Complementary, reverse genetic, protein–protein interaction and fusion approaches reveal the requirement of at least two interacting PPR proteins for the editing of a specific site in Arabidopsis plastids.
Abstract
After transcription, mRNA editing in angiosperm chloroplasts and mitochondria results in the conversion of cytidine to uridine by deamination. Analysis of Arabidopsis thaliana mutants affected in RNA editing have shown that many pentatricopeptide repeat proteins (PPRs) are required for specific cytidine deamination events. PPR proteins have been shown to be sequence-specific RNA binding proteins allowing the recognition of the C to be edited. The C-terminal DYW domain present in many editing factors has been proposed to catalyze C deamination, as it shows sequence similarities with cytidine deaminases in other organisms. However, many editing factors, such as the first to be discovered, CHLORORESPIRATORY REDUCTION4 (CRR4), lack this domain, so its importance has been unclear. Using a reverse genetic approach, we identified DYW1, an RNA editing factor acting specifically on the plastid ndhD-1 editing site recognized by CRR4. Unlike other known editing factors, DYW1 contains no identifiable PPR motifs but does contain a clear DYW domain. We were able to show interaction between CRR4 and DYW1 by bimolecular fluorescence complementation and to reconstitute a functional chimeric CRR4-DYW1 protein complementing the crr4 dyw1double mutant. We propose that CRR4 and DYW1 act together to edit the ndhD-1 site.
INTRODUCTION
RNA editing is a sequence-specific posttranscriptional modification leading to an insertion, deletion, or conversion of one or more nucleotides in a precursor RNA. Such modifications are observed in many organisms, including mammals, plants, bacteria, and protists. The first editing event to be described was the posttranscriptional addition of four nucleotides to the mitochondrial coxII transcript in trypanosomes (Benne et al., 1986). In plant organelles, RNA editing occurs as a pyrimidine exchange, resulting in a conversion of a cytidine into a uridine nucleotide (C to U) in mitochondria and plastids of virtually all land plants (Chateigner-Boutin and Small, 2010; Knoop, 2011). More rarely, uridine-to-cytidine (U to C) conversions are observed in organelles of some hornworts, lycopods, and ferns (Chateigner-Boutin and Small, 2010; Knoop, 2011). Thirty-four editing sites have been found in Arabidopsis thaliana chloroplasts (Chateigner-Boutin and Small, 2007), and more than 500 sites have been described in Arabidopsis mitochondria (Giegé and Brennicke, 1999; Bentolila et al., 2008; Zehrmann et al., 2008). RNA editing often restores conserved codons indispensable for synthesis of functional proteins (Bock et al., 1994).
Many thousands of editing events have been reported, but few editing factors have been identified at the molecular level. In mammals, the apoB transcript undergoes a C-to-U deamination that generates a stop codon (CAA to UAA) (Chen et al., 1987; Powell et al., 1987). The APOBEC-1 editing enzyme that catalyzes this modification contains a signature [C/HxE(x)nPCxxC] characteristic of a family of nucleotide deaminases (Teng et al., 1993; Wedekind and McKay, 2003; Iyer et al., 2011). The 11-nucleotide recognition sequence in the apoB transcript is named the “mooring sequence” and is localized 5 nucleotides after the edited cytidine. This sequence is highly conserved in mammals. The APOBEC1 complementation factor (ACF) was shown to specifically bind the mooring sequence (Mehta and Driscoll, 2002). The core editosome complex is constituted by interaction of the editing enzyme APOBEC-1 with the RNA/ACF complex (Blanc et al., 2001; Mehta and Driscoll, 2002).
Although first described over 20 years ago, mRNA editing in plant organelles is still not fully understood. It is observed in almost all land plant groups, but so far, not in algae (Steinhauser et al., 1999). A model similar to the mammalian model of apoB editing has been proposed, in which a specificity factor targeting the appropriate site in the RNA molecule and a catalytic factor carrying a C-to-U deamination catalytic activity could act together (Miyamoto et al., 2002). In tobacco (Nicotiana tabacum), it was demonstrated that 22 nucleotides around the editing site are essential for psbL editing (Chaudhuri and Maliga, 1996) and 14 nucleotides are necessary for ndhB editing (Bock et al., 1996). The first editing specificity factor identified in plants was CHLORORESPIRATORY REDUCTION4 (CRR4) (Kotera et al., 2005). Arabidopsis crr4 mutants are defective in editing of the ndhD transcript at the ndhD-1 site (Kotera et al., 2005). At this site, the ACG codon is converted into AUG to form the translation initiation codon of the NDHD protein, which is a subunit of the chloroplast NADH dehydrogenase-like complex (NDH) involved in cyclic electron flow around photosystem I (Shikanai et al., 1998; Yamamoto et al., 2011). It was proposed that the CRR4 protein is the ndhD-1 recognition factor, binding to a sequence of <36 nucleotides, but does not carry the catalytic activity that could perform the modification of the edited cytidine (Okuda et al., 2006). Subsequently, other factors have been found to be necessary for editing specific sites in plastids, including CHLOROPLAST BIOGENESIS19 (CLB19), necessary for the editing of two sites (Chateigner-Boutin et al., 2008) and many other proteins (Fujii and Small, 2011). In parallel, similar proteins have been found to be involved in mitochondrial RNA editing (Tasaki et al., 2010; Fujii and Small, 2011; Hammani et al., 2011b; Uchida et al., 2011).
All these proteins belong to the pentatricopeptide repeat (PPR) family (Small and Peeters, 2000). PPR proteins are involved in almost all stages of organellar gene expression, from transcription to translation (Andres et al., 2007; Schmitz-Linneweber and Small, 2008). Whereas it has been experimentally demonstrated only for very few of them, it is currently accepted that they act as sequence-specific RNA binding adaptors (Delannoy et al., 2007), and, more hypothetically, that they recruit effector enzymes to the target RNA (Okuda et al., 2006). Strikingly, all the PPR proteins required for specific editing events belong to the PLS subfamily, a particular plant-specific subfamily distinguished by arrays of characteristic variants of the canonical PPR motif and by additional C-terminal domains (Lurin et al., 2004). The PLS PPR proteins can be classified into two subgroups according to the last domain of the protein: the E/E+ subgroup and the DYW subgroup (Lurin et al., 2004; O’Toole et al., 2008). The DYW domain is generally preceded by the E/E+ domain (Lurin et al., 2004). The DYW consensus contains the cytidine deaminase signature HxE(x)nCxxC (Salone et al., 2007). In addition, the phylogenetic distribution of the DYW domain appears to exactly match the phylogenetic distribution of plant organellar RNA editing (Salone et al., 2007; Knoop, 2011; Rüdinger et al., 2011). These results supported the hypothesis that the DYW domain might contain the catalytic activity for editing in plant organelles (Salone et al., 2007). This model is still a matter for debate, and no RNA editing activity of the DYW domain has been demonstrated (Okuda et al., 2009). Moreover, not all plant editing factors contain a DYW domain. For example, CRR4, CRR21, and CLB19 belong to the PLS-E/E+ subgroup comprising 107 proteins in Arabidopsis, most of which are suspected to be editing factors, and all of which lack a terminal DYW domain (Kotera et al., 2005; Okuda et al., 2007; Chateigner-Boutin et al., 2008). Even more detrimentally for the hypothesis of the involvement of the DYW domain in editing, it was shown that deleting the DYW domains of the two editing factors CRR22 and CRR28 does not affect these factors’ ability to restore editing when expressed in the corresponding mutants (Okuda et al., 2009).
Here, we identified a unique Arabidopsis PPR protein (DYW1) composed of a plastid targeting sequence and a DYW domain without any identifiable intervening PPR motifs. The dyw1-1 knockout mutant completely lacks editing at the ndhD-1 site (i.e., is molecularly and physiologically identical to the crr4 mutant). We show that the DYW1 protein interacts in vivo with the CRR4 protein. Our findings suggest that DYW1, by interaction with CRR4, provides in trans an essential function required for editing of the ndhD-1 site.
RESULTS
At1g47580 Encodes a Unique DYW Protein
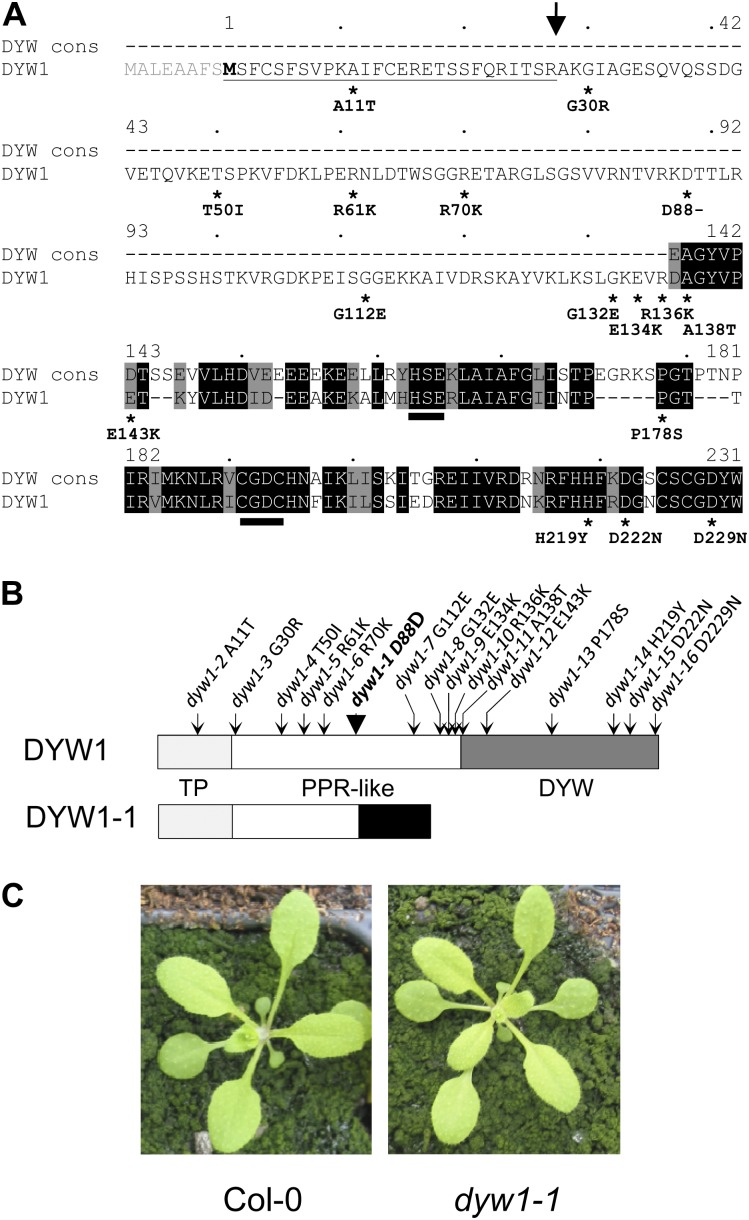
The At1g47580 gene (that we have named DYW1) attracted our attention because it encodes a protein unique in Arabidopsis with a clear DYW domain, characteristic of the PLS-DYW subgroup, but lacking any identifiable PPR motifs. The intronless At1g47580 locus consists of a 717-bp open reading frame (ORF) encoding a putative 239–amino acid protein. This protein is composed of a 110–amino acid region with weak similarity to PPR proteins and a 95–amino acid C-terminal DYW domain (Figure 1A). About 90 Arabidopsis proteins carry a DYW domain at the C terminus, but DYW1 is the only one with no identifiable PPR, E, or E+ motifs. By contrast, its DYW domain is highly conserved and is close to the DYW domain consensus (Figure 1A). This implies that this domain is under strong selection and that DYW1 is not simply a degenerating pseudogene. Two putatively full-length GSLT (GenoScope/LifeTechnologies) cDNAs that correspond to the At1g47580 model have been described (Castelli et al., 2004). Both cDNAs start just after the ATG codon of the AGI model (named hereafter ATG1), indicating that the ATG codon at position +24 (ATG2) is likely to be the translation start used in the plant cell.
Figure 1.
Structure of the Wild-Type DYW1 Protein and the Mutant Proteins Encoded by the Different Alleles.
(A) Alignment of the DYW1 sequence with the DYW domain consensus (Lurin et al., 2004). The DYW1 sequence was aligned against the DYW domain consensus (DYW cons) using the pairwise alignment software at http://pir.georgetown.edu/pirwww/search/pairwise.shtml. The sequence numbering is shown above the sequence according to M2. Identical residues are shaded black, and similar residues are shaded gray. The putative DYW1 targeting peptide is underlined, and the position of a potential cleavage site indicated by an arrow. The HxExnCxxC deaminase signature identified in DYW domains (Salone et al., 2007) is indicated by two black bars. Positions of the 16 substitutions identified in DYW1 coding sequence are indicated by stars under the DYW1 protein sequence. The sequence modification resulting from each mutation is indicated at the protein sequence level (amino acid identity in wild-type sequence/position of the amino acid in the protein sequence/ amino acid identity in the mutant protein).
(B) Structure of the 231–amino acid protein encoded by the DYW1 gene and positions of the EMS mutations. The 27–amino acid targeting peptide (TP) is indicated in light gray and the 95–amino acid DYW domain in dark gray. The PPR-like region in the middle is indicated in white. The PPR-like region of DYW1 is not recognized by any PPR detection software but presents a low similarity to PPR proteins when searching by BLAST. Positions of the mutations are indicated by arrows on the DYW1 protein. The sequence modification resulting from each mutation is indicated at the protein sequence level (amino acid identity in wild-type sequence/position of the amino acid in the protein sequence/amino acid identity in the mutant protein). The structure of the putative DYW1-1 is indicated below the wild-type DYW1 protein. The additional protein sequence unrelated to wild-type DYW1 protein is indicated in black.
(C) Phenotype of a dyw1-1 mutant and Col-0 wild-type plant after 18 d in soil.
[See online article for color version of this figure.]
The dyw1-1 Mutant Has a Frameshift in the First Half of the DYW1 Coding Sequence
Two apparent insertion mutants in the At1g47580 locus are listed in the T-DNA Express database of the Salk Institute (Alonso et al., 2003). After sequence verification of the insertions, we found that the T-DNA in Salk_012425 is inserted 97 bp upstream of the ATG2 codon, whereas the T-DNA in Salk_123655 is inserted ∼150 bp downstream of the ORF. As neither insertion disrupts the DYW1 ORF, we requested a screen for ethyl methanesulfonate (EMS)-induced mutations in the At1g47580 locus from the Seattle Arabidopsis TILLING Project (Till et al., 2003; http://tilling.fhcrc.org/). Forty-three different mutations across the 1500-bp locus were obtained. Among them, 19 were in noncoding regions, eight were in the ORF but did not change the protein sequence, and 16 mutations (dyw1-1 to dyw1-16) did alter the predicted protein sequence (Figure 1; see Supplemental Table 1 online). One of these, the dyw1-1 mutation, was a deletion of a guanidine at the position + 262 (after ATG2) and caused a frameshift in the ORF. The putative 118–amino acid protein encoded by this allele would be identical to the wild-type protein for the first 87 amino acids, then differ with a premature stop codon 31 amino acids after the mutation (Figure 1B). In particular, this truncated protein would completely lack the DYW domain. This mutant and the other alleles were backcrossed three times to Arabidopsis ecotype Columbia-0 (Col-0) to segregate other EMS-induced mutations. Homozygous mutant plants were identified after self-pollination of heterozygous plants obtained after backcrossing. All of them exhibited a macroscopic phenotype identical to wild-type Col-0 plants, as shown for homozygous dyw1-1 mutants (Figure 1C).
DYW1 Is Targeted to Plastids
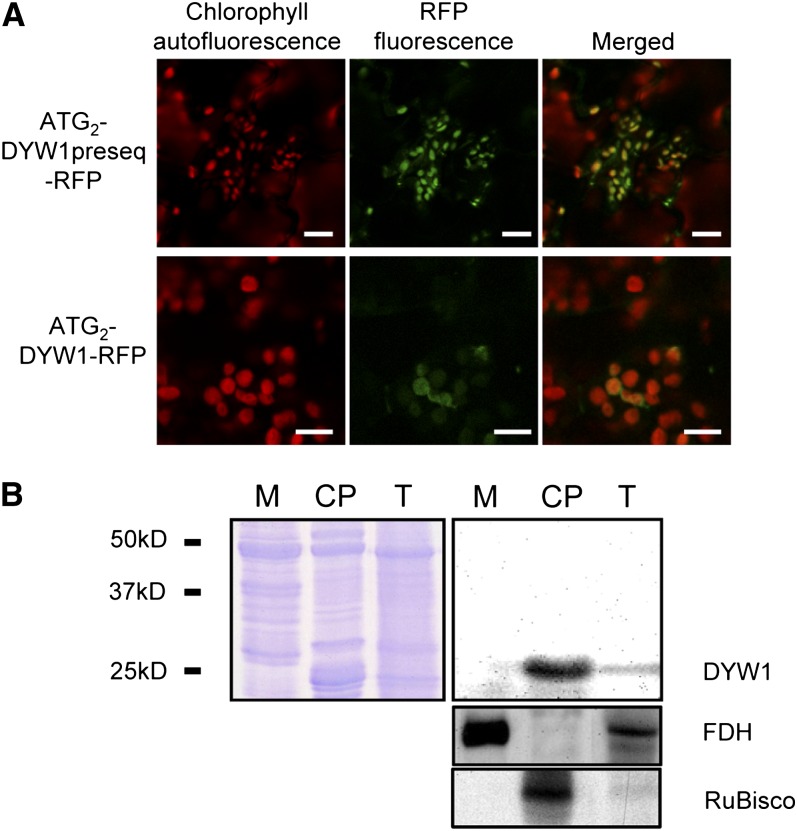
Using either ATG1 or ATG2 (encoding M1 and M2, respectively) as the start of the protein coding sequence, both TargetP (http://www.cbs.dtu.dk/services/TargetP/; Emanuelsson et al., 2007) and Predotar (http://urgi.versailles.inra.fr/predotar/predotar.html; Small et al., 2004) predict that the DYW1 protein is targeted to chloroplasts. The cleavage site is predicted to be after the 27th amino acid (counting from M2) leading to a protein of 22.8 kD after cleavage. To test this localization prediction, two fusion proteins were transiently expressed in Arabidopsis plantlets. The sequences encoding the first 108 or 100 amino acids of the protein (starting from M1 and M2, respectively) were fused in frame upstream of the red fluorescent protein (RFP) coding sequence. Expression of the M1 fusion protein did not lead to detectable fluorescence. By contrast, expression of the M2 fusion protein led to RFP-specific fluorescence signals in plastids (Figure 2A). This observation was further confirmed by expression of the full-length M2 protein fused to RFP (Figure 2A). The emission spectrum of RFP was confirmed for these signals and they were shown to colocalize with chlorophyll autofluorescence (Figure 2A).
Figure 2.
DYW1 Is Localized in Chloroplasts of Arabidopsis Cells.
(A) Fluorescence images of Arabidopsis plantlets transiently expressing DYW1-RFP fusion proteins. Either the first 100–amino acid polypeptide or the full-length DYW1 protein, starting both at the M2 Met encoded by the ATG2 codon, were expressed as a fusion with the RFP at the C terminus. Plantlets were observed by confocal microscopy 4 d after transformation. Bars = 10 μm.
(B) Immunoblot analysis of total extract (T), mitochondrial (M), and chloroplast (CP) protein fractions with antibodies directed against DYW1. Protein fractions from Arabidopsis were analyzed by immunoblots. Loading control (Coomassie blue staining; left panel) shows that equal amounts of total protein were loaded. Black bars represent molecular mass marker positions. The purity of the two fractions was tested with antibodies directed against the mitochondrial formate dehydrogenase (FDH) and the chloroplast large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCo).
A polyclonal antibody directed against DYW1 was used to detect the protein in subcellular fractions. Figure 2B shows that an ∼25-kD signal was observed in a total extract of leaf proteins of Arabidopsis and strongly increased in a subcellular fraction enriched in chloroplasts. By contrast, this signal was not observed in a mitochondrial protein extract.
Both the transient expression of RFP fusions and the analysis of subcellular fractions by immunoblot using a DYW1-specific antibody confirmed the bioinformatics predictions that the DYW1 protein is targeted to plastids.
dyw1-1 Is Not Able to Edit the ndhD-1 Site
Most DYW proteins investigated to date have been shown to be involved in RNA editing (Fujii and Small, 2011; Hammani et al., 2011b; Uchida et al., 2011), so we screened all 34 known chloroplast editing sites (Chateigner-Boutin and Small, 2007) in dyw1-1 by sequencing of RT-PCR products surrounding these sites. Among the 34 sites, a very strong defect was observed at the first editing site of the ndhD transcript (Figure 3A). None of the other sites were affected in the mutant (see Supplemental Figure 1 online). A complete lack of editing of ndhD-1 was confirmed by a quantitative and very sensitive poisoned primer extension (PPE) assay (Figure 3B). The editing of the ndhD-1 site was also characterized by RT-PCR product sequencing in the other 15 dyw1-x alleles. A defect in three lines (dyw1-11, dyw1-12, and dyw1-16) was observed with a reduction of 25 to 40% when compared with Col-0 (see Supplemental Figure 2 online).
Figure 3.
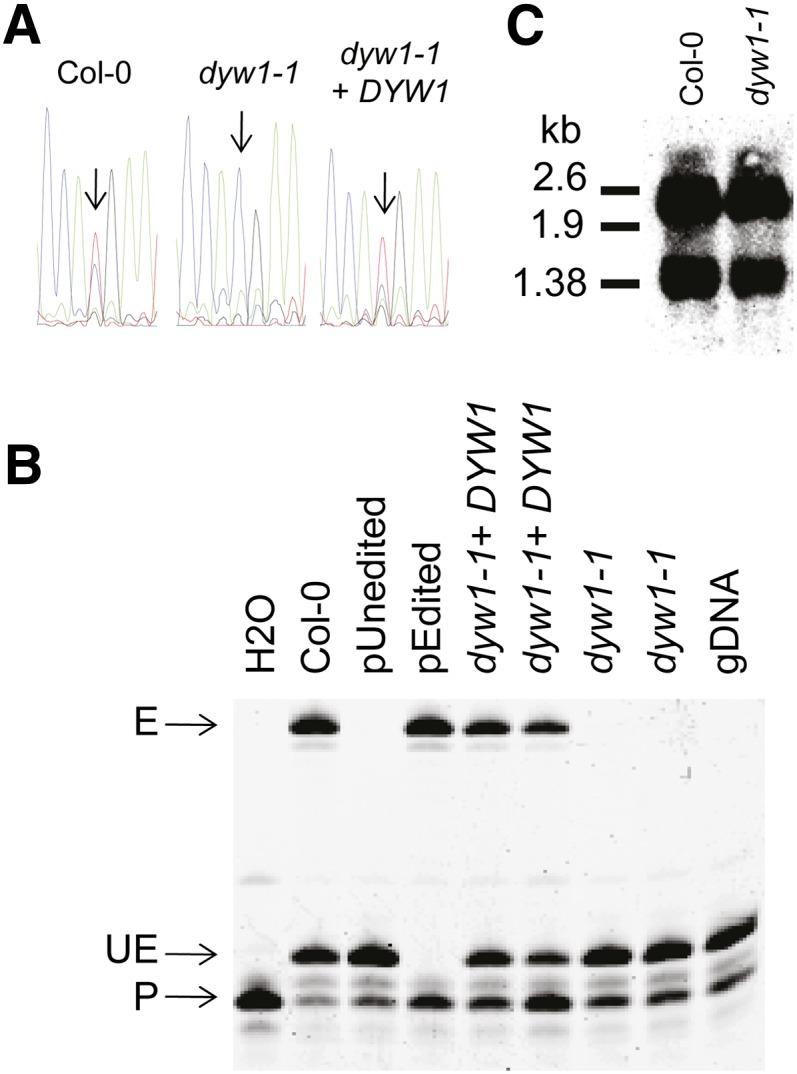
The dyw1-1 Mutant Lacks Editing of the ndhD-1 Site.
(A) Nucleotide sequences of RT-PCR products obtained from Col-0, dyw1-1, and complemented dyw1-1 cDNA are shown as sequencing chromatograms. An arrow pointing to the corresponding peak indicates the ndhD-1 editing site.
(B) PPE assays were conducted on the ndhD-1 (117166) editing site. RT-PCR products were obtained from Col-0, dyw1-1, and complemented dyw1-1 cDNA with primers surrounding the editing sites and these served as templates for the extension reaction from a 5′-labeled 6-carboxyfluorescein primer (P) that anneals next to the target editing site. The extension was stopped by the incorporation of 2′,3′-dideoxycytidine-5′-triphosphate at the location of the editing site for unedited molecules, producing a short unedited product (UE). The extension was stopped at the next C/G for the edited molecules, producing a longer edited product (E). PPE samples obtained on cloned edited (pEdited) and unedited (pUnedited) ndhD cDNA fragments were loaded as controls. gDNA, genomic DNA.
(C) RNA gel blot analysis of leaves of Arabidopsis Col-0 and dyw1-1 mutant using an ndhD probe. Polycistronic (∼2.6 kb) and monocistronic (∼1.38 kb) forms of the ndhD transcript were identified.
To confirm that the editing defect observed in dyw1-1 was a consequence of the frameshift in DYW1, the mutant was complemented with a 1502-bp genomic fragment comprising a region of 357 bp before ATG2, the DYW1 ORF (717 bp) and 428 bp downstream. After Agrobacterium tumefaciens transformation of dyw1-1 with this construct, two complemented lines were shown to be restored in the editing of ndhD-1 by the PPE assay (Figures 3A and 3B). This complementation experiment and the defects observed in weaker alleles demonstrated that the abolition of ndhD-1 editing, shown by sequencing and PPE, is due to the frameshift in DYW1.
To examine whether the loss of editing was a secondary effect of altered RNA processing or RNA stability, the ndhD transcript was analyzed by RNA gel blot analysis of the dyw1-1 mutant compared with the wild type. No variation in the pattern or level of the monocistronic (mature ndhD RNA) or polycistronic (precursor form of ndhD RNA) transcripts was observed (Figure 3C). This result supports a specific and direct role of DYW1 in the editing of the ndhD-1 site.
dyw1-1 Is Impaired in NDH Activity and Phenocopies the crr4 Editing Mutant
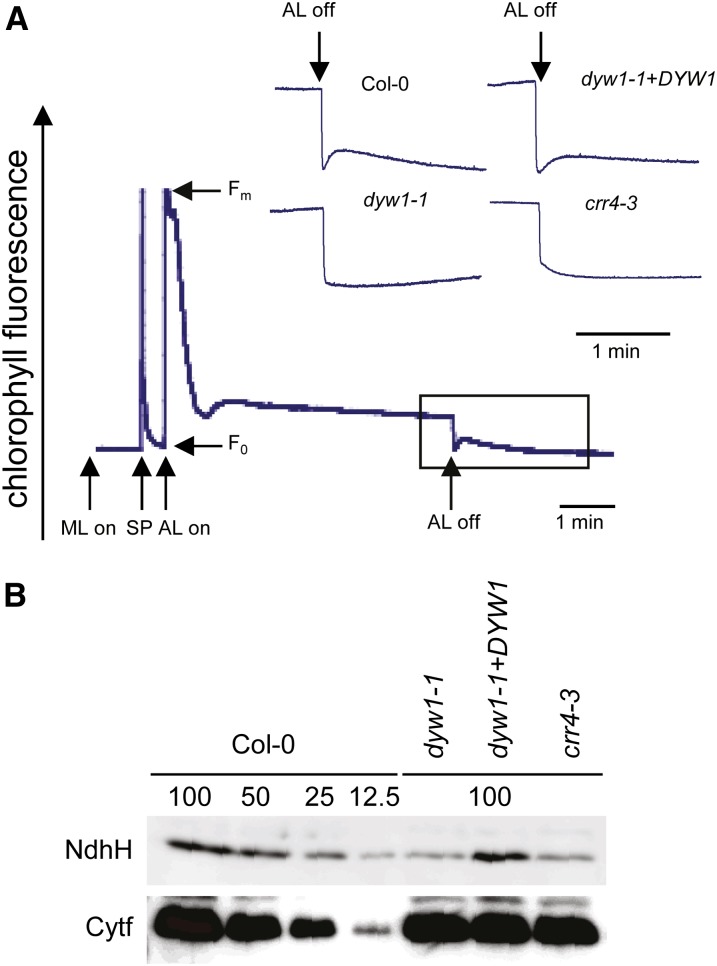
The ndhD-1 site is partially edited in the wild-type plant (Figures 3A and 3B, Col-0), and editing at this site converts a Thr codon (ACG) into a Met codon (AUG) that is the ndhD putative translation initiation codon. The chloroplast NDH complex catalyzes electron donation probably from ferredoxin to plastoquinone (Yamamoto et al., 2011). When NDH activity is present, a transient increase of chlorophyll fluorescence can be observed when actinic light (AL) is switched off (Shikanai et al., 1998). We analyzed this change of fluorescence to examine whether or not the NDH complex was affected in dyw1-1. Unlike the Col-0 control, the mutant showed no transient increase in fluorescence after the AL was turned off (Figure 4A). This defect was reverted in the complemented transgenic plants (Figure 4A). Immunoblot analysis using an antibody against NdhH showed a decrease in the level of the NDH complex in dyw1-1 (Figure 4B). The editing site impaired in the dyw1-1 mutant is exactly the same site that has been shown to be unedited in crr4 mutants (Kotera et al., 2005). To perform a fine comparison of the crr4 and dyw1-1 mutants, crr4-3 (encoding a truncated CRR4 protein) and dyw1-1 mutants were grown and characterized in parallel. The primary molecular defect (absence of editing of the ndhD-1 site), the resulting lack of NDH activity (Figure 4A), the decreases in the level of NDH complex (Figure 4B), and the macroscopic phenotypes of adult plants compared with the wild type (Figure 1C; Kotera et al., 2005) were identical in dyw1-1 and crr4-3.
Figure 4.
The dyw1-1 Mutant Is Impaired in NDH Activity.
(A) Monitoring NDH activity using chlorophyll fluorescence analysis after turning off AL. The bottom curve indicates a typical trace of chlorophyll fluorescence in the wild-type Col-0. The transient rise in fluorescence ascribed to NDH activity was monitored by chlorophyll fluorimetry. Insets are magnified traces from the boxed area. Fo, minimum fluorescent yield; Fm, maximum fluorescent yield; ML, measuring light; SP, saturating pulse.
(B) Immunoblot analysis of thylakoid proteins. Immunodetection of NDH (NdhH) and cytochrome b6f (Cytf) complexes. The lanes were loaded with a series of dilutions as indicated.
[See online article for color version of this figure.]
DYW1 Interacts with CRR4
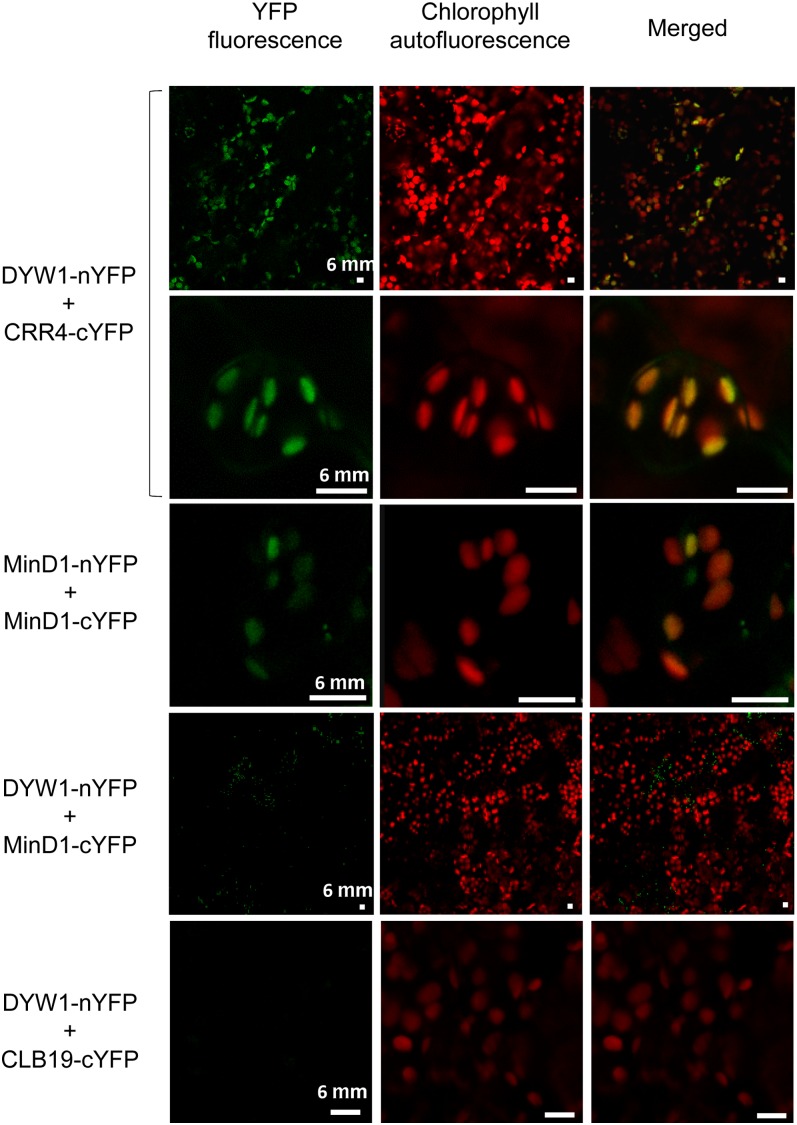
The dyw1-1 and crr4 mutants are indistinguishable by phenotype, indicating that the DYW1 and CRR4 proteins have essential roles in the same process. However, the common phenotype also indicates that CRR4 is not able to complement the DYW1 function in the dyw1 background, and, equally, DYW1 is not able to complement the CRR4 function in the crr4 background. This observation could suggest that the DYW1 protein interacts with the CRR4 protein to form a protein complex that functions in ndhD-1 editing. To test this hypothesis, we used bimolecular fluorescence complementation (BiFC) assays (Marion et al., 2008) to visualize any interaction between CRR4 and DYW1 in planta. In these experiments, one protein is fused to the N-terminal half of the yellow fluorescent protein (nYFP) and the potential partner is fused to the C-terminal half of YFP (cYFP). Arabidopsis plantlets were transiently cotransformed with pairs of plasmids encoding YFP fusions (Table 1). Because each half of the YFP is not intrinsically fluorescent, YFP fluorescence is observed only when intermolecular interactions occur between nYFP- and cYFP-tagged proteins (Citovsky et al., 2006; Marion et al., 2008). The emission spectrum of YFP was confirmed for each positive interaction (see Supplemental Figure 3 online). The results presented in Table 1 and Figure 5 show a positive interaction between CRR4-cYFP and DYW1-nYFP fusion proteins that was not observed when the opposite interaction (CRR4-nYFP X DYW1-cYFP) was assayed. Furthermore, no BiFC signal was observed for coexpressed DYW1-cYFP and DYW1-nYFP, suggesting that DYW1 does not dimerize, unlike known cytidine deaminases (Prochnow et al., 2007). To test the specificity of the interaction between DYW1 and CRR4, we tested the interaction between DYW1-nYFP and CLB19-cYFP. CLB19 is a plastid PLS-E protein necessary for the editing of clpP and rpoA sites (Chateigner-Boutin et al., 2008) and functionally and structurally very similar to CRR4. CLB19 did not interact with DYW1 in this assay as shown by the absence of YFP fluorescence in plant cells (Figure 5). In addition, several negative controls testing for interactions between either DYW1-nYFP or CRR4-cYFP and unrelated proteins that have been shown to interact in plastids using BiFC were tested: MinD1 × MinD1 (Maple et al., 2007) and FSD2 × FSD3 (Myouga et al., 2008). The consistent lack of YFP fluorescence in these control cells (Figure 5, Table 1) supports that the fluorescence observed in the BiFC assays resulted from a specific CRR4–DYW1 interaction.
Table 1. BiFC Analysis of in Vivo Interactions between DYW1, CRR4, and Control Proteins.
| Interaction Tested | DYW1-cYFP | CRR4-cYFP | CLB19-cYFP | FSD2-cYFP | MinD1-cYFP |
|---|---|---|---|---|---|
| DYW1-nYFP | − | + | − | − | − |
| CRR4-nYFP | − | ||||
| FSD3-nYFP | + | ||||
| MinD1-nYFP | + |
Arabidopsis plantlets were cotransformed with DYW1, CRR4, CLB19, FSD, and MinD1 fused to either the N- (rows) or C-terminal (columns) halves of YFP. Interactions were scored based on either the presence (+) or absence (−) of a BiFC (YFP) signal in the plastids. For each pair of plasmids tested, BiFC results were scored from at least two independent experiments. Blank cells correspond to untested interactions.
Figure 5.
In Planta Protein Interaction of DYW1 and CRR4 as Shown by BiFC.
BiFC of YFP in transiently transformed Arabidopsis plantlets. Left column, YFP fluorescent signal detection by confocal microscopy; middle column, chlorophyll autofluorescence; right column, merge of fluorescent signal and autofluorescence. Cotransformation of Arabidopsis plantlets with DYW1-nYFP and CRR4-cYFP (top lines) generates yellow fluorescence that colocalizes with autofluorescence of chlorophylls in plastids. Similar signals are observed when using the homodimerization of MinD1 (third panel) as a positive control (Maple et al., 2007). No signal was observed when plantlets were cotransformed with either DYW1-nYFP and MinD1-cYFP or DYW1-nYFP and CLB19-cYFP (bottom panels). Bars = 6 μm.
DYW1 Provides the Amino Acid Sequences Missing from CRR4
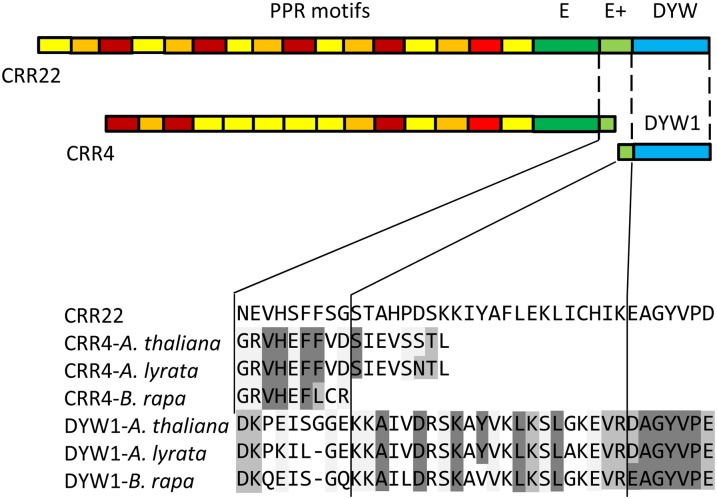
Approximately 50% of the editing factors identified so far contain the E, E+, and DYW motifs as defined by Lurin et al. (2004), whereas the other 50% lack these C-terminal domains to varying degrees. CRR4 lacks a DYW domain and the 14 C-terminal amino acids of the E+ motif. Interestingly, DYW1 contains not only a complete DYW domain, but also the C-terminal segment of the E+ motif (Figure 6). Thus, a complex of CRR4 and DYW1 would reconstitute all of the amino acid motifs found in full-length editing factors, such as CRR22 (Okuda et al., 2009).
Figure 6.
CRR4 and DYW1 Each Contain a Different Part of the E+ Domain.
The partial E+ domains of CRR4 and its putative orthologs, DYW1 and its putative orthologs, and the PPR-DYW editing factor CRR22 (Okuda et al., 2009) were aligned using ClustalW. Residues identical to those in CRR22 are shaded in dark gray, and residues similar to those in CRR22 are shaded in light gray. CRR4 contains the N-terminal segment of the E+ domain, whereas the C-terminal segment of the domain is found in DYW1. There is no apparent overlap of conserved sequence.
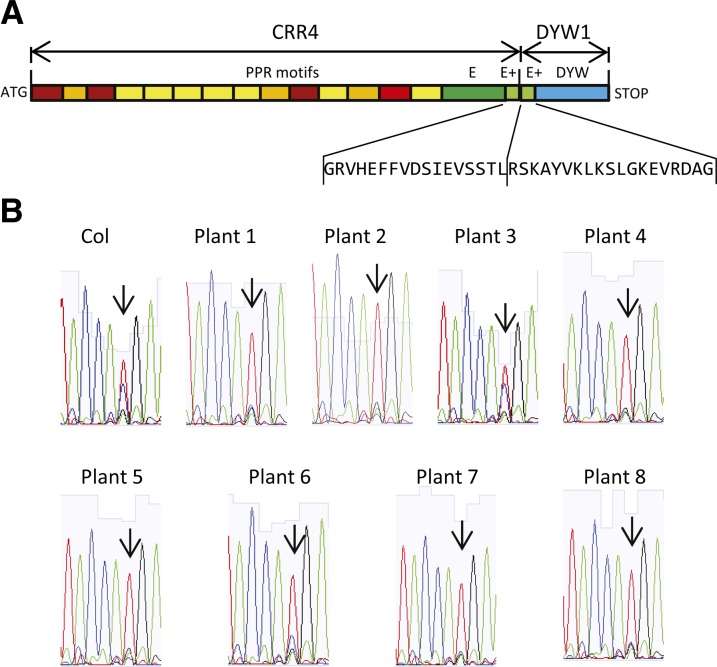
To test this model, we crossed the crr4-3 mutant with the dyw1-1 mutant to obtain a homozygous double mutant and then attempted to complement it with a chimeric construct consisting of the CRR4 PPR and E-E+ domains and the DYW1 E+-DYW domain (Figure 7). As one would expect, the double mutant completely lacks ndhD-1 editing. Eight lines complemented with the chimeric construct were tested and all were shown to be restored for the editing of ndhD-1 by cDNA sequencing (Figure 7). Seven of the eight plants tested showed a higher proportion of edited transcripts (more than 80%) than the wild type. Thus, a fusion of CRR4-DYW1 is functional, further evidence in favor of interaction between these two proteins.
Figure 7.
Complementation of the crr4-3 dyw1-1 Double Mutant by a CRR4-DYW1 Fusion.
(A) Structure of the CRR4-DYW1 construct. The PPR motifs and E/E+ domain of the CRR4 protein were fused to the E+ domain and DYW domain of DYW1.
(B) Sequencing chromatograms of RT-PCR products obtained from complemented crr4-3 dyw1-1 double mutants expressing the CRR4-DYW1 fusion. Each trace is from a different independent transformant. Arrows indicate the ndhD-1 editing site.
DISCUSSION
Editing of ndhD-1 Requires at Least Four Proteins
Through genetic studies, we have shown that DYW1 is necessary for editing of the ndhD-1 site in chloroplast transcripts of Arabidopsis, the same site that was shown previously to require CRR4. As the molecular process requiring these two proteins is the same and because they are both necessary for this function, the simplest model is that they interact to achieve this function. Indeed, we demonstrated an interaction between DYW1 and CRR4 using transgenic fusion proteins in Arabidopsis and showed that a fusion of CRR4 and part of DYW1 is capable of complementing a double mutant lacking both proteins.
Although PPR proteins have often been observed to be components of high molecular weight protein or RNA-protein complexes (Uyttewaal et al., 2008; Olinares et al., 2010; Klodmann et al., 2011), only a few specific protein–PPR interactions have been identified. The PPR protein GLUTAMINE-RICH PROTEIN23 was reported to interact with RNA polymerase II in the nucleus (Ding et al., 2006); similarly, PPR PROTEIN LOCALIZED TO THE NUCLEUS AND MITOCHONDRIA1 was shown to interact with two nuclear proteins, NUCLEOSOME ASSEMBLY PROTEIN and the transcription factor TCP8 (Hammani et al., 2011a). DELAYED GREENING1, a PPR protein, interacts with SIGMA FACTOR6, a cofactor required for the transcription of plastid-encoded RNA polymerase–dependent chloroplast genes in Arabidopsis cotyledons (Chi et al., 2010). In none of these cases has the functional relevance of the observed interactions been elucidated.
Unlike these prior examples, the CRR4/DYW1 interaction involves two PPR proteins implicated in the same molecular event on the same target RNA molecule, making it easier to understand the functional implications of the interaction. However, although clearly both proteins are required for editing to occur, we have not formally shown that their interaction is required for their function, and it is still possible that they could act sequentially. The proteins MORF2 and MORF9 are also required for ndhD-1 RNA editing (Takenaka et al., 2012), whereas a third member of the MORF family, RIP1 (=MORF8), facilitates editing at this site but is not strictly required (Bentolila et al., 2012). Based on the fact that several MORF–PPR interactions have been demonstrated (Bentolila et al., 2012; Takenaka et al., 2012), one or more of these MORF proteins are likely to interact with CRR4 and/or DYW1. The role of MORF proteins in editing is not clear yet.
Conservation of CRR4 and DYW1 across Species
In Arabidopsis, editing at the ndhD-1 site is necessary for correct synthesis and assembly of the NDH complex. This editing site is conserved across many dicots (Tsudzuki et al., 2001), suggesting that the editing factors should also be conserved. Hayes and Mulligan (2011) have recently shown a strict correlation between the presence of the ndhD-1 editing site in chloroplast transcripts and of an apparent CRR4 ortholog in the corresponding nuclear genome. However, the length of the E domain of these CRR4 homologs is not conserved (see Supplemental Figure 4 online); in Brassicaceae, this sequence is shorter than in the other dicots. In parallel, DYW1 proteins containing the missing E domain sequence and no PPR domain are only found in Brassicaceae (see Supplemental Table 2 and Supplemental Figure 5 online).
Is Association with DYW Proteins a General Feature of E-Class Editing Factors?
As discussed in the Introduction, 50% of known editing factors lack DYW domains. Do all these DYW-less proteins associate with DYW-containing partners, or is the CRR4/DYW1 association an isolated case? Currently, there are no published data that can help us decide either way, but it is tempting to speculate that other similar examples of such associations will be discovered.
Although the Arabidopsis genome contains 87 DYW proteins, DYW1 is unique in containing no E, E+, or PPR motifs. However, five other Arabidopsis DYW proteins contain only a few PPR motifs and a poorly conserved E domain; these make ideal candidates for interaction partners for E/E+ proteins. If all E/E+ proteins have DYW partners, then these associations would have to be less specific than that between CRR4 and DYW1. Our present results show that the dyw1-1 mutant is affected only in ndhD-1 editing and that the physical interaction between CRR4 and DYW1, which was not observed between CLB19 and DYW1, seems also to be specific.
What Does the Association of CRR4 and DYW1 Imply for the Editing Enzyme?
Our hypothesis is that the CRR4 protein interacts with the DYW1 protein to form a protein complex functionally equivalent to a PPR PLS-DYW protein that contributes to both specifically binding and editing the ndhD-1 site. This model is similar to the model in mammals in which ACF, the specificity factor, interacts with APOBEC-1, the enzyme, to edit the apoB transcript. The hypothesis that the DYW domain harbors the RNA editing activity is based on the presence of the conserved signature HxE(x)nCxxC, found in editing enzymes in other organisms (Salone et al., 2007), and broader similarities to a wide class of nucleotide deaminases (Iyer et al., 2011). This hypothesis was weakened by the discovery that many editing factors lack the DYW domain, and even those that contain it do not always require it in vivo (Okuda et al., 2009). However, we have shown that the DYW domain can be supplied in trans to the CRR4 protein and that in this case, it is essential for editing to occur. If, in the future, this phenomenon could be generalized to the other E/E+ editing factors, it would effectively eliminate one of the arguments against the DYW domain being the editing enzyme.
METHODS
The complete list of oligonucleotides used in this study is summarized in Supplemental Table 3 online.
Plant Material, Growth Conditions, and Complementation Analysis
Arabidopsis thaliana ecotype Col-0 was used in this study. Seeds were surface sterilized, vernalized at 4°C for 3 d, and grown on half-strength Murashige and Skoog media containing 3% Suc in vitro. Plates were placed in growth chambers under 16 h light/8 h dark, at 25°C, and with 45% humidity. Two-week-old seedlings were transferred onto soil and grown under 16 h light/8 h dark at 21°C and 65% humidity. A screening for EMS-induced mutations was ordered at the Seattle Arabidopsis TILLING Project (Till et al., 2003; http://tilling.fhcrc.org/) using two specific primers (TILING _LP and TILLING_RP) surrounding a 1509-bp region containing the At1g47580 locus. The identified EMS lines were obtained from the Nottingham Arabidopsis Stock Centre (see Supplemental Table 1 online). EMS mutants were backcrossed three times to Col-0 ecotype. EMS lines were genotyped by amplification of a 1502-bp product using DYW1_compF and DYW1_compR primers and sequencing using DYW1_genF and DYW1_genR primers. The crr4-3 mutant was provided by Toshiharu Shikanai (Kotera et al., 2005).
For complementation analysis, the complete DYW1 locus with its native promoter and terminator was amplified by PCR using DYW1_compF and DYW1_compR primers on genomic Arabidopsis Col-0 DNA, cloned into the pDNR207 vector by Gateway BP reaction (Invitrogen), and subcloned into pGWB1 vector (Nakagawa et al., 2007) by LR reaction. For the double mutant, CRR4 was amplified with CRR4_ATG_F and CRR4-DYW1_R and DYW1 was amplified with CRR4-DYW1_F and DYW1_STOP_R on Arabidopsis Col-0 genomic DNA. The fusion was achieved by pooling the purified PCR products and amplifying by PCR with CRR4_ATG_F and DYW1_STOP_R. The fusion product was cloned into the pDNR207 vector by Gateway BP reaction (Invitrogen) and subcloned into pGWB2.
Protein Expression and Antibody Production
The DYW1 coding region without its presequence was amplified using DYW1_internal and DYW1_stop primers and cloned into the pDNR207 vector by Gateway BP reaction (Invitrogen). LR recombination was done with the pDEST 17 destination vector (Invitrogen) allowing an N-terminal fusion with a 6His tag. Proteins were expressed for 5 h at 37°C in salt- inducible BL21-SI Escherichia coli induced with 0.3 M NaCl and purified by affinity to nickel-nitrilotriacetic acid agarose in denaturing conditions according to the manufacturer’s instructions (Qiagen). Purified DYW1 protein was used to immunize rabbits to produce polyclonal antibodies (Eurogentec).
Subcellular Localization
The full-length ORF or the 300 bp encoding the first 100 amino acids of DYW1 were amplified using DYW1_start, DYW1_end, and DYW1_preseq primers and subsequently cloned into the pDNR207 vector by BP reaction. The LR recombination was done with the pGreen 0229 destination vector containing the green fluorescent protein (GFP) or RFP gene (Lurin et al., 2004). C58C1 pSOUP Agrobacterium tumefaciens transformed with these binary plasmids was used for Arabidopsis transformation (Marion et al., 2008). Plantlets were observed 3 d after transformation by confocal microscopy (Leica SP2 AOBS 405) allowing the detection of GFP (excitation/emission 488/509nm, filter BP500/550), RFP (excitation/emission 543/583nm, filter BP580/620), and autofluorescence (excitation/emission 543/680nm, filter BP580/620).
For immunoblot analysis, Arabidopsis chloroplast and mitochondrial protein extracts were prepared as previously described (Hegeman et al., 2005; Sweetlove et al., 2007). Proteins were separated in 12.5% SDS-PAGE gel, transferred onto polyvinylidene difluoride membrane by electrotransfer, and incubated with the DYW1-specific antibody diluted in TBS-T (1/5000), the formate dehydrogenase–specific antibody (1/6000 in TBS-T) (Colas des Francs-Small et al., 1993), or the chloroplast large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase–specific antibody (1/20,000 in TBS-T) (supplied by Spencer Whitney, Australian National University). After incubation with the anti-rabbit IgG antibody (1/10,000 in TBS-T), immunoblots were analyzed using the ECL Western Blotting Analysis System reaction kit (GE Healthcare) and visualized with LAS-1000 (Fujifilm).
Analysis of RNA Editing
RNA from leaves of 18-d-old plantlets was extracted with the RNeasy plant mini kit (Qiagen). RNA was treated twice with DNase I (2 units/µL; Ambion) for 30 min at 37°C, and cDNA was synthesized using Superscript II (Invitrogen). RT-PCR products were obtained with NdhD_AT_For and NdhD_AT_rev primers surrounding the NdhD-1 editing site (117,166) and used as template for sequencing using the NdhD_AT_For primer.
Poisoned primer extension of RT-PCR products was performed as described by Chateigner-Boutin and Small (2007). RT-PCR products were obtained with NdhD_AT_For and NdhD_AT_rev primers and serve as templates for the extension reaction from 5 - Carboxyfluorescein-labeled ndhD_PPE_C primer purified on reverse phase cartridge (Sigma Genosys) that anneals next to the editing site. The extension was stopped by the incorporation of 2′,3′-dideoxycytidine-5′-triphosphate at the location of the editing site for unedited molecules, producing a short unedited product. The extension was stopped at the next G/C for the edited molecules, producing a longer edited product.
RNA Gel Blot Analysis
The ndhD RNA probe was labeled with biotinylated cytidine by in vitro transcription of a PCR product obtained and cloned in pGEM-T Easy vector (Promega) as described by Hammani et al. (2009). The PCR product served as a template for in vitro transcription with SP6 polymerase following the manufacturer’s instructions (Maxiscript Ambion). Ten micrograms of total RNA extracted from leaves of 14- and 28-d-old plantlets RNA (RNeasy plant mini kit; Qiagen) were separated on 1.2% (w/v) formaldehyde agarose gel and transferred onto Hybond N+ nylon membranes (GE Healthcare). After transfer, the membrane was stained with 0.04% methylene blue to check RNA integrity, loading, and transfer and subsequently hybridized with the biotinylated ndhD antisense RNA probe according to Hammani et al. (2009).
Chlorophyll Fluorescence Analysis
Chlorophyll fluorescence was measured using a MINI-PAM portable chlorophyll fluorometer (Waltz). The transient increase in chlorophyll fluorescence after turning off AL was monitored as previously described (Shikanai et al., 1998). Leaves were exposed to AL (50 µmol photons m−2 s−1) for 5 min. AL was turned off and the subsequent transient rise in fluorescence ascribed to NDH activity was monitored by chlorophyll fluorimetry.
Split-YFP Assay
The full-length ORFs of DYW1, CRR4, CLB19, FSD2, FSD3, and MinD1 were amplified without their stop codon using corresponding ORF_start and ORF_end primers and cloned into the pDNR207 vector. LR recombinations were done with split-YFP destination vectors. pBiFC1 and pBiFC4, coding for the N- and C-terminal YFP moieties, respectively, cloned at the 3′ end (C-terminal fusions) of the Gateway recombination sequence, were used (Azimzadeh et al., 2008). C58C1 Agrobacterium pCH32 containing these vectors was used to transform 15 to 20 seedlings of Landsberg erecta Arabidopsis seedlings grown in six-well plates as described previously (Marion et al., 2008). Cotyledons of transformed seedlings were observed 3 to 4 d after transformation using a confocal microscope (Leica SP2 AOBS diode 405 with two filters: GFP [band-pass], excitation filter band-pass 450 to 490, stop filter band-pass 500 to 550; I3 [long pass], excitation filter band-pass 450 to 490, stop filter band-pass 515).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: AT1G47580 (DYW1), AT2G45350 (CRR4), ATCG01050 (NDHD), AT5G24020 (MIND1), AT5G51100 (FSD2), AT5G23310 (FSD3), and AT1G11290 (CRR22).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Analysis of the Editotype of the dyw1-1 Mutant.
Supplemental Figure 2. Analysis of the Editing Rate of the ndhD-1 Site in dyw1-x Mutants.
Supplemental Figure 3. Fluorescence Emission Spectral Analysis of DYW1-nYFP X CRR4-cYFP BiFC Signals.
Supplemental Figure 4. Alignment of CRR4 Orthologs.
Supplemental Figure 5. Alignment of DYW1 Orthologs.
Supplemental Table 1. Complete List of EMS Mutations Identified in the DYW1 Locus by the TILLING Approach.
Supplemental Table 2. DYW1 Orthologs.
Supplemental Table 3. Complete List of Oligonucleotides Used in This Study.
Acknowledgments
We thank Etienne Delannoy (Unité de Recherche en Génomique Végétale, Evry, France) and Andéol Falcon de Longevialle (Unité de Recherche en Génomique Végétale, Evry, France) for valuable discussions and comments on the article and Olivier Grandjean (Institut Jean Pierre Bourgin, Versailles, France) and Lionel Gissot (Institut Jean Pierre Bourgin, Versailles, France) for their help with confocal microscopy. Research at the University of Western Australia was supported by Australian Research Council Grant CE0561495. We thank Tsuyoshi Endo (Kyoto University, Kyoto, Japan) and Amane Makino (Tohoku University, Sendai, Japan) for giving us anticytochrome b6f and NdhH polyclonal antibodies, respectively. Collaboration between the University of Western Australia and Unité de Recherche en Génomique Végétale was supported by Australian Government International Science Linkages Grants FR060030 and CG120098. K.H. is supported by a postgraduate scholarship from the University of Western Australia.
AUTHOR CONTRIBUTIONS
C.B. characterized the EMS lines, analyzed RNA editing, and drafted the article. V.S. characterized the T-DNA lines and localized DYW1 by immunoblot. C.B. and A.A. complemented the dyw1-1 mutant and the crr4 dyw1 double mutant. A.A. and R.B. localized DYW1 by confocal analysis and analyzed CRR4–DYW1 interaction by split-YFP assay. K.H., K.O., and T.S. measured the chlorophyll fluorescence of the dyw1-1 mutant and complemented plants. I.S. helped design the research, analyze the data, and write the article. C.L. conceived and coordinated the project and drafted the article.
Glossary
- ACF
APOBEC1 complementation factor
- NDH
NADH dehydrogenase-like complex
- ORF
open reading frame
- EMS
ethyl methanesulfonate
- Col-0
Columbia-0
- RFP
red fluorescent protein
- PPE
poisoned primer extension
- AL
actinic light
- BiFC
bimolecular fluorescence complementation
- YFP
yellow fluorescent protein
- GFP
green fluorescent protein
References
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Andres C., Lurin C., Small I.D. (2007). The multifarious roles of PPR proteins in plant mitochondrial gene expression. Physiol. Plant. 129: 14–22 [Google Scholar]
- Azimzadeh J., Nacry P., Christodoulidou A., Drevensek S., Camilleri C., Amiour N., Parcy F., Pastuglia M., Bouchez D. (2008). Arabidopsis TONNEAU1 proteins are essential for preprophase band formation and interact with centrin. Plant Cell 20: 2146–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne R., Van den Burg J., Brakenhoff J.P., Sloof P., Van Boom J.H., Tromp M.C. (1986). Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell 46: 819–826 [DOI] [PubMed] [Google Scholar]
- Bentolila S., Elliott L.E., Hanson M.R. (2008). Genetic architecture of mitochondrial editing in Arabidopsis thaliana. Genetics 178: 1693–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentolila S., Heller W.P., Sun T., Babina A.M., Friso G., van Wijk K.J., Hanson M.R. (2012). RIP1, a member of an Arabidopsis protein family, interacts with the protein RARE1 and broadly affects RNA editing. Proc. Natl. Acad. Sci. USA 109: E1453–E1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc V., Henderson J.O., Kennedy S., Davidson N.O. (2001). Mutagenesis of apobec-1 complementation factor reveals distinct domains that modulate RNA binding, protein-protein interaction with apobec-1, and complementation of C to U RNA-editing activity. J. Biol. Chem. 276: 46386–46393 [DOI] [PubMed] [Google Scholar]
- Bock R., Hermann M., Kössel H. (1996). In vivo dissection of cis-acting determinants for plastid RNA editing. EMBO J. 15: 5052–5059 [PMC free article] [PubMed] [Google Scholar]
- Bock R., Kössel H., Maliga P. (1994). Introduction of a heterologous editing site into the tobacco plastid genome: The lack of RNA editing leads to a mutant phenotype. EMBO J. 13: 4623–4628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli V., et al. (2004). Whole genome sequence comparisons and “full-length” cDNA sequences: A combined approach to evaluate and improve Arabidopsis genome annotation. Genome Res. 14: 406–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chateigner-Boutin A.L., Ramos-Vega M., Guevara-García A., Andrés C., de la Luz Gutiérrez-Nava M., Cantero A., Delannoy E., Jiménez L.F., Lurin C., Small I., León P. (2008). CLB19, a pentatricopeptide repeat protein required for editing of rpoA and clpP chloroplast transcripts. Plant J. 56: 590–602 [DOI] [PubMed] [Google Scholar]
- Chateigner-Boutin A.L., Small I. (2007). A rapid high-throughput method for the detection and quantification of RNA editing based on high-resolution melting of amplicons. Nucleic Acids Res. 35: e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chateigner-Boutin A.L., Small I. (2010). Plant RNA editing. RNA Biol. 7: 213–219 [DOI] [PubMed] [Google Scholar]
- Chaudhuri S., Maliga P. (1996). Sequences directing C to U editing of the plastid psbL mRNA are located within a 22 nucleotide segment spanning the editing site. EMBO J. 15: 5958–5964 [PMC free article] [PubMed] [Google Scholar]
- Chen S.H., et al. (1987). Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science 238: 363–366 [DOI] [PubMed] [Google Scholar]
- Chi W., Mao J., Li Q., Ji D., Zou M., Lu C., Zhang L. (2010). Interaction of the pentatricopeptide-repeat protein DELAYED GREENING 1 with sigma factor SIG6 in the regulation of chloroplast gene expression in Arabidopsis cotyledons. Plant J. 64: 14–25 [DOI] [PubMed] [Google Scholar]
- Citovsky V., Lee L.Y., Vyas S., Glick E., Chen M.H., Vainstein A., Gafni Y., Gelvin S.B., Tzfira T. (2006). Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. J. Mol. Biol. 362: 1120–1131 [DOI] [PubMed] [Google Scholar]
- Colas des Francs-Small C., Ambard-Bretteville F., Small I.D., Rémy R. (1993). Identification of a major soluble protein in mitochondria from nonphotosynthetic tissues as NAD-dependent formate dehydrogenase. Plant Physiol. 102: 1171–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delannoy E., Stanley W.A., Bond C.S., Small I.D. (2007). Pentatricopeptide repeat (PPR) proteins as sequence-specificity factors in post-transcriptional processes in organelles. Biochem. Soc. Trans. 35: 1643–1647 [DOI] [PubMed] [Google Scholar]
- Ding Y.H., Liu N.Y., Tang Z.S., Liu J., Yang W.C. (2006). Arabidopsis GLUTAMINE-RICH PROTEIN23 is essential for early embryogenesis and encodes a novel nuclear PPR motif protein that interacts with RNA polymerase II subunit III. Plant Cell 18: 815–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O., Brunak S., von Heijne G., Nielsen H. (2007). Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc. 2: 953–971 [DOI] [PubMed] [Google Scholar]
- Fujii S., Small I. (2011). The evolution of RNA editing and pentatricopeptide repeat genes. New Phytol. 191: 37–47 [DOI] [PubMed] [Google Scholar]
- Giegé P., Brennicke A. (1999). RNA editing in Arabidopsis mitochondria effects 441 C to U changes in ORFs. Proc. Natl. Acad. Sci. USA 96: 15324–15329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammani K., des Francs-Small C.C., Takenaka M., Tanz S.K., Okuda K., Shikanai T., Brennicke A., Small I. (2011b). The pentatricopeptide repeat protein OTP87 is essential for RNA editing of nad7 and atp1 transcripts in Arabidopsis mitochondria. J. Biol. Chem. 286: 21361–21371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammani K., Gobert A., Hleibieh K., Choulier L., Small I., Giegé P. (2011a). An Arabidopsis dual-localized pentatricopeptide repeat protein interacts with nuclear proteins involved in gene expression regulation. Plant Cell 23: 730–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammani K., Okuda K., Tanz S.K., Chateigner-Boutin A.L., Shikanai T., Small I. (2009). A study of new Arabidopsis chloroplast RNA editing mutants reveals general features of editing factors and their target sites. Plant Cell 21: 3686–3699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M.L., Mulligan R.M. (2011). Pentatricopeptide repeat proteins constrain genome evolution in chloroplasts. Mol. Biol. Evol. 28: 2029–2039 [DOI] [PubMed] [Google Scholar]
- Hegeman C.E., Hayes M.L., Hanson M.R. (2005). Substrate and cofactor requirements for RNA editing of chloroplast transcripts in Arabidopsis in vitro. Plant J. 42: 124–132 [DOI] [PubMed] [Google Scholar]
- Iyer L.M., Zhang D., Rogozin I.B., Aravind L. (2011). Evolution of the deaminase fold and multiple origins of eukaryotic editing and mutagenic nucleic acid deaminases from bacterial toxin systems. Nucleic Acids Res. 39: 9473–9497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klodmann J., Senkler M., Rode C., Braun H.P. (2011). Defining the protein complex proteome of plant mitochondria. Plant Physiol. 157: 587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop V. (2011). When you can’t trust the DNA: RNA editing changes transcript sequences. Cell. Mol. Life Sci. 68: 567–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotera E., Tasaka M., Shikanai T. (2005). A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts. Nature 433: 326–330 [DOI] [PubMed] [Google Scholar]
- Lurin C., et al. (2004). Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16: 2089–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maple J., Vojta L., Soll J., Møller S.G. (2007). ARC3 is a stromal Z-ring accessory protein essential for plastid division. EMBO Rep. 8: 293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion J., Bach L., Bellec Y., Meyer C., Gissot L., Faure J.D. (2008). Systematic analysis of protein subcellular localization and interaction using high-throughput transient transformation of Arabidopsis seedlings. Plant J. 56: 169–179 [DOI] [PubMed] [Google Scholar]
- Mehta A., Driscoll D.M. (2002). Identification of domains in apobec-1 complementation factor required for RNA binding and apolipoprotein-B mRNA editing. RNA 8: 69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T., Obokata J., Sugiura M. (2002). Recognition of RNA editing sites is directed by unique proteins in chloroplasts: Biochemical identification of cis-acting elements and trans-acting factors involved in RNA editing in tobacco and pea chloroplasts. Mol. Cell. Biol. 22: 6726–6734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myouga F., Hosoda C., Umezawa T., Iizumi H., Kuromori T., Motohashi R., Shono Y., Nagata N., Ikeuchi M., Shinozaki K. (2008). A heterocomplex of iron superoxide dismutases defends chloroplast nucleoids against oxidative stress and is essential for chloroplast development in Arabidopsis. Plant Cell 20: 3148–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Kurose T., Hino T., Tanaka K., Kawamukai M., Niwa Y., Toyooka K., Matsuoka K., Jinbo T., Kimura T. (2007). Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104: 34–41 [DOI] [PubMed] [Google Scholar]
- Okuda K., Chateigner-Boutin A.L., Nakamura T., Delannoy E., Sugita M., Myouga F., Motohashi R., Shinozaki K., Small I., Shikanai T. (2009). Pentatricopeptide repeat proteins with the DYW motif have distinct molecular functions in RNA editing and RNA cleavage in Arabidopsis chloroplasts. Plant Cell 21: 146–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Myouga F., Motohashi R., Shinozaki K., Shikanai T. (2007). Conserved domain structure of pentatricopeptide repeat proteins involved in chloroplast RNA editing. Proc. Natl. Acad. Sci. USA 104: 8178–8183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Nakamura T., Sugita M., Shimizu T., Shikanai T. (2006). A pentatricopeptide repeat protein is a site recognition factor in chloroplast RNA editing. J. Biol. Chem. 281: 37661–37667 [DOI] [PubMed] [Google Scholar]
- Olinares P.D., Ponnala L., van Wijk K.J. (2010). Megadalton complexes in the chloroplast stroma of Arabidopsis thaliana characterized by size exclusion chromatography, mass spectrometry, and hierarchical clustering. Mol. Cell. Proteomics 9: 1594–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole N., Hattori M., Andres C., Iida K., Lurin C., Schmitz-Linneweber C., Sugita M., Small I. (2008). On the expansion of the pentatricopeptide repeat gene family in plants. Mol. Biol. Evol. 25: 1120–1128 [DOI] [PubMed] [Google Scholar]
- Powell L.M., Wallis S.C., Pease R.J., Edwards Y.H., Knott T.J., Scott J. (1987). A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell 50: 831–840 [DOI] [PubMed] [Google Scholar]
- Prochnow C., Bransteitter R., Klein M.G., Goodman M.F., Chen X.S. (2007). The APOBEC-2 crystal structure and functional implications for the deaminase AID. Nature 445: 447–451 [DOI] [PubMed] [Google Scholar]
- Rüdinger M., Fritz-Laylin L., Polsakiewicz M., Knoop V. (2011). Plant-type mitochondrial RNA editing in the protist Naegleria gruberi. RNA 17: 2058–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salone V., Rüdinger M., Polsakiewicz M., Hoffmann B., Groth-Malonek M., Szurek B., Small I., Knoop V., Lurin C. (2007). A hypothesis on the identification of the editing enzyme in plant organelles. FEBS Lett. 581: 4132–4138 [DOI] [PubMed] [Google Scholar]
- Schmitz-Linneweber C., Small I. (2008). Pentatricopeptide repeat proteins: A socket set for organelle gene expression. Trends Plant Sci. 13: 663–670 [DOI] [PubMed] [Google Scholar]
- Shikanai T., Endo T., Hashimoto T., Yamada Y., Asada K., Yokota A. (1998). Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I. Proc. Natl. Acad. Sci. USA 95: 9705–9709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small I., Peeters N., Legeai F., Lurin C. (2004). Predotar: A tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 4: 1581–1590 [DOI] [PubMed] [Google Scholar]
- Small I.D., Peeters N. (2000). The PPR motif - A TPR-related motif prevalent in plant organellar proteins. Trends Biochem. Sci. 25: 46–47 [DOI] [PubMed] [Google Scholar]
- Steinhauser S., Beckert S., Capesius I., Malek O., Knoop V. (1999). Plant mitochondrial RNA editing. J. Mol. Evol. 48: 303–312 [DOI] [PubMed] [Google Scholar]
- Sweetlove L.J., Taylor N.L., Leaver C.J. (2007). Isolation of intact, functional mitochondria from the model plant Arabidopsis thaliana. Methods Mol. Biol. 372: 125–136 [DOI] [PubMed] [Google Scholar]
- Takenaka M., Zehrmann A., Verbitskiy D., Kugelmann M., Härtel B., Brennicke A. (2012). Multiple organellar RNA editing factor (MORF) family proteins are required for RNA editing in mitochondria and plastids of plants. Proc. Natl. Acad. Sci. USA 109: 5104–5109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki E., Hattori M., Sugita M. (2010). The moss pentatricopeptide repeat protein with a DYW domain is responsible for RNA editing of mitochondrial ccmFc transcript. Plant J. 62: 560–570 [DOI] [PubMed] [Google Scholar]
- Teng B., Burant C.F., Davidson N.O. (1993). Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science 260: 1816–1819 [DOI] [PubMed] [Google Scholar]
- Till B.J., et al. (2003). Large-scale discovery of induced point mutations with high-throughput TILLING. Genome Res. 13: 524–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudzuki T., Wakasugi T., Sugiura M. (2001). Comparative analysis of RNA editing sites in higher plant chloroplasts. J. Mol. Evol. 53: 327–332 [DOI] [PubMed] [Google Scholar]
- Uchida M., Ohtani S., Ichinose M., Sugita C., Sugita M. (2011). The PPR-DYW proteins are required for RNA editing of rps14, cox1 and nad5 transcripts in Physcomitrella patens mitochondria. FEBS Lett. 585: 2367–2371 [DOI] [PubMed] [Google Scholar]
- Uyttewaal M., Mireau H., Rurek M., Hammani K., Arnal N., Quadrado M., Giegé P. (2008). PPR336 is associated with polysomes in plant mitochondria. J. Mol. Biol. 375: 626–636 [DOI] [PubMed] [Google Scholar]
- Wedekind J.E., McKay D.B. (2003). Crystal structure of the leadzyme at 1.8 A resolution: Metal ion binding and the implications for catalytic mechanism and allo site ion regulation. Biochemistry 42: 9554–9563 [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Peng L., Fukao Y., Shikanai T. (2011). An Src homology 3 domain-like fold protein forms a ferredoxin binding site for the chloroplast NADH dehydrogenase-like complex in Arabidopsis. Plant Cell 23: 1480–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehrmann A., van der Merwe J.A., Verbitskiy D., Brennicke A., Takenaka M. (2008). Seven large variations in the extent of RNA editing in plant mitochondria between three ecotypes of Arabidopsis thaliana. Mitochondrion 8: 319–327 [DOI] [PubMed] [Google Scholar]