Abstract
It has been reported that carbonic anhydrase (CA) activity in plant leaves is decreased by Zn deficiency. We examined the effects of Zn deficiency on the activity of CA and on photosynthesis by leaves in rice plants (Oryza sativa L.). Zn deficiency increased the transfer resistance from the stomatal cavity to the site of CO2 fixation 2.3-fold and, consequently, the value of the transfer resistance relative to the total resistance in the CO2-assimilation process increased from 10% to 21%. This change led to a reduced CO2 concentration at the site of CO2 fixation, resulting in an increased gradient of CO2 between the stomatal cavity and this site. The present findings support the hypothesis that CA functions to facilitate the supply of CO2 from the stomatal cavity to the site of CO2 fixation. We also showed that the level of mRNA for CA decreased to 13% of the control level during Zn deficiency. This decrease resembled the decrease in CA activity, suggesting the possible involvement of the CA mRNA level in the regulation of CA activity.
CA (EC 4.2.1.1) catalyzes the reversible conversion of CO2 to HCO3, which can be dissolved more easily, and has been recognized as an important enzyme that is closely associated with photosynthesis. However, the application of inhibitors of CA to intact chloroplasts did not lower the rate of photosynthesis (Swader and Jacobson, 1972; Jacobson et al., 1975), suggesting that CA is not essential for the photosynthetic assimilation of CO2. It has also been reported that inactivation of CA mRNA by the introduction of antisense RNA had no significant negative effect on the photosynthetic assimilation of CO2 by tobacco leaves (Majeau et al., 1994; Price et al., 1994; Williams et al., 1996). In spite of experimental data showing the absence of an association of CA with photosynthesis, researchers showed that CA increased the CO2 concentration at the site of CO2 fixation in chloroplasts by 15 to 20 μL L−1, and that CA played a role in facilitating CO2 diffusion through the use of 13C and 18O in CO2 (Price et al., 1994; Williams et al., 1996).
In a previous study we tried to separate the rm into the rr and the rc by measuring the δ13C, and we succeeded in estimating the magnitude of the rr from the intercellular space of mesophyll cells to the site of CO2 fixation in the chloroplasts (Sasaki et al., 1996). The purpose of the present study was to elucidate the relationship between the activity of CA and each component of mesophyll resistance in an attempt to determine the mechanism that explains why CA activity is not related to photosynthetic performance.
CA is a Zn-containing enzyme, so Zn is essential for its catalytic activity (Bar-Akiva and Lavon, 1969; Silverman, 1991). Guliev et al. (1992) reported that removal of Zn from CA in vitro resulted in the irreversible loss of catalytic activity. Several authors have also reported that CA activity can be specifically inhibited by Zn deficiency in some plants without any significant reduction in the rate of photosynthesis (Edwards and Mohamed, 1973; Randall and Bouma, 1973; Ohki, 1976, 1978). Therefore, the second goal of this study was to elucidate the mechanism of the specific inhibition of CA activity by Zn deficiency at the level of expression of CA mRNA.
MATERIALS AND METHODS
Cultivation of Plants
Seeds of rice (Oryza sativa L. cv Hatsunishiki) were sown on wet quartz sand. Four weeks later, seedlings were transplanted to 40-L polyethylene containers filled with culture solution prepared as described by Yoshida et al. (1976) at a density of 56 plants per container. The solution contained 1.425 mm NH4NO3, 0.323 mm NaH2PO4, 0.513 mm K2SO4, 0.998 mm CaCl2, 0.998 mm MgSO4, 0.009 mm MnCl2, 0.075 μm (NH4)6Mo7O24, 0.019 mm H3BO3, 0.155 μm CuSO4, and 0.036 mm FeCl3. The plants were treated with 150 nm ZnCl2 from 4 weeks after germination (+Zn), with 15 nm ZnCl2 from 11 weeks after germination (±Zn), and without Zn (−Zn). Three containers were prepared for each treatment. The plants were grown in a naturally illuminated greenhouse that was air conditioned to give a day temperature of 30°C and a night temperature of 25°C.
Determination of Zn Content
The leaf area of the uppermost fully expanded leaf on the main stem was measured for 6 plants from each container (18 plants for each treatment) 13 weeks after germination, and these leaves were dried in an electric dryer. The dry weight was determined and the specific leaf weight was calculated. Samples of 0.1 g dry weight were digested overnight in 5.0 mL of concentrated HNO3 and 0.3 mL of 40% (w/v) HF in a 50-mL Teflon beaker, and wet ashed on the heater the following day. The Zn content was determined with an inductively coupled plasma atomic spectrometer (SPS-7000A, Seiko Instruments, Tokyo, Japan).
Measurement of the Gas-Exchange Rate
Leaf photosynthesis and transpiration were measured simultaneously for the uppermost fully expanded leaf on the main stem for 6 plants from each container (18 plants for each treatment) 13 weeks after germination using a handmade gas-exchange system with an IR gas analyzer (ZAP-AZ012, Fuji Electric Co. Ltd., Tokyo, Japan) and a humidity sensor (HMP111Y, Visala, Helsinki, Finland). A leaf (approximately 8–12 cm2) was clamped in the leaf chamber with a water jacket providing background cooling, and maintained under controlled conditions of temperature, light, humidity, and CO2 concentration. The measurements were made at a CO2 concentration of 340 μL L−1, an irradiance greater than 1400 μmol m−2 s−1 photon flux density under artificial light, and a leaf temperature of 30°C ± 3°C, after preillumination for 60 min to obtain steady-state readings of photosynthesis and transpiration.
Measurement of 13C Values of Soluble Sugars
Ten plants in each container were placed in darkness at 12 pm to starve the leaves of photosynthetic products. The next morning the leaves were exposed to artificial light at an irradiance greater than 1400 μmol m−2 s−1 photon flux density from 9 am to 12 pm in a 72-m3 laboratory ventilated at 2160 m3 h−1, and were then cut off from the plant. No one entered the laboratory during the exposure and there was no difference between the values of the isotopic composition of CO2 in the air in this room and outside. The accumulation of soluble sugars was conducted for three treatments at the same time. The soluble sugars were extracted and 13C was determined with a mass spectrometer (MAT-252, Finnigan MAT, San Jose, CA) as reported previously (Sasaki et al., 1996).
13C Determination
13C of soluble sugars was determined with a mass spectrometer according to the method of Sasaki et al. (1996). Δ can be expressed by the following equation (Hubick et al., 1986):
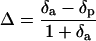 |
1 |
where δa and δp are the relative concentrations of 13C in the atmospheric air and in the photosynthetic products, respectively. The δa was determined as −8.0‰ ± 0.1‰ from the actual measurement of the air in our laboratory, and δp was determined by the mass-spectrometric method with soluble sugars extracted from the leaves. The Δ obtained was inserted into Equation 2 (Sasaki et al., 1996):
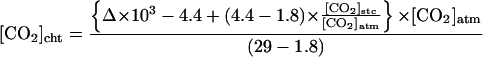 |
2 |
where 4.4 is the discrimination coefficient in the CO2-diffusion process through stomata in C3 plants, 1.1 is the discrimination coefficient in the CO2-dissolution process in water at 25°C, 0.7 is the discrimination coefficient in the CO2-diffusion process in the liquid phase at 25°C, and 29 is the discrimination coefficient in the CO2-carboxylation process by Rubisco. Carbon discrimination also occurred in the processes of dark respiration and photorespiration; however, we assumed the value of these factors to be zero because according to published reports the extent is almost negligible (Farquhar et al., 1982; Evans et al., 1986). [CO2]atm was maintained at about 340 μL L−1 in this experiment and [CO2]stc was obtained from the measurement of photosynthesis and transpiration. Therefore, [CO2]cht can be obtained from Equation 2 after inserting the value of Δ determined from Equation 1.
Calculation of Stomatal Transfer and Fixation Resistances
The rs and the rm were calculated using the following equations:
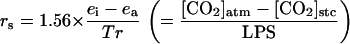 |
3 |
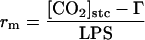 |
4 |
where ei and ea are the intercellular and atmospheric vapor pressures, respectively, Tr is transpiration, LPS is leaf photosynthesis, and Γ is the CO2-compensation point. The factor 1.56 is the ratio of the diffusivities of water vapor and CO2 in air. If [CO2]cht is obtained from Equation 2, the rr from the stomatal cavity to the site of CO2 fixation and the rc can be obtained using the following equations:
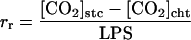 |
5 |
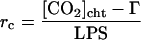 |
6 |
Determination of CA Activity
CA activity was measured in the uppermost fully expanded leaf on the main stem for 6 plants from each container (18 plants for each treatment). The detached leaves, which had been illuminated for 1 h at 1400 μmol m−2 s−1 photon flux density, were ground with a buffered solution (pH 8.3) that contained 50 mm barbital-H2SO4, 5 mm DTT, and 0.2% (w/v) PVP. The homogenate was centrifuged at 12,000g for 2 min, and the supernatant was used for the determination of CA activity, according to the method of Sasaki et al. (1996).
Determination of Rubisco Content and Activity
The content of Rubisco was determined in the uppermost fully expanded leaf on the main stem for six plants from each container (18 plants for each treatment). The leaves were excised immediately after the measurement of leaf photosynthesis and stored at −80°C in a freezer before the determinations. The content of Rubisco was determined by the method of Sasaki et al. (1996). The activity of Rubisco was measured in terms of the initial activity at 30°C according to the method described by Usuda (1985) for the uppermost fully expanded leaf on the main stem for 6 plants from each container (18 plants for each treatment) after illumination for 1 h at 1400 μmol m−2 s−1 photon flux density.
Quantitation of Soluble Protein
The amount of soluble protein in the supernatant prepared for the measurement of the content of Rubisco was determined as described by Lowry et al. (1951) using BSA as the standard.
Quantitation of Chlorophyll
The amount of chlorophyll in the homogenate prepared for the measurement of Rubisco was determined for 6 plants from each container (18 plants for each treatment) as described by Schmid (1971).
Isolation of RNA and Northern-Blot Hybridization
Total RNA was isolated from 0.2 g of frozen sample as described by Fromm et al. (1985). Total RNA (20 μg per lane) was fractionated on a 1.15% agarose gel that contained 1.85% (v/v) formaldehyde, transferred to a nylon membrane (Hybond-N, Amersham), and allowed to hybridize with radiolabeled DNA probes. After hybridization the blot was washed twice in 2× SSC (SSC contains 0.15 m NaCl and 0.015 m trisodium citrate) containing 0.1% SDS at 42°C for 5 min, and then washed again in 0.2× SSC containing 0.1% SDS at 45°C for 1 h. The signals attributable to the radiolabeled probe were visualized and quantified with a Bio-Imaging Analyzer (BAS 2000, Fujix, Tokyo, Japan). A 0.64-kb fragment of cDNA for rice chloroplastic CA (S. Suzuki and J.N. Burnell, unpublished data; accession no. U08404) and a full-length clone of the rice Rubisco small subunit (Matsuoka et al., 1988; accession no. D00644) were used as the probes.
RESULTS
Levels of Zn and Enzymes and Rates of Photosynthesis
The Zn contents per unit leaf area of the −Zn and ±Zn plants were as low as 0.19 and 0.23 mg m−2, respectively, compared with 0.51 mg m−2 in the +Zn plants (Table I). In spite of the great reduction in the Zn content of leaves, we observed no change in specific leaf weight or in chlorophyll content, which is considered to be a critical indicator of Zn deficiency. Therefore, −Zn and ±Zn plants were only moderately stressed. In contrast, although the CA activity in −Zn plants decreased dramatically to as little as 14% of that in +Zn plants, the Rubisco activity decreased only to 89% of that in +Zn plants. The levels of soluble protein and Rubisco increased slightly. Moreover, little change in the rate of photosynthesis was observed in the leaves with a reduced Zn content. These results indicated that Zn deficiency resulted in the specific inhibition of CA activity.
Table I.
Effects of Zn deficiency on Zn content, photosynthesis, δ13C value, chlorophyll content, and levels of CA and Rubisco in fully expanded leaves
| Parameter | +Zn | ±Zn | −Zn |
|---|---|---|---|
| Zn content (mg m−2) | 0.51 ± 0.05 | 0.23 ± 0.03 (45) | 0.19 ± 0.04 (37) |
| Specific leaf wt (g m−2) | 19.3 ± 2.0 | 20.2 ± 2.6 (105) | 19.9 ± 3.6 (103) |
| Chlorophyll content (g m−2) | 0.59 ± 0.09 | 0.49 ± 0.15 (82) | 0.45 ± 0.14 (78) |
| Photosynthesis (μmol CO2 m−2 s−1) | 22.9 ± 1.7 | 19.7 ± 3.6 (86) | 20.4 ± 2.3 (89) |
| Soluble protein (g m−2) | 6.5 ± 0.3 | 7.7 ± 0.3 (118) | 7.5 ± 0.2 (116) |
| CA activity (× 103 units m−2) | 473 ± 57 | 126 ± 23 (27) | 68 ± 36 (14) |
| Rubisco content (g m−2) | 3.5 ± 0.3 | 4.3 ± 0.3 (126) | 4.8 ± 0.4 (139) |
| Rubisco activity (μmol CO2 m−2 s−1) | 29.4 ± 2.6 | 23.5 ± 4.3 (80) | 26.3 ± 5.2 (89) |
| δ13C value (‰) | −25.83 ± 0.24 | −24.05 ± 1.27 | −23.55 ± 1.91 |
Values are expressed as means ± se; n = 18 except for the δ13C value (n = 3). Numbers in parentheses are percentages relative to values for +Zn plants.
Stomatal Transfer and Fixation Resistance
To clarify the contribution of CA activity to the assimilation process, we calculated the rc and the rr by measuring leaf photosynthesis and 13C in the soluble sugars extracted from the leaves (Table II). Although no significant difference was found in rc and rs in leaves of +Zn, −Zn, and ±Zn plants, we observed a 2.3-fold increase in rr, which increased from 1.2 mol−1 CO2 m2 s in +Zn plants to 2.7 mol−1 CO2 m2 s in −Zn plants. This corresponded to an increase from 10% to 21% when rr was calculated as a percentage of total resistance. This indicates that the Zn deficiency affected only the CO2-transfer step in the CO2-assimilation process.
Table II.
Effects of Zn deficiency on CO2-diffusion resistance
| Resistance | +Zn | ±Zn | −Zn |
|---|---|---|---|
| mol−1 CO2 m2 s | |||
| Stomatal | 4.7 ± 0.5 (40) | 5.7 ± 0.6 (42) | 5.2 ± 0.5 (40) |
| Mesophyll | 7.0 ± 0.5 (60) | 8.0 ± 1.6 (58) | 8.0 ± 1.2 (60) |
| CO2 transfer | 1.2 ± 0.3 (10) | 2.2 ± 0.9 (16) | 2.7 ± 0.7 (21) |
| CO2 fixation | 5.9 ± 0.4 (50) | 5.8 ± 0.8 (42) | 5.3 ± 0.6 (40) |
Values are expressed as means ± se; n = 18 for stomatal and mesophyll resistances and n = 3 for CO2-transfer and CO2-fixation resistances. Numbers in parentheses are percentages relative to the total resistance.
The Concentration of CO2 in Leaves
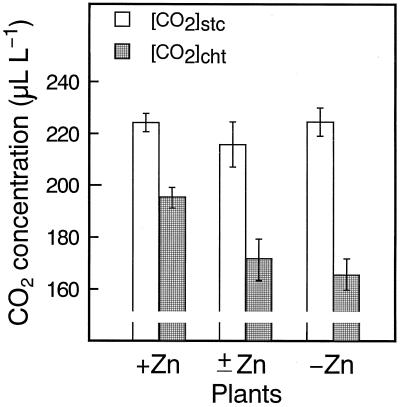
In all leaves examined [CO2]stc was maintained at about 220 μL L−1. In contrast, [CO2]cht in +Zn leaves was 195 ± 4 μL L−1, and [CO2]cht decreased with a reduction in the Zn content of leaves to 171 ± 6 and 166 ± 5 μL L−1 in ±Zn and −Zn leaves, respectively. The gradient of the CO2 concentration between the stomatal cavity and the site of CO2 fixation increased from 29 to 59 μL L−1 (Fig. 1). The resistance in CO2 flux with Zn deficiency and our findings support the hypothesis that CA plays a role in facilitating the supply of CO2 to the sites of carboxylation.
Figure 1.
[CO2]stc and [CO2]cht in plants with three different levels of Zn. Values are expressed as means ± se. See text for details.
Northern-Blot Analysis
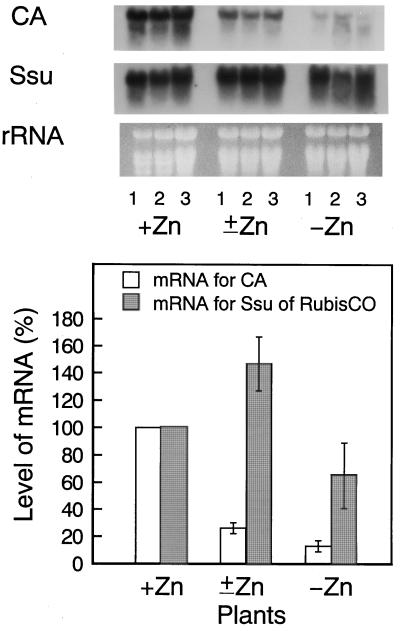
The results of northern-blot analysis of mRNAs for CA and Rubisco are shown in Figure 2. The level of the mRNA for CA decreased with the reduction in Zn content of the leaf, decreasing to 26% (±Zn) and 13% (−Zn) of that in control plants (+Zn). In contrast, the level of the mRNA for the small subunit of Rubisco showed no consistent trend. It seems likely that the reduction in CA activity was caused not by deactivation of the enzyme but, rather, by a decrease in the expression of the CA mRNA caused by Zn deficiency.
Figure 2.
Northern-blot analysis of the expression of mRNAs for CA and the small subunit of Rubisco in plants with three different levels of Zn. Total RNA, 20 μg per lane, was separated on 1.5% agarose containing formaldehyde, transferred to a nylon membrane, and hybridized to radiolabeled DNA probes. Each level of Zn included three lanes and each lane derived from one container. Levels of mRNAs in +Zn plants are set at 100%. Values are expressed as means ± se. See text for details. Ssu, Small subunit of Rubisco.
DISCUSSION
CA has been recognized as an important enzyme that is closely associated with photosynthesis. In C3 plants CA activity is located mainly in the mesophyll chloroplast, with much smaller activity in the cytosol. Chloroplastic CA activity was reported as 87% of total cellular activity in potato leaves (Rumeau et al., 1996), and from 86% to 95% in wheat, spinach, Moricandia arvensis, and Mesembryanthemum crystallinum (Tsuzuki et al., 1985). Recently, cytosolic and chloroplastic CA isoenzymes were isolated in Arabidopsis and potato, and a polyclonal antibody hybridized to two kinds of CA (Fett and Coleman, 1994; Rumeau et al., 1996). The hypothesis that an alternative processing occurred during transit-peptide removal has been proposed by Johansson and Forsman (1992). Furthermore, two Arabidopsis cDNA clones encoding putative cytosolic and chloroplastic CAs were isolated, and comparison of the predicted amino acid sequences indicated very similar genes (Fett and Coleman, 1994). In our experiment CA activity in the crude extract was measured and cDNA for rice chloroplastic CA was used as a probe for the northern-blot analysis. Considering the localization, percentage, and homology, although the CA activity and mRNA expression for CA in our study could not be distinguished from those for cytosolic and chloroplastic CA, they were considered to consist mainly of chloroplastic activity and mRNA expression for chloroplastic CA, respectively.
The main purpose of this study was to analyze the relationship between the activity of CA and each component of mesophyll resistance by using plants with reduced CA activity as a consequence of Zn deficiency. Major reductions in CA activity to 27% and 14% in ±Zn and −Zn plants, respectively, of that in +Zn plants were observed without any change in rs or rc. Therefore, we were able to detect a correlation between the transfer of CO2 from the stomatal cavity to the site of CO2 fixation and CA activity. It appeared that rr increased with the reduction in CA activity (Tables I and II). Although [CO2]stc was maintained at about 220 μL L−1 during photosynthesis, [CO2]cht decreased with the reduction in CA activity, and the gradient of the concentration of CO2 between the stomatal cavity and the site of CO2 fixation increased from 29 to 59 μL L−1 with the reduction in CA activity (Fig. 1). With antisense RNA for CA a great reduction in the levels of CA to as little as 2% of wild-type levels caused an increase in the gradient of the CO2 concentration of about 15 μmol mol−1 (Price et al., 1994; Williams et al., 1996), which is similar to our results. Consequently, we can conclude that a reduction in CA activity tampers with the transfer of CO2 from the stomatal cavity to the site of Rubisco and causes a low [CO2]cht. CA is mostly localized in chloroplasts in C3 plants. Therefore, our findings support the hypothesis that CA has a role in facilitating the supply of CO2 to the sites of carboxylation within the chloroplast.
The considerable reduction in CA activity was accompanied by the reduction in Zn content, but we observed no major change in the rate of photosynthesis. In the antisense analysis, rates of CO2 assimilation were also unaffected by low levels of CA in chloroplasts in transgenic tobacco plants (Majeau et al., 1994; Price et al., 1994). Such observations might be explained by the smaller contribution of the CO2-transfer process than the stomatal or CO2-fixation processes (Sasaki et al., 1996). Although [CO2]cht decreased with a reduction in the activity of CA from 195 to 166 μL L−1, we could not observe any marked decrease in the rate of photosynthesis as in [CO2]cht. If the CO2-compensation point and other factors were constant, it is possible that a 29 μL L−1 difference in [CO2]cht would cause an 18% to 20% decline in the rate of photosynthesis. We found that the rate of photosynthesis decreased only to 89% of that in +Zn plants. Although the initial activity of Rubisco was similar to the rate of photosynthesis, the Rubisco content unexpectedly increased slightly, from 3.5 to 4.8 g m−2, with a reduction in the Zn content of leaves (the increase of Rubisco content has not been shown in other experiments on Zn deficiency). We suggest that the absence of any additional decrease in photosynthesis might be explained by the increase in Rubisco content.
Zn is necessary for catalysis by CA (Bar-Akiva and Lavon, 1969; Silverman, 1991). In this study expression of the mRNA for CA decreased to 34% (±Zn) and 13% (−Zn) of that in control plants. This decrease resembled that in the CA activity in these plants with the reduction in the Zn content of leaves (Table I; Fig. 2). Therefore, we suggest that the reduction in CA activity attributable to Zn deficiency was a result not of failure to activate CA but of a decrease in the level of CA. Although modification of transcript abundance does not always indicate transcriptional regulation, it is possible that a feedback system balances the amount of CA synthesized with the available Zn in the cell.
Some studies on the levels of CA in intact leaves have suggested that coordinated regulation of the expression of CA and Rubisco might occur under some growth conditions, e.g. with changes in the supply of nitrogen (Makino et al., 1992) and in the level of CO2 (Porter and Grodzinski, 1984; Peet et al., 1986). Previously, we suggested that CA activity, most of which is found in the chloroplast, changes in association with changes in Rubisco activity in the chloroplast (Sasaki et al., 1996). Hudson et al. (1992) reported that CA activity changed in association with Rubisco activity in transgenic tobacco to which antisense Rubisco mRNA had been introduced. The activity ratio of CA to Rubisco is reportedly maintained in spite of differences in the levels of Rubisco and CA among cultivars and among leaf nitrogen contents in pea and wheat (Makino et al., 1992; Majeau and Coleman, 1994). Although such observations suggest the coordinated regulation of levels of CA and Rubisco, this relationship can easily be disrupted in low-CA plants (which are unusual), such as those in this study (Table I), which were induced by antisense RNA (Price et al., 1994). It seems likely that the level of CA is influenced by the level of Rubisco, but not vice versa, and although many studies show coordinated changes in CA and Rubisco, it appears that CA plays a role in facilitating the supply of CO2 to sites of carboxylation.
Abbreviations:
- CA
carbonic anhydrase
- [CO2]atm
CO2 concentration in the atmospheric air
- [CO2]cht
CO2 concentration at the site of CO2 fixation in the chloroplast
- [CO2]stc
CO2 concentration in the stomatal cavity
- Δ
carbon isotope discrimination by the plant
- δa
relative concentration of 13C in the atmospheric air
- δp
relative concentration of 13C in the photosynthetic products
- δ13C
relative concentration of 13C in total carbon atoms
- rc
CO2-fixation resistance
- rm
mesophyll CO2 resistance
- rr
CO2-transfer resistance
- rs
stomatal CO2 resistance
Footnotes
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Science, and Culture, Japan.
LITERATURE CITED
- Bar-Akiva A, Lavon R. Carbonic anhydrase activity as an indicator of zinc deficiency in citrus leaves. J Hortic Sci. 1969;44:359–362. [Google Scholar]
- Edwards GE, Mohamed AK. Reduction in carbonic anhydrase activity in zinc-deficient leaves of Phaseolus vulgaris L. Crop Sci. 1973;13:351–354. [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol. 1986;13:281–292. [Google Scholar]
- Farquhar GD, Ball MC, von Caemmerer S, Roksandic Z. Effect of salinity and humidity on 13C values of halophytes: evidence for diffusional isotope fractionation determined by the ratio of intercellular/atmospheric partial pressure of CO2 under different environmental conditions. Oecologia. 1982;52:121–124. doi: 10.1007/BF00349020. [DOI] [PubMed] [Google Scholar]
- Fett JP, Coleman JR. Characterization and expression of two cDNAs encoding carbon anhydrase in Arabidopsis thaliana. Plant Physiol. 1994;105:707–713. doi: 10.1104/pp.105.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm H, Devic M, Fluhr R, Edelman M. Control of psbA gene expression: in mature Spirodela oligorrhiza chloroplasts light regulation of 32 kD protein synthesis is independent of transcript level. EMBO J. 1985;4:291–295. doi: 10.1002/j.1460-2075.1985.tb03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guliev NM, Briramov SM, Aliev DA. Functional organization of carbonic anhydrase in higher plants. Sov Plant Physiol. 1992;39:537–544. [Google Scholar]
- Hubick KT, Farquhar GD, Shorter R. Correlation between water-use efficiency and carbon isotope discrimination in diverse peanut (Arachis) germplasm. Aust J Plant Physiol. 1986;13:803–816. [Google Scholar]
- Hudson GS, Evans JR, von Caemmerer S, Arvidsson YBC, Andrews TJ. Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase content by antisense RNA reduces photosynthesis in transgenic tobacco plants. Plant Physiol. 1992;98:294–302. doi: 10.1104/pp.98.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson BS, Fong F, Heath RL. Carbonic anhydrase of spinach. Studies on its location, inhibition and physiological function. Plant Physiol. 1975;55:468–474. doi: 10.1104/pp.55.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson IM, Forsman C. Processing of chloroplast transit peptide of pea carbonic anhydrase in chloroplasts and in Escherichia coli: identification of two cleavage sites. FEBS Lett. 1992;314:232–236. doi: 10.1016/0014-5793(92)81478-5. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Majeau N, Arnold MA, Coleman JR. Modification of carbonic anhydrase activity by antisense and over-expression constructs in transgenic tobacco. Plant Mol Biol. 1994;25:377–385. doi: 10.1007/BF00043867. [DOI] [PubMed] [Google Scholar]
- Majeau N, Coleman JR. Correlation of carbonic anhydrase and ribulose-1,5-bisphosphate carboxylase/oxygenase expression in pea. Plant Physiol. 1994;104:1393–1399. doi: 10.1104/pp.104.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Sakashita H, Hidema J, Mae T, Ojima K, Osmond B. Distinctive responses of ribulose-1,5-bisphosphate carboxylase and carbonic anhydrase in wheat leaves to nitrogen nutrition and their possible relationships to CO2-transfer resistance. Plant Physiol. 1992;100:1737–1743. doi: 10.1104/pp.100.4.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M, Kano-Murakami Y, Tanaka Y, Ozeki Y, Yamamoto N. Classification and nucleotide sequence of cDNA encoding the small subunit of ribulose-1,5-bisphosphate carboxylase from rice. Plant Cell Physiol. 1988;29:1015–1022. [Google Scholar]
- Ohki K. Effect of zinc nutrition on photosynthesis and carbonic anhydrase activity in cotton. Plant Physiol. 1976;38:300–304. [Google Scholar]
- Ohki K. Zinc concentration in soybean as related to growth, photosynthesis, and carbonic anhydrase activity. Crop Sci. 1978;18:79–82. [Google Scholar]
- Peet MM, Huber SC, Patterson DT. Acclimation to high CO2 in monoecious cucumber. Carbon exchange rates, enzyme activities, and starch and nutrient conditions. Plant Physiol. 1986;80:63–67. doi: 10.1104/pp.80.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter MA, Grodzinski B. Assimilation to high CO2 in bean: carbonic anhydrase and ribulose bisphosphate carboxylase. Plant Physiol. 1984;74:413–416. doi: 10.1104/pp.74.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, von Caemmerer S, Evans JR, Yu J-W, Lloyd J, Oja V, Kell P, Harrison K, Gallagher A, Badger MR. Specific reduction of chloroplast carbonic anhydrase activity by antisense RNA in transgenic tobacco plants has a minor effect on photosynthetic CO2 assimilation. Planta. 1994;193:331–340. [Google Scholar]
- Randall PJ, Bouma D. Zinc deficiency, carbonic anhydrase, and photosynthesis in leaves of spinach. Plant Physiol. 1973;52:229–232. doi: 10.1104/pp.52.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumeau D, Cuiné S, Fina L, Gault N, Nicole M, Peltier G. Subcellular distribution of carbonic anhydrase in Solanum tuberosum L. leaves. Planta. 1996;199:79–88. doi: 10.1007/BF00196884. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Samejima M, Ishii R. 13C measurement on mechanism of cultivar difference in leaf photosynthesis of rice (Oryza sativa L.) Plant Cell Physiol. 1996;37:1161–1166. [Google Scholar]
- Schmid GH. Origin and properties of mutant plants: yellow tobacco. Methods Enzymol. 1971;23:171–194. [Google Scholar]
- Silverman DN. The catalytic mechanism of carbonic anhydrase. Can J Bot. 1991;69:1070–1078. [Google Scholar]
- Swader JA, Jacobson BS. Acetazolamide inhibition of photosystem in isolated spinach chloroplasts. Phytochemistry. 1972;11:65–70. [Google Scholar]
- Tsuzuki M, Miyachi S, Edwards GE. Localization of carbonic anhydrase in mesophyll cells of terrestrial C3 plants in relation to CO2 assimilation. Plant Cell Physiol. 1985;26:881–891. [Google Scholar]
- Usuda H. The activation state of ribulose-1,5-bisphosphate carboxylase in maize (Zea mays) leaves in dark and light. Plant Cell Physiol. 1985;26:1455–1464. [Google Scholar]
- Williams TG, Flanagan LB, Coleman JR. Photosynthetic gas exchange and discrimination against 13CO2 and C18O16O in tobacco plants modified by an antisense construct to have low chloroplastic carbonic anhydrase. Plant Physiol. 1996;112:319–326. doi: 10.1104/pp.112.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Forno DA, Cock JH, Gomes KA (1976) Routine procedure for growing rice plants in culture solution. In Laboratory Manual for Physiological Studies of Rice. The International Rice Research Institute, Los Vanõs, Philippines, pp 61–66