Abstract
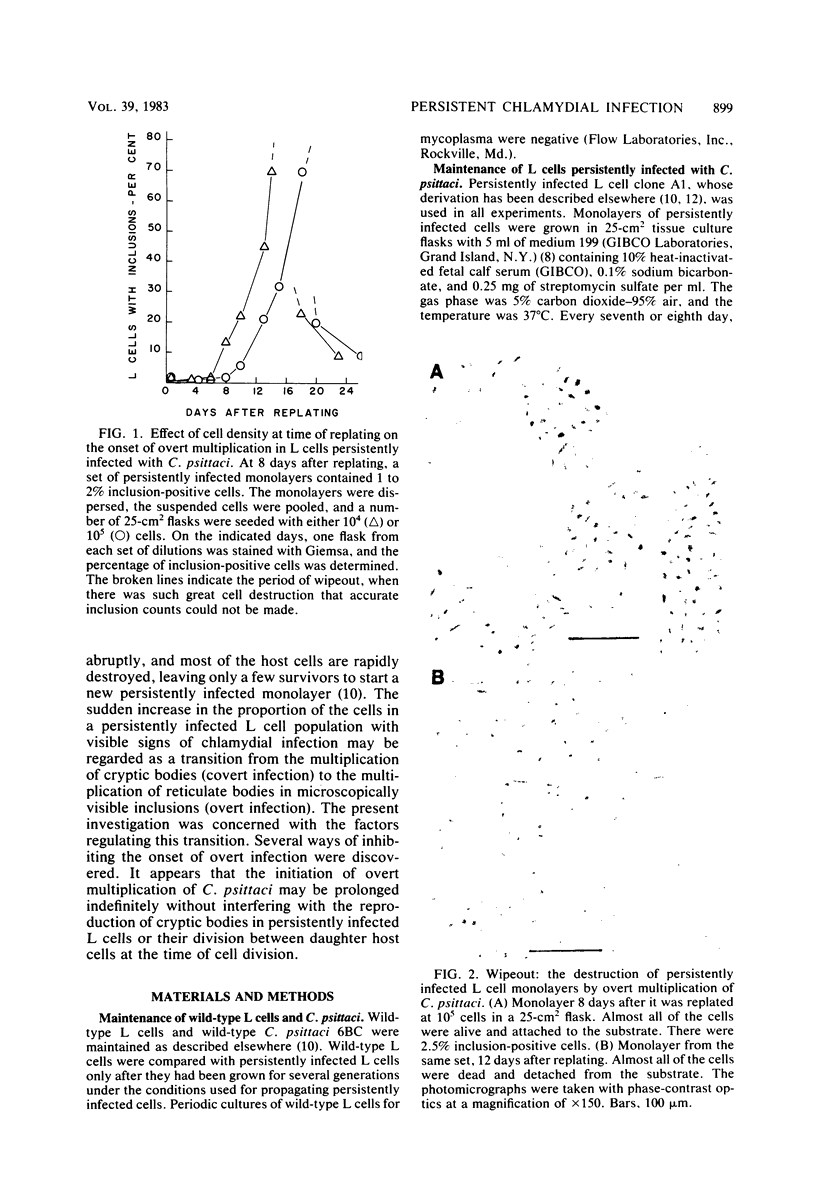
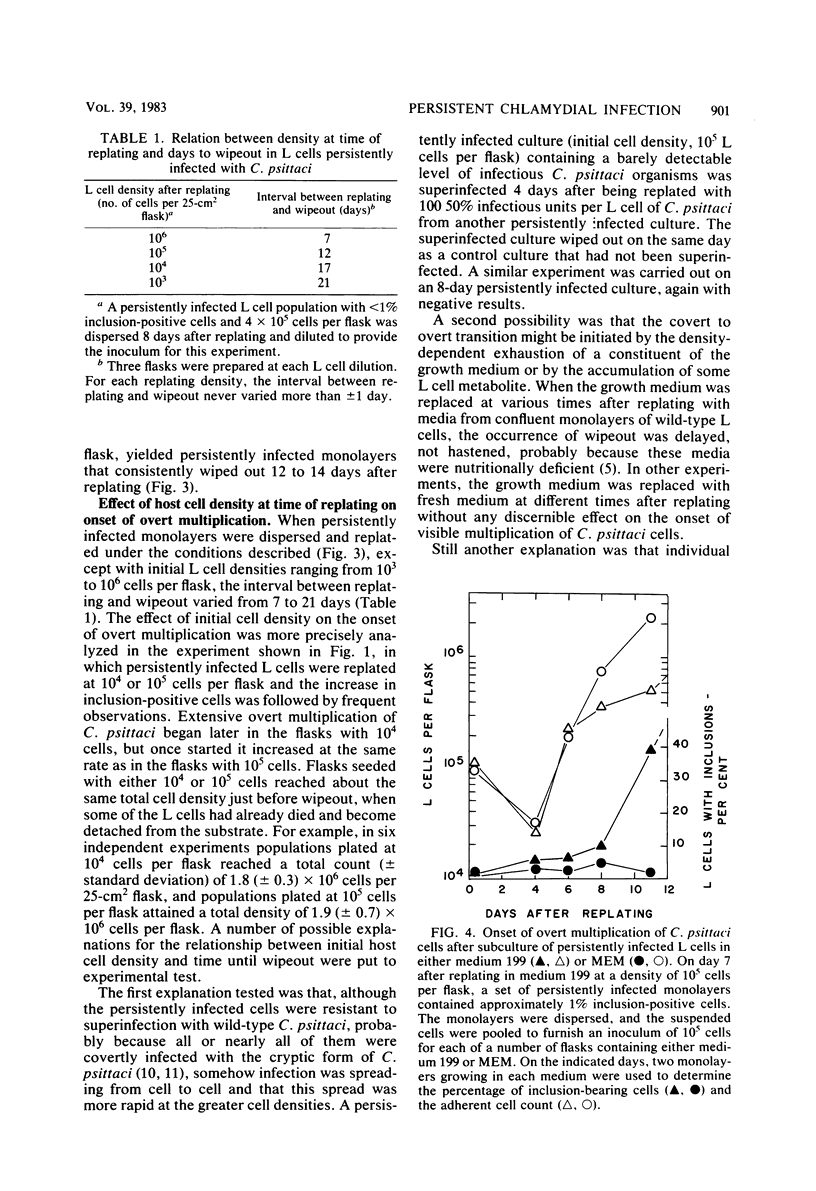
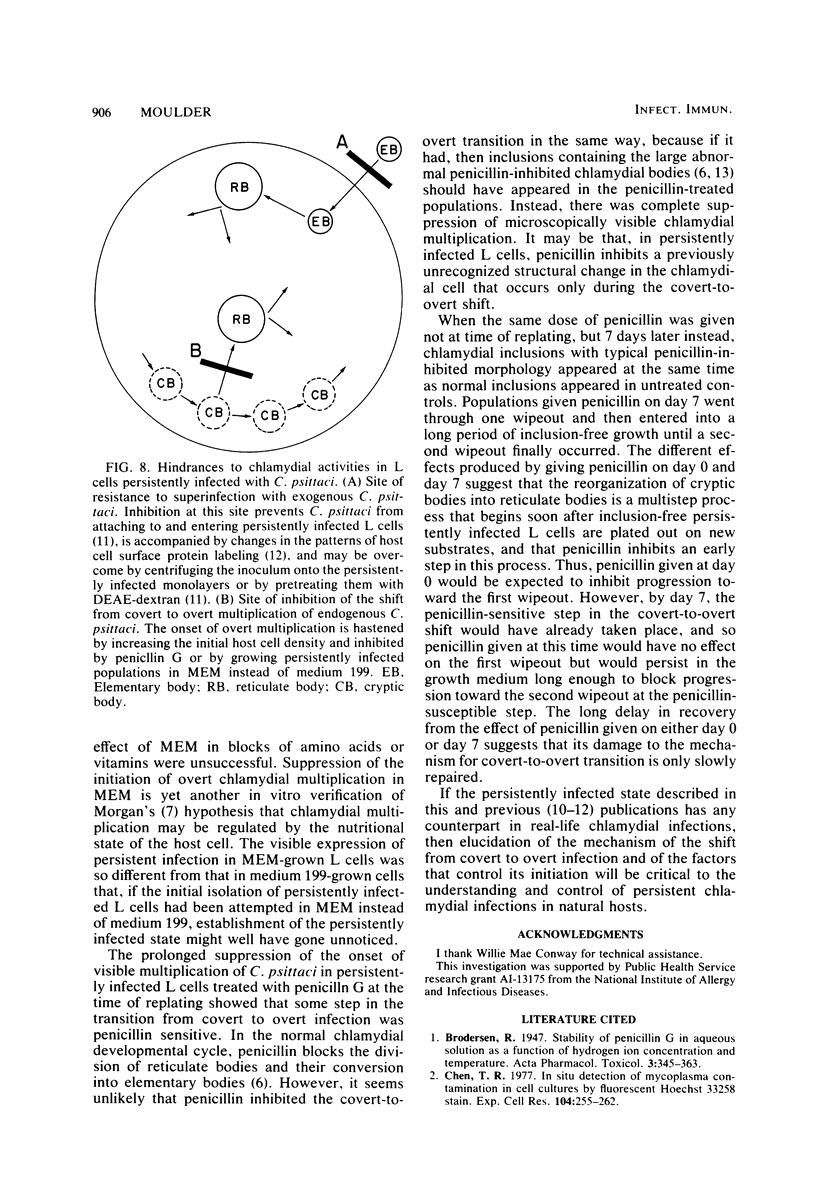
When monolayers of mouse fibroblasts (L cells) persistently infected with Chlamydia psittaci (strain 6BC) were dispersed in medium 199 and plated out in new flasks, the monolayers that grew out consisted almost exclusively of inclusion-free host cells that retained full resistance to superinfection with C. psittaci (covert infection). After a delay that was inversely proportional to the initial density of the newly transferred L cell population, the percentage of host cells containing visible chlamydial inclusions increased rapidly (overt infection), and most of the L cells were destroyed by extensive chlamydial multiplication (wipeout), leaving only a few survivors to start new persistently infected monolayers. When persistently infected L cell populations grown in medium 199 were transferred to Eagle minimal essential medium, the onset of overt multiplication was strongly suppressed although covert multiplication of C. psittaci continued unabated, as shown by host cell retention of resistance to superinfection and the prompt resumption of overt multiplication after transfer back into medium 199. The difference(s) between the two media responsible for the different expression of the persistently infected state was not determined. A single dose of 100 U of penicillin G per ml of medium 199 given at the time persistently infected monolayers were divided almost completely suppressed the appearance of visible signs of chlamydial infection for several weeks, although resistance to superinfection was retained at all times. The same amount of penicillin given 7 days after replating did not prevent the occurrence of the first expected wipeout, but there was a long period of inclusion-free L cell growth between the first wipeout and the second. It was concluded that covert multiplication of C. psittaci in persistently infected L cells may continue indefinitely without the appearance of visible signs of infection. The transition between covert and overt chlamydial multiplication appears to be a penicillin-sensitive, multistep process that is regulated, at least in part, by the host cell density and the composition of the growth medium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EAGLE H. The binding of penicillin in relation to its cytotoxic action. III. The binding of penicillin by mammalian cells in tissue culture (HeLa and L strains). J Exp Med. 1954 Jul 1;100(1):117–124. doi: 10.1084/jem.100.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN H. R. Latent viral infection of cells in tissue culture. I. Studies on latent infection of chick embryo tissues with psittacosis virus. J Exp Med. 1956 Jan 1;103(1):37–47. doi: 10.1084/jem.103.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Manire G. P. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol. 1970 Jan;101(1):278–285. doi: 10.1128/jb.101.1.278-285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton H. J. A survey of commercially available tissue culture media. In Vitro. 1970 Sep-Oct;6(2):89–108. doi: 10.1007/BF02616112. [DOI] [PubMed] [Google Scholar]
- Moulder J. W., Levy N. J., Schulman L. P. Persistent infection of mouse fibroblasts (L cells) with Chlamydia psittaci: evidence for a cryptic chlamydial form. Infect Immun. 1980 Dec;30(3):874–883. doi: 10.1128/iai.30.3.874-883.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Levy N. J., Zeichner S. L., Lee C. K. Attachment defect in mouse fibroblasts (L cells) persistently infected with Chlamydia psittaci. Infect Immun. 1981 Oct;34(1):285–291. doi: 10.1128/iai.34.1.285-291.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Zeichner S. L., Levy N. J. Association between resistance to superinfection and patterns of surface protein labeling in mouse fibroblasts (L cells) persistently infected with Chlamydia psittaci. Infect Immun. 1982 Mar;35(3):834–839. doi: 10.1128/iai.35.3.834-839.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I. Cell Wall Synthesis by Chlamydia psittaci Growing in L Cells. J Bacteriol. 1970 Dec;104(3):1176–1188. doi: 10.1128/jb.104.3.1176-1188.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]





