Summary
Current treatment for type I diabetes includes delivery of insulin via injection or pump, which is highly invasive and expensive. The production of chloroplast-derived proinsulin should reduce cost and facilitate oral delivery. Therefore, tobacco and lettuce chloroplasts were transformed with the cholera toxin B subunit fused with human proinsulin (A, B, C peptides) containing three furin cleavage sites (CTB-PFx3). Transplastomic lines were confirmed for site-specific integration of transgene and homoplasmy. Old tobacco leaves accumulated proinsulin up to 47% of total leaf protein (TLP). Old lettuce leaves accumulated proinsulin up to 53% TLP. Accumulation was so stable that up to ~40% proinsulin in TLP was observed even in senescent and dried lettuce leaves, facilitating their processing and storage in the field. Based on the yield of only monomers and dimers of proinsulin (3 mg/g leaf, a significant underestimation), with a 50% loss of protein during the purification process, one acre of tobacco could yield up to 20 million daily doses of insulin per year. Proinsulin from tobacco leaves was purified up to 98% using metal affinity chromatography without any His-tag. Furin protease cleaved insulin peptides in vitro. Oral delivery of unprocessed proinsulin bioencapsulated in plant cells or injectable delivery into mice showed reduction in blood glucose levels similar to processed commercial insulin. C-peptide should aid in long-term treatment of diabetic complications including stimulation of nerve and renal functions. Hyper-expression of functional proinsulin and exceptional stability in dehydrated leaves offer a low-cost platform for oral and injectable delivery of cleavable proinsulin.
Keywords: Diabetes, plant-made pharmaceuticals, Molecular farming, chloroplast biotechnology
Introduction
Type I diabetes is an autoimmune disorder that results from the T-cells of the body’s immune system attacking the β-cells of the islets of Langerhans in the pancreas. It represents 5%–10% of all diabetes cases. However, type 1 diabetes accounts for over 90% of diabetes cases in children (Giannini et al., 2009). As of 2007, it was reported that diabetes was the seventh leading cause of death in the USA. The main aetiology of type I diabetes is a drop in insulin levels due to the destruction of the insulin- producing pancreatic β-cells, and therefore this leads to abnormally high blood glucose levels. This can lead to symptoms of the disease such as frequent urination, extreme hunger and thirst, weight loss, fatigue and irritability. However, over time, sustained hyperglycaemia leads to a number of other more serious complications such as heart disease, high blood pressure, kidney disease and nervous system disease (CDC, 2008). It is these complications from diabetes that ultimately lead to death if left untreated.
Estimated annual costs in 2007 for diabetes care and other indirect costs for the United States alone totalled $174 billion with roughly $116 billion in excess medical expenditures comprised of $27 billion going to direct care, $58 billion to treat diabetic complications and $31 billion in excess general medical costs. It is estimated that approximately $1 out of every $10 health care dollars is attributed to diabetes (American Diabetes Association, 2008). As the prevalence of diabetes worldwide continues to increase, these costs are projected to be between $213 and $396 billion by the year 2025 (Giannini et al., 2009). In many developing countries, the annual cost for insulin exceeds 50% of the average annual income. In developed countries, the cost of insulin alone is around $100 per month (Raab et al., 2004). Therefore, there is a great need to reduce the economic burden on people dealing with this disease.
Insulin is a 51 amino acid peptide hormone with a molecular weight of 5.8 kDa which is derived from its precursor, proinsulin. Proinsulin is produced in the pancreas as a 110 amino acid polypeptide containing a 24 amino acid signal sequence and the 86 amino acid proinsulin molecule consisting of the B-chain (30 aa), C peptide (34 aa) and A-chain (21 aa). During insulin biosynthesis in the pancreas, the C-peptide is cleaved off via the action of prohormone convertases 2 and 3 at the B-chain/C-peptide and C-peptide/A-chain junctions, respectively, and insulin (51 aa) is formed via disulphide bonds between the A- and B-chains (Steiner, 1998; Shaw et al., 2002). Commercial insulin production first began in 1922 and consisted of insulin harvested from bovine or porcine pancreas. Thirty years later, the amino acid sequence of human insulin was determined, and by the 1970s scientists were able to recreate the sequence in order to produce recombinant human insulin. This eliminated previous problems with adverse immune reactions from animal-derived insulin. Recently, insulin analogues such as insulin lispro and insulin aspart have been manufactured to contain amino acid alterations that increase absorption time (Levinson, 2003). Commercial preparations of insulin are produced mainly in Escherichia coli (Swartz, 2001) or yeast (Kjeldsen, 2000) as fusion proteins. In E. coli, the A-chain and the B-chain genes are introduced into two separate colonies, and the fusion peptides are produced individually. Then, the two chains are mixed together and joined by disulphide bonding. The folded insulin is subsequently purified. Alternatively, insulin is produced from the proinsulin gene in yeast. However, C-peptide is removed from the final product.
Although insulin is predominantly made in bacteria or yeast for commercial production, there has been further research focusing on other methods for producing insulin including expression in plants. Earlier attempts at insulin expression in plant tissues have focused mainly on treating the autoimmune aspect of the disease by induction of oral tolerance. Both CTB-insulin produced in potato tubers (Arakawa et al., 1998) and cholera toxin B subunit (CTB) fused with three copies of the insulin B-chain expressed in the tobacco nuclear genome (Li et al., 2006) showed expression levels of only 0.1% of total soluble protein. A recent review article reports expression of various proinsulin constructs with or without C-peptide in Arabidopsis seeds that accumulated up to 1% total seed protein (Boothe et al., 2010). However, there is no reference for oral delivery of insulin expressed in plant cells, and there is a need to increase expression levels in large biomass crops in order to further advance this concept. Therefore, we have expressed in plant chloroplasts a CTB-proinsulin fusion protein containing three furin cleavage sites, one of which is located at the point of fusion, and two other sites flank either end of the C-peptide (CTB-PFx3). The vector DNA sequence can be seen in figure S1.
Chloroplast genetic engineering in plants offers several advantages over nuclear transformation. Some of these advantages include higher copy number and expression levels of the transgene. Each chloroplast may contain up to 100 genomes, while each plant cell may contain up to 100 chloroplasts. Therefore, each plant cell may contain as many as 10 000 chloroplast genomes which results in high expression levels of proteins expressed via the chloroplast genome. Expression levels of up to 70% total soluble protein (Oey et al., 2009) and 72% total leaf protein (Ruhlman et al., 2010) of biopharmaceutical proteins have been reported in transgenic chloroplasts, which are the highest levels reported in published literature in this field. Chloroplasts offer gene containment through maternal inheritance, as the chloroplast genome is not transferred through pollen unlike nuclear genomic DNA (Daniell, 2007). The chloroplast vectors are designed with flanking regions that are homologous to the chloroplast genome, and this prevents unwanted positional effects or gene silencing. Furthermore, chloroplasts have the ability to transcribe polycistronic RNA (Quesada-Vargas et al., 2005) and can perform the correct processing of eukaryotic proteins including the ability to carry out post-translational modifications such as disulphide bonding, assembly of multimers and lipid modifications (Arlen et al., 2007, 2008; Bally et al., 2008; Daniell et al., 2009a,b; Lee et al., in press). Finally, plants offer the benefit of low-cost production of proteins and the protection of antigen via bioencapsulation (Limaye et al., 2006; Arlen et al., 2008; Daniell et al., 2009a; Davoodi-Semiromi et al., 2009; Verma et al., 2010). These advantages make the chloroplast ideal for production of biopharmaceutical proteins and vaccines as well as an ideal vehicle for oral delivery. Ideally, expression of insulin in chloroplasts should provide a low-cost means to produce biologically functional insulin, which could be administered through both the injectable and oral delivery methods. Correct processing of proinsulin involves production of three intramolecular disulphide bonds, whereas CTB is required for assembly into pentamers in order to be successfully absorbed into the circulation via the GM1 receptors on the gut mucosa (Limaye et al., 2006). Chloroplasts are capable of performing necessary post-translational modifications, which are essential for the functionality of CTBPFx3 (Limaye et al., 2006; Arlen et al., 2008; Bally et al., 2008; Daniell et al., 2009a; Davoodi-Semiromi et al., 2009; Verma et al., 2010; Lee et al., in press). Efficient transformation of plastids of edible crops was recently achieved (Ruhlman et al., 2010), further facilitating the oral delivery of therapeutic proteins.
The production of chloroplast-derived, orally deliverable functional insulin should provide many benefits including a lower cost of production and the possibility of delivering the C-peptide, which is currently lacking in commercially available insulin. This is of great importance because type 1 diabetic patients fail to produce C-peptide, an essential molecule that once was thought to be biologically inactive. C-peptide is located in between the B-chain and A-chain of the proinsulin molecule. Only recently, C-peptide has been shown to function independently of insulin. C-peptide acts via G-protein activation, which signals the cell to open Ca2+ channels allowing for an influx of cellular Ca2+. This leads to stimulation of eNOS, Na+, K+-ATPase and the MAP kinase pathway (Wahren, 2004). This action of C-peptide is thought to be the mechanism behind the beneficial effects seen upon restoration of C-peptide levels in type 1 diabetic animals and humans (Johansson et al., 2000; Sima and Li, 2005; Hills and Brunskill, 2009). Stimulation of the activities of Na+, K+-ATPase and endothelial nitric oxide synthase (eNOS) is essential for nerve function (Ekberg et al., 2003; Wahren et al., 2007), and the stimulation of Na+, K+-ATPase also leads to correction of renal structure and function (Rebsomen et al., 2008).
Furin is an endoprotease present in the constitutive secretory pathway and cell surface of virtually all cells (Taylor et al., 2003). The consensus sequence for furin cleavage is the C-terminal arginine in the amino acid sequence Arg-X-Lys/Arg-Arg (Thomas, 2002). The introduction of furin consensus sequences at the B-chain/C-peptide and the C-peptide/A-chain has been demonstrated to increase the processing of proinsulin to mature insulin in a wide variety of non-neuroendocrine cells, including fibroblasts, myoblasts, epithelial cells and lymphocytes (Groskreutz et al., 1994; Fujimoto et al., 2005; Tatake et al., 2007). In contrast, native proinsulin is processed in the pancreatic β-cells via prohormone convertases PC2/PC3. Non-neuroendocrine cells therefore do not possess the ability to process proinsulin into mature insulin. This processing is necessary for function of insulin, so addition of furin cleavage sites is necessary to facilitate processing in the gut. Although several different furin cleavage sites have been engineered, there seems to be the best processing (87%) of human proinsulin to unmodified mature human insulin using the Arginine Arginine Lysine Arginine (RRKR) furin cleavage sequence in adult fibroblasts (Shaw et al., 2002). Therefore, this sequence was engineered into proinsulin. Tobacco was used as a model system to quantify the production and facilitate the purification of CTB-PFx3 as well as monitor its functionality. Subsequentially, CTB-PFx3 was produced in lettuce for oral delivery studies.
Results
Chloroplast vectors for expression of cleavable proinsulin
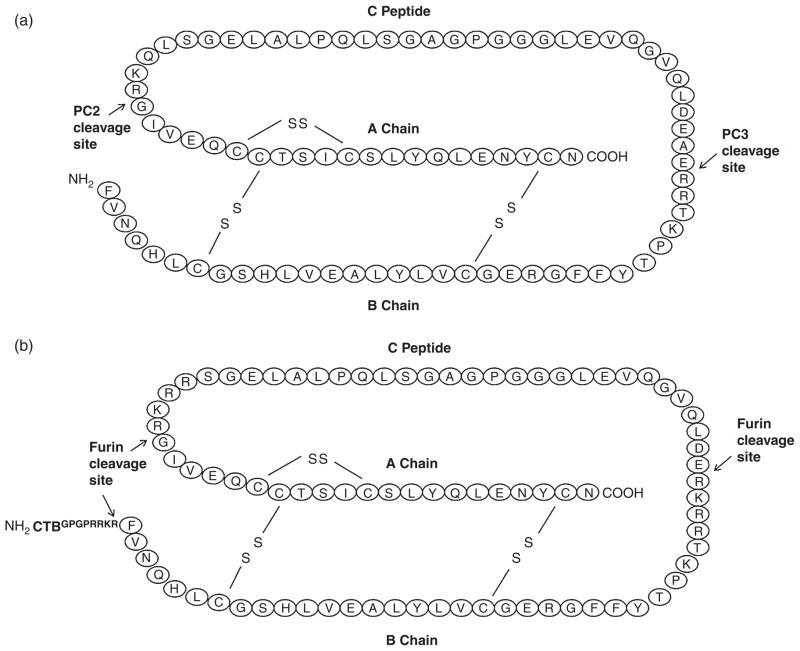
The CTB-PFx3 gene construct was inserted into the pLD vector as previously established by the Daniell laboratory (Verma and Daniell, 2007; Verma et al., 2008) or the pLsLF vector (Ruhlman et al., 2007, 2010) under the control of the light-regulated psbA region located within the 5′ UTR. Both vectors contain the aadA gene that confers resistance to spectinomycin and are driven by the upstream prrn promoter. Chloroplast vectors include native tobacco or lettuce DNA flanking regions (trnI/trnA) in order to facilitate homologous recombination. The CTB gene was separated from the proinsulin gene via a Glycine–Proline–Glycine– Proline (GPGP) hinge to avoid steric hindrance, followed by a furin cleavage site (RRKR) to enable removal of the CTB following delivery. Two additional furin cleavage sites were engineered at the B-chain/C-peptide and the C-peptide/A-chain junctions, replacing the native PC2 and PC3 cleavage sites as previously described (Shaw et al., 2002). The location of engineered cleavage sites and disulphide bonds are compared with native proinsulin in Figure 1. The location of CTB fusion is also shown.
Figure 1.
Location of furin cleavage sites. N and C termini are indicated. (a) Native proinsulin. (9 kDa) (b) Proinsulin molecule was modified to include furin cleavage sites and fused to cholera toxin B subunit (CTB). MW of CTB is 11 kDa. MW of modified CTB-proinsulin molecule is 22 kDa. Fusion proteins are expressed by the CTB-Fx3Pris coding region of the pLDutr-CTB-Fx3Pris or pLsLF-CTB-Fx3Pris chloroplast vector.
Chloroplast transformation and evaluation of homoplasmy
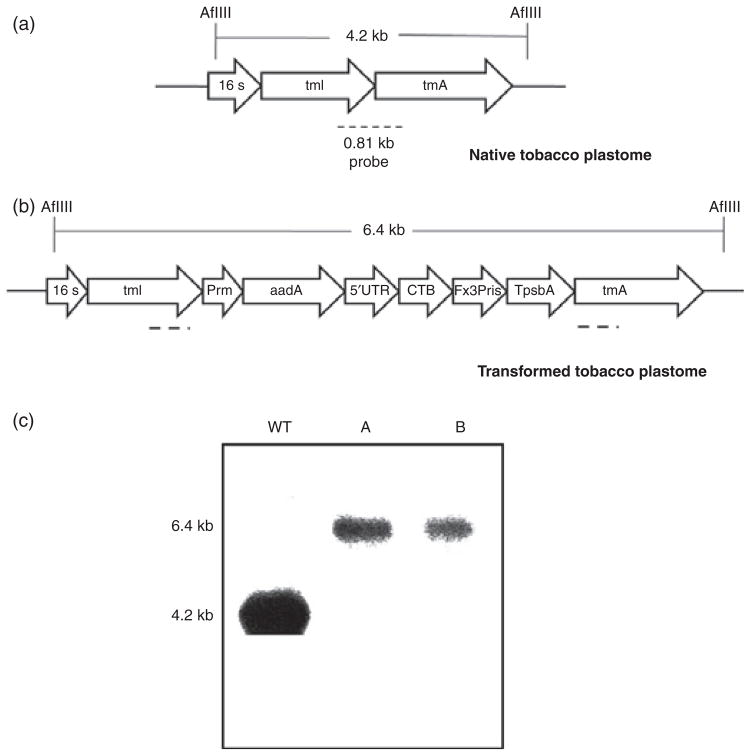
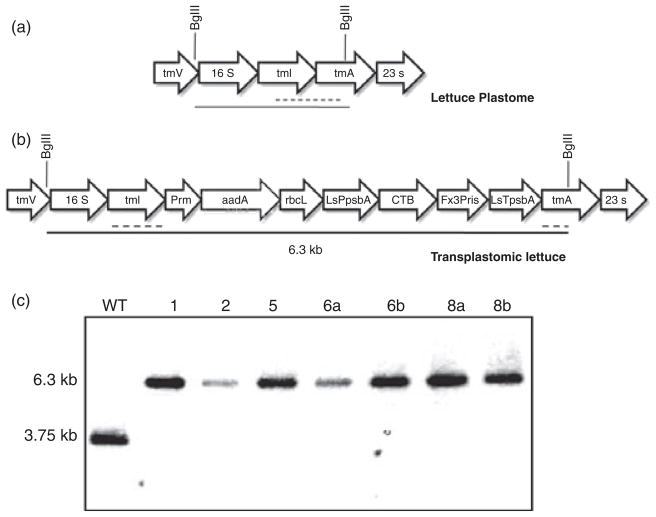
Chloroplast vectors pLDutr-CTB-Fx3Pris and pLsLF-CTB-Fx3Pris were bombarded into tobacco and lettuce leaves, respectively. After 4–6 weeks, green shoots appeared and were subjected to three rounds of selection. Homoplasmy was confirmed by Southern blots. Transformed and untransformed tobacco DNA was digested with AflIII and probed with a 0.81-kb sequence complimentary to the trnI/trnA region of the native chloroplast genome. This produced a 4.2-kb fragment in the untransformed tobacco (Figure 2a) and a 6.4-kb fragment in the transplastomic tobacco (Figure 2b). Both transplastomic lines A and B showed only one hybridizing fragment of 6.4-kb size and did not show a fragment of the same size as the untransformed plant, indicating that all the chloroplast genomes had integrated the foreign gene and confirmed homoplasmy (Figure 2c). Similarly, transformed and untransformed lettuce DNA was digested with BglII which produced a 3.75-kb fragment in the untransformed lettuce (Figure 3a) and a 6.3-kb fragment in the transplastomic lettuce (Figure 3b). Only the 6.3-kb band was present in all transplastomic lines tested, confirming homoplasmy (Figure 3c).
Figure 2.
Southern analysis of transgenic tobacco containing cholera toxin B subunit (CTB)-Proinsulin with three furin cleavage sites. (a) Map of untransformed tobacco plastome. Digestion with AflIII yields a 4.2-kb fragment. Broken line represents probe annealing site. (b) Map of transformed tobacco plastome. Digestion with AflIII yields a 6.4-kb fragment. The 5′UTR represents the light-regulated psbA promoter. (c) Southern analysis from T1 generation. A and B represent independent transplastomic lines. WT, untransformed plant.
Figure 3.
Southern analysis of transgenic lettuce containing cholera toxin B subunit (CTB)-proinsulin with three furin cleavage sites. (a) Map of untransformed lettuce plastome. Digestion with BglII yields a 3.75-kb fragment. Broken line represents probe annealing site. (b) Map of transformed lettuce plastome. Digestion with BglII yields a 6.3-kb fragment. (c) Southern analysis from T0 generation. 1, 2, 5, 6 and 8 represent independent transplastomic lines. Lowercase letters a and b represent different samples from the same line. WT, untransformed plant.
Detection and quantification of cleavable proinsulin
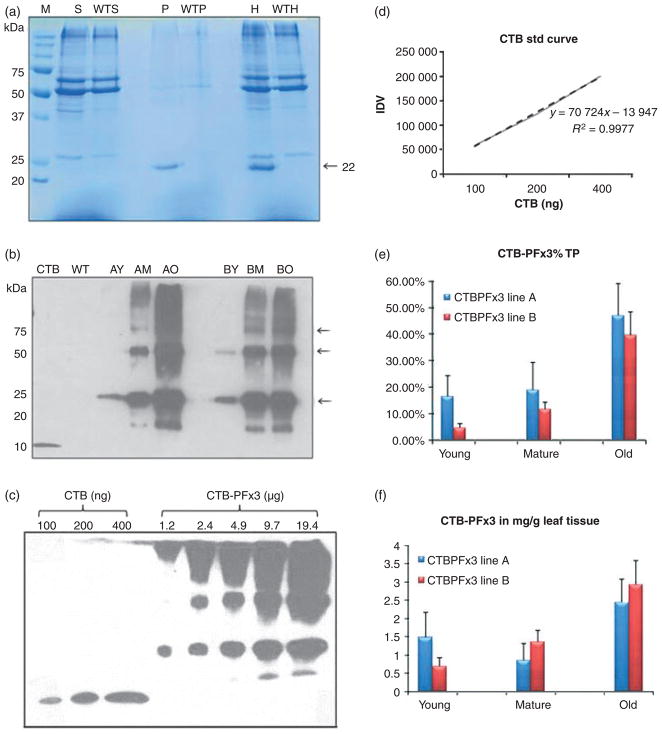
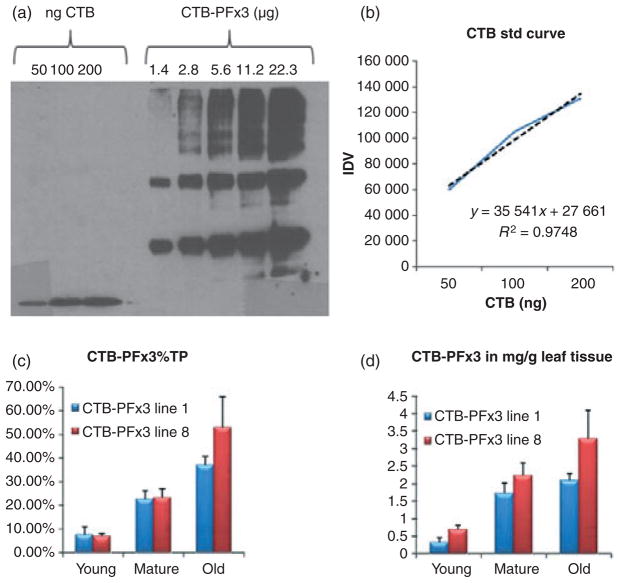
Coomassie staining and immunoblotting were used to determine whether the transgenic plants were expressing CTB-PFx3. Transplastomic and untransformed young, mature and old leaves were ground to a fine powder in liquid nitrogen, and protein was extracted with the addition of 300–500 μL plant extraction buffer. Young leaves are the new, light green, smaller leaves growing at the top of the plant. Mature leaves are large, dark green and grow towards the middle of the plant. Old leaves are the bottom-most senescent leaves that are beginning to turn brown. Samples were spun down, and the resulting supernatant, pellet and homogenate fractions were collected and run on a 12% SDS–PAGE gel (Figure 4a). A polypeptide was seen prominently at 22 kDa in the pellet and homogenate fractions, but not in the supernatant, indicating that the CTB-PFx3 accumulated in the form of insoluble inclusion bodies. Immunoblots were performed using anti-CTB antibody to further confirm transgene expression. Young, mature and old homogenate samples were loaded in equal protein concentrations along with wild-type homogenate and recombinant CTB protein (Figure 4b). A prominent band at 22 kDa represents the monomer size of the CTB-PFx3, but it is clear that the protein forms dimers, trimers, tetramers, pentamers and multimers of 44 kDa, 66 kDa, 88 kDa and 110 kDa sizes. Varying dilutions of the CTB-PFx3 homogenate were loaded onto a 12% SDS–PAGE gel along with known concentrations of recombinant CTB protein, and spot densitometry was performed in order to quantify expression levels (Figure 4c). Different concentrations of CTB were loaded onto the gel in order to generate a standard curve (Figure 4d). CTB-PFx3 was expressed in levels up to 47% in old leaves (Figure 4e) and as much as 2.92 mg/g leaf tissue (Figure 4f).
Figure 4.
Characterization of cholera toxin B subunit (CTB)-proinsulin expressed in tobacco chloroplasts. (a) Coomassie stain. M, marker; S, supernatant; P, pellet; H, homogenate; WT, untransformed plant. S and H: 30 μg per lane. P: 7.5 μg per lane. (b) Western blot probed with anti-CTB antibody showing CTB-PFx3 expression of young, mature and old leaf total protein. 1 μg per lane. CTB: 100 ng CTB standard. Arrows represent the monomer, dimer and trimer bands, respectively. (c) CTB-PFx3 total protein was loaded in varying concentrations and compared to known quantities of CTB standard protein using densitometry. Blot was probed using anti-CTB antibody. (d) Plot of integrated density values (IDV) for quantification of CTBPFx3 based on standard curve. Broken line shows data points. Solid line: trend line. (e) Per cent total protein for young, mature and old leaves based on densitometry values. (f) Quantification of young, mature and old leaves in mg CTB-PFx3 per gram total leaf tissue.
Transgenic lettuce plants were prepared in the same way as tobacco, and varying dilutions of the samples were loaded onto a 12% SDS–PAGE gel for spot densitometric analysis of protein concentration (Figure 5a). Different concentrations of recombinant CTB protein were loaded onto the gel in order to create a standard curve (Figure 5b). Of the four lines tested, CTB-PFx3 was found to account for up to 53% of the total leaf protein (Figure 5c) and as much as 3.28 mg/g leaf tissue (Figure 5d). The majority of expressed protein was found in the old leaves, and expression levels were comparable to levels observed in tobacco.
Figure 5.
Characterization of cholera toxin B subunit (CTB)-proinsulin expressed in lettuce chloroplasts. (a) CTB-PFx3 total protein was loaded in varying concentrations and compared to known quantities of CTB standard protein using densitometry. Blot was probed using anti-CTB antibody. (b) Plot of integrated density values (IDV) for quantification of CTB-PFx3 based on standard curve. Broken line shows data points. Solid line: trend line. (c) Per cent total protein for young, mature and old leaves based on densitometry values. (d) Quantification of young, mature and old leaves in mg CTBPFx3 per gram total leaf tissue.
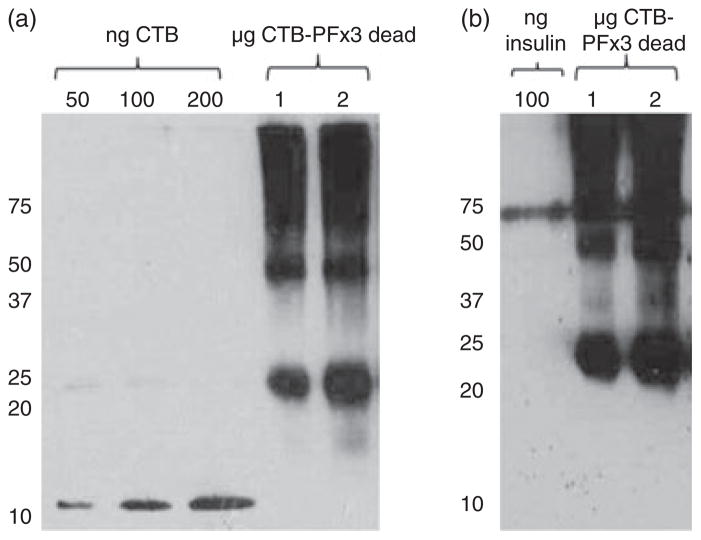
Additionally, owing to the high levels of expression observed in old leaves, senescent and dried lettuce leaves were tested to investigate stability of CTB-PFx3. Senescent leaves that were completely brown and dry were harvested from transplastomic lettuce plants, and protein was extracted as previously described. Leaf homogenate ranging from 0.125 to 2 μg total leaf protein was loaded on a 12% SDS–PAGE gel and probed with CTB or insulin antibodies (Figure 6, a and b). High levels of CTB-PFx3 expression, up to 39% total leaf protein, were detected even in senescent lettuce leaf material, further demonstrating that owing to the insolubility of CTB-PFx3, it is highly stable and protected from proteolytic degradation.
Figure 6.
Expression of cholera toxin B subunit (CTB)-proinsulin in dried senescent lettuce leaves. (a) CTB-PFx3 total protein from dried leaf homogenate was loaded in 1 or 2 μg concentrations along with varying dilutions of CTB standard protein. Immunoblot was probed with anti-CTB antibody. (b) CTB-PFx3 total protein from dried leaf homogenate was loaded in 1 or 2 μg concentrations along with varying dilutions of insulin standard protein (shown as multimers). Immunoblot was probed with anti-insulin antibody.
Solubilization and furin cleavage of proinsulin
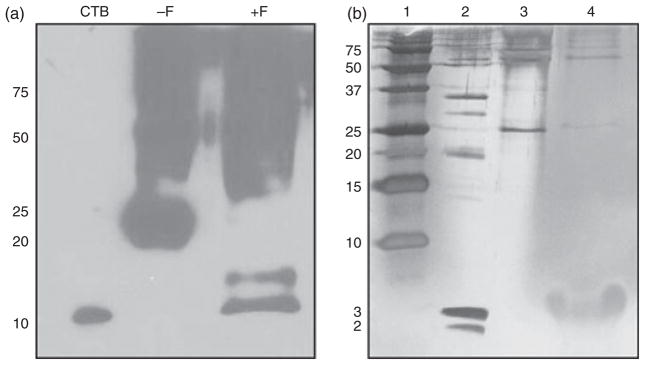
To prepare the CTB-PFx3 for purification and cleavage assays, it was feasible to remove this protein as inclusion bodies and convert into a soluble form. Previously, in our laboratory, insoluble inclusion bodies of human serum albumin (HSA) expressed in tobacco chloroplasts were solubilized and successfully purified (Fernandez-San Millan et al., 2003). Solubilization was achieved using high quantities of guanidine hydrochloride (Gu-HCl) and DTT. DTT was added to pellet resuspended in plant extraction buffer containing 6 M Gu-HCl. No CTB-PFx3 was found in the supernatant fraction after extraction, and this protein was entirely seen in the pellet fraction. Addition of 100 mM DTT achieved some solubility, but addition of 200 or 300 mM DTT achieved almost complete solubility of CTB-PFx3. Therefore, 300 mM DTT was used for all subsequent solubilizations. After solubilization, the denatured protein was refolded in 20 mM sodium phosphate pH 7.8 and 500 mM NaCl. To determine whether the engineered cleavage sites were functional, a furin cleavage assay was performed (Figure 7a). Upon addition of furin and immunoblot visualization with CTB antibody, it is seen that the 22 kDa polypeptide representing the size of the CTB-PFx3 monomer disappears, and a polypeptide is seen at ~11 kDa representing CTB alone as well as a polypeptide at ~14 kDa which most likely represents an incomplete cleavage between CTB and proinsulin consisting of CTB fused to the B-chain of proinsulin, with cleavage occurring at the B-chain/C peptide junction. The proinsulin 2–3 kDa cleavage products were not detected by immunoblot with insulin antibody most likely because of the small size of the protein. Silver stain was then performed to visualize the 2–3 kDa products (Figure 7b). In this gel, the monomer of CTB-PFx3 is seen to decrease after addition of furin, and a large band appears at the 2–3 kDa size. One large band is visualized because the A-chain, C peptide and B-chain cleavage products of proinsulin are 2 kDa, 2.5 kDa and 3 kDa, respectively, which are too close in size to be resolved independently.
Figure 7.
Furin cleavage assays. Samples were solubilized with 6 M Gu-HCl and 300 mM DTT and subsequently dialysed in 20 mM Na+ PO4− buffer with 500 mM NaCl before addition of Furin protease. (a) Furin digestion of CTB-PFx3. CTB, 100 ng CTB standard protein. −F, CTB-PFx3 before furin cleavage. +F, CTB-PFx3 after addition of furin. 1 μg per lane. (b) Silver stained gel after affinity purification of CTB-PFx3. Lane 1, precision plus ladder. Lane 2, Mark 12 low molecular weight ladder. Lane 3, purified CTB-PFx3 1 μg per lane. Lane 4, purified CTB-PFx3 after addition of furin. 1 μg per lane.
Purification and enrichment of proinsulin using nickel affinity chromatography
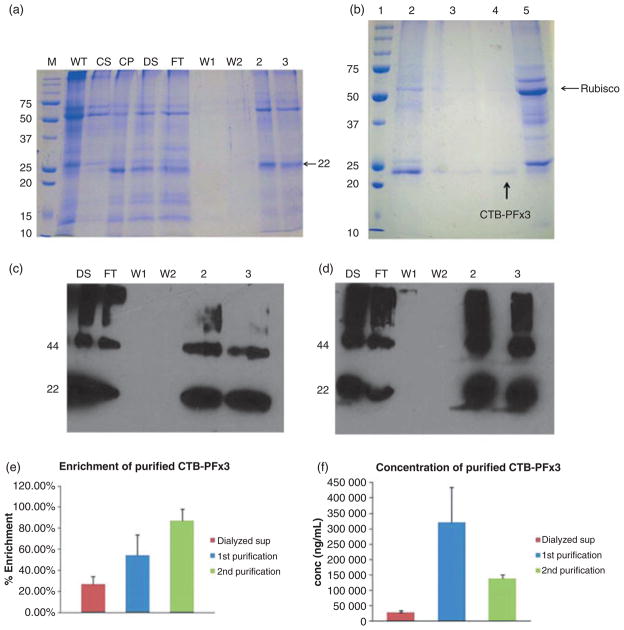
Solubilized and dialysed plant extracts containing CTB-PFx3 were passed over a Ni-NTA column to facilitate purification. Fractions were eluted and run on an SDS–PAGE gel and visualized by coomassie staining (Figure 8a). The 55-kDa Rubisco large subunit is seen prominently in the wild-type sample. This is typical as Rubisco is the most abundant protein in plants and probably the most abundant protein on earth (Jensen, 2000). The 22-kDa band of CTB-PFx3 is seen prominently in the control pellet (CP), but not in the control supernatant (CS), again showing that the protein is in the form of inclusion bodies. After solubilization and dialysis, CTB-PFx3 is detected in the dialysed supernatant fraction (DS). Approximately 30 mL of dialysed supernatant was passed over the Ni-NTA column. No protein is seen to elute in the wash fractions (W1 and W2). The two eluted fractions with the highest optical density (OD) at 280 nm were run on the gel. The 22 kDa polypeptide representing CTB-PFx3 is seen prominently along with Rubisco, but it is seen that the contaminating protein bands are much less abundant; therefore, total CTB-PFx3 protein content was purified and compared with the starting material.
Figure 8.
Purification of cholera toxin B subunit (CTB)-proinsulin by affinity chromatography. (a) Coomassie stained gel. DS = dialysed supernatant; FT = flow through; W1 = wash 1; W2 = wash 2; 2,3 = purified fractions; WT = untransformed plant material; CS = control supernatant; CP = control pellet (b) Coomassie stained gel showing second purification of CTB-PFx3: Lane 1: Marker. Lane 2: control pellet (before purification). Lane 3: Purified CTB-PFx3, Lane 4: Purified CTB-PFx3 after phytate precipitation to remove Rubisco. Lane 5: Control supernatant. All 10 μg load. (c) 1 μg of purified CTB-PFx3 samples were loaded using CTB primary antibody. DS = dialysed supernatant; FT = flow through; W1 = wash 1; W2 = wash 2; 2, 3 = purified fractions (d) 1 μg of purified CTB-PFx3 samples were loaded using insulin primary antibody. DS = dialysed supernatant; FT = flow through; W1 = wash 1; W2 = wash 2; 2, 3 = purified fractions (e) Densitometry shows that after purification, CTB-PFx3 makes up ~57% of the eluted fractions after the first purification and ~87% after the second purification as compared to ~27% of the dialysed solubilized supernatant before passing over the nickel column. (f) The concentration of CTB-PFx3 increases roughly 10-fold (~320 000 ng/mL) after the first purification and 4.6-fold (~140 000 ng/mL) after the second purification over nickel column than the initial concentration of dialysed solubilized supernatant (~30 000 ng/mL).
Purification was repeated in order to achieve a greater level of purity by removing Rubisco. In order to enhance purification, old leaves were prepared in the same way as in the previous purification as they contain less Rubisco, and samples were run over a Ni-NTA column. After purification, additional Rubisco was removed using sodium phytate precipitation (Krishnan and Natarajan, 2009), and samples were loaded on an SDS–PAGE gel for coomassie staining (Figure 8b).
The resultant purified fractions were loaded in equal quantity onto an SDS–PAGE gel for immunoblot analysis along with the dialysed supernatant, flow through and wash fractions. Blots were probed with either anti-CTB antibody (Figure 8c) or anti-insulin antibody (Figure 8d). In both blots, the purified CTB-PFx3 is shown to be highly enriched compared to the dialysed supernatant. Additionally, the CTB-PFx3 monomer is enhanced, which would provide the functional, cleavable proinsulin. Densitometric analysis showed that the original dialysed supernatant contained 27% CTB-PFx3, whereas after the first purification, this increased to an average of 57% purity (range determined by densitometry to be between 42% and 72%), a nearly twofold increase. After the second step of purification followed by sodium phytate precipitation, the sample was shown to be about 87% pure (range determined by densitometry to be between 75% and 98%), over a threefold increase from the original dialysed supernatant and a 1.5-fold increase from the first purification (Figure 8e). The starting concentration of the CTB-PFx3 in the dialysed supernatant was ~30 000 ng/mL (0.03 mg/mL) which increased to ~320 000 ng/mL (0.32 mg/mL) after the first purification and to ~140 000 ng/mL (0.14 mg/mL) after the second purification representing a total enrichment greater than 10-fold and 4.5-fold, respectively (Figure 8f). The lower concentration in the second purification is most likely due to the fact that less total sample volume was loaded onto the column resulting in a lower concentration but achieving higher purity.
Functional evaluation of cleavable proinsulin
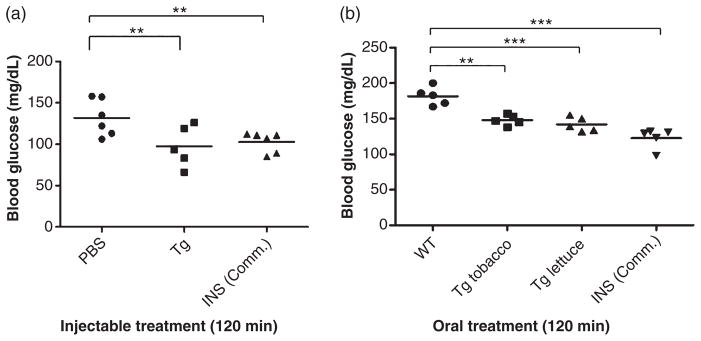
CTB-PFx3 purified from transplastomic tobacco as well as commercially produced purified insulin (Roche, Basel, Switzerland) was injected into female C57BL/6 mice along with PBS as a negative control. Blood glucose measurements were made by tail vein bleed before intraperitoneal (IP) injection and at 15-, 30-, 60-, 120- and 180-min time intervals. Another injection was given after the 180-min time point, and blood glucose measurements were again made at 15-, 30-, 60-, 120- and 180-min time points after the second injection. Blood glucose levels were found to be significantly lower than PBS control in all groups at the 120-min time point after the second injection (Figure 9a), similar to commercial insulin.
Figure 9.
Functional evaluation of cholera toxin B subunit (CTB)-proinsulin in mice delivered by injection or oral gavage. ANOVA (Dunnett’s multiple comparison test) was performed on all groups of mice. (a) Mice were administered two intraperitoneal (IP) injections of PBS only (PBS), purified CTB-PFx3 (Tg) or commercial Insulin (INS Comm.). Blood glucose levels were measured after the first 15 min and each hour (up to three hours) after each injection. Blood glucose levels were statistically lower (**P < 0.01) 2 h after the second injection in all groups compared to the PBS only negative control. (b) Mice were administered two oral gavages of untransformed lettuce (WT), tobacco expressing CTB-PFx3 (Tg tobacco), lettuce expressing CTB-PFx3 (Tg lettuce) or two IP injections of commercial Insulin (INS Comm.). Blood glucose levels were measured after the first 15 min and each hour (up to three hours) after each injection. Blood glucose levels were statistically lower (**P < 0.01; ***P < 0.001) 2 h after the second injection in all groups compared to the untransformed (WT) control.
Additionally, transplastomic lettuce and tobacco plants along with untransformed lettuce were delivered to mice by oral gavage. Commercially produced purified insulin was injected as a control. Blood glucose measurements were taken by tail vein bleed before gavage or injection and at 15-, 30-, 60-, 120- and 180-min time intervals. Another dose was given after the 180-min time point, and blood glucose measurements were again taken at 15-, 30-, 60- and 120-min time points after the second dose. Blood glucose levels were found to be significantly lower than untransformed control in all groups at the 120-min time point after the second injection (Figure 9b). Food pellets were removed from cages for the duration of both studies.
Discussion
Existing treatments for type 1 diabetes are painful, costly, and currently available insulin does not include the C-peptide. Here, we have expressed CTB-proinsulin with three furin cleavage sites in both tobacco and lettuce chloroplasts in order to produce insulin that may be processed in most cells in the body. This will significantly reduce the cost of production, as well as facilitate the possibility of delivering the C-peptide, which should aid in the treatment of diabetic complications. One acre of farm land can yield about 40 metric tons of tobacco with three harvests (Arlen et al., 2007) or 11 metric tons of lettuce biomass in a single harvest. Assuming the expression level of CTB-PFx3 is around 3 mg/g of leaf tissue, and taking into consideration a 50% loss of protein during the purification process, this could yield up to 20 million daily doses of insulin. However, the efficiency of refolding would actually determine yield of functional protein, and this has not been quantified in this study. It is estimated that 220 million people worldwide have diabetes, 10% of these having type 1 diabetes. Therefore, one acre of biomass could produce enough insulin to treat a large type 1 diabetic population, which would be a major economic advantage.
There are many disadvantages to insulin injections which are currently the most widespread form of administration. These include, pain, itching, allergy and lipodystrophy at the injection site. Additionally, these disadvantages often cause noncompliance of patients with their insulin regimen. Therefore, alternative approaches to insulin delivery are necessary to help diabetic populations meet their medical needs. Some of the recent approaches to address these problems include buccal spray insulin, inhalable insulin, oral anti-diabetic drugs and oral delivery of insulin. Buccal spray insulin is absorbed by the mucosa of the mouth and has a faster onset than injectable insulin. Limitations of this approach include a shorter duration of action and a dosage requirement that is 5–7 times higher than injectable insulin as well as mild side effects such as dizziness (Pozzilli et al., 2010). Inhalable insulin faces similar challenges. The FDA approved inhalable insulin, Exubera, was pulled off the market because of poor sales and adverse side effects such as reduced lung function (Siekmeier and Scheuch, 2008). Oral anti-diabetic drugs are used mainly to treat type 2 diabetics as they improve insulin production, so there is still a need to deliver functional insulin to people with type 1 diabetes and type 2 diabetics who require insulin. The main challenges that arise in advancing the concept of oral delivery of insulin are protection from enzymatic degradation in the stomach and efficient absorption into the gut-associated lymphoid tissue (GALT, Agarwal and Khan, 2001). Approaches to overcome these challenges include encapsulation of insulin using nanoparticles and the delivery of insulin along with a protease inhibitor (Iyer et al., 2010). A novel approach to circumvent these obstacles is the expression of insulin in plant cells.
Insulin has never been produced without a fusion protein, as it is highly unstable and is prone to N-terminal degradation (Boothe et al., 2010). Therefore, we fused proinsulin with CTB in order to protect the N-terminus of insulin from degradation as well as provide a means to facilitate purification and uptake of orally delivered insulin into the GALT. CTB binds to GM1 receptors in the lumen of the gut (Tsuji et al., 1995; de Haan et al., 1998), and it has been shown that fusion to the C-terminus of CTB is ideal for pentamerization and GM1 binding (Liljeqvist et al., 1997; Limaye et al., 2006). Recently, CTB fused with blood clotting factor IX was successfully expressed and delivered orally to aid in treatment of haemophilia B. After delivery, the antigen was found in different tissues of the GALT, specifically the ileum, Peyer’s patches, liver and plasma within two hours (Verma et al., 2010). In order to successfully obtain purified, functional insulin, the CTB-PFx3 must be solubilized in high quantities of reducing agent. We achieved this by using 6 M GuHCl and 300 mM DTT. After solubilizing, it was necessary to refold the denatured protein. This was achieved by dialysis in 20 mM sodium phosphate buffer containing 500 mM NaCl. The refolded CTB-PFx3 was then run over the Ni2+ column. Proper refolding was necessary in order to achieve purification because the lack of a His-tag made it necessary to use the refolded CTB moiety to bind to the Ni2+ column. CTB contains three histidine residues that are in close enough proximity when properly folded, which is enough to allow binding to anion exchange resins such as Ni2+ or Co2+ (Dertzbaugh and Cox, 1998). This way, purification of the CTB-PFx3 is facilitated by the CTB fusion. Additionally, purification is enhanced by the fact that a majority of the plant proteins are discarded with the supernatant, thus the initial sample to be purified contains only those proteins that were insoluble, eliminating a majority of endogenous plant soluble proteins. When expressed in plants, the insulin is also protected from enzymatic degradation in the stomach via bioencapsulation (Limaye et al., 2006; Davoodi-Semiromi et al., 2010; Verma et al., 2010). Therefore, advancing the concept of oral delivery of insulin by expressing it in plant cells should reduce its cost, facilitate purification and processing, eliminate the need for cold storage and simplify transportation and delivery (Daniell et al., 2009a).
Additionally, none of the alternative or currently used approaches to insulin delivery include the C-peptide. C-peptide is generally used as a marker to determine the extent of insulin release in patients. A large portion of diabetic patients who undergo extensive insulin therapy to maintain normal blood glucose levels still develop long-term complications such as neuropathy and nephropathy. C-peptide has been shown to alleviate these complications in various studies, and this suggests that diabetic patients should receive supplemental C-peptide in addition to insulin (Hills and Brunskill, 2009). For these reasons, we have created cleavable proinsulin, which is able to be processed into properly folded and functional insulin as shown by functionality studies in mice. Furin cleavage sites were chosen owing to the fact that furin is an ubiquitous protease found in all human cells and tissue types. We have worked with furin protease in previous animal studies involving oral tolerance, and no adverse immunogenic effects have been seen (Verma et al., 2010). Furthermore, furin cleavage has been used in several studies to process human proinsulin to achieve safe and long-term expression (Suda et al., 2006). Upon cleavage, C-peptide is available for circulation, where it may interact with its G-protein- coupled receptor and in turn activate the Na+/K+ ATPase and MAPK pathways, which are partly responsible for the beneficial effects seen in alleviating diabetic complications. This is the first time that the complete proinsulin gene has been expressed in plants with the possibility of in vivo processing outside the pancreas, into functional insulin and C-peptide.
Upon hyper-expression of cleavable proinsulin in tobacco and lettuce chloroplasts, we noticed expression in the form of insoluble inclusion bodies similar to insulin inclusion bodies observed in E. coli (Williams et al., 1982) or plants (Boothe et al., 2010). The functionality of CTB stems from its ability to pentamerize in order to bind to GM1 receptors (Liljeqvist et al., 1997) and has been demonstrated to form functional pentamers in chloroplasts (Daniell et al., 2001; Limaye et al., 2006; Verma et al., 2010). Additionally, insulin and proinsulin may form self-aggregates of monomers, dimers, tetramers and hexamers (Pekar and Frank, 1972), which may further contribute to their aggregation in chloroplasts. However, formation of pentamers, multimeric forms and inclusion bodies caused the proinsulin to be highly stable by conferring protection from proteases. The majority of proinsulin accumulation was seen in older leaves as opposed to mature leaves, which is often observed among foreign (soluble) proteins expressed in chloroplasts (Tsuji et al., 1995; Koya et al., 2005; Arlen et al., 2008; Daniell et al., 2009b). This may account for the high levels of expression seen in old leaves, as proinsulin may be protected from proteases (Martin and Thimann, 1972; Vierstra, 1993). Stability of CTB-PFx3 was even seen in senescent and dried leaves that should facilitate harvest, field drying, storage and transportation of this valuable protein. This is the first report of stable accumulation of a therapeutic protein in dried senescent leaves.
Attempts made to determine protein quantification by means of enzyme-linked immunosorbent assay (ELISA) were unsuccessful because of the high quantity of protein aggregates, which either inhibited binding to the ELISA plate or reduced the ability of the CTB primary antibody from binding to the proper epitopes on the CTB molecule. Addition of DTT to the sample extraction buffer or boiling of samples prior to coating of the ELISA plate with CTB-PFx3 improved the detection of signal, but not enough to yield an accurate quantification. The denaturation of the CTB-PFx3 monomer and multimers during the immunoblot procedure most likely facilitated better binding of the CTB primary antibody, allowing access to the desired epitopes. For densitometry studies, only the monomer and dimer bands were used for the quantification, as the higher multimers tended to form less defined bands. Therefore, the percentages reported are likely an underestimation of the total expressed CTB-proinsulin.
Mice that were given oral CTB-PFx3 experienced changes in blood glucose levels, but levels were significantly lower at 120 min after the second dose when compared to mice given untransformed lettuce. This demonstrates that proinsulin is delivered to the circulation and is properly cleaved and folded. In a recently published study, we observed blood clotting factor IX fused with CTB in the circulatory system 2 h after oral delivery (the earliest time point in this study) of CTB-FIX expressed in chloroplasts (Verma et al., 2010). Therefore, detection of plant-derived insulin activity in less than two hours is quite reasonable. At this point, the percentage of orally delivered protein absorbed by the GALT and delivered into the circulatory system has not been fully investigated, although several bioencapsulated chloroplast-derived therapeutic proteins have been orally delivered and shown to be fully functional. Additionally, injected CTB-PFx3 must be processed into CTB and insulin when compared with the commercial insulin, which is already in its correct functional conformation. However, the efficiency of refolding has not been quantified in our study. Therefore, determining the actual amount of CTB-PFx3 mice received and time required for processing would require further investigations. Future studies would investigate long-term effects of the C-peptide delivery on diabetic complications in addition to the effects of CTB-PFx3 on blood glucose levels. Although we investigated solubilization of multimers of proinsulin into monomers for effective delivery of functional insulin, a recent study has shown that injection of aggregates of insulin oligomers facilitates sustained and prolonged release, up to 140 days (Gupta et al., 2010). Therefore, further studies would investigate injection of chloroplast-derived oligomeric aggregates without solubilization into the monomeric form.
Experimental procedures
Vector construction
The CTB-PFx3 construct was created from pLD-5′UTR-CTB-Pins as previously described (Ruhlman et al., 2007). The proinsulin gene was modified in order to include furin cleavage sites in between the B-chain/C-peptide and the C-peptide/A-chain junctions as well as between the CTB/B-chain fusion site. The pLD-5′UTR-CTB- Pins vector was digested with NcoI and SmaI, and the resulting portion containing the 5′UTR was ligated into the pGEM-Pris( fx3) vector containing the mutated proinsulin. This was subsequently digested with EcoRI and NotI and subcloned into the pLD-g10-RecA vector, replacing the g10-RecA gene with the 5′Utr CTB-Pris(fx3) gene. The final vector was designated pLDutr-CTB-Fx3Pris. To create the lettuce vector, pLDutr-CTB-Fx3Pris was digested with SmaI and XbaI, and the resulting Fx3Pris fragment was ligated into the 12.13.9.11 CTB-GPGP-FIX vector containing the lettuce-specific psbA 3′ and 5′ UTRs, replacing the FIX gene. To create the final vector, the pLspsbACTB-Fx3 Pris was digested with NdeI and NotI and was cloned into pLsLFHPAG vector containing the lettuce flanking regions, and this vector was designated as pLsLF-CTB-Fx3Pris. After vector construction, the CTBPFx3 genes were sequenced (GENEWIZ). Sequences were confirmed to be correct using BLAST search.
Bombardment and selection
Bombardment was done using the Bio-Rad PDS-1000/He gene gun as described previously (Verma et al., 2008). Sterile tobacco leaves were placed abaxial side up, whereas sterile lettuce leaves were placed abaxial side down on Murashige and Skoog (MS) medium and were bombarded under sterile conditions using pLDutr-CTB-Fx3Pris or pLsLF-CTB-Fx3Pris coated with gold particles prepared according to (Kumar and Daniell, 2004). Bombarded leaf pieces were incubated in the dark for 2 days at room temperature, and then leaf segments were transferred to regeneration media of plants (RMOP) containing 500 mg/L spectinomycin for tobacco or regeneration media of lettuce (RMOL) containing 100 mg/L spectinomycin (Ruhlman et al., 2010). The appearance of green shoots was seen after 4– 6 weeks. These shoots were allowed to grow, and the leaves were cut and subsequently transferred to RMOP or RMOL containing 500 mg/L or 100 mg/L spectinomycin, respectively, for the second round of selection. After new shoots formed, these were transferred for the third round of selection to MS containing 500 mg/L spectinomycin for tobacco or 100 mg/L spectinomycin for lettuce and allowed to establish roots. Plants were then transferred to the greenhouse.
Southern analysis to confirm homoplasmy
Transgenic and untransformed tobacco DNA was digested using AflIII, and lettuce DNA was digested using BglII in a reaction mixture containing 2 μL 10× buffer (New England Biolabs, Ipswich, MA), 3 μg plant genomic DNA, 2 μL BSA and 1 μL enzyme made up to 20 μL with dH2O. The reactions were incubated overnight at 37 °C. Southern analysis was performed according to laboratory protocol (Kumar and Daniell, 2004). The entire 20 μL restriction digestion samples were separated on a 0.8% agarose gel at 50V for 4 h in Tris-acetic acid-EDTA (TAE) buffer. The gel was depurinated for 15 min in 0.25N HCl, rinsed two times for 5 min each in dH2O and soaked in transfer buffer (0.4N NaOH, 1 M HCl) for 20 min. All steps were performed with gentle rocking. Capillary transfer was allowed to take place over night. The next day, the membrane was rinsed with 2× SSC (0.3 M NaCl, 0.03 M Sodium Citrate) two times for 5 min each, and DNA was cross-linked to the membrane at 150 mJ in GS Gene Linker (Bio-Rad, Hercules, CA). Flanking sequence probe was generated by digesting the pUC-CT vector (Verma et al., 2008) with BglII and BamHI in order to generate a 0.81-kb fragment corresponding to the trnI/trnA region of the native chloroplast genome. Probe was labelled with 32P using Ready-to-Go DNA labelling beads (General Electric, Fairfield, MA). Hybridization was carried out using Stratagene Quick-Hyb solution and protocol. Afterwards, the membrane was placed in a cassette and exposed to X-ray film at −80 °C.
Protein extraction and quantitation
Young, mature and old leaf tissue was ground to a fine powder in liquid nitrogen using a mortar and pestle. Five hundred microlitres of plant extraction buffer (100 mM NaCl, 200 mM Tris-HCl pH 8.0, 0.1% Triton-X, 400 mM sucrose, and Roche complete mini protease inhibitor cocktail tablet) was added to 100 mg of frozen leaf tissue and vortexed for 10 min at 4 °C. Plant tissue homogenates were further separated into supernatant and pellet fractions via centrifugation (20 000 g for 5 min at 4 °C), and the pellet was resuspended in 500 μL plant extraction buffer for analysis. Total leaf protein concentration was determined using the Bio-Rad protein assay dye reagent concentrate. Dye was diluted 1 : 5 in water and filtered through Whatman filter paper. The standard curve was made using 0.4 mg/mL BSA serially diluted down to 0.006 mg/mL. Plant homogenate, supernatant and pellet samples were diluted in water 1 : 5, 1 : 10, 1 : 20 and 10 μL of sample, and BSA standard was loaded in duplicate to a 96-well plate. Diluted Bio-Rad dye (200 μL) was added to each sample and standard and the plate was read on a plate reader at 595 nm.
SDS–PAGE and immunoblot analysis
Homogenate, supernatant and pellet samples were diluted in 2× sample loading buffer (3.55 mL dH2O, 1.25 mL 0.5 M Tris-HCl pH 6.8, 2.5 mL glycerol, 2 mL 10% SDS, 0.2 mL 0.5% bromophenol blue) and loaded onto a 12% SDS–PAGE (Bio-Rad) gel. Samples were separated at 100–150V until the dye front reached the bottom of the gel. After separation, the gel was either stained with Coomassie Brilliant Blue R-250 (Bio-Rad) or transferred to a nitrocellulose membrane (Bio-Rad) for immunoblot analysis. Transfer was carried out using a Bio-Rad transfer cassette at 85V for 1 h. Membranes were briefly rinsed in dH2O and blocked in PBS-Tween-20 + 3% dry milk (PTM) for 30 min to 1 h. CTB-PFx3 was detected using anti-CTB primary antibody (Sigma, St. Louis, MO) diluted 1 : 4000 for 1.5 h followed by goat anti-rabbit IgG-HRP (Southern Biotech, Birmingham, AL) at a dilution of 1 : 5000 for 1 h. Proteins were subsequently detected on autoradiography film using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, Waltham, MA).
Densitometric quantification of CTB-proinsulin
Immunoblots for quantification were performed using known concentrations of CTB protein standard (Sigma) of 25, 50, 100, 200 and 400 ng in order to create a standard curve. Total protein concentration was determined via the Bradford assay (Bio-Rad). Varying concentrations of CTB-PFx3 plant homogenate were directly compared to the standard curve using Alphaimager and Alphaease FC software. The percentage of CTB-PFx3 relative to the concentration of total leaf protein and mg CTB-PFx3 per gram leaf tissue were calculated according to the formula published in (Verma et al., 2008).
Protein solubilization and furin cleavage
CTB-PFx3 homogenate was separated into supernatant and pellet fractions. The pellet fraction containing the CTB-PFx3 in an insoluble form was resuspended in 1.5 mL of plant extraction buffer per 100 mg ground plant tissue along with 100–300 mM dithiothreitol (DTT, Promega Fitchburg, WI) and 6 M guanidine hydrochloride (Gu-HCl, Sigma). Samples were rocked for 16– 18 h at 4 °C. The samples were then centrifuged at 14 000 rpm for 5 min at 4 °C, and the supernatant containing soluble CTB-PFx3 was separated from the pellet fraction. The samples were subsequently dialysed using Spectra/Por molecularporous membrane tubing (Fisher Scientific, Waltham, MA) in 20 mM sodium phosphate pH 7.8, 500 mM NaCl overnight at 4 °C. For cleavage with furin protease, 1 μg of dialysed sample was incubated with 100 mM HEPES pH 7.5, 0.5% Triton X-100, 1 mM CaCl2, 1 mM 2-mercaptoethanol, and 2 units furin (New England Biolabs) at 25 °C for 16 h.
Affinity purification
After solubilization and dialysis of the CTB-PFx3, the resulting supernatant was passed over a column (Clontech, Mountain View, CA) consisting of 2 mL Ni-NTA Agarose (Qiagen, Venlo, the Netherlands) which was equilibrated with 20 mM sodium phosphate pH 7.8 and 500 mM NaCl (Binding buffer). The column was subsequently washed with 30 mL binding buffer and then with 30 mL wash buffer (20 mM sodium phosphate pH 6.0 and 500 mM NaCl). Samples were eluted from the column in 1 mL fractions using 10 mL elution buffer (20 mM sodium phosphate pH 6.0, 500 mM NaCl, and 250 mM imidazole). All steps were carried out at a flow rate of 0.5 mL/min. Fractions were assayed for protein concentration via absorbance units at 280 nm using the SmartSpec 3000 (Biorad) and Bradford assay to determine the concentration in mg/mL. Purified CTB-PFx3 (1 μg) was incubated with furin, and cut and uncut samples were run on a 16% Tricine-SDS gel. Samples were also subsequently run on 12% SDS–PAGE gel and subjected to coomassie stain and immunoblot in order to determine the per cent purity.
Sodium phytate precipitation
Sodium phytate precipitation was performed to remove Rubisco from purified samples. Purified samples were treated with 10 mM CaCl2 and 10 mM sodium phytate (phytic acid sodium salt hydrate from Sigma) for 10 min at 37 °C. Afterwards, samples were spun in a tabletop centrifuge at room temperature for 10 min at 14 000 rpm. Supernatant was removed, placed in a fresh tube and stored at −20 °C until use.
Silver stain
Tricine-SDS gel was incubated in fixative (45% dH2O, 45% methanol, 10% acetic acid) for 40 min. The gel was then washed with 50% methanol 2× for 5 min each and then with dH2O 2× for 5 min each. Next, the gel was rocked in Hypo solution (20 mg Na2S2O3 in 100 mL dH2O) for exactly 1 min and then washed in dH2O 3× for 5 min each. Silver solution (200 mg AgNO3 and 100 μL formaldehyde in 100 mL dH2O) was added and the gel was rocked for 30 min. The gel was washed in dH2O 4× for 5 min each and then developer solution (6 g Na2CO3, 100 μL of hypo, 100 μL formaldehyde in 300 mL dH2O) was added. The gel was vigorously shaken in developer solution until protein bands appeared, and then the reaction was stopped by addition of 5% acetic acid.
Animal studies to determine functionality
All procedures performed in this study are based on an approved protocol in accordance with UCF-IACUC. Female C57BL/6 mice weighing 17–20 g each were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice were divided into four groups with six mice per group. Samples were prepared for delivery via IP injection by dilution in sterile PBS. Commercially purified insulin and purified CTB-PFx3 derived from transgenic tobacco were normalized via immunoblot and subsequently diluted to give each mouse a dosage of 0.04 U/kg of commercially prepared insulin (Roche) and 8 μg/mouse of plant-derived purified CTB-PFx3. IP injections were delivered in a volume of 100 μL. Blood glucose levels were measured by bleeding of the tail vein before sample administration and again at 15, 30, 60, 120 and 180 min after sample administration (Accu-Chek, Aviva; Roche, Basel, Switzerland). A second injection was given after 180 min, and blood glucose measurements were taken at 15, 30, 60, 120 and 180 min after the second IP injection. Food pellets were removed for the duration of the study. For the oral study, female C57BL/6 mice were divided into four groups with five mice per group. Commercially purified insulin was diluted in sterile PBS and given at a dose of 0.04 U/kg. IP injections were delivered in a volume of 100 μL. Oral samples were prepared from material ground in liquid nitrogen and stored at −80 °C until the day of the experiment. Prior to gavage, 3 g of wild-type and transgenic leaf material were mixed with 500 μL 20× PBS and homogenized using a General Laboratory Homogenizer (GLH-2596, Omni International, Kennesaw, GA) for 5 min. Two hundred microlitres (~200 mg) of leaf material containing approximately 0.5 mg CTB-PFx3 was administered orally to mice via a 20-gauge gavage needle. Blood serum was taken by bleeding of the tail vein before sample administration and again at 15, 30, 60, 120 and 180 min after sample administration. A second dose was given after 180 min, and blood glucose measurements were taken at 15, 30, 60 and 120 min after the second dose.
Supplementary Material
Acknowledgments
Investigations reported here were supported by NIH R01 GM 63879 and USDA 3611-21000-021-02S grants to Henry Daniell. Authors are thankful to Andrew Devine for creation of pLDutr-CTB-Fx3Pris vector, Dr. Dheeraj Verma for assistance in creating pLsLF-CTB-Fx3Pris, Dr. Kiran Velpula for assistance with protein purification, and Dr. Abdolreza-Davoodi Semiromi and Robert Banks for their help with the animal studies.
Footnotes
Additional Supporting information may be found in the online version of this article:
Figure S1 DNA sequence of 5′-3′ strand of cholera toxin B subunit (CTB)-Proinsulin containing three furin cleavage sites gene.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Agarwal V, Khan M. Current status of the oral delivery of insulin. Pharm Technol. 2001 Oct;:76–90. [Google Scholar]
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Yu J, Chong DK, Hough J, Engen PC, Langridge WH. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat Biotechnol. 1998;16:934–938. doi: 10.1038/nbt1098-934. [DOI] [PubMed] [Google Scholar]
- Arlen PA, Falconer R, Cherukumilli S, Cole A, Cole AM, Oishi KK, Daniell H. Field production and functional evaluation of chloroplast-derived interferon-alpha2b. Plant Biotechnol J. 2007;5:511–525. doi: 10.1111/j.1467-7652.2007.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlen PA, Singleton M, Adamovicz JJ, Ding Y, Davoodi-Semiromi A, Daniell H. Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infect Immun. 2008;76:3640–3650. doi: 10.1128/IAI.00050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally J, Paget E, Droux M, Job C, Job D, Dubald M. Both the stroma and thylakoid lumen of tobacco chloroplasts are competent for the formation of disulphide bonds in recombinant proteins. Plant Biotechnol J. 2008;6:46–61. doi: 10.1111/j.1467-7652.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- Boothe J, Nykiforuk C, Shen Y, Zaplachinski S, Szarka S, Kuhlman P, Murray E, Morck D, Moloney MM. Seed-based expression systems for plant molecular farming. Plant Biotechnol J. 2010;8:588–606. doi: 10.1111/j.1467-7652.2010.00511.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- Daniell H. Transgene containment by maternal inheritance: effective or elusive? Proc Natl Acad Sci USA. 2007;104:6879–6880. doi: 10.1073/pnas.0702219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Lee SB, Panchal T, Wiebe PO. Expression of the Native Cholera Toxin B Subunit Gene and Assembly as Functional Oligomers in Transgenic Tobacco Chloroplasts. J Mol Biol. 2001;311:1001–1009. doi: 10.1006/jmbi.2001.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Singh N, Mason H, Streatfield SJ. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009a;14:669–679. doi: 10.1016/j.tplants.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell H, Ruiz G, Denes B, Sandberg L, Langridge W. Optimization of codon composition and regulatory elements for expression of human insulin like growth factor-1 in transgenic chloroplasts and evaluation of structural identity and function. BMC Biotechnol. 2009b;9:33. doi: 10.1186/1472-6750-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodi-Semiromi A, Samson N, Daniell H, et al. The green vaccine: a global strategy to combat infectious and autoimmune diseases. Hum Vaccin. 2009;5:488–493. doi: 10.4161/hv.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodi-Semiromi A, Schreiber M, Nalapalli S, Verma D, Singh ND, Banks RK, Chakrabarti D, Daniell H. Chloroplast-derived vaccine antigens confer dual immunity against cholera and malaria by oral or injectable delivery. Plant Biotechnol J. 2010;8:223–242. doi: 10.1111/j.1467-7652.2009.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dertzbaugh MT, Cox L. The affinity of cholera toxin for Ni2 + ion. Protein Eng. 1998;11:577–581. doi: 10.1093/protein/11.7.577. [DOI] [PubMed] [Google Scholar]
- Ekberg K, Brismar T, Johansson BL, Jonsson B, Lindström P, Wahren J. Amelioration of sensory nerve dysfunction by C-Peptide in patients with type 1 diabetes. Diabetes. 2003;52:536–541. doi: 10.2337/diabetes.52.2.536. [DOI] [PubMed] [Google Scholar]
- Fernandez-San Millan A, Minego-Castel A, Mingo-Castel A, Miller M, Daniell H. A chloroplast genetic approach to hyper-express and purify human serum albumin, a protein highly susceptible to proteolytic degradation. Plant Biotechnol J. 2003;1:71–79. doi: 10.1046/j.1467-7652.2003.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K, Sasaki T, Nemoto M, Nakai N, Sakai K, Yamasaki K, Hiki Y, Ohashi T, Eto Y, Tajima N. Enhanced insulin secretion from engineered 3T3-L1 preadipocytes by induction of cellular differentiation. Mol Cell Biochem. 2005;268:1–8. doi: 10.1007/s11010-005-0608-8. [DOI] [PubMed] [Google Scholar]
- Giannini C, Mohn A, Chiarelli F, et al. Technology and the issue of cost/benefit in diabetes. Diabetes Metab Res Rev. 2009;25(Suppl 1):S34–44. doi: 10.1002/dmrr.986. [DOI] [PubMed] [Google Scholar]
- Groskreutz DJ, Sliwkowski M, Gorman CM. Genetically engineered proinsulin constitutively processed and secreted as mature, active insulin. J Biol Chem. 1994;269:6241–6245. [PubMed] [Google Scholar]
- Gupta S, Chattopadhyay T, Singh MP, Surolia A. Supramolecular insulin assembly II for a sustained treatment of type 1 diabetes mellitus. Proc Natl Acad Sci USA. 2010;107:13246–13251. doi: 10.1073/pnas.1005704107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan L, Verweij W, Agsteribbe E, Wilschut J. The role of ADP-ribosylation and GM1-binding activity in the mucosal immunogenicity and adjuvanticity of the Escherichia coli heat-labile enterotoxin and Vibrio cholerae cholera toxin. Immunol Cell Biol. 1998;76:270–279. doi: 10.1046/j.1440-1711.1998.00745.x. [DOI] [PubMed] [Google Scholar]
- Hills CE, Brunskill N. Cellular and physiological effects of C-peptide. Clin Sci (Lond) 2009;116:565–574. doi: 10.1042/CS20080441. [DOI] [PubMed] [Google Scholar]
- Iyer H, Khedkar A, Verma M. Oral insulin – a review of current status. Diabetes Obes Metab. 2010;12:179–185. doi: 10.1111/j.1463-1326.2009.01150.x. [DOI] [PubMed] [Google Scholar]
- Jensen R. Activation of Rubisco regulates photosynthesis at high temperature and CO2. Proc Natl Acad Sci. 2000;97:12937–12938. doi: 10.1073/pnas.97.24.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BL, Borg K, Fernqvist-Forbes E, Kernell A, Odergren T, Wahren J. Beneficial effects of C-peptide on incipient nephropathy and neuropathy in patients with Type 1 diabetes mellitus. Diabet Med. 2000;1:181–189. doi: 10.1046/j.1464-5491.2000.00274.x. [DOI] [PubMed] [Google Scholar]
- Kjeldsen T. Yeast secretory expression of insulin precursors. Appl Microbiol Biotechnol. 2000;54:277–286. doi: 10.1007/s002530000402. [DOI] [PubMed] [Google Scholar]
- Koya V, Moayeri M, Leppla SH, Daniell H. Plant-based vaccine: mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect Immun. 2005;73:8266–8274. doi: 10.1128/IAI.73.12.8266-8274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan HB, Natarajan S. A rapid method for depletion of Rubisco from soybean (Glycine max) leaf for proteomic analysis of lower abundance proteins. Phytochemistry. 2009;70:1958–1964. doi: 10.1016/j.phytochem.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Kumar S, Daniell H. Engineering the chloroplast genome for hyperexpression of human therapeutic proteins and vaccine antigens. Methods Mol Biol. 2004;267:365–383. doi: 10.1385/1-59259-774-2:365. [DOI] [PubMed] [Google Scholar]
- Lee SB, Li B, Jin S, Daniell H. Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol J. 2011 doi: 10.1111/j.1467-7652.2010.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson PD. Eighty years of insulin therapy: 1922–2002. Med Health R I. 2003;86:101–106. [PubMed] [Google Scholar]
- Li D, O’Leary J, Huang Y, Huner NP, Jevnikar AM, Ma S. Expression of cholera toxin B subunit and the B chain of human insulin as a fusion protein in transgenic tobacco plants. Plant Cell Rep. 2006;25:417–424. doi: 10.1007/s00299-005-0069-2. [DOI] [PubMed] [Google Scholar]
- Liljeqvist S, Stahl S, Andréoni C, Binz H, Uhlén M, Murby M. Fusions to the cholera toxin B subunit: influence on pentamerization and GM1 binding. J Immunol Methods. 1997;210:125–135. doi: 10.1016/s0022-1759(97)00170-1. [DOI] [PubMed] [Google Scholar]
- Limaye A, Koya V, Samsam M, Daniell H. Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. FASEB J. 2006;20:E37–E46. doi: 10.1096/fj.05-5134fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Thimann K. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972;49:64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oey M, Lohse M, Scharff LB, Kreikemeyer B, Bock R. Plastid production of protein antibiotics against pneumonia via a new strategy for high-level expression of antimicrobial proteins. Proc Natl Acad Sci USA. 2009;106:6579–6584. doi: 10.1073/pnas.0813146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekar AH, Frank B. Conformation of proinsulin. A comparison of insulin and proinsulin self-association at neutral pH. Biochemistry. 1972;11:4013–4016. doi: 10.1021/bi00772a001. [DOI] [PubMed] [Google Scholar]
- Pozzilli P, Raskin P, Parkin CG. Review of clinical trials: update on oral insulin spray formulation. Diabetes Obes Metab. 2010;12:91–96. doi: 10.1111/j.1463-1326.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- Quesada-Vargas T, Ruiz O, Daniell H. Characterization of heterologous multigene operons in transgenic chloroplasts: transcription, processing, and translation. Plant Physiol. 2005;138:1746–1762. doi: 10.1104/pp.105.063040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab R, Fezeu L, Mbanya JC. Cost and availability of insulin and other diabetes supplies: IDF survey 2003–2003. Diabetes Voice. 2004;49:24–29. [Google Scholar]
- Rebsomen L, Khammar A, Raccah D, Tsimaratos M. C-peptide effects on renal physiology and diabetes. Exp Diabetes Res. 2008:1–5. doi: 10.1155/2008/281536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H. Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts–oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol J. 2007;5:495–510. doi: 10.1111/j.1467-7652.2007.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlman T, Verma D, Samson N, Daniell H. The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol. 2010;152:2088–2104. doi: 10.1104/pp.109.152017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JAM, Delday M, Hart AW, Docherty HM, Maltin CA, Docherty K. Secretion of bioactive human insulin following plasmid-mediated gene transfer to non-neuroendocrine cell lines, primary cultures and rat skeletal muscle in vivo. J Endocrinol. 2002;172:653–672. doi: 10.1677/joe.0.1720653. [DOI] [PubMed] [Google Scholar]
- Siekmeier R, Scheuch G. Inhaled insulin–does it become reality? J Physiol Pharmacol. 2008;59(Suppl 6):81–113. [PubMed] [Google Scholar]
- Sima AAF, Li ZG. The effect of C-peptide on cognitive dysfunction and hippocampal apoptosis in type 1 diabetic rats. Diabetes. 2005;54:1497–1505. doi: 10.2337/diabetes.54.5.1497. [DOI] [PubMed] [Google Scholar]
- Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- Suda T, Katoh M, Hiratsuka M, Takiguchi M, Kazuki Y, Inoue T, Oshimura M. Heat regulated production and secretion of insulin from a human artificial chromosome vector. Biochem Biophys Res Commun. 2006;340:1053–1061. doi: 10.1016/j.bbrc.2005.12.106. [DOI] [PubMed] [Google Scholar]
- Swartz JR. Advances in Escherichia coli production of therapeutic proteins. Curr Opin Biotechnol. 2001;12:195–201. doi: 10.1016/s0958-1669(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Tatake RJ, O’neill MM, Kennedy CA, Reale VD, Runyan JD, Monaco KA, Yu K, Osborne WR, Barton RW, Schneiderman RD. Glucose-regulated insulin production from genetically engineered human non-beta cells. Life Sci. 2007;81:1346–1354. doi: 10.1016/j.lfs.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Taylor NA, Van De Ven W, Creemers JW. Curbing activation: proprotein convertases in homeostasis and pathology. FASEB J. 2003;17:1215–1227. doi: 10.1096/fj.02-0831rev. [DOI] [PubMed] [Google Scholar]
- Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Watanabe K, Miyama A. Monomer of the B Subunit of Heat-Labile Enterotoxin from Enterotoxigenic Escherichia coli Has Little Ability to Bind to GM1 Ganglioside Compared to Its Coligenoid. Microbiol Immunol. 1995;39:817–819. doi: 10.1111/j.1348-0421.1995.tb03262.x. [DOI] [PubMed] [Google Scholar]
- Verma D, Daniell H. Chloroplast Vector Systems for Biotechnology Applications. Plant Physiol. 2007;145:1129–1143. doi: 10.1104/pp.107.106690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D, Samson N, Koya V, Daniell H. A protocol for expression of foreign genes in chloroplasts. Nat Protoc. 2008;3:739–758. doi: 10.1038/nprot.2007.522. [DOI] [PubMed] [Google Scholar]
- Verma D, Moghimi B, LoDuca PA, Singh HD, Hoffman BE, Herzog RW, Daniell H. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc Natl Acad Sci USA. 2010;107:7101–7106. doi: 10.1073/pnas.0912181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra RD. Protein Degradation in Plants. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:385–410. [Google Scholar]
- Wahren J. C-peptide: new findings and therapeutic implications in diabetes. Clin Physiol Funct Imaging. 2004;24:180–189. doi: 10.1111/j.1475-097X.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- Wahren J, Ekberg K, Jörnvall H. C-peptide is a bioactive peptide. Diabetologia. 2007;50:503–509. doi: 10.1007/s00125-006-0559-y. [DOI] [PubMed] [Google Scholar]
- Williams DC, Van Frank RM, Muth WL, Burnett JP. Cytoplasmic inclusion bodies in Escherichia coli producing biosynthetic human insulin proteins. Science. 1982;215:687–689. doi: 10.1126/science.7036343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.