Introduction
The treatment of diseased tissues and organs is an ongoing healthcare problem that could be solved with artificial tissues.1,2 Functional tissue constructs could also decrease the time and the cost spent on the drug discovery process, which could have a direct effect on discovering cures for various diseases.1,2 Tissue engineering holds great promise for developing methods to create tissue models with the structural organization of native tissues, which could be useful for regenerative medicine and pharmaceutical research.
Native tissues contain different cell types, with each cell type having its own unique three-dimensional (3-D) extracellular matrix (ECM) environment, and mechanical properties. To recreate such complexity in engineered tissues various approaches have been used. In one such approach, cells are seeded on a degradable scaffold on which they reorganize into engineered tissues. Alternatively, cells are used to create modular tissue units with microscale polymers or without any scaffolds.3 These modular tissues can later be assembled,4 stacked,5,6 or rolled7 to form large-scale tissue constructs by mimicking the native architecture.
To generate tissue modules, photolithographic4 and soft lithographic methods8 have been used to encapsulate cells within microgels with different shapes and geometries. These cell-encapsulated microgels can be further assembled into defined geometries.3 Rigid9,10 or soft microfabricated templates11,12 can also be used to form scaffold-free tissue modules made from clusters of cells. Furthermore, tissue monolayers can be generated on 2-D substrates, which can be further stacked or rolled to fabricate macroscale tissues.5–7
Each of these techniques have their own limitations. For example, photolithography is only applicable to photocrosslinkable materials,13 whereas most soft lithographic methods rely on static microstructures, that limit the range of microgel shapes that can be fabricated.13 Also, the pattern geometries and surface properties of static microstructures cannot be changed. These static features may be limited in creating biomimetic microtissues and the retrieval of tissues from these platforms in a controlled manner. Furthermore, 2-D templates with nonswitchable surfaces require the use of enzymes or physical forces to detach monolayer of tissues, which is not desirable.14,15
Dynamic microstructures with controllable features and switchable surface properties are emerging as useful tools for creating biomimetic and retrievable modular tissues. Poly(N-isopropylacrylamide) (PNIPAAm) is a well-known stimuli-responsive polymer, which responds to temperature by changing its hydrophilicity and swelling.16–18 Properties of PNIPAAm make it favorable to fabricate dynamic platforms to overcome the static features of previous technologies. This review will highlight the current developments in PNIPAAm-based thermoresponsive platforms for tissue engineering and regenerative medicine.
Characteristics of PNIPAAm and its derivatives
Stimuli-responsive polymers show great potential in several fields, including tissue engineering and drug delivery, due to their controllable hydrogel properties, such as swelling/deswelling and surface energy. PNIPAAm is one of such polymers with a lower critical solution temperature (LCST) of ~32°C.16,17 It shrinks and becomes hydrophobic at temperatures above its LCST, and turns into swollen and hydrophilic state below its LCST (Figure 1).16 The entropic gain of the system produces this responsive behavior by segregating water molecules from isopropyl chains at temperatures above the phase temperature (LCST) (Figure 1).16 The entropic gain of the system in aqueous environment is higher than the enthalpic gain of the bonds between water molecules and PNIPAAm chains at this phase transition.16
Figure 1. Schematic of the thermoresponsive behavior of PNIPAAm hydrogel.
It swells and becomes hydrophilic at temperatures below LCST. Raising the temperature above LCST results in PNIPAAm shrinking and increased hydrophobicity.16 Reproduced by permission of The Royal Society of Chemistry.
The LCST of PNIPAAm can be tailored by incorporating hydrophilic or hydrophobic comonomers into the polymer structure.16 For example, phase temperature can be adjusted to around physiological temperature (37°C) to tailor its use for biological applications.16 To alter the LCST of PNIPAAm, hydrophilic comonomers, such as Acrylamide (AAm), N-methyl-N-vinylacetamide (MVA), N-vinylacetamide (NVA), and N-vinyl-2-pyrrolidinone (VPL), have been crosslinked with N-isopropylacrylamide (NIPAAm) via free-radical polymerization.19 At lower concentrations of these comonomers, LCST point has been adjusted to between 32 and 37°C.19 Incorporation of slight amounts of ionic comonomers in PNIPAAm hydrogels can raise the LCST.20,21 Copolymers of PNIPAAm with two distinct LCSTs can be fabricated by using oligomers, such as carboxy-terminated oligo NIPAAm, oligo(N-vinylcaprolactam) (VCL) and a random co-oligomer of NIPAAm and AAm.22 These modifications on PNIPAAm hydrogels can be useful for controlled release applications in drug delivery and tissue engineering.
PNIPAAm-based hydrogels can be used for temperature-controlled drug delivery, in two ways. In the first approach, drugs can be released from the hydrogel structure as a result of PNIPAAm shrinking at temperatures above LCST.23 In the second approach, drugs can be dissociated from swollen polymer network by decreasing the temperature below LCST.23 Incorporation of hydrophilic comonomers within PNIPAAm structure could speed up the deswelling kinetics of the hydrogels.24 Acrylic acid (AAc) or methacrylic acid (MAAc) can be crosslinked with NIPAAm to generate hydrogels with rapid thermoresponsiveness.24 Faster release kinetics can also be achieved by synthesizing a comb-type PNIPAAm hydrogel network, which has been demonstrated to have faster deswelling kinetics than linear type PNIPAAm hydrogel networks.25,26
Additional functionalities, such as pH responsiveness and partial degradability, can be given to PNIPAAm hydrogels by incorporating comonomers and proteins into the polymer network. For example, temperature and pH responsive hydrogels were generated by copolymerizing NIPAAm with AAc.27 Crosslinked and random copolymers of NIPAAm and MAAc also showcase the temperature and pH responsiveness.28,29 Graft copolymers of these configurations demonstrate higher temperature- and pH-dependent swelling kinetics than random copolymers.18,27 Furthermore, biodegradable materials, such as zein protein30 and alginate,31 were incorporated within PNIPAAm networks while maintaining thermoresponsiveness. In addition, gelatin was used to generate interpenetrating networks with PNIPAAm without chemical crosslinking.32
Due to its switchable hydrophilicity and tunable hydrogel properties, PNIPAAm is highly favorable for use in cell culture platforms.18,33 Hydrophobic surfaces, such as polystyrene culture dishes and elastomeric templates, are attractive for protein adhesion and subsequent cell attachment.18 However, detachment of cells and tissues from these templates requires either enzymatic reaction or physical scraping, both of which can damage cells and cell-ECM interactions.14,15 Cell culture platforms have been increasingly functionalized with PNIPAAm to first induce cell attachment at temperatures above LCST, and then trigger controlled cell detachment from the surface by decreasing the temperature below LCST. Micro- and nanofabrication techniques have also been used to create PNIPAAm-based platforms for tissue engineering applications.
Two-dimensional thermoresponsive platforms
Cell-sheet engineering has been a growing approach to generate functional tissues for biomedical applications. In this approach cell are initially induced to form a monolayer upon adhesion to hydrophobic surfaces, such as polystyrene culture dishes and elastomeric substrates. The resulting monolayers, also called cell sheets, can be removed from the substrates to generate functional tissues.
Thermoresponsive surfaces are useful for controlling the cell attachment and detachment to produce intact cell sheets. A pioneering study on thermoresponsive surfaces was published in 1990 which demonstrated the use of PNIPAAm grafted culture substrates for temperature-dependent detachment of cell sheets.34 Cells adhered to these substrates at 37°C since PNIPAAm was hydrophobic at these temperatures (Figure 2A).35 Enzymatic recovery damages cell-cell junction proteins and cell-ECM interactions, preventing retrieval of monolayer tissues (Figure 2B).35 To maintain intact structure, detachment of tissues was induced at ambient temperatures as the substrate becomes hydrophilic, inhibiting cell adhesion (Figure 2C).35 Cell sheets generated using this process have been used for various tissue engineering applications, including cardiac,6,35 hepatic,36 skin,37 kidney,38 and corneal.39 Here we review some of these studies.
Figure 2. Controlled cell adhesion and detachment of cell sheets from 2-D thermoresponsive platforms.
(A) Cells adhere on hydrophobic thermoresponsive surface at physiological temperature and attach to each other through cell-cell junctions. (B) Digestive enzymes destroy cell-cell and cell-ECM interactions, inhibiting the recovery of cell monolayer. (C) Cell monolayers are detached without disturbing cell-cell and cell-ECM interactions by using hydrophilicity of thermoresponsive surface at temperatures below LCST.35 Adapted from Ref. 35, Copyright (2003), with permission from Elsevier.
PNIPAAm coating on cell-culture substrates
Various methods have been used to create PNIPAAm coated substrates.14,15,17,40 Some of these surfaces were successfully used to generate cell sheets. However, some PNIPAAm coated surfaces were not suitable for the formation of tissue monolayers as they inhibit cell attachment at temperatures above LCST.41,42
The most common method to coat cell culture substrates with PNIPAAm are by the use of electron-beam (e-beam) irradiation.6,35–39,41 Controlled cell adhesion and further tissue retrieval were reported for limited grafting densities of 1.4–2 µg/cm2 and for limited PNIPAAm thicknesses ranging from 15 to 20 nm.36,37,41 Increasing PNIPAAm thickness above 30 nm did not allow cell attachment on these surfaces for grafting densities of 2.9–3 µg/cm2.41,42
Plasma polymerization was also employed to induce covalent bonding of NIPAAm on cell-culture substrates.14,43 Plasma coating of PNIPAAm showed similar properties of crosslinked polymers as it maintained its NIPAAm structure and phase transition behavior.44 Cell attachment and detachment process showed similar trend for different plasmacoated PNIPAAm surfaces with different PNIPAAm thicknesses.14,43 Cell adhesion and detachment for plasma-coated PNIPAAm substrates was shown to be independent from PNIPAAm thickness.17
Ultraviolet (UV) irradiation has also been used to coat cell culture substrates with a copolymer of PNIPAAm.45 Cell attachment and detachment process for these substrates were independent from copolymer thickness for grafting densities in the range of 2.4–6.9 µg/cm2.45 Photopolymerization was also used to to form micropatterns of thermoresponsive polymer on substrates.46,47 Cells were selectively attached on the thermoresponsive micropatterns, and disassociated from these regions at 10°C.46,47 These micro-patterned thermoresponsive surfaces could be useful for coculture of different cell types with controlled spatial distribution.17
Cell adhesion on thermoresponsive substrates and controlled detachment of tissues
Even though many thermoresponsive cell culture surfaces have been generated using different methods, only some of these are suitable for cell adhesion,6,35–39,41 in contrast many others have failed to be cell adhesive even above their LCST.41,42 For example, cells did not adhere to PNIPAAm coated surfaces with a thickness over 30 nm,41,42 methylenebis(acrylamide) (MBAAm) crosslinked PNIPAAm hydrogels,41,42 and noncrosslinked PNIPAAm hydrogels.34 Adsorption of serum proteins, such as fibronectin, on the substrates can promote further cell attachment,17 however, fibronectin was not able to adsorb significantly on e-beam polymerized PNIPAAm with the highest density or on crosslinked PNIPAAm polymers.41,42
Static water contact angle measurements of polymer based surfaces were used to explain cell adhesion properties. As expected, the contact angle suitable for cell attachment was found to be ~70°.48 Cell repellent behavior of MBAAm crosslinked PNIPAAm hydrogels was correlated with low static water contact angles of these hydrogels.41 Although similar low contact angles were reported for surfaces with plasma polymerized PNIPAAm,44,49 these surfaces were suitable for cell attachment and detachment.44 These findings suggest that in addition to surface wettability there are other factors, such as grafting density and polymer thickness that influence cell adhesion for different PNIPAAm polymerization methods. For example, increasing the thickness of ebeam polymerized PNIPAAm did not change the contact angles of the substrates, although cell attachment on these surfaces decreased significantly compared to low thicknesses.17 This shows that cell adhesion depends on the PNIPAAm thickness for surfaces coated with e-beam irradiation. Furthermore, for e-beam deposited PNIPAAm surfaces, although high grafting densities (2.9 µg/cm2) demonstrated similar contact values with low grafting densities (1.4 µg/cm2 and 1.6 µg/cm2), cell attachment significantly decreased for high grafting densities,41 suggesting cell adhesion also depends on the grafting density of the PNIPAAm.
Swelling ratio and molecular mobility of the polymer films can also affect the cell adhesion behavior of the surfaces.17,50 Crosslinking PNIPAAm on a surface affects its chain mobility and swelling properties.17,50 Decreasing the grafting thickness decreases the swelling ratio of e-beam grafted PNIPAAm films.17,41 PNIPAAm chains are less independent at the interface of the substrate due to the high hydrophobic interactions, resulting in a dehydrated and aggregated layer at the inner most layer of the film.50 Above this aggregated layer, there is somewhat hydrated and hydrophobic layer, supporting cell adhesion and detachment. It was reported that the thickness of this cell adhesive layer can be between 15 nm and 20 nm.50 At the outermost layer of e-beam grafted PNIPAAm films, chains have more mobility and are dehydrated, which were reported as nonsupportive for cell adhesion.50 Cells cannot adhere to PNIPAAm chains for e-beam grafted surfaces with a film thickness of above 30 nm.41,42 Thus, cell adhesion on PNIPAAm coated surfaces depends on various factors, including chain mobility, grafting density, swelling ratio, and wettability.17
Adhered cells spread and proliferate on thermoresponsive surfaces at physiological temperatures.50 Adhesion and further cell morphology on the substrate require metabolic activities, such as ATP synthesis.50,51 High cell confluence leads to the formation of monolayers of tissues on the substrates. Mechanical or enzymatic removal of these tissues can destroy cell-cell interactions and, subsequently, the intact structure of the tissue. Cell sheets can be dissociated from the surface by decreasing the temperature below LCST, facilitating the hydration of the PNIPAAm layer.50 Different cell types require different retrieval temperatures, such as 10°C for hepatocytes and 20°C for endothelial cells.50,52 Detachment process can be suppressed by treating cells with an ATP synthesis inhibitor or with a tyrosine kinase inhibitor, suggesting that cell retrieval process depends on cell metabolic activities.50,51
Temperature controlled cell detachment method maintains the intact structure of the tissue with its natural ECM. The content of the ECM depends on the cultured cell types as different cell types produce different ECM proteins.17,50 Also, previously deposited proteins on the substrate, such as fibronectin, can be detached with cell sheets from the thermoresponsive surface at low temperatures.53 Recovered tissues leave a small amount of ECM proteins on the surface after the retrieval process.14,43 This may be due to weak interactions between cells and remaining ECM proteins on the surface.14 As the amount of remaining protein is small, the resulting monolayer tissues retain their intact structure.
Patterned coculture platforms
Control of the spatial distribution of different cell types in close proximity can be used to replicate native tissues in vitro. Patterned cocultures of different cell types can generate functional tissue constructs. Merging patterning techniques with thermoresponsive substrates can provide not only tissue retrieval but also controlled coculture platforms. To develop a platform to generate cell sheets with multiple cell types, masked e-beam irradiation was used to create circular PNIPAAm domains on polystyrene dishes.54,55 The first cell type was attached on cell culture dishes at 37°C. As the temperature was decreased to 20°C, cells on PNIPAAm domains were detached due to the hydration of PNIPAAm, but cells on polystyrene parts remained attached. PNIPAAm domains were then used to culture another cell type. This patterning technique controlled spatial arrangements of two different cell types in a coculture platform.54,55
In another approach, cell-culture substrates with dual thermoresponsiveness were fabricated with e-beam irradiation to culture different cell types in a spatial arrangement.50,56 The LCST can be reduced by copolymerization of n-butyl-methacrylate (BMA), a hydrophobic monomer, on PNIPAAm grafted surfaces with e-beam polymerization.57 Micropatterns of BMA were grafted on PNIPAAm coated culture dishes, producing a dual thermoresponsive substrate.56 Hepatocytes and endothelial cells were cultured on micropatterned dual thermoresponsive substrates to fabricate cocultured cell sheets.56
Cell-sheet technology
Thermoresponsive platforms have been successfully employed to generate intact monolayers of tissues. Cell sheets can be easily recovered with their natural ECM from the substrates by exploiting the hydration property of PNIPAAm at temperatures below LCST.50 As this method does not require the use of enzymes, tissues preserve their structural integrity. This gives additional mechanical strength to cell sheets, enabling further manipulation of tissues.6,36–38 As a cell sheet is transferred to another substrate or a physiological environment, the ECM proteins induce its attachment to the new environment.53
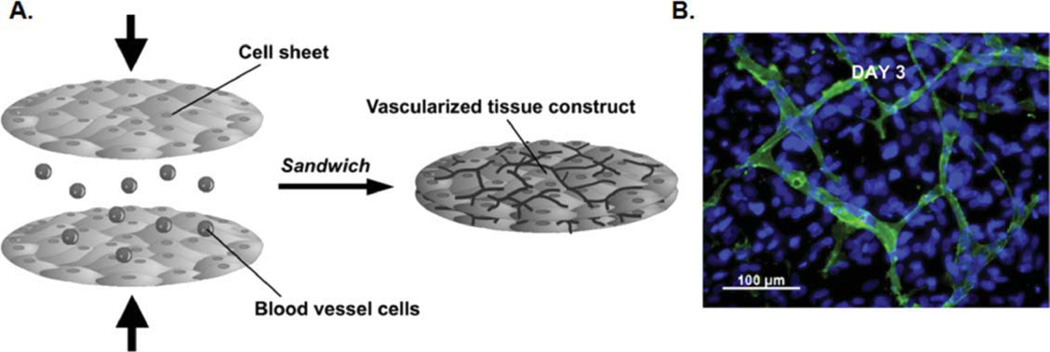
Cell sheets can be implanted in vivo and attach to the new tissue through ECM proteins, eliminating the use of sutures.58 Cell sheets also do not require any scaffolding material, which may minimize inflammation and immune rejection in vivo.17 Larger tissue constructs can be fabricated by stacking cell sheets.59 For example, seeding endothelial cells between two layers of cell sheets leads to the formation of vessels, resulting in prevascularized tissues (Figure 3).59 Cell-sheet technology has been used to fabricate different types of tissues, such as myocardial and hepatic.
Figure 3. Formation of vascularized tissues by cell-sheet stacking.
(A) Schematic of vascularized tissue fabrication by culturing endothelial cells between two layers of cell sheets. (B) Endothelial cells formed a vascular network between two monolayer tissues within 3 days. Green color shows antihuman CD31 staining for endothelial cells within the vascular network. Blue color represents cell nuclei stained with Hoechst 33342.59 Adapted from Ref. 59, Copyright (2009), with permission from Elsevier.
Cardiac cell sheets
Attaining tight cell-cell connections is important for engineering cardiac tissues with functional features in vitro.50 Thermoresponsive platforms have been used to fabricate cardiac cell sheets with an electrical functionality. In this approach, cardiomyocytes were cultured on thermoresponsive surfaces, producing an intact cardiac cell sheet with tight cell-cell junctions.6,35 These junctions are critical for electrical communication between cardiomyocytes, facilitating the contraction and beating of the monolayer tissues.50 Three-dimensional cardiac tissues were also fabricated by stacking cardiac cell sheets.6,35,60 The gap junctions between stacked cardiac cell sheets produced an electrical communication between layered monolayer tissues.50
To assess the functionality of the cell-sheet cardiac tissue, stacked monolayers of cocultured cardiac cells with endothelial cells were implanted into nude rats.6,35 It was observed that the implanted tissues formed a vascular connection with the host tissue,61 facilitating the connection with the native microenvironment.6,35,61 Cell-sheet technology also enables the recovery of intact cardiac cell sheets with their own ECM and high cell-cell interactions.50 Vascular network and cardiac functionalities result from these tight cell-cell interactions and secreted proteins within the stacked cardiac tissues.6,35,50 Controlling the cell-cell interactions within the cardiac cell sheets can lead to functional vascularized cardiac tissues,50 which could be useful for in vitro studies and to treat impaired hearts.
Cardiac cell sheets were also used to fabricate cell-based components of microdevices. For example, cardiac cell-sheet–based micropumps were integrated on a chip for flow control applications.62 A microspherical heart-like pump was also fabricated from monolayer cardiac tissues with contractile properties.63 These cell-sheet–based applications could lead to self-actuated microdevices. Furthermore, myocardial tubes were fabricated by rolling cardiac cell sheets around a thoracic aorta of an adult rat, which were then implanted into nude rats for a circulatory assist.7 Implanted tubes exhibited integration with the native tissue within 4 weeks after the procedure.7 Given these results, engineering cardiac cell sheets shows promise for regenerative therapies and in vitro studies.
Hepatic cell sheets
Hepatic tissue engineering attempts to create liver-like tissue constructs for use either in regenerative therapies or in vitro studies, such as drug toxicity screening. Some of the previous approaches encapsulated hepatocytes within ECM proteins64 or biodegradable scaffolds65 to fabricate implantable liver tissues. As an alternative approach, scaffold-free liver tissues were also generated by culturing hepatocytes on thermoresponsive culture dishes to form monolayers of hepatic tissues, which were then recovered with a temperature change.5 Hepatic cell sheets that were implanted into the subcutaneous spaces of mice maintained their functionality for more than 200 days.5 In other experiments, hepatic cell sheets were also layered to form thick hepatic tissues.5
Heterotypic cell-cell interactions play an important role to produce hepatic tissues with improved functionalities.56 To meet this demand, dual thermoresponsive surfaces were fabricated for coculture of hepatocytes and endothelial cells.56 Copolymerization of BMA with PNIPAAm was used to reduce the LCST.57 Micropatterns of BMA were grafted on PNIPAAm coated substrate, resulting in a dual thermoresponsive surface.56 Hepatocytes attached on hydrophobic BMA grafted domains on the surface at 27°C (Figure 4A).66 Endothelial cells were placed on hydrophobic PNIPAAm coated regions at 37°C, producing cocultured hepatic cell sheets (Figure 4B).66 Intact hepatic cell sheets were successfully recovered from the substrates at 20°C (Figure 4C,D).66 Coculture of hepatocytes with endothelial cells demonstrated improved functionalities, such as albumin synthesis and ammonium metabolism.56,66 Furthermore, endothelial cell sheets were placed on top of hepatic cell sheets to generate layered cocultured hepatic tissues.36 These studies show that thermoresponsive cell-culture surfaces were successfully employed to produce monocultured or cocultured hepatic tissue sheets for transplantation and in vitro use.
Figure 4. Schematic of fabrication of cocultured hepatic cells sheets by exploiting the dual thermoresponsiveness of patterned surfaces.
(A) Hepatocytes were seeded and adhered on hydrophobic domains of the surface at 27°C. P(IPAAm-BMA) represents co-grafting of NIPAAm and BMA. PIPAAm indicates poly(N-isopropylacrylamide) coated regions. (B) Cocultured cell sheets were produced by seeding endothelial cells as the second cell type at 37°C. (C, D) Cocultured hepatic tissues were recovered from dual thermoresponsive surface at 20°C when whole surface became hydrophilic.66 Adapted from Ref. 66, Copyright (2006), with permission from Elsevier.
Three-Dimensional thermoresponsive platforms
Thermoresponsive templates have also been used to directly generate 3-D tissue structures which can mimic native tissues. Previously, polymers were microengineered to create rigid or soft 3-D platforms to fabricate microdevices, in which microtissues can be generated with different shapes, such as stripe9 and spherical.10 These platforms can also be integrated within microfluidic devices for high-throughput analysis. For example, hydrogels, such as poly(ethylene glycol) (PEG)12 and chitosan,11 were microengineered to fabricate 3-D templates to generate microtissues for potential use in regenerative therapies and in vitro studies. All of these microstructures have a static nature, meaning that their surface properties and pattern areas cannot be changed after fabrication. We have used the switchable hydrophilicity and swelling/deswelling properties of PNIPAAm to fabricate dynamic microstructures. These microstructures were used to control the orientations and the alignment of cells to form geometrically controlled microtissues. Switchable surface properties of these microstructures allowed for controlled attachment and further detachment of cells from patterned areas. Temperature-dependent patterned areas were also used to recover microtissues or encapsulate cells into multicompartment microgels.
PNIPAAm coated microstructures
Structural organization of cells is a critical issue in the formation of functional tissues. Micropatterned substrates can be used to control the alignment and elongation of cells to induce cytoskeletal organization of the cells which can lead to physiologically active modular tissues. These microstructures can be fabricated from various materials, such as poly (dimethylsiloxane) (PDMS) and polystyrene. However, these surfaces demonstrate constant hydrophobicity, preventing controlled detachment of tissues from the substrates. To overcome this problem, microtextured surfaces were coated with PNIPAAm, generating a temperature-dependent switchable surface.67–69 Microtextured polystyrene templates were coated with a thin film of PNIPAAm by employing e-beam irradiation.67 Smooth muscle cells were seeded on PNIPAAm coated microtextured substrates and nonpatterned polystyrene surfaces. Cell orientations on patterned substrates were significantly higher compared to nontextured surfaces.67 Hydration of the PNIPAAm film at 20°C allowed for the retrieval of tissues with an intact structure.67 These functional microtextured templates enabled not only the cytoskeletal organization of cells but also the controlled detachment of tissues.
Controlled capillary formation is important to fabricate vascularized microtissues, which can overcome oxygen and diffusion limitations.68 Micropatterned surfaces have been shown to trigger capillary formation.70 PNIPAAm coated microtextured substrates were also employed to induce capillary network formation.68 Microgroove patterns of poly(urethane acrylate) were generated with soft lithographic methods by using photoresist patterned silicon wafers as molding templates.68 PNIPAAm was covalently grafted on these patterns by using e-beam polymerization. The ridges demonstrated rounded shapes and the aggregation of PNIPAAm coating has been observed in the grooves.68 As the thick PNIPAAm graft in the grooves prevents cell adhesion, endothelial cells moved through to top of the ridges and formed capillary networks within 3 weeks. Tubular endothelial networks were easily recovered from the groove substrates after reducing the temperature to 20°C.68
Previous coating methods employed liquid phase polymerization, which may not easily coat all the surfaces of a microfabricated platform. Nonconformal coatings can change the shapes of the device patterns, which can affect the structural organization of tissues. Recently, initiated chemical vapor deposition was used to generate conformal PNIPAAm films on PDMS microgrooves (Figure 5A).69 Cells have been selectively immobilized into grooves and elongated toward the groove direction (Figure 5B).69 These thermoresponsive microgrooves allowed the recovery of striped tissues at ambient temperature. The template could also enable the formation of different modular tissues, such as cardiac and skeletal muscle, and be merged with microfluidic applications.
Figure 5. Three-Dimensional thermoresponsive platforms.
(A) PDMS microgrooves were coated with a thin and conformal PNIPAAm film by employing initiated chemical vapor deposition. Monomer, initiator, and crosslinker were sent in a vapor phase into the reactor, and initiated free-radical polymerization on the surface.69 (B) Cells were cultured within conformally coated thermoresponsive microgrooves, resulting in stripe tissues with elongated cells after 3 days of culture. Red color represents phalloidin staining to show F-actins in the cells.69 (A) and (B) were adapted with permission from Ref. 69, copyright (2011) American Chemical Society. (C) Responsive micromolds were used to fabricate hydrogel biocomposites, encapsulating two different cell types in a spatial arrangement.13 Adapted with permission from Ref. 13, Copyright (2011) American Chemical Society.
Hydrogel–based platforms
Soft lithographic and photolithographic methods can be employed to fabricate hydrogel–based structures with a wide variety of shapes. For example, photocrosslinkable materials were used to generate microwell structures, which were then used to form size-controlled embryonic bodies12 and microtissues.11 Microwells can be fabricated with a glass bottom or with a polymer bottom.71 Glass bottomed microwells facilitate the stable formation of microtissues within the microwells, enabling high-throughput manipulation. However, it is challenging to recover these tissues from the microwells because of the adhesive bottom.71 Soft lithographically fabricated PNIPAAm-based microwells were used to overcome this problem.72 These microwells demonstrated shape changes with varying temperature. The dynamic behavior of PNIPAAm-based microwells creates a physical force on microtissues, inducing their recovery from the microwells for further experimentations.
Photolithographic methods were employed to create cell-encapsulating multicompartment hydrogels,4 but these methods only work for photocrosslinkable polymers. Micromolds were also used to fabricate microgels.8 However, most of the micromolds have static structures, inhibiting the sequential patterning of hydrogels. Recently, thermoresponsive micromolds were generated from PNIPAAm and employed to create multicompartment microgels.13 These dynamic micromolds enabled the spatial immobilization of two different cell types within a multicompartment microgel (Figure 5C).13 This method can ease the fabrication of 3-D modular tissues, mimicking the native tissue complexity. In another approach, PNIPAAm gel molds were fabricated by polymerizing PNIPAAm in other solid molds.73 Cells were then placed on these gels molds to form 3-D tissues with different geometries. Tissue constructs were recovered from PNIPAAm gel molds by inducing the volume change of the gel molds at ambient temperature.73 As such these cell-based tissue constructs could potentially be useful for tissue engineering applications.
Conclusion and future outlook
Thermoresponsive platforms overcome static properties of previous cell-culture surfaces and microstructures by providing the ability to form geometrically controlled and retrievable biomimetic tissue constructs in a temperature-dependent manner without the use of digestive enzymes. Two-dimensional thermoresponsive templates are useful to obtain intact monolayers of tissues with high cell-cell interactions and facilitate their further use for different regenerative therapies by creating thick tissues or prevascularized tissue constructs with stacking methods. Three-dimensional thermoresponsive platforms give the opportunity to control tissue geometries in a 3-D manner by mimicking the native tissue architecture, and enable their further retrieval. It is still challenging to obtain shapes of native tissues and organs, and to preserve their functionalities in the long term. Dynamic microstructures could be further engineered to control spatial orientation of various cell types within microgels to create more complex tissues by replicating the native tissue architectures. Another current problem in tissue engineering is the lack of suitable cell source to fabricate complex tissues. Stem cell research could resolve this problem by producing different cell types from either embryonic stem cells or adult stem cells with reprogramming techniques. Dynamic behavior of 3-D thermoresponsive platforms could also be employed to control the differentiation of embryonic stem cells. Merging stem cell research with dynamic microstructures could provide an opportunity to fabricate patient specific complex tissues, or tissue models for drug discovery.
Acknowledgments
We would like to acknowledge financial support from U.S. Army Research Office through the Institute for Soldier Nanotechnologies at MIT under the project DAAD-19-02-D-002, the NIH (DE013023 and DE016516 (R.L.), HL092836, AR057837, DE021468, and HL099073 (A.K.)), NSF, and the Office of Naval Research.
Contributor Information
Halil Tekin, Dept. of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA 02139; Dept. of Medicine, Center for Biomedical Engineering, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115; David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139.
Jefferson G. Sanchez, Dept. of Medicine, Center for Biomedical Engineering, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115 Dept. of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139.
Tonia Tsinman, Dept. of Medicine, Center for Biomedical Engineering, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115; Dept. of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139.
Robert Langer, David H. Koch Institute for Integrative Cancer Research, Massachusetts Institute of Technology, Cambridge, MA 02139; Dept. of Chemical Engineering and Dept. of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139.
Ali Khademhosseini, Dept. of Medicine, Center for Biomedical Engineering, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115; Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139; Dept. of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139; Wyss Institute for Biologically Inspired Engineering, Harvard Medical School, Boston, MA 02138.
Literature Cited
- 1.Khademhosseini A, Vacanti JP, Langer R. Progress in tissue engineering. Sci. Am. 2009;300(5):64–71. doi: 10.1038/scientificamerican0509-64. [DOI] [PubMed] [Google Scholar]
- 2.Griffith LG, Naughton G. Tissue engineering - Current challenges and expanding opportunities. Science. 2002;295(5557):1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 3.Nichol JW, Khademhosseini A. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter. 2009 2009;5(7):1312–1319. doi: 10.1039/b814285h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du YA, Ghodousi M, Qi H, Haas N, Xiao WQ, Khademhosseini A. Sequential assembly of cell-laden hydrogel constructs to engineer vascular-like microchannels. Biotechnol Bioeng. 2011;108(7):1693–1703. doi: 10.1002/bit.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohashi K, Yokoyama T, Yamato M, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nature Med. 2007;13(7):880–885. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu T, Yamato M, Isoi Y, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circulation Res. 2002;90(3):E40–E48. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 7.Sekine H, Shimizu T, Yang J, Kobayashi E, Okano T. Pulsatile myocardial tubes fabricated with cell sheet engineering. Circulation. 2006;114:I87–I93. doi: 10.1161/CIRCULATIONAHA.105.000273. [DOI] [PubMed] [Google Scholar]
- 8.Franzesi GT, Ni B, Ling Y, Khademhosseini A. A controlled-release strategy for the generation of cross-linked hydrogel microstructures. J Am Chem Soc. 2006;128(47):15064–15065. doi: 10.1021/ja065867x. [DOI] [PubMed] [Google Scholar]
- 9.Motlagh D, Hartman TJ, Desai TA, Russell B. Microfabricated grooves recapitulate neonatal myocyte connexin43 and N-cadherin expression and localization. J Biomed Mater Res, Part A. 2003;67A(1):148–157. doi: 10.1002/jbm.a.10083. [DOI] [PubMed] [Google Scholar]
- 10.Nakazawa K, Izumi Y, Fukuda J, Yasuda T. Hepatocyte spheroid culture on a polydimethylsiloxane chip having microcavities. J Biomater Sci Polym Ed. 2006;17(8):859–873. doi: 10.1163/156856206777996853. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda J, Khademhosseini A, Yeo Y, Yang X, Yeh J, Eng G, Blumling J, Wang CF, Kohane DS, Langer R. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006;27(30):5259–5267. doi: 10.1016/j.biomaterials.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 12.Moeller H-C, Mian MK, Shrivastava S, Chung BG, Khademhosseini A. A microwell array system for stem cell culture. Biomaterials. 2008;29(6):752–763. doi: 10.1016/j.biomaterials.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tekin H, Tsinman T, Sanchez JG, Jones BJ, Camci-Unal G, Nichol JW, Langer R, Khademhosseini A. Responsive micromolds for sequential patterning of hydrogel microstructures. J Am Chem Soc. 2011;133(33):12944–12947. doi: 10.1021/ja204266a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canavan HE, Cheng XH, Graham DJ, Ratner BD, Castner DG. Cell sheet detachment affects the extracellular matrix: A surface science study comparing thermal lift-off, enzymatic, and mechanical methods. J Biomed Mater Res, Part A. 2005;75A(1):1–13. doi: 10.1002/jbm.a.30297. [DOI] [PubMed] [Google Scholar]
- 15.Yamada N, Okano T, Sakai H, Karikusa F, Sawasaki Y, Sakurai Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol Chemie Rapid Commun. 1990;11(11):571–576. [Google Scholar]
- 16.Alarcon CDH, Pennadam S, Alexander C. Stimuli responsive polymers for biomedical applications. Chem Soc Rev. 2005;34(3):276–285. doi: 10.1039/b406727d. [DOI] [PubMed] [Google Scholar]
- 17.Da Silva RMP, Mano JF, Reis RL. Smart thermoresponsive coatings and surfaces for tissue engineering: switching cell-material boundaries. Trends Biotechnol. 2007;25(12):577–583. doi: 10.1016/j.tibtech.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Euro J Pharma Biopharma. 2000;50(1):27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 19.Eeckman F, Moes AJ, Amighi K. Synthesis and characterization of thermosensitive copolymers for oral controlled drug delivery. Euro Polym J. 2004;40(4):873–881. [Google Scholar]
- 20.Hirotsu S, Hirokawa Y, Tanaka T. Volume-phase transitions of ionized N-isopropylacrylamide gels. J Chem Phys. 1987;87(2):1392–1395. [Google Scholar]
- 21.Beltran S, Baker JP, Hooper HH, Blanch HW, Prausnitz JM. Swelling equilibria for weakly ionizable, temperature-sensitive hydrogels. Macromolecules. 1991;24(2):549–551. [Google Scholar]
- 22.Inoue T, Chen GH, Nakamae K, Hoffman AS. Temperature sensitivity of a hydrogel network containing different LCST oligomers grafted to the hydrogel backbone. Polym Gels Networks. 1997;5(6):561–575. [Google Scholar]
- 23.Allan SH. Applications of thermally reversible polymers and hydrogels in therapeutics and diagnostics. J Controlled Release. 1987;6(1):297–305. [Google Scholar]
- 24.Kaneko Y, Nakamura S, Sakai K, Aoyagi T, Kikuchi A, Sakurai Y, Okano T. Rapid deswelling response of poly(N-isopropylacrylamide) hydrogels by the formation of water release channels using poly(ethylene oxide) graft chains. Macromolecules. 1998;31(18):6099–6105. [Google Scholar]
- 25.Yoshida R, Uchida K, Kaneko Y, Sakai K, Kikuchi A, Sakurai Y, Okano T. Comb-type grafted hydrogels with rapid deswelling response to temperature changes. Nature. 1995;374(6519):240–242. [Google Scholar]
- 26.Kaneko Y, Nakamura S, Sakai K, Kikuchi A, Aoyagi T, Sakurai Y, Okano T. Deswelling mechanism for comb-type grafted poly(N-isopropylacrylamnide) hydrogels with rapid temperature responses. Polym Gels Networks. 1998;6(5):333–345. [Google Scholar]
- 27.Chen GH, Hoffman AS. Graft copolymers that exhibit temperature-induced phase-transitions over a wide-range of pH. Nature. 1995;373(6509):49–52. doi: 10.1038/373049a0. [DOI] [PubMed] [Google Scholar]
- 28.Diez-Pena E, Quijada-Garrido I, Barrales-Rienda JM. On the water swelling behaviour of poly(N-isopropylacrylamide) P(N-iPAAm), poly(methacrylic acid) P(MAA), their random copolymers and sequential interpenetrating polymer networks (IPNs) Polymer. 2002;43(16):4341–4348. [Google Scholar]
- 29.Diez-Pena E, Quijada-Garrido I, Frutos P, Barrales-Rienda JM. Thermal properties of cross-linked poly(N-isopropylacrylamide) P(N-iPAAm), poly(methacrylic acid) P(MAA), their random copolymers P(N-iPAAm-co-MAA), and sequential interpenetrating polymer networks (IPNs) Macromolecules. 2002;35(7):2667–2675. [Google Scholar]
- 30.Bromberg L. Zein-poly(N-isopropylacrylamide) conjugates. J Phys Chem B. 1997;101(4):504–507. 23. [Google Scholar]
- 31.Ju HK, Kim SY, Lee YM. pH/temperature-responsive behaviors of semi-IPN and comb-type graft hydrogels composed of alginate and poly(N-isopropylacrylamide) Polymer. 2001;42(16):6851–6857. [Google Scholar]
- 32.Dhara D, Rathna GVN, Chatterji PR. Volume phase transition in interpenetrating networks of poly(N-isopropylacrylamide) with gelatin. Langmuir. 2000;16(6):2424–2429. [Google Scholar]
- 33.Gil ES, Hudson SM. Stimuli-reponsive polymers and their bioconjugates. Progr Polym Sci. 2004;29(12):1173–1222. [Google Scholar]
- 34.Takezawa T, Mori Y, Yoshizato K. Cell-culture on a thermoresponsive polymer surface. Bio Technol. 1990;8(9):854–856. doi: 10.1038/nbt0990-854. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003;24(13):2309–2316. doi: 10.1016/s0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 36.Harimoto M, Yamato M, Hirose M, Takahashi C, Isoi Y, Kikuchi A, Okano T. Novel approach for achieving double-layered cell sheets co-culture: overlaying endothelial cell sheets onto monolayer hepatocytes utilizing temperature-responsive culture dishes. J Biomed Mater Res. 2002;62(3):464–470. doi: 10.1002/jbm.10228. [DOI] [PubMed] [Google Scholar]
- 37.Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001;7(4):473–480. doi: 10.1089/10763270152436517. [DOI] [PubMed] [Google Scholar]
- 38.Kushida A, Yamato M, Kikuchi A, Okano T. Two-dimensional manipulation of differentiated Madin-Darby canine kidney (MDCK) cell sheets: The noninvasive harvest from temperature-responsive culture dishes and transfer to other surfaces. J Biomed Mater Res. 2001;54(1):37–46. doi: 10.1002/1097-4636(200101)54:1<37::aid-jbm5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Nishida K, Yamato M, Hayashida Y, Watanabe K, Maeda N, Watanabe H, Yamamoto K, Nagai S, Kikuchi A, Tano Y, Okano T. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsieve cell culture surface. Transplantation. 2004;77(3):379–385. doi: 10.1097/01.TP.0000110320.45678.30. [DOI] [PubMed] [Google Scholar]
- 40.Ista LK, Mendez S, Perez-Luna VH, Lopez GP. Synthesis of poly(N-isopropylacrylamide) on initiator-modified self-assembled monolayers. Langmuir. 2001;17(9):2552–2555. [Google Scholar]
- 41.Akiyama Y, Kikuchi A, Yamato M, Okano T. Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir. 2004;20(13):5506–5511. doi: 10.1021/la036139f. [DOI] [PubMed] [Google Scholar]
- 42.Yamato M, Konno C, Koike S, Isoi Y, Shimizu T, Kikuchi A, Makino K, Okano T. Nanofabrication for micro-patterned cell arrays by combining electron beam-irradiated polymer grafting and localized laser ablation. J Biomed Mater Res Part A. 2003;67A(4):1065–1071. doi: 10.1002/jbm.a.10078. [DOI] [PubMed] [Google Scholar]
- 43.Canavan HE, Cheng XH, Graham DJ, Ratner BD, Castner DG. Surface characterization of the extracellular matrix remaining after cell detachment from a thermoresponsive polymer. Langmuir. 2005;21(5):1949–1955. doi: 10.1021/la048546c. [DOI] [PubMed] [Google Scholar]
- 44.Cheng XH, Canavan HE, Stein MJ, Hull JR, Kweskin SJ, Wagner MS, Somorjai GA, Castner DG, Ratner BD. Surface chemical and mechanical properties of plasmapolymerized N-isopropylacrylamide. Langmuir. 2005;21(17):7833–7841. doi: 10.1021/la050417o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Recum HA, Kim SW, Kikuchi A, Okuhara M, Sakurai Y, Okano T. Novel thermally reversible hydrogel as detachable cell culture substrate. J Biomed Mater Res. 1998;40(4):631–639. doi: 10.1002/(sici)1097-4636(19980615)40:4<631::aid-jbm15>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 46.Chen GP, Imanishi Y, Ito Y. Effect of protein and cell behavior on pattern-grafted thermoresponsive polymer. J Biomed Mater Res. 1998;42(1):38–44. doi: 10.1002/(sici)1097-4636(199810)42:1<38::aid-jbm6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 47.Ito Y, Chen GP, Guan YQ, Imanishi Y. Patterned immobilization of thermoresponsive polymer. Langmuir. 1997;13(10):2756–2759. [Google Scholar]
- 48.Tamada Y, Ikada Y. Fibroblast growth on polymer surfaces and biosynthesis of collagen. J Biomed Mater Res. 1994;28(7):783–789. doi: 10.1002/jbm.820280705. [DOI] [PubMed] [Google Scholar]
- 49.Bullett NA, Talib RA, Short RD, McArthur SL, Shard AG. Chemical and thermo-responsive characterisation of surfaces formed by plasma polymerisation of N-isopropyl acrylamide. Surf Interface Anal. 2006;38(7):1109–1116. [Google Scholar]
- 50.Matsuda N, Shimizu T, Yamato M, Okano T. Tissue engineering based on cell sheet technology. Adv Mater. 2007;19(20):3089–3099. [Google Scholar]
- 51.Yamato M, Okuhara M, Karikusa F, Kikuchi A, Sakurai Y, Okano T. Signal transduction and cytoskeletal reorganization are required for cell detachment from cell culture surfaces grafted with a temperature-responsive polymer. J Biomed Mater Res. 1999;44(1):44–52. doi: 10.1002/(sici)1097-4636(199901)44:1<44::aid-jbm5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 52.Okano T, Yamada N, Okuhara M, Sakai H, Sakurai Y. Mechanism of cell detachment from temperature-modulated, hydrophilic–hydrophobic polymer surfaces. Biomaterials. 1995;16(4):297–303. doi: 10.1016/0142-9612(95)93257-e. [DOI] [PubMed] [Google Scholar]
- 53.Kushida A, Yamato M, Konno C, Kikuchi A, Sakurai Y, Okano T. Decrease in culture temperature releases monolayer endothelial cell sheets together with deposited fibronectin matrix from temperature-responsive culture surfaces. J Biomed Mater Res. 1999;45(4):355–362. doi: 10.1002/(sici)1097-4636(19990615)45:4<355::aid-jbm10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 54.Yamato M, Konno C, Utsumi M, Kikuchi A, Okano T. Thermally responsive polymer-grafted surfaces facilitate patterned cell seeding and co-culture. Biomaterials. 2002;23(2):561–567. doi: 10.1016/s0142-9612(01)00138-7. [DOI] [PubMed] [Google Scholar]
- 55.Yamato M, Kwon OH, Hirose M, Kikuchi A, Okano T. Novel patterned cell coculture utilizing thermally responsive grafted polymer surfaces. J Biomed Mater Res. 2001;55(1):137–140. doi: 10.1002/1097-4636(200104)55:1<137::aid-jbm180>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 56.Tsuda Y, Kikuchi A, Yamato M, Nakao A, Sakurai Y, Umezu M, Okano T. The use of patterned dual thermoresponsive surfaces for the collective recovery as co-cultured cell sheets. Biomaterials. 2005;26(14):1885–1893. doi: 10.1016/j.biomaterials.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Tsuda Y, Kikuchi A, Yamato M, Sakurai Y, Umezu M, Okano T. Control of cell adhesion and detachment using temperature and thermoresponsive copolymer grafted culture surfaces. J Biomed Mater Res Part A. 2004;69A(1):70–78. doi: 10.1002/jbm.a.20114. [DOI] [PubMed] [Google Scholar]
- 58.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, Okano T, Tano Y. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. NE J Med. 2004;351(12):1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 59.Sasagawa T, Shimizu T, Sekiya S, Haraguchi Y, Yamato M, Sawa Y, Okano T. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials. 2010;31(7):1646–1654. doi: 10.1016/j.biomaterials.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 60.Shimizu T, Yamato M, Akutsu T, Shibata T, Isoi Y, Kikuchi A, Umezu M, Okano T. Electrically communicating three-dimensional cardiac tissue mimic fabricated by layered cultured cardiomyocyte sheets. J Biomed Mater Res. 2002;60(1):110–117. doi: 10.1002/jbm.1284. [DOI] [PubMed] [Google Scholar]
- 61.Sekiya S, Shimizu T, Yamato M, Kikuchi A, Okano T. Bioengineered cardiac cell sheet grafts have intrinsic angiogenic potential. Biochem Biophys Res Commun. 2006;341(2):573–582. doi: 10.1016/j.bbrc.2005.12.217. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka Y, Morishima K, Shimizu T, Kikuchi A, Yamato M, Okano T, Kitamori T. An actuated pump on-chip powered by cultured cardiomyocytes. Lab on a Chip. 2006;6(3):362–368. doi: 10.1039/b515149j. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka Y, Sato K, Shimizu T, Yamato M, Okano T, Kitamori T. A micro-spherical heart pump powered by cultured cardiomyocytes. Lab on a Chip. 2007;7(2):207–212. doi: 10.1039/b612082b. [DOI] [PubMed] [Google Scholar]
- 64.Yokoyama T, Ohashi K, Kuge H, Kanehiro H, Iwata H, Yamato M, Nakajima Y. In vivo engineering of metabolically active hepatic tissues in a neovascularized subcutaneous cavity. Am J. Transplant. 2006;6(1):50–59. doi: 10.1111/j.1600-6143.2005.01155.x. [DOI] [PubMed] [Google Scholar]
- 65.Lee HM, Cusick RA, Utsunomiya H, Ma PX, Langer R, Vacanti JP. Effect of implantation site on hepatocytes heterotopically transplanted on biodegradable polymer scaffolds. Tissue Eng. 2003;9(6):1227–1232. doi: 10.1089/10763270360728134. [DOI] [PubMed] [Google Scholar]
- 66.Tsuda Y, Kikuchi A, Yamato M, Chen GP, Okano T. Heterotypic cell interactions on a dually patterned surface. Biochem Biophys Res Commun. 2006;348(3):937–944. doi: 10.1016/j.bbrc.2006.07.138. [DOI] [PubMed] [Google Scholar]
- 67.Isenberg BC, Tsuda Y, Williams C, Shimizu T, Yamato M, Okano T, Wong JY. A thermoresponsive, microtextured substrate for cell sheet engineering with defined structural organization. Biomaterials. 2008;29(17):2565–2572. doi: 10.1016/j.biomaterials.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsuda Y, Yamato M, Kikuchi A, Watanabe M, Chen G, Takahashi Y, Okano T. Thermoresponsive microtextured culture surfaces facilitate fabrication of capillary networks. Adv Mater. 2007;19(21):3633–3636. [Google Scholar]
- 69.Tekin H, Ozaydin-Ince G, Tsinman T, Gleason KK, Langer R, Khademhosseini A, Demirel MC. Responsive microgrooves for the formation of harvestable tissue constructs. Langmuir. 2011;27(9):5671–5679. doi: 10.1021/la200183x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Co CC, Wang YC, Ho CC. Biocompatible micropatterning of two different cell types. J Am Chem Soc. 2005;127(6):1598–1599. doi: 10.1021/ja044382a. [DOI] [PubMed] [Google Scholar]
- 71.Khademhosseini A, Yeh J, Jon S, Eng G, Suh KY, Burdick JA, Langer R. Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab on a Chip. 2004;4(5):425–430. doi: 10.1039/b404842c. [DOI] [PubMed] [Google Scholar]
- 72.Tekin H, Anaya M, Brigham MD, Nauman C, Langer R, Khademhosseini A. Stimuli-responsive microwells for formation and retrieval of cell aggregates. Lab on a Chip. 2010;10(18):2411–2418. doi: 10.1039/c004732e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sasaki J-I, Asoh T-A, Matsumoto T, Egusa H, Sohmura T, Alsberg E, Akashi M, Yatani H. Fabrication of three-dimensional cell constructs using temperature-responsive hydrogel. Tissue Eng Part A. 2010;16(8):2497–2504. doi: 10.1089/ten.TEA.2009.0523. [DOI] [PubMed] [Google Scholar]