of the phenotypes we have observed, could indicate that the plant GTGs may have a similar role. A role independent of G protein activation would be consistent with the observation that GTGs are present in a number of eukaryotes, such as the green algae, that lack heterotrimeric G proteins (Anantharaman et al., 2011). It would be interesting in the future to determine whether the plant GTGs have channel activity when expressed in a lipid bilayer and whether they can rescue the defect in Golgi acidification seen in the GPHR-deficient mutant cell line.
METHODS
Plant Material
Plants were grown in soil containing equal proportions of vermiculite, Levingtons M2, and John Innes No. 2 compost (Fargro) in 8-cm pots in a growth room in 16 h white light (WL) (cool-white fluorescent tubes with a fluence rate of 120 μmol m−2 s−1) at 23°C with an 8-h dark (D) period at 18°C. For growth on media, seeds were surface sterilized with 70% ethanol for 1 min and 10% (v/v) bleach for 10 min, washed five times in sterile distilled water, and plated on growth media plates containing 0.8% (w/v) agar and 0.5× MS salt medium (Sigma-Aldrich) unless otherwise specified.
Seeds of potential Arabidopsis thaliana mutants gtg1-2, gtg2-2, gtg1-3, and gtg2-3 were originally obtained from the NASC (http://nasc.nott.ac.uk) and the Institut National de la Recherche Agronomique (France) (http://dbsgap.versailles.inra.fr/publiclines), respectively. Isolation and confirmation of T-DNA insertional mutants was performed following the procedure previously described (Mills et al., 2005, 2008). Briefly, seeds were grown and selected on kanamycin to obtain homozygous lines. The T-DNA insertion sites were confirmed by PCR using the following cycling parameters: 95°C for 2 min, followed by 35 cycles of 95°C, 30 s; 56°C, 30 s; and 72°C 2 min using Arabidopsis genomic DNA that was prepared using the DNAMITE plant kit (Microzone). The resulting products were sequenced (Geneservice) using a T-DNA left-border primer and gene-specific primers (see Supplemental Table 4 online). Homozygous lines were crossed to generate two double mutants, gtg1-2 gtg2-2 and gtg1-3 gtg2-3, in the wild-type ecotypes Col and Ws, respectively.
Germination Assay
The ABA germination assay was performed as previously described (Pandey et al., 2009). Sterilized seeds were plated on 0.5× MS media, 1% (w/v) Suc, and 0.8% (w/v) agar containing final concentrations of 0, 1, 2, 4, and 6 μM ABA (AG Scientific). Following plating, seeds were stratified in the dark at 4°C for 2 d and then transferred into 16 h WL (120 μmol m−2 s−1)/8 h D cycles at 22°C. Germination was scored as the emergence of the radicle at 72 h from 25 seeds per genotype per plate with three technical replicates for each of three biological repeats.
Root and Hypocotyl Growth Analysis
For root experiments, sterilized seeds were placed individually on growth media plates containing 0.8% (w/v) agar and 0.5× MS salts with modifications for some experiments as specified in results. Plated seeds were placed in D at 4°C for 2 d and then transferred into WL (120 μmol m−2 s−1) for 6 to 14 d at 23°C. For experiments using ABA, the root length assay described previously (Pandey et al., 2009) was used. Sterilized seeds were plated on 0.5× MS with 1% (w/v) Suc and 0.8% (w/v) agar, placed in D at 4°C for 2 d, and then placed vertically into 16 h WL/8 h D cycles at 23°C for 72 h. Seedlings were then transferred to new media containing 0, 10, 20, and 30 μM ABA and grown vertically for a further 10 d. For hypocotyl experiments seeds were sown as above, but transferred from 4°C to WL for 2 h before being placed in D for 5 d or transferred to WL after 22 h D at 23°C. For experiments with low WL, a neutral density filter 211/210 (Led Filters) was used to obtain 10 μmol m−2 s−1. For analysis, photographic images of plates were taken and seedling root and hypocotyl lengths measured using ImageJ software (http://rsbweb.nih.gov/ij/).
Determining Effect of Auxin on Hypocotyl and Root Growth
The distribution of auxin in the wild type and double mutants was monitored by determining the expression of the IAA2pro:GUS reporter (Swarup et al., 2007). The construct was kindly supplied by M.J. Bennett (Nottingham, UK) and transformed into plants as described later.
Pollen Tube Growth Assays
Pollen was harvested and germinated exactly as previously described (Boavida and McCormick, 2007). For germination assays, pollen tubes were imaged at 2 and 16 h postgermination using a Leica MZ16F dissection microscope (Leica Microsystems). For the in vivo pollen tube assay, pistils were chosen from 2-d-old early stage flowers when the petals had just opened and were prepared for staining by fixation in acetic acid:chloroform:ethanol (10:30:60; v:v:v) for 2 h before being transferred to 4 M NaOH for 10 min and washed three times with 50 mM KPO4 buffer, pH 7.5. The cleared pistils were stained overnight with decolorized 0.1% (w/v) aniline blue solution in 50 mM KPO4 adjusted to pH 10.0 with NaOH. Pistils were then pressed gently between a slide and cover slip and imaged on a Zeiss Axiovert 200 inverted microscope (Carl) with 436/20-nm excitation and 535/30-nm emission band-pass filters.
Cloning of GTG1 and GTG2 and Production of Expression Constructs
GTG1 and GTG2 were isolated from wild-type Col cDNA generated by RT-PCR from whole seedling RNA or genomic DNA. GTG1 and GTG2 cDNAs were PCR amplified using specific primer pairs: Gate GTG1 F and Gate GTG1 R, and Gate GTG2 P2 F and Gate GTG2 R, respectively (see Supplemental Table 4 online for all primer sequences). The PCR products were TA cloned into pENTR/D-TOPO (Invitrogen). Genomic sequences were PCR amplified using HiFi Taq (Invitrogen) and gene-specific primers. The 5′ promoter sequences of 1289 bp for GTG1 and 952 bp for GTG2 were amplified using the gene-specific primer pairs GTG1 Pro F1 and GTG1 Pro R, and GTG2 Pro F1 and GTG2 Pro R before TA cloning into pENTR/D-TOPO. Genomic sequences of 6970 bp for GTG1 and 6003 bp for GTG2, including the 5′ promoter, introns, and exons, were amplified using gene-specific primer pairs attB1GTG1 Pro F1 and GTG1 Pro R, and attB1GTG2 Pro F1 and GTG2 Pro R, respectively, and the products were BP cloned into pDONRZeo (Invitrogen). All construct sequences were confirmed by sequencing (Geneservice). Confirmed entry clones were LR recombined into destination vectors as required following the manufacturer’s instructions (Invitrogen). cDNA clones were recombined into pMDC32 and pMDC83, while promoter and genomic clones were transferred into pMDC107 (GFP) and pMDC163 (GUS) (Curtis and Grossniklaus, 2003). Promoters of 1289 bp for GTG1 and 952 bp for GTG2 were introduced into pMDC163 to create the GUS reporter constructs GTG1pro:GUS and GTG2pro:GUS, respectively, while 6970 bp of GTG1 and 6003 bp of GTG2 genomic sequence were introduced into pMDC163 to create the GTG1pro:GTG1-GUS and GTG2pro:GTG2-GUS constructs.
Protein Topology Prediction Analysis
Hydropathy analysis was conducted according to Kyte and Doolittle (1982) (http://gcat.davidson.edu/DGPB/kd/kyte-doolittle.htm). Predicted protein topology was generated using the ConPred_v2 transmembrane domain prediction software (available at http://bioinfo.si.hirosaki-u.ac.jp/∼ConPred2).
Phylogenetic Analysis
The evolutionary history was inferred using the neighbor-joining method (Saitou and Nei, 1987). The bootstrap consensus tree inferred from 1000 replicates is taken to represent the evolutionary history of the taxa analyzed and was generated using MEGA5 (http://www.megasoftware.net/mega.html) (Tamura et al., 2011). Branches corresponding to partitions reproduced in <70% bootstrap replicates are collapsed to give the condensed tree. The multiple sequence alignment was made with the ClustalW module within MEGA5 using default parameters:gap opening penalty = 11; gap extension penalty = 1; protein weight matrix = BLOSUM with residue specific and hydrophylic penalties; gap separation distance = 5, and a 30% delay divergent cutoff. The sequences and alignment used to generate the tree are available as Supplemental Data Set 1 online.
Plant Transformation
Stable transformation of Arabidopsis was performed as previously described (Mills et al., 2010) using the floral dipping method (Clough and Bent, 1998) and with the appropriate selection. Homozygous T3 plants were used for analysis. For tobacco (Nicotiana tabacum) transient transformation, Agrobacterium tumefaciens GV3101 containing GTG1 and GTG2 cDNA clones in pMDC83 were used with tobacco leaf cells as described previously (Sparkes et al., 2006).
Transfection of Arabidopsis Protoplasts for GFP Imaging
GTG1 and GTG2 cDNAs were cloned into mGFP pART7 and transfected into protoplasts using the method of Yoo et al. (2007). To determine localization of GFP signal in protoplasts, a Leica confocal microscope was used (Leica TCS SP2; Leica Microsystems).
Microarray Analysis
Approximately 50 gtg1-2 gtg2-2 mutant and wild-type Col seedlings were grown on 0.5× MS for 6 d. Total RNA was isolated from ∼300 mg of Arabidopsis seedling tissue for the mutant and wild type as described previously (Stephenson and Terry, 2008). Affymetrix chip analysis was performed through the NASC using the Arabidopsis ATH1 Genome Array. Three biological repeats were performed. Expression signal values were generated for each chip, and these were normalized using signal values for control probes contained in each chip by NASC (Craigon et al., 2004). Chip data for a pair (a wild type and a mutant) were considered as one repeat. If probe signal values for both the wild type and mutant samples fell to <50 in at least two out of three repeats, then these data were not considered for analysis and removed. Genes exhibiting twofold or more enhanced or reduced expression over the three repeats (P ≤ 0.1) were considered for further analysis using MapMan software (http://mapman.gabipd.org/web/guest/home).
Real-Time Quantitative PCR and Standard RT-PCR
First-strand synthesis used 0.5 or 1 μg total RNA, which was reverse transcribed using the SuperScript system (Invitrogen) and oligo(dT) primer according to the manufacturer’s instructions. Standard 20-μL PCR reactions were performed using BioMix Red (Bioline), forward (F) and reverse (R) primers with 1 μL of first-strand synthesis cDNA. Reactions were incubated at 95°C for 5 min followed by either 27, 30, or 33 cycles of 95°C for 30s, 56°C for 30s, and 72°C for 90s with a final 72°C extension step of 5 min in a PTC-200 Peltier Thermal Cycler (MJ Research).
Real-time PCR was performed as described previously in a 96-well plate using 0.25 μL cDNA per 10 μL reaction (Stephenson and Terry, 2008). SYBR Green (Finnzymes) was used to monitor cDNA amplification with an Opticon DNA Engine Continuous Fluorescence Detector (GRI Ltd.). Specific F and R primers (see Supplemental Table 4 online) were designed to quantify expression of each gene. PCR was performed at 95°C for 10 min followed by 35 cycles of 95°C for 15 s and 60°C for 1 min. All data were standardized by normalizing to Yellow-Leaf-Specific gene8 (YLS8) or Actin2 expression and analyzed using Opticon software.
For experiments on ABA-induced gene expression, seedlings were grown and treated with 50 μM ABA (AG Scientific) (or mock solvent control) as previously described (Pandey et al., 2009). RNA isolation and real-time RT-PCR conditions and primers for ABA experiments were also exactly as previously described (Pandey et al., 2009).
Microscopy
Plant tissue images were made using a Leica MZ16F dissecting microscope and digital images taken using a Canon Powershot S80. For light microscopy of tissue sections, seedlings were fixed as described previously (Terry et al., 2001). Sections (0.5 µm) were cut on a Leica OMU 3 ultramicrotome and stained with 1% (w/v) toluidine blue in 1% borax and imaged using a Zeiss Axiovert 200 microscope (Carl Zeiss) with a Nikon Coolpix 4500 digital camera. Localization of GFP in transiently and stably transformed plant tissue was done using either a Leica TCS SP2 confocal microscope (Leica Microsystems) or a Zeiss LSM 510 META confocal microscope (Carl Zeiss). GFP excitation was with the 488-nm line of an argon ion laser, and emission was detected between 505 and 530 nm. ST-mRFP localization was done by excitation of mRFP using the 543 line of a HeNe laser, and emission was detected between 560 and 615 nm.
Histochemical GUS Assays
Arabidopsis tissue containing GTGpro:GUS and GTGpro:GTG-GUS were immersed in GUS staining solution (40 mg/mL 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid in 100 mM sodium phosphate buffer, pH 7.0, 10 mM Na2EDTA, 0.5 mM potassium ferrocyanide, 0.5 mM potassium ferricyanide, and 0.1% [v/v] Triton X-100) and incubated at 37°C for 3 h or overnight. Samples were treated with 70% (v/v) ethanol to remove chlorophyll and examined using a Leica MZ16F dissection microscope (Leica Microsystems).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: GTG1 (HM776216, At1g64990); GTG2 (HM776217, At4g27630); RT-PCR control genes: 40S rRNA (At5g18380) and YLS8 (At5g08290). Genes analyzed in Figure 12B are as follows: GLY17 (At1g15380), LKR (At4g33150), XTR6 At4g25810, PTR3 (At5g46050), WRKY25 (At2g30250), PDF2 (At4g04890), SHY2 (At1g04240), TOE2 (At5g60120), EXPA8 (At2g40610), JAL22 (At2g39310), and THI2.2 (At5g36910).See Supplemental Table 2 online for the accession numbers of the proteins used in the phylogenetic analysis in Figure 1B. T-DNA insertion mutants are as follows: gtg1-2 (SALK_128150), gtg1-3 (FLAG_055FO1), gtg2-2 (SALK_055330), and gtg2-3 (FLAG_586C01). The raw microarray data are available at http://affymetrix.Arabidopsis.info/narrays/experimentpage.pl?experimentid=477.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Sequence Alignment of At GTG1 from Arabidopsis and GTG/GPHRs from Neurospora crassa and Saccharomyces cerevisiae.
Supplemental Figure 2. Isolation of T-DNA Insertional Mutants for GTG1 and GTG2 in Ws Background.
Supplemental Figure 3. gtg1-3 gtg2-3 Mutants Exhibit Cellular Distortion.
Supplemental Figure 4. Localization of GFP-Tagged GTG1 and GTG2 at the Periphery and in the Interior of Mesophyll Protoplasts.
Supplemental Figure 5. Functional Groups for Genes Expressed and for Those Changing in the Mutant.
Supplemental Figure 6. Auxin Distribution and Responses in gtg1 gtg2 Mutants.
Supplemental Figure 7. Localization of GTG1-GFP in Arabidopsis and Tobacco.
Supplemental Table 1. Species and Relatedness of Identified GTG Proteins to GTG1 (AAL49849).
Supplemental Table 2. The GenBank Accession Numbers of the Proteins Used in the Phylogenetic Analysis.
Supplemental Table 3. Predicted Protein Structure.
Supplemental Table 4. Primers Used in This Study.
Supplemental Table 5. Crosses between the Wild Type and gtg1 gtg2 Double Mutant Were Made to Observe Pollen Tube Extension in Vivo.
Supplemental Data Set 1. Text File of the Alignment Used for the Phylogenetic Analysis in Figure 1B.
Supplemental Data Set 2. Genes Showing Twofold Up- and Downregulation (P < 0.1) in gtg1 gtg2 Mutant Seedlings Grown for 6d in White Light Compared with the Wild Type.
Supplemental Data Set 3. Genes Belonging to Functional Groups That Have a Significantly Higher Percentage of Genes Changing in the Mutant.
Supplemental Movie 1. Expression of GTG1pro:GTG1-GFP in Arabidopsis Root Tip Cells Showing Golgi Localization.
Supplemental Movie 2. Transient Expression of 35Spro:GTG1-GFP in Tobacco Leaf Cells Showing Golgi Localization.
Acknowledgments
We thank Anton Page (University of Southampton, UK) for tissue sectioning, Michael Duke (University of Southampton, UK) for technical help with growth experiments, Jon Jerram (University of Southampton, UK) for some preliminary analysis, Richard J. Edwards (University of Southampton, UK) for advice on the statistical analysis of microarray data, and Haruko Okamoto (Iwate Medical University, Japan) for critical reading of the article. The IAA2pro:GUS construct was kindly supplied by Malcolm J. Bennett (University of Nottingham, UK). The Biotechnology and Biological Sciences Research Council is gratefully acknowledged for grant support (Grants BB/E008968/1 and BB/F014074/1) and studentship (G.-E.C.F.).
AUTHOR CONTRIBUTIONS
F.W.J., G.-E.C.F., and J.R. performed research, analyzed the data, and contributed to writing the article. B.M.V. performed research. M.J.T. designed the research, analyzed the data, and wrote the article. L.E.W. designed the research, performed research, analyzed the data, and wrote the article.
Glossary
- GTG
G protein–coupled receptor-type G protein
- GPHR
Golgi pH regulator
- ABA
abscisic acid
- GPCR
G protein–coupled receptor
- Col
Columbia
- Ws
Wassilewskija
- MS
Murashige and Skoog
- GFP
green fluorescent protein
- GUS
β-glucuronidase
- NASC
Nottingham Arabidopsis Stock Centre
- IAA
indole-3-acetic acid
- ER
endoplasmic reticulum
- WL
white light
- D
dark
References
- Abe M., Katsumata H., Komeda Y., Takahashi T. (2003). Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 130: 635–643 [DOI] [PubMed] [Google Scholar]
- Anantharaman V., Abhiman S., de Souza R.F., Aravind L. (2011). Comparative genomics uncovers novel structural and functional features of the heterotrimeric GTPase signaling system. Gene 475: 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann S.M. (2002). Heterotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell 14 (suppl.): S355–S373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero J.M., Piqueras P., González-Guzmán M., Serrano R., Rodríguez P.L., Ponce M.R., Micol J.L. (2005). A mutational analysis of the ABA1 gene of Arabidopsis thaliana highlights the involvement of ABA in vegetative development. J. Exp. Bot. 56: 2071–2083 [DOI] [PubMed] [Google Scholar]
- Boavida L.C., McCormick S. (2007). Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J. 52: 570–582 [DOI] [PubMed] [Google Scholar]
- Brüx A., Liu T.Y., Krebs M., Stierhof Y.D., Lohmann J.U., Miersch O., Wasternack C., Schumacher K. (2008). Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth Inhibition in Arabidopsis. Plant Cell 20: 1088–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Collett C.E., Harberd N.P., Leyser O. (2000). Hormonal interactions in the control of Arabidopsis hypocotyl elongation. Plant Physiol. 124: 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.M., Swain S.M. (2006). Localised and non-localised promotion of fruit development by seeds in Arabidopsis. Funct. Plant Biol. 33: 1–8 [DOI] [PubMed] [Google Scholar]
- Craigon D.J., James N., Okyere J., Higgins J., Jotham J., May S. (2004). NASCArrays: A repository for microarray data generated by NASC’s transcriptomics service. Nucleic Acids Res. 32 (Database issue): D575–D577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis M.D., Grossniklaus U. (2003). A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler S.R., Rodriguez P.L., Finkelstein R.R., Abrams S.R. (2010). Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 61: 651–679 [DOI] [PubMed] [Google Scholar]
- Desnos T., Orbović V., Bellini C., Kronenberger J., Caboche M., Traas J., Höfte H. (1996). Procuste1 mutants identify two distinct genetic pathways controlling hypocotyl cell elongation, respectively in dark- and light-grown Arabidopsis seedlings. Development 122: 683–693 [DOI] [PubMed] [Google Scholar]
- Ding L., Pandey S., Assmann S.M. (2008). Arabidopsis extra-large G proteins (XLGs) regulate root morphogenesis. Plant J. 53: 248–263 [DOI] [PubMed] [Google Scholar]
- Finkelstein R.R., Gampala S.S.L., Rock C.D. (2002). Abscisic acid signaling in seeds and seedlings. Plant Cell 14 (suppl.): S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Zeng Q., Guo J., Cheng J., Ellis B.E., Chen J.G. (2007). Genetic characterization reveals no role for the reported ABA receptor, GCR2, in ABA control of seed germination and early seedling development in Arabidopsis. Plant J. 52: 1001–1013 [DOI] [PubMed] [Google Scholar]
- Gookin T.E., Kim J., Assmann S.M. (2008). Whole proteome identification of plant candidate G-protein coupled receptors in Arabidopsis, rice, and poplar: Computational prediction and in-vivo protein coupling. Genome Biol. 9: R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Zeng Q., Emami M., Ellis B.E., Chen J.G. (2008). The GCR2 gene family is not required for ABA control of seed germination and early seedling development in Arabidopsis. PLoS ONE 3: e2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hála M., Soukupová H., Synek L., Zárský V. (2010). Arabidopsis RAB geranylgeranyl transferase beta-subunit mutant is constitutively photomorphogenic, and has shoot growth and gravitropic defects. Plant J. 62: 615–627 [DOI] [PubMed] [Google Scholar]
- Jaillais Y., Chory J. (2010). Unraveling the paradoxes of plant hormone signaling integration. Nat. Struct. Mol. Biol. 17: 642–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P.J., Hangarter R.P., Estelle M. (1998). Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol. 116: 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C.A., Taylor J.P., Gao Y., Kimple A.J., Grigston J.C., Chen J.G., Siderovski D.P., Jones A.M., Willard F.S. (2007a). GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc. Natl. Acad. Sci. USA 104: 17317–17322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C.A., Temple B.R., Chen J.G., Gao Y., Moriyama E.N., Jones A.M., Siderovski D.P., Willard F.S. (2007b). Comment on “A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid”. Science 318: 914. [DOI] [PubMed] [Google Scholar]
- Jones A.M., Ecker J.R., Chen J.G. (2003). A reevaluation of the role of the heterotrimeric G protein in coupling light responses in Arabidopsis. Plant Physiol. 131: 1623–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.S., Mizoi J., Yoshida T., Fujita Y., Nakajima J., Ohori T., Todaka D., Nakashima K., Hirayama T., Shinozaki K., Yamaguchi-Shinozaki K. (2011). An ABRE promoter sequence is involved in osmotic stress-responsive expression of the DREB2A gene, which encodes a transcription factor regulating drought-inducible genes in Arabidopsis. Plant Cell Physiol. 52: 2136–2146 [DOI] [PubMed] [Google Scholar]
- Klingler J.P., Batelli G., Zhu J.K. (2010). ABA receptors: The START of a new paradigm in phytohormone signalling. J. Exp. Bot. 61: 3199–3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopffleisch K., et al. (2011). Arabidopsis G-protein interactome reveals connections to cell wall carbohydrates and morphogenesis. Mol. Syst. Biol. 7: 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R.F. (1982). A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157: 105–132 [DOI] [PubMed] [Google Scholar]
- Liu X., Yue Y., Li B., Nie Y., Li W., Wu W.H., Ma L. (2007). A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science 315: 1712–1716 [DOI] [PubMed] [Google Scholar]
- Ma Y., Szostkiewicz I., Korte A., Moes D., Yang Y., Christmann A., Grill E. (2009). Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Maeda Y., Ide T., Koike M., Uchiyama Y., Kinoshita T. (2008). GPHR is a novel anion channel critical for acidification and functions of the Golgi apparatus. Nat. Cell Biol. 10: 1135–1145 [DOI] [PubMed] [Google Scholar]
- Marchant A., Bhalerao R., Casimiro I., Eklöf J., Casero P.J., Bennett M., Sandberg G. (2002). AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14: 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourt P., Creelman R. (2008). The ABA receptors — We report you decide. Curr. Opin. Plant Biol. 11: 474–478 [DOI] [PubMed] [Google Scholar]
- Mills R.F., Doherty M.L., Lopez-Marques R.L., Weimar T., Dupree P., Palmgren M.G., Pittman J.K., Williams L.E. (2008). ECA3, a Golgi-localized P-2A-type ATPase plays a crucial role in manganese nutrition in Arabidopsis. Plant J. 146: 116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills R.F., Francini A., Ferreira da Rocha P.S., Baccarini P.J., Aylett M., Krijger G.C., Williams L.E. (2005). The plant P1B-type ATPase AtHMA4 transports Zn and Cd and plays a role in detoxification of transition metals supplied at elevated levels. FEBS Lett. 579: 783–791 [DOI] [PubMed] [Google Scholar]
- Mills R.F., Valdes B., Duke M., Peaston K.A., Lahner B., Salt D.E., Williams L.E. (2010). Functional significance of AtHMA4 C-terminal domain in planta. PLoS ONE 5: e13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama E.N., Strope P.K., Opiyo S.O., Chen Z., Jones A.M. (2006). Mining the Arabidopsis thaliana genome for highly-divergent seven transmembrane receptors. Genome Biol. 7: R96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller A.H., Hansson M. (2009). The barley magnesium chelatase 150-kd subunit is not an abscisic acid receptor. Plant Physiol. 150: 157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N., Sarkeshik A., Nito K., Park S.Y., Wang A., Carvalho P.C., Lee S., Caddell D.F., Cutler S.R., Chory J., Yates J.R., Schroeder J.I. (2010). PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J. 61: 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Göbel C., Capper R.G., Saunders N., Feussner I., Knight M.R. (2009). The α-subunit of the heterotrimeric G-protein affects jasmonate responses in Arabidopsis thaliana. J. Exp. Bot. 60: 1991–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Matsui M., Deng X.W. (2001). Overexpression of the heterotrimeric G-protein α-subunit enhances phytochrome-mediated inhibition of hypocotyl elongation in Arabidopsis. Plant Cell 13: 1639–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S., Chen J.-G., Jones A.M., Assmann S.M. (2006). G-protein complex mutants are hypersensitive to abscisic acid regulation of germination and postgermination development. Plant Physiol. 141: 243–256 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pandey S., Nelson D.C., Assmann S.M. (2009). Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell 136: 136–148 [DOI] [PubMed] [Google Scholar]
- Park M.Y., Chung M.S., Koh H.S., Lee D.J., Ahn S.J., Kim C.S. (2009). Isolation and functional characterization of the Arabidopsis salt-tolerance 32 (AtSAT32) gene associated with salt tolerance and ABA signaling. Physiol. Plant. 135: 426–435 [DOI] [PubMed] [Google Scholar]
- Rahman A., Bannigan A., Sulaman W., Pechter P., Blancaflor E.B., Baskin T.I. (2007). Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J. 50: 514–528 [DOI] [PubMed] [Google Scholar]
- Razem F.A., El-Kereamy A., Abrams S.R., Hill R.D. (2006). The RNA-binding protein FCA is an abscisic acid receptor. Nature 439: 290–294 Retracted. Nature 456: 824. [DOI] [PubMed] [Google Scholar]
- Razem F.A., El-Kereamy A., Abrams S.R., Hill R.D. (2008). Retraction. The RNA-binding protein FCA is an abscisic acid receptor. Nature 456: 824. [DOI] [PubMed] [Google Scholar]
- Risk J.M., Day C.L., Macknight R.C. (2009). Reevaluation of abscisic acid-binding assays shows that G-Protein-Coupled Receptor2 does not bind abscisic Acid. Plant Physiol. 150: 6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risk J.M., Macknight R.C., Day C.L. (2008). FCA does not bind abscisic acid. Nature 456: E5–E6 [DOI] [PubMed] [Google Scholar]
- Rosenbaum D.M., Rasmussen S.G., Kobilka B.K. (2009). The structure and function of G-protein-coupled receptors. Nature 459: 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425 [DOI] [PubMed] [Google Scholar]
- Santiago J., Rodrigues A., Saez A., Rubio S., Antoni R., Dupeux F., Park S.Y., Márquez J.A., Cutler S.R., Rodriguez P.L. (2009). Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. Plant J. 60: 575–588 [DOI] [PubMed] [Google Scholar]
- Shen Y.Y., et al. (2006). The Mg-chelatase H subunit is an abscisic acid receptor. Nature 443: 823–826 [DOI] [PubMed] [Google Scholar]
- Sparkes I.A., Runions J., Kearns A., Hawes C. (2006). Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1: 2019–2025 [DOI] [PubMed] [Google Scholar]
- Stephenson P.G., Terry M.J. (2008). Light signalling pathways regulating the Mg-chelatase branchpoint of chlorophyll synthesis during de-etiolation in Arabidopsis thaliana. Photochem. Photobiol. Sci. 7: 1243–1252 [DOI] [PubMed] [Google Scholar]
- Stowe-Evans E.L., Harper R.M., Motchoulski A.V., Liscum E. (1998). NPH4, a conditional modulator of auxin-dependent differential growth responses in Arabidopsis. Plant Physiol. 118: 1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R., Perry P., Hagenbeek D., Van Der Straeten D., Beemster G.T.S., Sandberg G., Bhalerao R., Ljung K., Bennett M.J. (2007). Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19: 2186–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple B.R.S., Jones A.M. (2007). The plant heterotrimeric G-protein complex. Annu. Rev. Plant Biol. 58: 249–266 [DOI] [PubMed] [Google Scholar]
- Terry M.J., Ryberg M., Raitt C.E., Page A.M. (2001). Altered etioplast development in phytochrome chromophore-deficient mutants. Planta 214: 314–325 [DOI] [PubMed] [Google Scholar]
- Trusov Y., Zhang W., Assmann S.M., Botella J.R. (2008). Ggamma1 + Ggamma2 not equal to Gbeta: Heterotrimeric G protein Ggamma-deficient mutants do not recapitulate all phenotypes of Gbeta-deficient mutants. Plant Physiol. 147: 636–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki T., Takahashi K., Inoue S., Okigaki Y., Tomiyama M., Hossain M.A., Shimazaki K.I., Murata Y., Kinoshita T. (2011). Mg-chelatase H subunit affects ABA signaling in stomatal guard cells, but is not an ABA receptor in Arabidopsis thaliana. J. Plant Res. 124: 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H., Chen J.G., Temple B., Boyes D.C., Alonso J.M., Davis K.R., Ecker J.R., Jones A.M. (2003). The beta-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 15: 393–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H., Chen J.G., Young J.C., Im K.H., Sussman M.R., Jones A.M. (2001). Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292: 2066–2069 [DOI] [PubMed] [Google Scholar]
- Wang X.-Q., Ullah H., Jones A.M., Assmann S.M. (2001). G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292: 2070–2072 [DOI] [PubMed] [Google Scholar]
- Yoo S.D., Cho Y.H., Sheen J. (2007). Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zheng Z., Mosher S.L., Fan B., Klessig D.F., Chen Z. (2007). Functional analysis of Arabidopsis WRKY25 transcription factor in plant defense against Pseudomonas syringae. BMC Plant Biol. 7: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]