Abstract
Active delivery of recombinant autoantigens or allergens at the intestinal mucosa by genetically modified Lactococcus lactis (LL) provides a novel therapeutic approach for the induction of tolerance. Celiac disease is associated with either HLA-DQ2 or HLA-DQ8 restricted responses to specific antigenic epitopes of gliadin, and may be treated by induction of antigen-specific tolerance. We investigated whether oral administration of LL-delivered DQ8-specific gliadin epitope induces antigen-specific tolerance.
L. lactis
was engineered to secrete a deamidated DQ8 gliadin epitope (LL-eDQ8d) and the induction of antigen-specific tolerance was studied in NOD AB° DQ8 transgenic mice. Tolerance was assessed by delayed-type hypersensitivity reaction, cytokine measurements, eDQ8d-specific proliferation and regulatory T cell analysis. Oral administration of LL-eDQ8d induced suppression of local and systemic DQ8 restricted T-cell responses in NOD AB° DQ8 transgenic mice. Treatment resulted in an antigen-specific decrease of the proliferative capacity of inguinal lymph node cells and lamina propria cells. Production of IL-10 and TGF-β and a significant induction of Foxp3+ regulatory T-cells were associated with the eDQ8d-specific suppression induced by LL-eDQ8d.
These data provide support for the development of effective therapeutic approaches for gluten-sensitive disorders using orally administered antigen-secreting LL. Such treatments may be effective even in the setting of established hypersensitivity.
Introduction
The chronic small intestinal inflammation that defines celiac disease is characterized by flattening of the villous architecture and massive infiltration of T cells which release pro-inflammatory cytokines, such as IFN-γ and IL-2. Celiac disease is caused by a loss of tolerance to ingested dietary gliadin and is mediated by HLA-DQ2 or HLA-DQ8 restricted T-cell response (1). Effective treatment can only be reached by a socially restrictive diet that requires lifelong abstinence from foods that contain gliadin present in wheat or proteins from related cereals like rye or barley. While a strict gluten free diet can lead to healing of the intestine, the intolerance to gluten is permanent (2) and better therapeutic options are needed (3).
Oral tolerance is defined as the induction of antigen-specific suppression of immune responses to an antigen by its prior oral feeding and is an attractive therapeutic approach for treatment of allergic, autoimmune and inflammatory diseases. In contrast to most other autoimmune diseases, the trigger, the genetic association, and the highly specific humoral response have been well characterized (4) for celiac disease. Because disease activity is strongly correlated to the presence and dosage of antigen, the induction of antigen-specific oral tolerance is an attractive therapeutic approach. Oral tolerance is mediated by multiple mechanisms such as anergy, deletion and/or active suppression of antigen-specific effector T cells by regulatory T cells (Tregs). The efficacy of oral tolerance in preventing the induction of autoimmune and allergic diseases has been clearly demonstrated in several animal models, but unfortunately previous clinical attempts to induce oral tolerance for therapeutic purposes have failed (5,6). These failures are related to the source, the purity, and the amount of (auto)antigen needed and the mode of presentation of the antigen to the mucosal immune system (7). Previously, a delivery system (TopAct™) based on living Lactococcus lactis strains (ActoBiotics™) for the oral administration of biopharmaceuticals has been described and validated in preclinical experiments as well as a clinical trial (8–11). More recently, we have reported that active in situ synthesis and mucosal delivery of ovalbumin (OVA) by genetically engineered L. lactis (LL) induces antigen specific oral tolerance in OVA T-cell receptor transgenic mice (DO11.10), by the induction of CD4+CD25− regulatory T cells that function through a TGF-β dependent mechanism (12). In the present study we further developed this approach to investigate the possible induction of antigen-specific tolerance in a well-established genotypic celiac disease mouse model (13). We therefore genetically engineered LL to secrete a deamidated DQ8-specific gliadin epitope (DQ8d) that is immunodominant for DQ8 mediated T-cell responses (LL-eDQ8d) and subsequently studied its oral supplementation in DQ8d-immunized NOD AB° DQ8 transgenic mice. NOD AB° DQ8 is a mouse model that utilizes the NOD background which contributes to autoimmunity and pathogenesis in combination with a human DQ8 MHC class II transgene, which contributes to the sensitivity to gliadin (13).
Here, we report that the active mucosal delivery of DQ8d by genetically modified LL induces suppression of systemic DQ8-specific T cell responses in NOD AB° DQ8 transgenic mice and provide a method for the induction of DQ8d antigen-specific tolerance. Moreover this approach provides a method to deliver the right antigens in an adequate manner to the intestinal mucosal immune system in the context of a non-colonizing, non pathogenic bacterium (7) and has the potential for being an effective and non-toxic treatment of celiac disease.
Materials and Methods
Bacteria and media
The genetically modified Lactococcus lactis MG1363 (LL) strain was used throughout this study (14). Bacteria were cultured in GM17E medium, consisting of M17 broth (Difco Laboratories) supplemented with 0.5% glucose and 5μg/ml erythromycin (Abbott). Stock suspensions of LL strains were stored at −20°C in 50% glycerol in GM17E medium. Stock suspensions were diluted 200-fold in GM17E medium and incubated at 30°C overnight. Within 16 h of culture, a saturation density of 2×109 colony forming units (CFU) per ml was reached. Bacteria were harvested by centrifugation and 10-fold concentrated in BM9 inoculation buffer at 2 × 109 bacteria/100 μl. The dose used in all experiments was 100 μl of this suspension administered daily by intragastric catheter.
Construction of genetically modified LL-eDQ8d
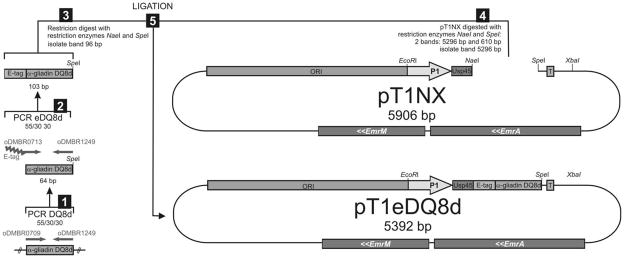
A genetically engineered L. lactis strain, designated LL-eDQ8d, was constructed to secrete an immunodominant DQ8d (Fig. 1). The sequence encoding the DQ8d was retrieved from published data (15). In summary, two glutamine residues within the alpha-gliadin peptide were changed into glutamic acids to stimulate the deamidated immunodominant alpha-gliadin response for DQ8 carrying celiac disease patients, and this epitope is recognized by T cells of NOD AB° DQ8 mice (16) (Table. 1). The DQ8d cDNA fragment was synthetically constructed (Operon) and amplified by Polymerase Chain Reaction (PCR) using the following forward and reverse primers 5′caatacccatcaggtgaaggttc3′ and 5′cgactagttaagcttgtgggttttcttgtgat3′. For detection purposes an e-tag (e) was attached to the fragment (Table. 1). To add the e-tag to the 5′ end of DQ8d gene, we used the PCR product that was produced in step 1 (DQ8d) as template in a PCR with oligonucleotides 5′ggtgctccagttccatacccagatccacttgaaccacgtcaatacccatca3′ and 5′cgactagttaagcttgtgggttttcttgtgat3′. The amplified fragment was fused to the Usp45 secretion signal of the erythromycin resistant pT1NX vector, downstream of the lactococcal P1 promotor (17,18). MG1363 strains transformed with plasmids carrying eDQ8d cDNA were designated LL-eDQ8d. The LL-pT1NX, which is MG1363 containing the empty vector (pT1NX) served as control. The coding sequence of the circular vector was confirmed by sequence analysis and constitutive eDQ8d secretion did not alter the growth rate of L. lactis.
FIGURE 1.
Lactococcus lactis secreting eDQ8d (LL-eDQ8d). A PCR amplified eDQ8d cDNA fragment was ligated in the L. lactis specific pT1NX vector (pT1eDQ8d), and subsequently transformed in MG1363 strains producing designated Lactococcus lactis secreting eDQ8d (LL-eDQ8d).
Functional analysis of secreted epitopes
For functional analysis of the secreted eDQ8d epitope a proliferation assay with human T cell clones derived from the intestines of celiac disease patients was performed. Bacteria were grown overnight as described before, diluted 1:50 and grown for another 4 or 6 hours, respectively. T cell clones specific for gluten were generated from a small intestinal biopsy taken from an adult Dutch celiac patient that had been on a gluten-free diet for several years as described (19). The patient gave informed consent to the study, which was approved by the hospital ethics committee. The patient was typed serologically to be HLA-DR3/4, DQ2/8, thus carrying both celiac disease-associated DQ dimers. T cell clone II29 was found to respond to an alpha-gliadin derived peptide with a minimal 9 amino acid core QGSFQPSQQ, when bound to HLA-DQ8. Deamidation of the P1 and/or P9 glutamine residue (Q) into glutamic acid (E) by the activity of tissue transglutaminase was found to substantially enhance the T cell stimulatory capacity of this gluten peptide (20). Proliferation assays were performed in duplicate or triplicate in 150 μl culture medium (Iscoves) in 96-well flat-bottomed plates (Falcon) using 104 T cells stimulated with 105 HLA-DQ-matched and 3000 RAD irradiated peripheral blood mononuclear cells in the absence or presence of supernatant at several concentrations. After 48 hours, cultures were pulsed with 0.5 μCi of 3H-thymidine, harvested 18 hours thereafter upon which 3H-thymidine incorporation was determined as a measure for proliferation.
Mice
Transgenic mice that express HLA-DQ8 in an endogenous MHC II-deficient background (AB° DQ8) were backcrossed to NOD mice for 10 generations and intercrossed to produce congenic NOD AB° DQ8 mice, as described previously (13). NOD AB° DQ8 transgenic and NOD AB° mice were previously generated and bred at the Department of Immunology, Mayo Clinic, Rochester, MN, USA. Seven to sixteen week old mice were used for the experiments. Mice were weaned and maintained on gluten free chow and were kept in a conventional animal facility until 8–12 weeks of age. All experiments performed were approved by the Institutional Animal Care and Use Committee of the Mayo Clinic College of Medicine.
Antigens and Antibodies
DQ8d with and without e-tag (GAPVPYPDPLEPRQYPSGEGSFQPSQENPQA) as well as irrelevant peptides AHSLERVCHCLGKWLGHPDK (PLP-14) (21) and GDIYRRWIVLGLNKMVK (HIV) (22) were synthesized by the Mayo Proteomics Research Center (MPRC) at Mayo Clinic, Rochester. For T-cell phenotyping; CD4 and CD25 antibodies were purchased from BD Biosciences, and APC anti-Foxp3 staining kits were purchased from eBiosciences. Anti-IL-10 neutralizing monoclonal antibody (1 μg/ml, clone JES052A5), TGF-β neutralizing monoclonal antibody (1 μg/ml, clone 1D11) and LAP (latency associated peptide) neutralizing antibodies (1 μg/ml, clone 27235) were obtained from R&D systems.
Oral feeding and Delayed-type hypersensitivity (DTH) reaction
NOD AB° DQ8 mice on a gluten free chow were sensitized by subcutaneous injection of 100 μg eDQ8d peptide in 100 μl of a 1:1 CFA (Complete Freund’s Adjuvant, purchased from Difco of Becton, Dickinson and Company) saline solution in the tail base at day 1 (23). The peptide used for the sensitization had the same sequence as the secreted epitope. Mice were fed BM9 as a negative control, LL-pT1NX or LL-eDQ8d at days 1–10, dissolved in 100 μl BM9. Feedings were performed by intragastric administrations of BM9 or bacterial suspensions using an 18-gauge stainless gavage needle. Ten days after immunization, antigen-specific DTH responses were assessed. Twenty-four hours thereafter, DTH measurements were performed. For measurement of antigen-specific DTH responses, baseline ear-thickness was measured using an engineer’s micrometer (Mitutoyo). Mice were then injected with 10 μg eDQ8d in 10 μl saline in the auricle of the ear. The ear-thickness was measured again in a blinded fashion at 24 h after challenge. DTH responses were expressed as the difference in the baseline ear-thickness and the ear-thickness 24 hours after eDQ8d injection. Subsequently mice were sacrificed, spleen and lymph nodes were harvested and cells were assessed for DQ8d-specific proliferation and cytokine production. To test for e-tag interference NOD AB° DQ8 mice were immunized with 100 μg DQ8d peptides with (eDQ8d) or without e-tag (DQ8d) in 100 μl of 1:1 CFA solution in the tail base at day 1. At day 7, mouse DTH measurements were performed as described above with 10 μg DQ8d with or without e-tag, corresponding to the peptide used for the immunization.
Isolation of Lamina Propria Cells
Lamina propria cells were isolated using the protocol from Current Protocols in Immunology (24). Briefly, mice were euthanized by CO2 inhalation, and the small intestine removed and cut longitudinally and laterally into 2cm long pieces using a scalpel blade. The tissue was then washed six times using CMF/HEPES solution (1X Hanks balanced salt solution, 15mMHEPES, 2%FBS) followed by a one hour incubation in CMF/FBS/EDTA solution (1X Hank’s balanced salt solution, 10% FBS, 15mM HEPES, 5mM EDTA, 100μg Penicillin/Streptomycin) to remove epithelial cells. A subsequent collagenase (100U/ml) digestion was performed for one hour to release the lamina propria lymphocytes.
Cell cultures, proliferation and cytokine production assays
Cell suspensions of spleen and lymph nodes were prepared at day 11 of the experiment by homogenizing the tissue with a tissue grinder (VWR International, Inc.) in 1X PBS. Erythrocytes were removed from the spleen cell suspensions by incubation with Ammonium Chloride/Potassium lysis buffer. Cells were incubated in 96-well microtiter plates at 5×105 cells/well in 0.2-ml volumes at 37°C in RPMI 1640 (1.5% Hepes, 1% Penstrep and 10% FBS) with supplements containing either medium alone, 10 μg/ml Con A, 50 μg/ml irrelevant peptide (HIV), or 50 μg/ml eDQ8d epitope. In a separate experiment IL-10, TGF-β, IL10&TGF-β or LAP neutralizing antibodies were added to splenocytes of LL-eDQ8d treated mice. After 24 h, proliferation was assessed by addition of 1 μCi/well [3H]-thymidine for the last 24 h of culture. DNA-bound radioactivity was harvested onto glass fiber filter mats (Perkin Elmer) and thymidine-incorporation measured on a scintillation counter (Perkin Elmer). Results were expressed as mean counts per minute (CPM) of triplicate wells. For the neutralizing antibody proliferation assay the results were expressed as the percentage of LL-eDQ8d proliferation compared to the BM9 treated group. For cytokine measurements, supernatants of the cell cultures used in the different proliferation assays, described above, were collected after 24 h of culture and frozen at −20°C until cytokine analysis was performed. Cytokine production was quantified using the Mouse Inflammation Cytometric Bead Assay (BD Biosciences).
T cell Proliferation Assay Using Lamina Propria Cells
Lamina Propria cells were isolated from mice that had been administered medium (BM9), LL-pT1NX, or LL-eDQ8d. These cells (5×10e6 cells/ml) were then incubated with medium (RPMI, Sigma-Aldrich), eDQ8d epitope (50 μg/ml) or irrelevant peptide (PLP-14) (50 μg/ml), for 24 hours before the addition of [3H]-thymidine for an additional 24 hours. Cells were then harvested in a similar fashion as the splenocytes.
Flow cytometric analysis
Spleens and gut-associated lymph node tissue (GALT) of BM9, LL-pT1NX or LL-eDQ8d treated mice were isolated, prepared as described above and stained for CD4, CD25 and Foxp3. Intracellular staining was performed for Foxp3 according to the manufacturer’s instructions (eBiosciences) and subsequently measured using flow cytometry on a Becton Dickinson FACSCaliburs (Mayo Clinic College of Medicine Flow Cytometry/Optical Morphology Core Facility). Cells were gated on CD4+CD25+ and CD4+CD25− subpopulations and within these populations Foxp3 histograms were used to determine Mean Fluorescence Intensity (MFI).
Statistical analysis
Results from cytokine measurements are expressed as mean ± SEM. eDQ8d-specific proliferation, ear-thickness, and cytokine measurements were tested for significance using one-way ANOVA followed by the student’s t-test comparison to determine the differences between individual groups. For all tests a P value <0.05 *, <0.01 ** was used to indicate statistical significance.
Results
Synthesis and secretion of functional DQ8d
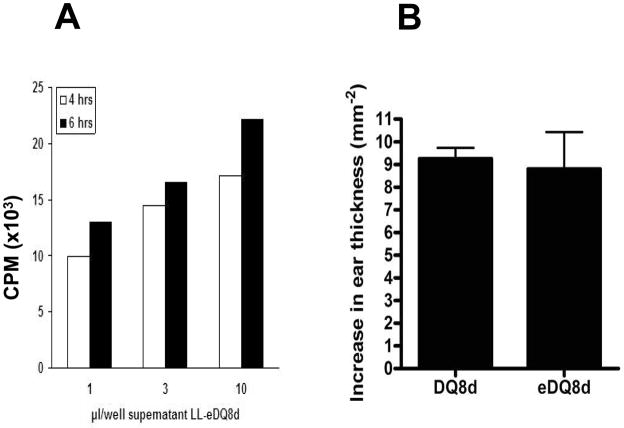
In vitro synthesis and functionality of the secreted DQ8d was confirmed by a proliferation assay with human DQ8 T cell clones derived from the intestine of celiac disease patients. T cell clones derived from the intestine of celiac patients were stimulated with supernatant of a LL-eDQ8d culture at different concentrations (Fig. 2A). Control (LL-pT1NX) supernatant did not induce proliferation (data not shown). The secreted immunodominant DQ8d peptide contains an amino-terminal e-tag for detection purposes. To exclude a possible interference of the e-tag with the functional properties of the peptide, NOD AB° DQ8 transgenic mice were immunized with DQ8d epitopes with or without e-tag at day 0. At day 7, DTH response measurements were performed with the injection of 10 μg DQ8d epitope. The presence of an e-tag did not change the DTH response in DQ8d immunized mice (Fig. 2B). Immediately after the DTH measurement, bulk splenocytes and bulk inguinal lymph node cells were isolated and stimulated ex vivo with DQ8d epitopes with or without e-tag. Again, the presence of the e-tag did not change the immune-stimulating properties of the DQ8d epitope (data not shown). These data demonstrate that L. lactis-derived eDQ8d is fully bioactive and that the addition of the e-tag does not interfere with its functionality.
FIGURE 2.
L. lactis -derived eDQ8d exhibits bioactivity as it induces proliferation of human HLA DQ8 T cell clones with no e-tag interference. (A) Human DQ8 T cells were derived as described in the material and methods. LL-eDQ8d was grown overnight and diluted 1:50, 4 and 6 hours thereafter supernatant was collected. Cells were stimulated with 1, 3 or 10 μl supernatant of a LL-eDQ8d culture. (B) For detection purposes an e-tag was attached. To exclude any possible interference of the e-tag, NOD AB° DQ8 transgenic mice were immunized by subcutaneous injection of 100 μg DQ8d with or without e-tag in CFA at day 1. At day 7, mice baseline ear-thickness was measured and mice were challenged with 10 μg DQ8d with or without e-tag, corresponding to the peptide used for the immunization, in 10 μl saline in the auricle of the ear. DTH responses were expressed as the difference in ear-thickness 24 h after the peptide injection minus the ear-thickness before injection. Data represent mean (± SEM) increase in ear thickness of 1 experiment including 6 mice per group.
Suppression of the DTH and proliferative response by LL- eDQ8d
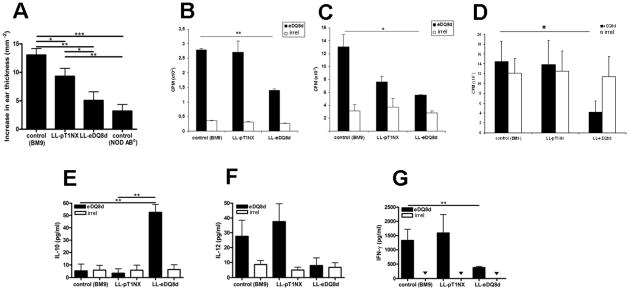
We subsequently investigated the effect of oral administration of LL-eDQ8d on the eDQ8d-induced DTH (25). NOD AB° DQ8 transgenic mice were immunized by eDQ8d and fed BM9 (inoculation buffer, as a negative control), LL-pT1NX (empty vector control) or LL-eDQ8d intra-gastrically for 10 consecutive days. On day 10, mouse ears were injected with 10 μg eDQ8d and 24 hours later ear-thickness measurements were performed. Control mice (fed BM9) were clearly immunized to eDQ8d. Daily intra-gastric administration of LL-eDQ8d significantly reduced the DTH response (13.1×10−2 mm vs. 5.1×10−2 mm, P=0.003) (Fig. 3A). Ear swelling was also slightly reduced in LL-pT1NX-treated mice compared to controls (9.3×10−2 mm vs. 13.1×10−2 mm, P=0.034) but to a much lesser degree than in LL-eDQ8d treated mice. NOD AB° mice (without DQ8 transgene) showed only a minor increase in ear thickness (3.2×10−2 mm). These data indicate that orally administered LL-eDQ8d suppresses systemic inflammatory T-cell responses in immunized NOD AB° DQ8 transgenic mice and that the secreted antigen is necessary for induction of a significant tolerogenic effect.
FIGURE 3.
Mucosal delivery of eDQ8d epitopes by L. lactis significantly decreases the DQ8d-induced DTH response and proliferative capacity of bulk spleen and inguinal lymph node cells and lamina propria cells. NOD AB° DQ8 transgenic mice were immunized by s.c. injection of 100 μg eDQ8d in CFA at day 1. Mice were orally treated with LL-eDQ8d or LL-pT1NX at days 1–10. Control mice received BM9. At day 10, mice were challenged with 10 μg eDQ8d in 10 μl saline in the auricle of the ear. DTH responses are expressed as the mean (±SEM) increase in ear thickness from baseline, 24 hours after injection (A). After the DTH measurements, spleens, inguinal lymph nodes, and lamina propria cells of the BM9 (control), LL-pT1NX and LL-eDQ8d groups were isolated and ex vivo stimulated with 50 μg/ml eDQ8d peptide or 50 μg/ml irrelevant peptide (irrel) (white bars: 3b–3d). eDQ8d-specific proliferative response of bulk splenocytes (P=0.048) (B) and inguinal lymph node cells (P=0.002) (C) and lamina propria cells (P=0.044) (D) were studied by thymidine incorporation, expressed as the mean (±SEM) cpm.
Cytokine measurements in the supernatant of splenocytes (3e-3f) and inguinal lymph node cells (3g) were performed 24 hours after ex vivo eDQ8d (black bars) or irrelevant peptide (irrel)(white bars) stimulation. Results represent the mean (±SEM) of cytokine secretion in pg/ml for at least two individual experiments including 6 mice in each group. ▼ indicates not detected (below detection threshold).
Peripheral immune responses were further analyzed by investigating eDQ8d-specific proliferation of spleen and draining inguinal lymph node cells (ILN). Splenocytes of mice treated with BM9, LL-pT1NX or LL-eDQ8d were isolated on day 11 after immunization, and the eDQ8d-specific proliferative response was assessed by ex vivo stimulation with eDQ8d peptides or irrelevant peptide. Splenocytes of immunized mice showed a high eDQ8d-specific proliferative response that was significantly suppressed by daily intra-gastric administration of LL-eDQ8d when compared to the control BM9 fed mice (CPM: 13.1×103 vs. 5.6×103, P=0.048) (Fig. 3B). However, the inguinal lymph nodes are the primary antigen recognition site in this immunization protocol; therefore, we also examined the proliferative capacity of these lymphocytes. Proliferation of inguinal lymph node cells was significantly decreased in the LL-eDQ8d treated group as compared to the BM9 treated group (1.4 ×103 vs 2.8×103 CPM, P= 0.002) (Fig. 3C). Splenocytes and inguinal lymph node cells did not show any proliferative response with addition of an irrelevant control peptide in all three treatment groups.
To determine if antigen-specific suppression exists in the lamina propria cells, these cells were isolated from mice administered LL-eDQ8d as well as mice administered BM9 alone or LL-pT1NX. These cells were then treated in vivo with medium, eDQ8d, or an irrelevant control peptide. A high background of proliferation was observed in lamina propria cells in all three groups of treated mice. However, the proliferation was suppressed with the addition of the eDQ8d peptide only in mice treated with LL-eDQ8d (Fig. 3D). Proliferation in the lamina propria was not diminished with the addition of irrelevant control peptide. This demonstrates that in the lamina propria, the suppression induced by the feeding of the LL-eDQ8d is specific to eDQ8d peptide.
To investigate the mechanisms behind the reduction of antigen-induced T cell proliferation, we determined cytokine profiles of ex vivo stimulated splenocytes or inguinal lymph node cells. Ex vivo eDQ8d stimulated spleen cells showed a significant up-regulation of IL-10 (52.6 vs 5.4 pg/ml, P= 0.002) and a downregulation of IL-12 production (8.0 vs 27.6 pg/ml) only in the LL-eDQ8d treated group compared to the negative control (BM9) (Fig. 3 E,F). Moreover LL-eDQ8d treatment significantly reduced the eDQ8d-induced IFN-γ production (380 vs 1328 pg/ml, P=0.009) in the inguinal lymph nodes compared to the negative control (BM9) treated mice (Fig 3G). There was no difference between the cytokine levels across treatment groups when we stimulated the splenocytes, inguinal lymph node cells with irrelevant peptide. Addition of irrelevant peptide also did not change the levels of IL-10 and IL-12p70 from the media in three treatment groups (BM9, LL-pT1NX and LL-eDQ8d). Together, the proliferative and cytokine data indicate that LL-eDQ8d treatment is able to suppress T cell responses systemically in NOD AB° DQ8 transgenic mice in an antigen-specific manner.
Critical role for both TGF-β and IL-10 in LL-eDQ8d mediated suppression
The functional importance of TGF-β, IL-10, and LAP (membrane-associated TGF-β) for the eDQ8d-specific splenic proliferative response of splenocytes from LL-eDQ8d treated mice compared to the BM9 control group was assessed using neutralizing antibodies. The individual neutralization of IL-10-, TGF-β- or LAP did not significantly interfere with the decreased splenic proliferative response of LL-eDQ8d treated mice, but adding a combination of TGF-β and IL-10 neutralizing monoclonal antibodies completely abolished the decreased eDQ8d-specific proliferative capacity of splenocytes (4.9×103 vs 1.6×103 CPM, P< 0.01) (Fig. 4). These data strongly suggest that the T cell activation suppression mediated by LL-eDQ8d treatment is dependent upon interplay between IL-10 and TGF-β.
FIGURE 4.
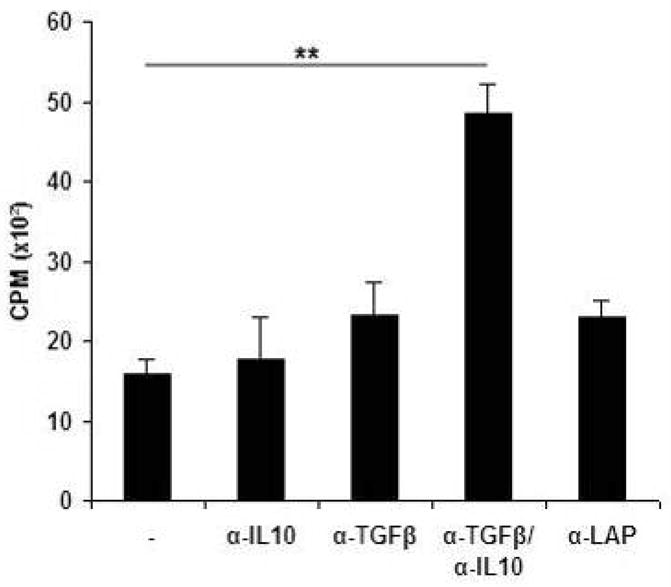
Decreased splenic eDQ8d-specific proliferation depends on IL-10 and TGF-β. Mice were fed BM9, LL-pT1NX and LL-eDQ8d and DTH measurements were performed as described above. We next investigated the functional importance of cytokines such as TGF-β, IL-10, TGF-β in combination with IL-10 and LAP on the eDQ8d-specific splenic proliferative response using neutralizing antibodies. To do so, bulk spleen cells of LL-eDQ8d treated mice were isolated and 5 × 105 cells were stimulated ex vivo with 50 μg/ml eDQ8d with or without neutralizing antibodies. Proliferative responses are expressed as percentage of proliferation compared to the BM9 treated group. Results are representative of two individual experiments with 6 mice each per group.
Increase in Foxp3 expression by CD4+CD25+ and CD4+CD25− T cells
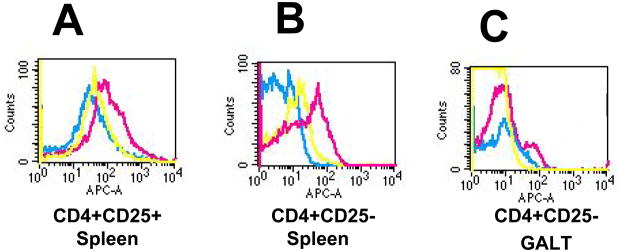
To analyze the role of regulatory T cells (Tregs) in the induction of LLeDQ8d-induced tolerance, we investigated the expression of Foxp3 within the CD4+ T cell population by FACS analysis. A significant increase in the number of Foxp3+ CD4+CD25+ cells as well as Foxp3+ CD4+CD25− cells was seen in the spleen of LL-eDQ8d treated mice as compared to the control group (BM9) (MFI 171 vs. 61 and 35 vs. 6, respectively) (Fig. 5A and 5B). An increased number of Foxp3+CD4+CD25−cells was also detected in the gut-associated lymph node tissue (GALT) of the LL-eDQ8d treated mice as compared to the BM9 treated (MFI in M1 73 vs. 30) (Fig. 6C), but not in the GALT CD4+CD25+ population. LL-pT1NX feeding also increased the number of Foxp3+CD4+CD25− cells in the spleen, but this was to a lesser extent than the LL-eDQ8d treated mice (MFI 15 vs. 35, respectively). These data suggest a strong association between the increase in Foxp3+ T-cells and the suppression of the immune response to gliadin by the oral administration of the LL-eDQ8d bacteria.
FIGURE 5.
LL-eDQ8d treatment significantly increases splenic and GALT Foxp3 expression. At day 11, spleens and gut associated lymph node tissue (GALT) of mice treated with BM9 (blue), LL-pT1NX (yellow) or LL-eDQ8d (pink) were isolated and stained for CD4, CD25 and intracellular Foxp3. Flow cytometry was performed on the splenic CD4+CD25+ (A), CD4+CD25− (B) and GALT CD4+CD25− (C) subpopulations. Data represent 1 experiment with 6 mice per group.
Discussion
Our data demonstrate that genetically modified L. lactis can be used for mucosal delivery of functional immunodominant antigens, and that this approach suppresses inflammatory antigen-specific T cell responses in gliadin-sensitized NOD AB° DQ8 transgenic mice. Furthermore, our results suggest that the induced suppression is antigen-specific and mediated by Foxp3+ CD4+CD25+ and CD25− regulatory T cells that possibly function through an IL-10 and TGF-β dependent mechanism.
Successful clinical application of antigen-specific mucosal tolerance for the treatment of human diseases has been difficult to achieve and critically depends on several factors, including the purity, source, dose and the mode of antigen presentation to the mucosal immune system (7). Several protocols for induction of oral tolerance, including oral administration of the antigen with IL-10, have been shown to induce antigen-specific Tr1 cells that suppress undesired immune responses toward self-antigens, allergens, and food antigens. Although a previous clinical attempt to induce tolerance in celiac disease by the administration of rhIL-10 in refractory celiac disease patients was ineffective (26), strategies to boost the number and/or function of Ag-specific Tr-1 cells may offer new therapeutic opportunities. This notion is supported by the finding that gliadin-specific mucosal regulatory T cells from celiac disease patients are able to suppress proliferation of pathogenic Th0 cells (27,28).
Both dendritic cells (DC) and regulatory T cells are critically involved in tolerance induction (29,30). We recently demonstrated that exposure to L. lactis alters DC phenotype and function, which in the presence of simultaneous exposure to a DC-presented antigen might result in the generation of an antigen-specific regulatory T cell subset (Huibregtse et al submitted and (12)). We hypothesize that induction of antigen-specific regulatory T cells in our experiments was mediated by altered presentation of the immunodominant peptide by dendritic cells, and our observation that LL-eDQ8d treatment interfered with IL-12 production of splenocytes, suggests that at least part of the tolerogenic effect is DC-mediated. However, it should be noted that the spleen is not the primary antigen-recognition site in our model, and alternatively, activation of regulatory T cells may have resulted in a reduced activation of antigen-presenting cells. In fact, our results with the lamina propria cells demonstrate that the GALT is a site of induction of regulatory cells in response to mucosal administration of L. Lactis treatments.
We further demonstrated that LL-eDQ8d treatment reduced peripheral DTH responses as well as eDQ8d-specific proliferation of lamina propria cells, bulk splenocytes and inguinal lymph node cells. LL-pT1NX treatment also somewhat reduced the DTH and splenic proliferative capacity but less pronounced than the LL-eDQ8d treated mice. The LL-eDQ8d treatment-mediated IL-10 secretion and the reduction of IL-12 and IFN-γ production that was found after ex vivo stimulation of splenocytes and inguinal lymph node cells respectively and was not observed in the LL-pT1NX treated mice. Moreover splenocytes of sensitized LL-eDQ8d treated mice were not sensitive to stimulation with an irrelevant antigen. These data confirm our previous findings that the tolerogenic effect, at least in part is L. lactis mediated but that the co-delivery of low-dose antigen, in this case DQ8d, greatly enhances the induction of antigen-specific oral tolerance.
In recent years it has become apparent that Tregs play a critical role in the induction and maintenance of oral tolerance (31,32). Still many questions need to be answered concerning the phenotype and complexity of Tregs as well as the precise role and different overlaps in oral tolerance. Several phenotypically and functionally distinct Treg subsets have been implicated in suppression of intestinal inflammation and induction of oral tolerance, including adaptive Tregs (aTregs), comprising Th3 and Tr1 cells, and naturally occurring Tregs (nTregs), which maintain tolerance to self-antigen under normal physiological conditions. Although it is probable that nTregs play a central role in regulating gut immune homeostasis, their precise function remains to be characterized (5,33,34). Furthermore, recently a separate category of Tregs has been described that acquires Foxp3 upon TGF-β stimulation. These so-called inducible Tregs (iTregs) have regulatory functions both in vitro and in vivo (35,36). This recently discovered subset mimics the Tregs induced in this model, as both subsets are induced in the periphery, express Foxp3 and are critically dependent on TGF-β/IL-10.
To map the Tregs that mediated oral tolerance in our experiments, we studied the functional importance of TGF-β, IL-10, and LAP (membrane-associated TGF-β) on the eDQ8d-specific splenic proliferative response using neutralizing antibodies. Interestingly only the combined neutralization of IL-10 and TGF-β interfered with the proliferative capacity of the splenocytes suggesting an interactive role for both anti-inflammatory cytokines. The exact mechanism by which these cytokines function is not completely understood but both cytokines frequently interact during regulatory Treg responses (37–39).
Furthermore we found a significant Foxp3 upregulation in both the mucosal and the splenic CD4+ T-cell population. It is known that antigen-specific TGF-β producing Th3 cells drive the differentiation of antigen-specific Foxp3+ regulatory cells in the periphery (40). Furthermore TGF-β-dependent conversion of peripheral CD4+CD25−T cells into CD25+, CD45RB−/low suppressor cells has been reported (35). Unfortunately specific markers for distinguishing between naturally occurring Tregs and inducible Tregs are lacking. Our data therefore suggest that either mucosal CD4+CD25−Foxp3+ cells induced by LL-eDQ8d treatment play a regulatory role or they can be eventually converted into CD4+CD25+ Tregs (41). It has been shown that oral tolerance induced by CTB-conjugated Ag is associated with increased TGF-β production and the generation of Foxp3+CD25+CD4+ and both Foxp3+ and Foxp3−CD25−CD4+ Tregs (42). The relationship between thymus derived natural CD4+CD25+Foxp3+ Tregs and other subpopulations induced in the periphery, such as peripherally generated CD4+ Foxp3+ cells, as well as Tr1 and Th3 cells needs clarification, but these data suggest a significant overlap and interactive function in the induction of mucosal tolerance.
Direct in vivo induction of tolerogenic DC or Treg is a major target for immunotherapy for allergic, autoimmune and several inflammatory diseases and can be achieved by exposing the mucosal immune system to low doses of antigen (7,43,44).
We here report that oral supplementation of a genetically modified L. lactis secreting DQ8d peptides greatly reduces systemic immune responses induced by that antigen in DQ8d-immunized NOD AB° DQ8 transgenic mice. The suppression is mediated by the induction of Foxp3+ Tregs that are dependent on both TGF-β and IL-10. These observations support the development of gut delivered bacteria enhanced oral tolerance for the treatment of both mucosal and systemic autoimmune, inflammatory or allergic diseases by specific antigen-secreting L. lactis. With such studies, this type of therapy may be considered a viable option for treating gluten-sensitive disorders such as celiac disease.
Table 1.
Sequences; E-tag & Secreted Peptide
| Coding sequence: | E-tag : ggt gct cca gtt cca tac cca gat cca ctt gaa cca cgt DQ8d : caa tac cca tca ggt gaa ggt tca ttc caa cca tca caa gaa aac cca caa gct |
| Protein sequence: | E-tag: GAPVPYPDPLEPR DQ8d: QYPSGEGSFQPSQENPQA |
Acknowledgments
We thank Dr. Robin Patel, M.D. and the Microbiology Research Facility at the Mayo Clinic Rochester for their expertise, help, and advice and we thank M.S. ten Brink and J.B. Daalhuisen for their technical support.
This study was supported by National Institute of Health grant DK071003. The trademarks TopAct™ and ActoBiotics™ are used with the kind permission of ActoGeniX.
Abbreviations used in this paper
- Ag
antigen
- APC
antigen presenting cell
- DC
dendritic cell
- DTH
delayed type hypersensitivity
- DQ8d
deamidated DQ8-specific gliadin epitope
- eDQ8d
deamidated DQ8-specific gliadin epitope with e-tag
- Foxp3
forkhead box P3
- GALT
gut-associated lymphoid tissue
- HLA
Human leukocyte antigen
- IL
interleukin
- ILN
inguinal lymph node
- LAP
latency associated peptide
- LL
Lactococcus lactis
- LL-pT1NX
Lactococcus lactis with empty vector
- LL-eDQ8d
Lactococcus lactis secreting deamidated DQ8-specific gliadin epitope
- LL-OVA
Lactococcus lactis secreting OVA
- MFI
mean fluorescence intensity
- MLN
mesenterial lymph node
- TGF-β
Transforming growth factor-B
- Treg
regulatory T-cell
References
- 1.Stepniak D, Koning F. Celiac disease--sandwiched between innate and adaptive immunity. Hum Immunol. 2006;67:460. doi: 10.1016/j.humimm.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Kagnoff MF. Celiac disease: pathogenesis of a model immunogenetic disease. J Clin Invest. 2007;117:41. doi: 10.1172/JCI30253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sollid LM, Khosla C. Future therapeutic options for celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:140. doi: 10.1038/ncpgasthep0111. [DOI] [PubMed] [Google Scholar]
- 4.Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:516. doi: 10.1038/ncpgasthep0582. [DOI] [PubMed] [Google Scholar]
- 5.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraus TA, Mayer L. Oral tolerance and inflammatory bowel disease. Curr Opin Gastroenterol. 2005;21:692. doi: 10.1097/01.mog.0000182862.88798.28. [DOI] [PubMed] [Google Scholar]
- 7.Weiner HL. Current issues in the treatment of human diseases by mucosal tolerance. Ann N Y Acad Sci. 2004;1029:211. doi: 10.1196/annals.1309.053. [DOI] [PubMed] [Google Scholar]
- 8.Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 9.Steidler L, Neirynck S, Huyghebaert N, Snoeck V, Vermeire A, Goddeeris B, Cox E, Remon JP, Remaut E. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol. 2003;21:785. doi: 10.1038/nbt840. [DOI] [PubMed] [Google Scholar]
- 10.Vandenbroucke K, Hans W, Van Huysse J, Neirynck S, Demetter P, Remaut E, Rottiers P, Steidler L. Active delivery of trefoil factors by genetically modified Lactococcus lactis prevents and heals acute colitis in mice. Gastroenterology. 2004;127:502. doi: 10.1053/j.gastro.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Braat H, Rottiers P, Hommes DW, Huyghebaert N, Remaut E, Remon JP, van Deventer SJ, Neirynck S, Peppelenbosch MP, Steidler L. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:754. doi: 10.1016/j.cgh.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 12.Huibregtse IL, Snoeck V, de CA, Braat H, De Jong EC, Van Deventer SJ, Rottiers P. Induction of ovalbumin-specific tolerance by oral administration of Lactococcus lactis secreting ovalbumin. Gastroenterology. 2007;133:517. doi: 10.1053/j.gastro.2007.04.073. [DOI] [PubMed] [Google Scholar]
- 13.Marietta E, Black K, Camilleri M, Krause P, Rogers RS, III, David C, Pittelkow MR, Murray JA. A new model for dermatitis herpetiformis that uses HLA-DQ8 transgenic NOD mice. J Clin Invest. 2004;114:1090. doi: 10.1172/JCI21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasson MJ. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzarella G, Maglio M, Paparo F, Nardone G, Stefanile R, Greco L, van de WY, Kooy Y, Koning F, Auricchio S, Troncone R. An immunodominant DQ8 restricted gliadin peptide activates small intestinal immune response in in vitro cultured mucosa from HLA-DQ8 positive but not HLA-DQ8 negative coeliac patients. Gut. 2003;52:57. doi: 10.1136/gut.52.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black KE, Murray JA, David CS. HLA-DQ determines the response to exogenous wheat proteins: a model of gluten sensitivity in transgenic knockout mice. J Immunol. 2002;169:5595. doi: 10.4049/jimmunol.169.10.5595. [DOI] [PubMed] [Google Scholar]
- 17.Vanasseldonk M, Rutten G, Oteman M, Siezen RJ, Devos WM, Simons G. Cloning of Usp45, A Gene Encoding A Secreted Protein from Lactococcus-Lactis Subsp Lactis Mg1363. Gene. 1990;95:155. doi: 10.1016/0378-1119(90)90428-t. [DOI] [PubMed] [Google Scholar]
- 18.Waterfield NR, Lepage RWF, Wilson PW, Wells JM. The Isolation of Lactococcal Promoters and Their Use in Investigating Bacterial Luciferase Synthesis in Lactococcus-Lactis. Gene. 1995;165:9. doi: 10.1016/0378-1119(95)00484-n. [DOI] [PubMed] [Google Scholar]
- 19.van de Wal Y, Kooy YM, van Veelen PA, Pena SA, Mearin LM, Molberg O, Lundin KE, Sollid LM, Mutis T, Benckhuijsen WE, Drijfhout JW, Koning F. Small intestinal T cells of celiac disease patients recognize a natural pepsin fragment of gliadin. Proc Natl Acad Sci U S A. 1998;95:10050. doi: 10.1073/pnas.95.17.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Wal Y, Kooy Y, van Veelen VP, Pena S, Mearin L, Papadopoulos G, Koning F. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J Immunol. 1998;161:1585. [PubMed] [Google Scholar]
- 21.Mangalam AK, Khare M, Krco C, Rodriguez M, David C. Identification of T cell epitopes on human proteolipid protein and induction of experimental autoimmune encephalomyelitis in HLA class II-transgenic mice. Eur J Immunol. 2004;34:280. doi: 10.1002/eji.200324597. [DOI] [PubMed] [Google Scholar]
- 22.Kelleher AD, Long C, Holmes EC, Allen RL, Wilson J, Conlon C, Workman C, Shaunak S, Olson K, Goulder P, Brander C, Ogg G, Sullivan JS, Dyer W, Jones I, McMichael AJ, Rowland-Jones S, Phillips RE. Clustered mutations in HIV-1 gag are consistently required for escape from HLA-B27-restricted cytotoxic T lymphocyte responses. J Exp Med. 2001;193:375. doi: 10.1084/jem.193.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tobagus IT, Thomas WR, Holt PG. Adjuvant costimulation during secondary antigen challenge directs qualitative aspects of oral tolerance induction, particularly during the neonatal period. J Immunol. 2004;172:2274. doi: 10.4049/jimmunol.172.4.2274. [DOI] [PubMed] [Google Scholar]
- 24.Lefrancois L, Lycke N. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer’s patch, and lamina propria cells. Curr Protoc Immunol. 2001;Chapter 3(Unit) doi: 10.1002/0471142735.im0319s17. [DOI] [PubMed] [Google Scholar]
- 25.Unger WW, Hauet-Broere F, Jansen W, van Berkel LA, Kraal G, Samsom JN. Early events in peripheral regulatory T cell induction via the nasal mucosa. J Immunol. 2003;171:4592. doi: 10.4049/jimmunol.171.9.4592. [DOI] [PubMed] [Google Scholar]
- 26.Mulder CJ, Wahab PJ, Meijer JW, Metselaar E. A pilot study of recombinant human interleukin-10 in adults with refractory coeliac disease. Eur J Gastroenterol Hepatol. 2001;13:1183. doi: 10.1097/00042737-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Gianfrani C, Levings MK, Sartirana C, Mazzarella G, Barba G, Zanzi D, Camarca A, Iaquinto G, Giardullo N, Auricchio S, Troncone R, Roncarolo MG. Gliadin-specific type 1 regulatory T cells from the intestinal mucosa of treated celiac patients inhibit pathogenic T cells. J Immunol. 2006;177:4178. doi: 10.4049/jimmunol.177.6.4178. [DOI] [PubMed] [Google Scholar]
- 28.Salvati VM, Mazzarella G, Gianfrani C, Levings MK, Stefanile R, De GB, Iaquinto G, Giardullo N, Auricchio S, Roncarolo MG, Troncone R. Recombinant human interleukin 10 suppresses gliadin dependent T cell activation in ex vivo cultured coeliac intestinal mucosa. Gut. 2005;54:46. doi: 10.1136/gut.2003.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faria AM, Weiner HL. Oral Tolerance and TGF-beta-Producing Cells. Inflamm Allergy Drug Targets. 2006;5:179. doi: 10.2174/187152806778256034. [DOI] [PubMed] [Google Scholar]
- 30.Kelsall BL, Leon F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol Rev. 2005;206:132. doi: 10.1111/j.0105-2896.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 31.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 32.Strober W, Fuss I, Boirivant M, Kitani A. Insights into the mechanism of oral tolerance derived from the study of models of mucosal inflammation. Ann N Y Acad Sci. 2004;1029:115. doi: 10.1196/annals.1309.029. [DOI] [PubMed] [Google Scholar]
- 33.Lider O, Santos LM, Lee CS, Higgins PJ, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. II. Suppression of disease and in vitro immune responses is mediated by antigen-specific CD8+ T lymphocytes. J Immunol. 1989;142:748. [PubMed] [Google Scholar]
- 34.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+ J Exp Med. 2003;198:1875. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+ J Immunol. 2004;172:5149. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 37.Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168:900. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- 38.Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. Transforming growth factor (TGF)-beta1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-beta1-mediated fibrosis. J Exp Med. 2003;198:1179. doi: 10.1084/jem.20030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol. 2005;174:3237. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 40.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 Cells in Peripheral Tolerance. I. Induction of Foxp3-Positive Regulatory T Cells by Th3 Cells Derived from TGF-beta T Cell-Transgenic Mice. J Immunol. 2007;178:179. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- 41.You S, Leforban B, Garcia C, Bach JF, Bluestone JA, Chatenoud L. Adaptive TGF-beta-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc Natl Acad Sci U S A. 2007;104:6335. doi: 10.1073/pnas.0701171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun JB, Raghavan S, Sjoling A, Lundin S, Holmgren J. Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+CD25+ and Foxp3−. J Immunol. 2006;177:7634. doi: 10.4049/jimmunol.177.11.7634. [DOI] [PubMed] [Google Scholar]
- 43.Tang Q, Bluestone JA. Regulatory T-cell physiology and application to treat autoimmunity. Immunol Rev. 2006;212:217. doi: 10.1111/j.0105-2896.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 44.van Duivenvoorde LM, van Mierlo GJ, Boonman ZF, Toes RE. Dendritic cells: vehicles for tolerance induction and prevention of autoimmune diseases. Immunobiology. 2006;211:627. doi: 10.1016/j.imbio.2006.05.014. [DOI] [PubMed] [Google Scholar]