Abstract
Background/Aims
Three types of open-angle glaucoma (OAG) – primary, pigmentary, and pseudoexfoliative – are frequently encountered. The aim of this study was to compare demographic, ocular, and systemic medical information collected on people with these three OAG types at diagnosis, and determine if the OAG type affected prognosis.
Methods
Information on 607 participants of the Collaborative Initial Glaucoma Treatment Study was accessed. Descriptive statistics characterized their demographic, ocular, and medical status at diagnosis. Comparisons were made using analysis of variance (ANOVA), and chi-square or Fisher exact tests. Multinomial, mixed, and logistic regression analyses were also performed.
Results
Relative to people with primary OAG, those with pigmentary OAG were younger, more likely to be white, less likely to have a family history of glaucoma, and were more myopic. Those with pseudoexfoliative OAG were older, more likely to be white, more likely to be female, less likely to have bilateral disease, and presented with higher IOP and better VA. The type of glaucoma was not associated with intraocular pressure or visual field progression during follow-up.
Conclusion
Characteristics of newly-diagnosed enrollees differed by the type of OAG. While some of these differences relate to the pathogenesis of OAG type, other differences are noteworthy for further evaluation within population-based samples of subjects with newly-diagnosed OAG.
Keywords: Glaucoma, Epidemiology
Introduction
In 2004, open-angle glaucoma (OAG) was estimated to affect 2.22 million people in the United States, and the authors estimated that by 2020, there will be more than 3 million people with OAG in the United States.(1) While primary OAG (POAG) is the most common type of OAG encountered in the United States,(2) a number of other OAG types exist, among which include pigmentary OAG and pseudoexfoliative OAG.
POAG is defined as a group of ocular diseases that cause characteristic, progressive changes in the optic nerve head, visual field loss, or both.(2) These changes may be associated with elevated intraocular pressure (IOP), but often can occur with IOPs below the population mean. The term “primary” indicates that there is no overt cause such as trauma, inflammation, excessive anterior chamber pigment dispersion, or pseudoexfoliation of the lens capsule underlying this glaucoma. Risk factors commonly associated with POAG include elevated IOP, older age, African descent, and a family history of POAG.(3–7)
Pigmentary OAG (PIGM) characteristically develops in young myopic patients with pigment dispersion syndrome, which is characterized by melanin pigment liberation from the iris pigment epithelium. Sugar noted a predominance of males and myopes among 137 cases of PIGM he reviewed,(8) a finding which Lichter and Shaffer also noted in 102 patients with PIGM they reviewed. They also reported an association of younger age at diagnosis with higher degrees of myopia.(9)
Pseudoexfoliation syndrome (PXS) has been termed the most common “identifiable” cause of open-angle glaucoma.(10) PXS results in deposition and accumulation of exfoliative material on the lens, iris, and other intraocular surfaces. While not all people with PXS develop glaucoma, those that develop pseudoexfoliative glaucoma (PEXG) tend to have a higher IOP at diagnosis than those with POAG, so that achieving success in treating PEXG can be more difficult than in treating POAG.(11)
In this study, we used the data collected during the Collaborative Initial Glaucoma Treatment Study (CIGTS) (12) to compare the three types of OAG (primary, pigmentary, and pseudoexfoliative) that were included in the enrollment criteria for the CIGTS. Our aim is to describe differences in subjects who presented with these three types of OAG, using information obtained on patients when they were newly diagnosed with OAG, and to determine if these different OAG types responded differently to treatment.
Materials and Methods
CIGTS investigators at 14 clinical centers in the United States enrolled 607 OAG patients between October 1993 and April 1997. Eligibility criteria included being newly diagnosed with one of three types of OAG in one or both eyes: POAG, PIGM, or PEXG, an age between 25 and 75 years, and lack of prior treatment for glaucoma. Details on these criteria have been described.(12) The objective of CIGTS was to determine whether patients with newly diagnosed OAG demonstrated better control of their glaucoma by initial treatment with topical medication(s) or by immediate filtration surgery. In the present study, we analyzed the baseline data that CIGTS investigators gathered at the study participants’ baseline visits at the clinical centers. A study eye was designated at baseline as the first eye to be treated for glaucoma, and only data from this eye were analyzed.
Statistical methods
Descriptive statistics were used to characterize the demographic and clinical characteristics of the CIGTS participants at enrollment. We compared the distribution of variables within the three types of glaucoma using analysis of variance (ANOVA) for continuous variables and chi-square or Fisher exact tests for categorical variables. Pair-wise comparisons were made with adjustment for multiple comparisons via the Tukey test. Following bivariate analyses, a multinomial logistic regression model was developed to characterize the extent to which variables were associated with specific types of OAG. Backward selection was used to identify variables of significance after adjustment for all other variables that had significant associations.
Mixed linear regression was used to evaluate associations of perimetric mean deviation (MD, from Humphrey 24–2 full threshold tests) and IOP (from Goldmann applanation tonometry) during follow-up with OAG type. Glaucoma diagnosis was added to previously published models of baseline risk factors for MD(13) and IOP(14) to test whether diagnosis added predictive strength. For the MD model, variables included were: treatment (surgery vs. medicine), race (White & Other vs. Black), age, diabetes, baseline MD, cataract surgery within previous year, time from randomization, the range of 6 baseline IOP measures, as well as 5 interaction terms. For the IOP model, variables included were treatment (surgery vs. medicine), smoking status (current smoker vs. other), interaction between treatment and smoking, baseline IOP, baseline MD, education, hypertension, time since randomization (time and time squared), and center. A heterogeneous toeplitz structure was used to model the correlation among repeated measures (MD or IOP) of a subject. The model for IOP also used a random subject intercept and slope. Statistical analyses were performed using SAS version 9.2 software (SAS Institute, Cary, NC).
Results
The descriptive characteristics of subjects with the three types of OAG are shown in Table 1. Out of 607 participants, 550 (90.6%) were newly diagnosed with POAG, 28 (4.6%) with PIGM, and 29 (4.8%) with PEXG. The mean ages of the subjects in these three groups differed significantly (p<0.0001). POAG subjects (58.0 years) were nine years older on average than those with PIGM (48.9 years) and seven years younger on average than those with PEXG (65.1 years). The distribution of males and females among the three types of glaucoma did not differ significantly (p=0.335). 41.5% (228) of POAG subjects indicated their race was black, whereas only 7.1% of PIGM subjects and 3.5% of PEXG subjects reported that their race was black (p<0.0001). Educational achievement varied somewhat between the groups (p=0.055), with the POAG subjects having the highest percentage (22.6%) with less than a high school education relative to PIGM subjects (3.6%) and PEXG subjects (10.3%). Follow-up time did not significantly differ for the POAG (7.2 years), PIGM (7.2 years), and PEXG (7.7 years) subjects (p=0.447).
Table 1.
Descriptive characteristics of subjects with the three types of OAG.
| Continuous Variables | POAG (n=550) | PIGM (n=28) | PEXG (n=29) | P-value* | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 58.02 | 10.73 | 48.87 | 11.58 | 65.13 | 8.30 | <0.0001 |
| Follow-up (years) | 7.18 | 2.34 | 7.75 | 2.00 | 7.24 | 2.02 | 0.4474 |
| IOP (mmHg) | 27.25 | 5.45 | 28.11 | 6.77 | 31.93 | 5.42 | <0.0001 |
| VA (letters) | 85.57 | 5.73 | 85.64 | 4.98 | 87.48 | 5.59 | 0.2124 |
| Spherical Equivalent (D) | −0.87 | 2.54 | −3.81 | 3.03 | −0.15 | 2.34 | <0.0001 |
| MD (dB) | −5.48 | 4.26 | −5.38 | 4.73 | −4.71 | 4.29 | 0.6435 |
| PSD (dB) | 5.68 | 3.49 | 6.04 | 3.85 | 4.95 | 3.26 | 0.4596 |
| CPSD (dB) | 5.04 | 3.67 | 5.41 | 4.06 | 4.22 | 3.34 | 0.4297 |
| SF (dB) | 2.09 | 0.73 | 2.06 | 0.73 | 2.08 | 0.86 | 0.9788 |
| CDR - Vertical | 0.69 | 0.17 | 0.70 | 0.16 | 0.68 | 0.16 | 0.9273 |
| Categorical Variables | N | Percent | N | Percent | N | Percent | P-value** |
|---|---|---|---|---|---|---|---|
| Race | |||||||
| White | 284 | 51.6 | 26 | 92.9 | 27 | 93.1 | <0.0001 |
| Black | 228 | 41.5 | 2 | 7.1 | 1 | 3.5 | |
| Other | 38 | 6.9 | 0 | 0.0 | 1 | 3.5 | |
| Sex | |||||||
| Male | 303 | 55.1 | 18 | 64.3 | 13 | 44.8 | 0.3346 |
| Female | 247 | 44.9 | 10 | 35.7 | 16 | 55.2 | |
| Education | |||||||
| <HS | 124 | 22.6 | 1 | 3.6 | 3 | 10.3 | 0.0545 |
| HS | 150 | 27.3 | 8 | 28.6 | 9 | 31.0 | |
| >HS | 276 | 50.2 | 19 | 67.9 | 17 | 58.6 | |
| Smoking Status | |||||||
| Non-smoker | 434 | 78.9 | 20 | 71.4 | 26 | 89.7 | 0.2222 |
| Smoker | 116 | 21.1 | 8 | 28.6 | 3 | 10.3 | |
| Diabetes | |||||||
| No | 450 | 81.8 | 27 | 96.4 | 28 | 96.6 | 0.0136 |
| Yes | 100 | 18.2 | 1 | 3.6 | 1 | 3.5 | |
| Hypertension | |||||||
| No | 338 | 61.5 | 22 | 78.6 | 22 | 75.9 | 0.0630 |
| Yes | 212 | 38.6 | 6 | 21.4 | 7 | 24.1 | |
| Other vascular disease | |||||||
| No | 467 | 84.9 | 26 | 92.9 | 23 | 79.3 | 0.3763 |
| Yes | 83 | 15.1 | 2 | 7.1 | 6 | 20.7 | |
| Hemorrhage | |||||||
| No | 533 | 96.9 | 27 | 96.4 | 27 | 93.1 | 0.3559 |
| Yes | 17 | 3.1 | 1 | 3.6 | 2 | 6.9 | |
| Immediate Family Hx | |||||||
| No | 304 | 61.2 | 24 | 92.3 | 16 | 72.7 | 0.0016 |
| Yes | 193 | 38.8 | 2 | 7.7 | 6 | 27.3 | |
| Distant Family Hx | |||||||
| No | 312 | 74.6 | 21 | 87.5 | 16 | 84.2 | 0.2684 |
| Yes | 106 | 25.4 | 3 | 12.5 | 3 | 15.8 | |
| Bilaterality | |||||||
| No | 111 | 20.4 | 7 | 25.0 | 16 | 55.2 | <0.0001 |
| Yes | 433 | 79.6 | 21 | 75.0 | 13 | 44.8 |
Analysis of variance;
Chi-square test when all cell counts of a table are ≥ 5, or Fisher’s exact test when at least 1 cell has a count of < 5; note: missing data account for sums that are less than the total
SD=standard deviation; IOP=intraocular pressure; VA=visual acuity; D=diopters; MD=mean deviation; dB=decibels; PSD=pattern standard deviation; CPSD=corrected pattern standard deviation; SF=short term fluctuation; CDR=cup to disk ratio; HS=high school; Hx=history
Those with newly diagnosed POAG tended to have more non-ocular co-morbidities than the other two OAG subtypes. Diabetes was found significantly more frequently (p=0.014) among subjects with POAG (18.2%) vs. subjects with PIGM (3.6%) or PEXG (3.5%). 38.6 % (212) of POAG subjects had systemic hypertension, whereas 21.4% of PIGM subjects and 24.1% of PEXG subjects had hypertension (p=0.063). The percentages of subjects with other vascular or cardiac diseases did not significantly differ among the three groups (p=0.376). In terms of family history of glaucoma, 7.7 % of PIGM subjects had a history of glaucoma within the immediate family whereas 38.8% of POAG and 27.3% of PEXG subjects reported this (p=0.002). The distribution of history of glaucoma in the distant family among subjects with the three types of glaucoma did not differ significantly (p=0.268), nor did smoking status (p=0.222).
Ophthalmic examination findings showed some significant differences among the three OAG subtypes. The mean IOPs at baseline of POAG, PIGM, and PEXG participants’ study eyes were 27.3, 28.1, and 31.9 mmHg, respectively (p<0.0001). Post hoc pairwise comparisons showed a significantly higher mean IOP in those with PEXG relative to either of the other OAG diagnoses, but no difference between POAG and PIGM. Those diagnosed with PIGM were significantly (p<0.0001) more myopic on average (spherical equivalent mean value of −3.81 diopters, D) than the other two groups, whose mean values were −0.87 D (POAG) and −0.15 D (PEXG). The mean visual acuities at baseline, results from Humphrey 24–2 visual field testing, vertical cup to disc ratio, and the presence of disc hemorrhage among the three types of glaucoma were not significantly different. In terms of bilaterality, those with PEXG were more likely to present at diagnosis with only one eye involved (p<0.0001).
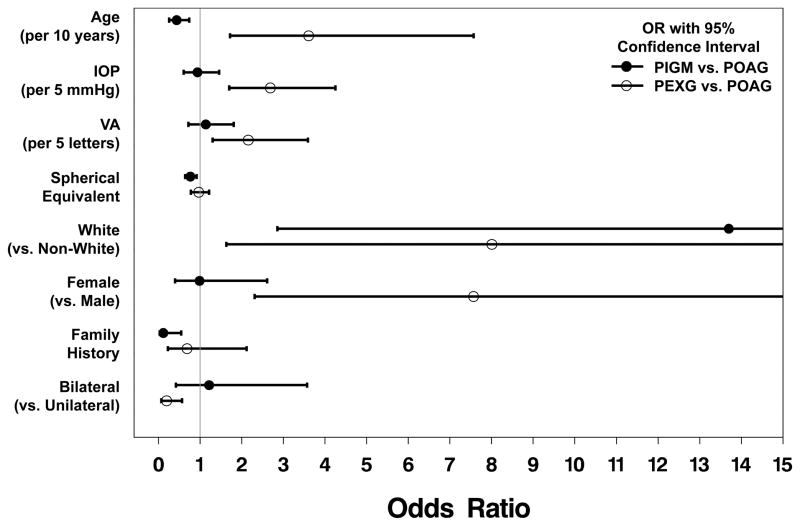
The multinomial logistic regression model results (Figure 1) identified four factors that were significantly associated with a diagnosis of PIGM versus a diagnosis of POAG: age, race, history of glaucoma in the immediate family, and spherical equivalent. Relative to POAG, those with PIGM were younger (odds ratio (OR)=0.44 for a 10-year increment in age), more likely to be white (OR=13.70), less likely to have an immediate family history of glaucoma (OR=0.12), and more likely to have a more negative (myopic) spherical equivalent value (OR= 0.77 for a 1 D increment).
Figure 1.
Six factors were significantly associated with having a diagnosis of PEXG relative to POAG: age, sex, race, visual acuity, IOP, and bilaterality. Relative to POAG, CIGTS enrollees with newly diagnosed PEXG were significantly older (OR=3.61 for a 10 year increment), more likely to be female (OR=7.57), and more likely to be white (OR=8.01). PEXG subjects had higher IOP than POAG subjects (OR = 2.69 for a 5 mmHg increment), and their visual acuity at baseline was somewhat better (OR=2.16 for a 5-letter increment). PEXG subjects were less likely to have bilateral disease relative to those with POAG (OR=0.20).
In two binary logistic regression models (PIGM vs. POAG and PEXG vs. POAG) where non-significant effects were stepped-out, multi-variable results showed similar effects to that of the multinomial logistic regression results. Univariable results were also similar for all but the gender effect. This effect was weakened due to its collinearity with baseline IOP (r=−0.19) – males had significantly higher baseline IOP than females. See supplementary online Table 1 for a comparison of effects between the multinomial logistic regression, multi-variable binary logistic regression, and univariable binary logistic regression results.
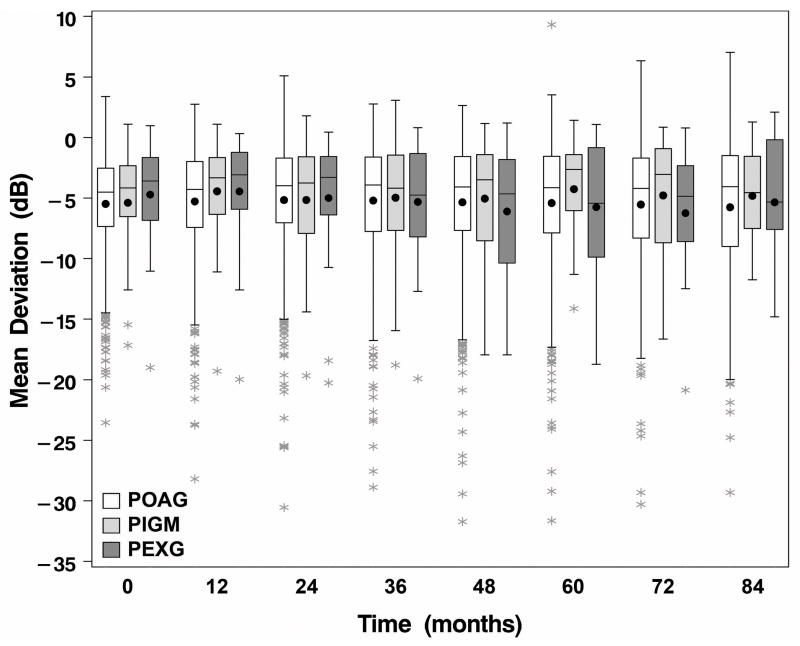
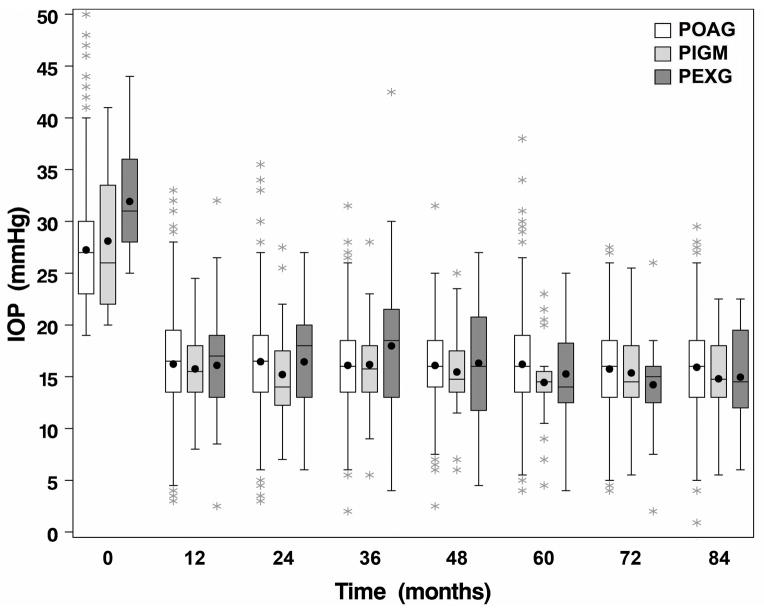
The relationship of glaucoma diagnosis with two treatment outcomes (MD and IOP) was evaluated over seven years of follow-up. Boxplots of these two outcomes over time by diagnosis are shown in Figures 2 and 3. The association of glaucoma diagnosis with these outcomes was investigated using repeated measures linear regression. Glaucoma diagnosis was added to previously published models of baseline risk factors(13, 14) to determine if diagnosis added predictive strength. Glaucoma diagnosis was not associated with MD over time (p=0.627). There were no significant interactions between diagnosis and either treatment or time. Glaucoma diagnosis was also not associated with IOP measures during follow-up (p=0.310), with no significant interactions between diagnosis and treatment or time. Since baseline IOP was significantly higher in those with PEXG than in POAG or PIGM, we evaluated and found a significant interaction (p=0.030) between baseline IOP and glaucoma diagnosis on follow-up IOP. The interaction resulted from differing trends in IOP reduction over time relative to baseline IOP in patients with PEXG, in whom the higher the baseline IOP, the lower the follow-up IOP, whereas in patients with POAG and PIGM, baseline IOP and follow-up IOP were directly related.
Figure 2.
Figure 3.
Discussion
Of the three types of OAG eligible for enrollment in the CIGTS, POAG is the most frequent and thereby has an extensive literature on characteristics found in people with this condition. Among numerous reviews of OAG epidemiology, Tielsch’s review(15) focuses on POAG and identifies demographic factors (older age and black race), ocular factors (elevated IOP and myopia), and a positive family history as consistently associated with POAG, whereas gender and systemic co-morbidities (diabetes and hypertension) were less consistently reported associations with POAG across studies. Within the CIGTS enrollees, who do not constitute a representative population of newly diagnosed subjects, we identified three unique characteristics of our POAG enrollees relative to those with the other two types of OAG. Enrollees diagnosed with POAG were more likely to report their race as black, presented more frequently with two systemic co-morbidities (diabetes and hypertension), and were more likely to report a history of glaucoma in their immediate family.
PIGM has been reported to primarily affect young myopic males, and is usually bilateral. The average age of the onset of PIGM is between the third or fourth decades.(8) These associations are also found in the results we report, as the mean age of the onset of PIGM was 48.9 years, 64% were males, and the mean spherical equivalent at baseline was −3.8 D. While a study eye was identified at baseline for comprehensive follow-up in the CIGTS, the fellow eye of those with PIGM in the study eye shared this diagnosis in 75% (21/28) of enrollees. PIGM is said to be prevalent in whites, with a lower prevalence in blacks, Latinos and Asians.(8) While our finding of PIGM in two blacks and 26 whites is of interest, enrollment of study participants at clinical trial centers does not yield prevalence estimates, and so population-based findings are best used to evaluate this.
The proportion of open angle glaucoma accounted for by PEXG varies considerably across the world, and is affected by the prevalence of exfoliation syndrome in the particular area. In the United States, most estimates range from 2% to 12% for the proportion of OAG accounted for by PEXG,(16–18) whereas in the eastern region of the Arabian peninsula, 77% of all OAG was reported to be associated with exfoliation syndrome.(19) A recent evaluation of two large cohorts of health professionals in the United States identified increasing latitude as a risk factor for PEXG (as well as exfoliation syndrome).(20) Very few PEXG cases have been reported among blacks.(10) Only one CIGTS enrollee was black among the 29 CIGTS participants who had PEXG. Teus and colleagues reported that IOP at diagnosis is usually higher in patients with PEXG than POAG,(11) which we found as well. PEXG study participants had a mean IOP at baseline (31.9 mmHg) that was higher than those with POAG (27.3 mmHg) or PIGM (28.1 mmHg). Our finding that those newly diagnosed with PEXG were significantly older than either of the other OAG groups fits well with the strong association reported between older age and risk of exfoliation glaucoma by Kang et al.(20) In contrast to the persons with POAG and PIGM included in the CIGTS, less than 50% of those with PEXG presented with bilateral disease.
Budde and Jonas evaluated the frequency of a positive family history (in a 1st or 2nd degree relative) among subjects with POAG, PIGM, and PEXG.(21) They found that the frequency of a positive family history of glaucoma among subjects with PIGM and PEXG was not different compared to that among subjects with POAG when adjusted for age. Our unadjusted and adjusted findings indicate that POAG has a much stronger association with a history of glaucoma in the immediate (1st degree) family relative to those with PIGM, but not relative to those with PEXG.
One of the strengths of this study is that the CIGTS included carefully standardized examinations by glaucoma subspecialists at 14 centers around the United States. Even so, some caveats should be noted in considering these findings. The diagnostic criteria used to identify those with POAG, PIGM, and PEXG were left to the discretion of the examining ophthalmologist, which may have led to some variation in diagnoses of glaucoma subtypes. CIGTS was not designed to enroll a representative sample of newly diagnosed open-angle glaucoma, and so our 607 enrollees may be quite different from such a sample. Finally, given the relatively few enrollees identified with PIGM and PEXG, small sample sizes limit our ability to be definitive about factors that were associated with these two conditions.
Conclusions
Characteristics of newly-diagnosed enrollees into a clinical trial of OAG treatment differed by the type of OAG. While some of these differences relate to the underlying pathogenesis of the specific type of OAG, such as higher IOP among those with PEXG, the associations we found with type of OAG should be evaluated within population-based samples of subjects with newly-diagnosed OAG.
Supplementary Material
Acknowledgments
This research was supported by NIH/NEI Grant EY020912 and a departmental grant from Research to Prevent Blindness (RPB), New York, NY. Dr. Musch is a recipient of the RPB Lew R. Wasserman Merit Award.
Footnotes
This research was presented in part at the annual meeting of ARVO, Ft. Lauderdale, FL, May 2010.
The authors have no competing interests.
All named authors have contributed to the writing and/or revision of the manuscript we are submitting. Dr. Musch and Ms. Niziol were involved in the analyses that were required for this study, and all authors took part in interpreting the results of these analyses.
References
- 1.Eye Diseases Prevalence Research Group. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–8. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 3.Tielsch JM, Sommer A, Katz J, et al. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Study. JAMA. 1991;266:369–74. [PubMed] [Google Scholar]
- 4.Tielsch JM, Katz J, Sommer A, et al. Family history and risk of primary open angle glaucoma. The Baltimore Eye Survey. Arch Ophthalmol. 1994;112:69–73. doi: 10.1001/archopht.1994.01090130079022. [DOI] [PubMed] [Google Scholar]
- 5.Quigley HA, Enger C, Katz J, et al. Risk factors for the development of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol. 1994;112:644–9. doi: 10.1001/archopht.1994.01090170088028. [DOI] [PubMed] [Google Scholar]
- 6.Leske MC, Connell AMS, Wu S–Y, et al. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch Ophthalmol. 1995;113:918–24. doi: 10.1001/archopht.1995.01100070092031. [DOI] [PubMed] [Google Scholar]
- 7.Le A, Mukesh BN, McCarty CA, et al. Risk factors associated with the incidence of open-angle glaucoma: the Visual Impairment Project. Invest Ophthalmol Vis Sci. 2003;44:3783–9. doi: 10.1167/iovs.03-0077. [DOI] [PubMed] [Google Scholar]
- 8.Sugar HS. Pigmentary glaucoma: a 25 year review. Am J Ophthalmol. 1966;62:499–507. doi: 10.1016/0002-9394(66)91330-4. [DOI] [PubMed] [Google Scholar]
- 9.Lichter PR, Shaffer RN. Diagnosis and prognostic signs in pigmentary glaucoma. Trans Am Acad Ophthalmol Otolaryngol. 1970;74:984–98. [PubMed] [Google Scholar]
- 10.Schlotzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141:921–37. doi: 10.1016/j.ajo.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 11.Teus MA, Castejón MA, Calvo MA, et al. Intraocular pressure as a risk factor for visual field loss in pseudoexfoliative and in primary open-angle glaucoma. Ophthalmology. 1998;105:2225–9. doi: 10.1016/S0161-6420(98)91220-9. Discussion: 2229–30. [DOI] [PubMed] [Google Scholar]
- 12.Musch DC, Lichter PR, Guire KE, et al. The Collaborative Initial Glaucoma Treatment Study: study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999;106:653–62. doi: 10.1016/s0161-6420(99)90147-1. [DOI] [PubMed] [Google Scholar]
- 13.Musch DC, Gillespie BW, Lichter PR, et al. Visual field progression in the Collaborative Initial Glaucoma Treatment Study. The impact of treatment and other baseline factors. Ophthalmology. 2009;116:200–7. doi: 10.1016/j.ophtha.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musch DC, Gillespie BW, Niziol LM, et al. Factors associated with intraocular pressure before and during 9 years of treatment in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2008;115:927–33. doi: 10.1016/j.ophtha.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tielsch JM. The epidemiology and control of open angle glaucoma: a population-based perspective. Ann Rev Pub Health. 1996;17:121–36. doi: 10.1146/annurev.pu.17.050196.001005. [DOI] [PubMed] [Google Scholar]
- 16.Layden WE, Shaffer RN. Exfoliation syndrome. Am J Ophthalmol. 1974;78:835–41. [PubMed] [Google Scholar]
- 17.Roth M, Epstein DL. Exfoliation syndrome. Am J Ophthalmol. 1980;89:477–81. doi: 10.1016/0002-9394(80)90054-9. [DOI] [PubMed] [Google Scholar]
- 18.Cashwell LF, Jr, Shields MB. Exfoliation syndrome in the southeastern United States. I. Prevalence in open-angle glaucoma and non-glaucoma populations. Acta Ophthalmol Suppl. 1988;184:99–102. doi: 10.1111/j.1755-3768.1988.tb02637.x. [DOI] [PubMed] [Google Scholar]
- 19.Bialasiewicz AA, Wali U, Shenoy R, et al. Patients with secondary open-angle glaucoma in pseudoexfoliation (PEX) syndrome among a population with high prevalence of PEX. Clinical findings and morphological and surgical characteristics. Ophthalmologe. 2005;102:1064–8. doi: 10.1007/s00347-005-1226-2. [DOI] [PubMed] [Google Scholar]
- 20.Kang JH, Loomis S, Wiggs JL, et al. Demographic and geographic features of exfoliation glaucoma in 2 United States-based prospective cohorts. Ophthalmology. 2012;119:27–35. doi: 10.1016/j.ophtha.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budde WM, Jonas JB. Family history of glaucoma in the primary and secondary open-angle glaucomas. Graefes Arch Clin Exp Ophthalmol. 1999;237:554–7. doi: 10.1007/s004170050278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.