Abstract
The opportunistic human pathogen Pseudomonas aeruginosa is able to utilize a wide range of carbon and nitrogen compounds, allowing it to grow in vastly different environments. The uptake and catabolism of growth substrates are organized hierarchically by a mechanism termed catabolite repression control (Crc) whereby the Crc protein establishes translational repression of target mRNAs at CA (catabolite activity) motifs present in target mRNAs near ribosome binding sites. Poor carbon sources lead to activation of the CbrAB two-component system, which induces transcription of the small RNA (sRNA) CrcZ. This sRNA relieves Crc-mediated repression of target mRNAs. In this study, we have identified novel targets of the CbrAB/Crc system in P. aeruginosa using transcriptome analysis in combination with a search for CA motifs. We characterized four target genes involved in the uptake and utilization of less preferred carbon sources: estA (secreted esterase), acsA (acetyl-CoA synthetase), bkdR (regulator of branched-chain amino acid catabolism) and aroP2 (aromatic amino acid uptake protein). Evidence for regulation by CbrAB, CrcZ and Crc was obtained in vivo using appropriate reporter fusions, in which mutation of the CA motif resulted in loss of catabolite repression. CbrB and CrcZ were important for growth of P. aeruginosa in cystic fibrosis (CF) sputum medium, suggesting that the CbrAB/Crc system may act as an important regulator during chronic infection of the CF lung.
Introduction
Pseudomonas aeruginosa is a metabolically versatile organism, which is able to grow in a variety of different ecological niches. Its natural habitats can be nutrient-poor (e.g., aqueous environments) or nutrient-rich (e.g., the rhizosphere or infected animal tissues). The CbrAB/Crc carbon catabolite control system helps to ensure a metabolic optimization process by allowing preferential utilization of good energy sources and establishing a healthy carbon (C) to nitrogen (N) balance [1]. The CbrAB/Crc system involves a regulatory protein (Crc), which is required to establish translational repression of target mRNAs at CA (catabolite activity) motifs, usually located in the vicinity of ribosome binding sites [1]–[4]. The core sequence of the CA motif consists of eight nucleotides, AAnAAnAA, where n stands for any nucleotide. However, inspection of RNAs that are under Crc control suggests that n = C or U may be preferred over n = A or G in CA motifs [1], [2]. A small RNA (sRNA) termed CrcZ, which possesses several conserved CA motifs and appears to bind to Crc, antagonizes translational repression mediated by the Crc protein [1]. CrcZ levels are low in the presence of a good carbon source (e.g., succinate), elevated in the presence of an intermediate carbon source (e.g., glucose) and high when a less preferred carbon compound is used as the sole energy source (e.g., mannitol). This regulation is established by the activity of the two-component system CbrAB consisting of the membrane-bound sensor CbrA and the response regulator CbrB, which are required for the expression of crcZ, together with the alternative sigma factor RpoN and IHF protein [1], [5]. CbrB binds to an upstream activating sequence (UAS) in the crcZ promoter region [5]. Mutants affected in cbrB or crcZ have similar, but not identical phenotypes, indicating that CrcZ mediates most, but not all, output of the CbrAB system [1], [5]. The CbrAB/Crc system is involved in the regulation of swarming, biofilm formation, cytotoxicity and antibiotic resistance in P. aeruginosa [6], [7]. However, in these interactions the regulatory mechanisms have not been investigated in molecular detail.
While the effects of the CbrAB/Crc system occur essentially at a post-transcriptional level, we asked whether transcript abundance could be used to reveal targets of this system. Previous experience with the mechanistically similar GacSA/RsmA pathway in P. aeruginosa [8]–[10] and P. fluorescens Pf-5 [11] suggests that during translational repression transcript abundance tends to be low, whereas transcript levels may be elevated during derepression, presumably reflecting effects of translational control on mRNA stability. Based on these observations, we decided to compare the transcriptome of the P. aeruginosa wild type PAO1 with that of its crc, crcZ and cbrB mutants. To find new targets of the CbrAB/Crc system, we focussed on several mRNAs having appropriately located CA motifs. The targets chosen are all involved in the utilization of relatively poor C sources. Furthermore, we found that growth of cbrB and crcZ mutants was severely impaired in an artificial cystic fibrosis (CF) sputum medium, an effect that is most likely due to repression of amino acid uptake and catabolism by the Crc protein. Thus, the CbrAB/Crc system may be an important regulatory system enabling P. aeruginosa to thrive in the CF lung.
Results
Transcriptome analysis of genes regulated by Crc – To assess the impact of the CbrAB/Crc pathway on global gene expression of P. aeruginosa, we performed a transcriptome analysis of P. aeruginosa PAO1 wild type and its crc, crcZ and cbrB deletion mutants using commercially available genome-wide DNA microarrays (Affymetrix). First, we grew cells to late exponential phase in Luria Broth (LB) as a nutrient rich medium, which mainly contains amino acids, peptides and small amounts of sugars and nucleotides [12] and which creates conditions of moderate carbon catabolite repression [3]. Second, we used a defined basal salts medium (BSM) amended with 40 mM succinate, which establishes strong catabolite repression in strain PAO1 [1]. Under both conditions, Crc mediates catabolite repression of amiE, which encodes short-chain aliphatic amidase and has previously served as a reporter gene ([1]; Figure S1). We expected that genes whose expression is translationally repressed by the CbrAB/Crc system might show elevated transcript levels in a crc mutant, whereas down-regulated transcript levels might be seen in both cbrB and crcZ mutants, due arrest of translation.
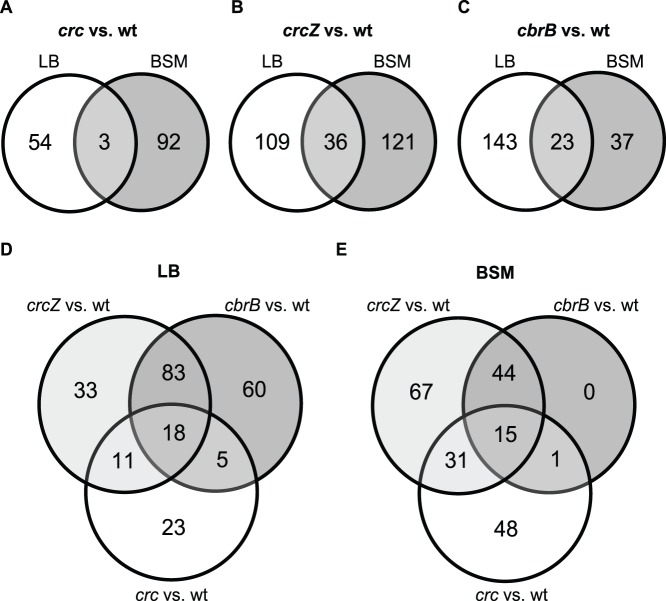
In LB-grown cells, we found 57 transcripts to be differentially (≥2.0-fold) expressed in the crc mutant, by comparison with the wild type PAO1; 19 transcripts were up-regulated and 38 were down-regulated at least two-fold (Table S1). After growth in BSM containing succinate, 95 transcripts showed altered expression; 53 transcripts were up-regulated and 42 were down-regulated (Table S1). The overlap of genes that were differentially affected under both conditions was small (Figure 1A). The probable reason is that transcriptional expression of catabolic genes varies greatly between the two media, making it difficult to detect the same effects of Crc on transcript abundance. Nevertheless, the combined data from both media enabled us to see a considerable number of transcripts influenced by Crc. As a control, we checked amiE transcript levels. They were elevated in the Δcrc mutant cultivated in LB (Table 1 and Table S1), which is consistent with increased amiE translation (Figure S1) and, presumably, with concomitantly enhanced amiE mRNA stability in the absence of Crc-mediated catabolite repression. By contrast, the Δcrc mutation had no effect on amiE transcript abundance in cells grown in BSM, which lacks inducing amides and hence does not permit transcriptional expression of the amiE operon [1], [13]. These results suggest that transcriptome analysis may be used to identify targets of the CbrAB/Crc system provided that sufficient transcriptional expression of the target genes takes place.
Figure 1. Venn diagrams summarizing changes in transcript abundance in mutants affected in the CbrAB/Crc cascade.
Data are taken from Tables S1 and S2. Changes in transcript levels from LB vs. BSM-succinate cultures are shown for (A) PAO1Δcrc, (B) PAO1ΔcrcZ and (C) PAO1ΔcbrB, by comparison with the wild-type strain. Overlaps between the differentially regulated transcripts in the crc, crcZ and cbrB mutants are shown (D) for LB cultures and (E) for BSM-succinate cultures.
Table 1. Selection of targets of the CbrAB/Crc system implicated by the transcriptome analysis.
| ORFa | Gene | LBb | BSMb + succinate | Description | CA motif (location)c | ||||
| crc vs. wt | cbr vs. wt | crcZ vs. wt | crc vs. wt | cbrB vs. wt | crcZ vs. wt | ||||
| PA0755 | opdH | 5,9 | 2,05 | cis-aconitate porin OpdH | AAGAACAA (−25 to −18) | ||||
| PA0866 | aroP2 | 4,73 | aromatic amino acid transport protein | AACAAUAA (−33 to −12) | |||||
| PA0887 | acsA | −3,41 | −2,51 | −3,27 | 7,98 | 2,88 | 3,62 | acetyl-coenzyme A synthetase | AACAAAAACAA (−35 to −25) |
| PA0996 | pqsA | −3,55 | −3,49 | −2,01 | −18,34 | −16,67 | probable coenzyme A ligase | ||
| PA0997 | pqsB | −3,72 | −3,49 | −2,11 | −33,37 | −56,5 | PqsB protein | ||
| PA0998 | pqsC | −3,73 | −3,89 | −2,01 | −25,21 | −40,64 | PqsC protein | ||
| PA0999 | pqsD | −3,77 | −3,38 | −2 | −23,39 | −34,65 | 3-oxoacyl-[acyl-carrier-protein] synthase III | ||
| PA1000 | pqsE | −2,79 | −2,58 | −2,09 | −18,62 | −22,18 | quinolone signal response protein | ||
| PA1001 | phnA | −3,58 | −3,28 | −2,08 | −18,69 | −23,34 | anthranilate synthase component I | ||
| PA1002 | phnB | −2,04 | −2,01 | −11,69 | −11,46 | anthranilate synthase component II | |||
| PA1003 | pqsR | −2,48 | transcriptional regulator PqsR (MvfR) | ||||||
| PA1070 | braG | −2,28 | −3,76 | −3,22 | branched-chain amino acid transport protein BraG | ||||
| PA1071 | braF | −2,37 | −4,02 | 2,08 | −2,91 | branched-chain amino acid transport protein BraF | |||
| PA1072 | braE | −2,21 | −3,11 | 2,33 | −3,05 | branched-chain amino acid transport protein BraE | |||
| PA1073 | braD | −2,88 | 2,5 | −5,56 | branched-chain amino acid transport protein BraD | ||||
| PA1074 | braC | −2,11 | −3,6 | 2,14 | −2,41 | branched-chain amino acid transport protein BraC | |||
| PA1342 | −2,68 | −2,38 | probable binding protein component ofABC transporter | AAUAAAAA (−33 to −26) | |||||
| PA1617 | 2,44 | probable AMP-binding enzyme | AACAACAACAA (−23 to −13) | ||||||
| PA1894 | 5,83 | −4,24 | hypothetical protein | ||||||
| PA1895 | 4,27 | −3,07 | hypothetical protein | ||||||
| PA1984 | exaC | −2,76 | 3,84 | −3,37 | NAD+ dependent aldehyde dehydrogenase ExaC | ||||
| PA1985 | pqqA | 11,34 | 8,74 | pyrroloquinoline quinone biosynthesis protein A | |||||
| PA1986 | pqqB | 2,47 | 2,52 | pyrroloquinoline quinone biosynthesis protein B | |||||
| PA1987 | pqqC | 3,86 | 3,7 | pyrroloquinoline quinone biosynthesis protein C | |||||
| PA1988 | pqqD | 3,7 | 4,02 | pyrroloquinoline quinone biosynthesis protein D | |||||
| PA1989 | pqqE | 2,72 | 2,77 | pyrroloquinoline quinone biosynthesis protein E | |||||
| PA2247 | bkdA1 | −2,36 | −3,81 | 3,23 | 2-oxoisovalerate dehydrogenase (alpha subunit) | ||||
| PA2248 | bkdA2 | −2,6 | −3,51 | 2-oxoisovalerate dehydrogenase (beta subunit) | |||||
| PA2249 | bkdB | −2,48 | −3,22 | branched-chain alpha-keto acid dehydrogenase | |||||
| PA2250 | lpdV | −2,62 | −3,07 | lipoamide dehydrogenase-Val | |||||
| PA2533 | 2,13 | probable sodium:alanine symporter | AACAAGAAUAA (−20 to −10) | ||||||
| PA3038 | −4,13 | 16,75 | 2,11 | probable porin | AAUAACAA (−7 to +1) | ||||
| PA3190 | −7,43 | −7,81 | probable binding protein component ofABC sugar transporter | AAUAACAA (−24 to −17) | |||||
| PA3366 | amiE | 4,65 | 2,04 | aliphatic amidase | AACAACAA (−20 to −13) | ||||
| PA3452 | mqoA | 4,87 | 3,12 | malate:quinone oxidoreductase | |||||
| PA3570 | mmsA | 2,23 | methylmalonate-semialdehyde dehydrogenase | AACAAUAA (−37 to −30) | |||||
| PA3875 | narG | −6,01 | −4,46 | −4,79 | respiratory nitrate reductase alpha chain | AAGAAGAA (+34 to +41) | |||
| PA4139 | −3,37 | 2,87 | 4,72 | hypothetical protein | |||||
| PA4147 | acoR | 3,38 | transcriptional regulator AcoR | AACAACAA (−30 to −23) | |||||
| PA4150 | acoA | 2,77 | probable dehydrogenase E1 component | AACAACAA (−9 to −2) | |||||
| PA4151 | acoB | 4,1 | acetoin catabolism protein AcoB | AACAAGAA (−22 to −15) | |||||
| PA4306 | flp | −2,9 | −6,66 | −8,64 | Type IVb pilin, Flp | AACAAGAA (−22 to −15) | |||
| PA4496 | −2,54 | −2,55 | 2,9 | −2,73 | −8,18 | probable binding protein component of ABC transporter | |||
| PA4500 | −3,29 | −3,94 | 2,03 | probable binding protein component of ABC transporter | AAAAAGAAAAAA (−22 to −11) | ||||
| PA4501 | opdD | −2,53 | 2,64 | glycine-glutamate dipeptide porin OpdP | AACAAUAA (−37 to −30) | ||||
| PA4770 | lldP | 2,96 | L-lactate permease | AACAACAA (−25 to −18) | |||||
| PA4913 | 6,89 | −2,07 | probable binding protein component of ABC transporter | AACAACAA (−53 to −46) | |||||
| PA5112 | estA | −2,25 | 2 | −2,23 | esterase EstA | AAAAACAA (−24 to −17) | |||
| PA5153 | −2.42 | −2.55 | 2.92 | −2.28 | probable periplasmic binding protein | ||||
| PA5167 | dctP | −4,35 | −4,05 | 3,37 | −6,85 | probable c4-dicarboxylate-binding protein | AAGAACAA (−20 to −13) | ||
| PA5168 | dctQ | −2,19 | −2,12 | 6,54 | −6,4 | probable dicarboxylate transporter | AAUAAGAA (−20 to −13) | ||
| PA5169 | dctM | −2,41 | −2,2 | 6,72 | −10,69 | probable C4-dicarboxylate transporter | |||
| PA5220 | 2,38 | −4,08 | hypothetical protein | AAGAACAACAAGAA (−31 to −18) | |||||
| PA5348 | 5,54 | −3,03 | probable DNA-binding protein | AACAACAA (−26 to −19) | |||||
The numbers represents ORF according to www. pseudomonas.com. [45].
Fold changes observed after growth in LB and BSM + succinate, respectively. Positive values indicate that transcripts were more abundant in the mutant than in the wild type. Negative values indicate that transcripts were less abundant in the mutant than in the wild type.
The locations of the CA-motif are given according to the start codon (A of the ATG = +1).
Transcriptome analysis may reveal both and indirect effects of the CbrAB/Crc system. Direct effects are assumed to involve at least one CA motif (AAnAAnAA) in the region of −50 to +50 nucleotides relative to the translational start site in target mRNAs. We found 29 Crc-regulated transcripts having an appropriately located putative CA motif (Table S1). With the exception of four transcripts (three of them in LB and one in BSM), all mRNAs harbouring a CA motif were up-regulated in the crc mutant.
Transcriptome analysis of crcZ and cbrB mutants - As mentioned above, the Crc protein and the CrcZ sRNA, which requires CbrB to be transcribed, have opposite regulatory effects; thus one might expect that transcripts that are up-regulated in a crc mutant would be down-regulated in crcZ and cbrB mutants and vice versa. To investigate this assumption, we performed transcriptome analyses with a crcZ and a cbrB mutant under the same conditions as above. In LB-grown cells, 145 transcripts were differentially expressed by a factor of ≥2.0 in PAO1ΔcrcZ, whereas 166 transcripts were differentially expressed in PAO1ΔcbrB, by comparison with the wild type PAO1 (Table S2). In BSM-grown cells, 157 transcripts showed differential regulation in the crcZ mutant and 60 transcripts in the cbrB mutant (Table S2). Again, the number of genes showing a similar expression pattern in both media was low (Figure 1B and 1C). However, the crcZ and cbrB mutations had largely parallel effects; both mutations affected 101 out of 210 transcripts and 59 out of 158 transcripts in LB (Figure 1D) and in BSM (Figure 1E), respectively. Among the transcripts that were affected by both mutations, a majority was down-regulated (Table S2). Some transcripts seemed to be only regulated by CbrB and not by CrcZ. However, preliminary attempts to identify CbrB binding sites upstream of the corresponding ORFs have not been successful. Apart from the crcZ promoter, known direct CbrB targets include the lipA and hutU promoters [5], [14]. However, these genes did not appear in our transcriptome analysis, presumably because of lack of transcriptional inducers in the growth media. Some transcripts whose abundance appeared to be only regulated by CrcZ, but not by CbrB, were also seen, but were considered as questionable since crcZ expression strictly depends on CbrB [1]. Taken together, our transcriptome analysis is consistent with the current model stating that the sRNA CrcZ mediates most effects of the CbrAB two-component system.
Somewhat unexpectedly, the overlap between transcripts whose abundance was affected by Crc as well as by CrcZ and/or CbrB in an opposite manner was low in BSM-grown cells (17 out of 47 genes; Figures 1D and 1E, Table 1) and absent in LB-grown cells. Among the genes that did show the anticipated transcript pattern were estA (encoding a secreted esterase), the dctPMQ operon (required for C4-dicarboxylate transport at low concentrations), braC and the braDEFG operon (which are important for transport of branched chain amino acids) (Table 1). Moreover, estA, dctP and braC possess CA motifs indicating that they may be targets of the CbrAB/Crc system.
Transcripts that were strongly up-regulated in LB in the absence of Crc, but not down-regulated in the absence of CrcZ or CbrB, included opdH (encoding a cis-aconitate porin) and aroP2 (encoding an aromatic amino acid transporter) (Table 1). For opdH and aroP2 candidate CA motifs were found. Transcripts that were strongly up-regulated in BSM in the absence of Crc included acsA (encoding acetyl-CoA synthetase required for acetate catabolism), opdH, braC, braDEFG, bkdA1 (first gene of an operon involved in branched-chain amino acid assimilation), acoR and acoAB (for acetoin catabolism). CA motifs were found in all of these transcripts except for braDEFG and bkdA1 (Table 1), which provides evidence that these genes may be targets of the CbrAB/Crc system.
Some striking effects of the CbrAB/Crc system on the transcriptome were not associated with CA motifs. For example, the pqsABCDE and phnAB operons were strongly down-regulated in the crcZ and cbrB mutants, especially in minimal medium with succinate (Table 1). Both operons are required for the biosynthesis of the Pseudomonas quinolone signal (PQS) and 2-heptyl-4-hydroxyquinoline, which act as quorum sensing signal molecules together with the transcriptional regulator PqsR [15]. Transcript abundance of pqsR was also affected, but to a lesser extent (Table 1). As none of these genes showed elevated transcript levels in the crc mutant, their regulation by the CbrAB/Crc system is probably indirect. Moreover, several genes and operons involved in the anaerobic denitrification pathway were affected by the CbrAB/Crc system at the transcript level (Tables S1 and S2), although the cells had been grown aerobically. However, except for narG, which has a poorly conserved CA motif (Table 1), none of the corresponding transcripts showed a CA motif, suggesting indirect regulation by CbrAB/Crc. Finally, in cells cultivated in LB, pqqA (encoding the pyrroloquinoline quinone [PQQ] biosynthesis protein A) was one of the most strongly up-regulated transcripts in the crcZ and cbrB mutants (Table 1). P. aeruginosa when growing on ethanol uses a PQQ-dependent ethanol oxidation system (encoded by the exaA and exaBC genes), which converts ethanol to acetate. Acetyl-CoA synthetase (encoded by the acsA gene) is also essential for ethanol utilization [16]. The transcripts of the pqqABCDE operon and of the acsA and exaC genes were all differentially expressed in LB when either crcZ or cbrB was mutated (Table 1). However, as mentioned above, only the acsA transcript was also affected by a crc mutation. Thus, regulation of PQQ biosynthesis by the CbrAB/Crc system appears to be mostly indirect. The agmR gene encoding a positive regulator of PQQ biosynthesis [17] is a good candidate as it exhibits a CA motif (AUAACGACAAUAA).
Validation of candidate targets - In the remainder of this study, we will focus on transcripts (estA, acsA, and aroP2) that have increased abundance in a crc mutant and all contain a putative CA motif (i.e. AAnAAnAA). In addition, we investigated the role of the CbrAB/Crc system on bkdR and bkdA1, as the bkdA1 transcript was highly upregulated in the crc mutant and the cognate regulatory gene bkdR contains a putative CA-motif in the vicinity of its ribosome binding site. All these genes are involved in the utilization of less preferred carbon sources. Our initial concern was to validate the microarray results by an independent method. Data obtained by RT-qPCR (Table 2) were in full agreement with the microarray data (Table 1). In particular, the RT-qPCR measurements obtained in LB-grown cells proved to be more sensitive and hence possibly more accurate than the microarray data.
Table 2. Fold changes of transcript abundance in Δcrc, ΔcrcZ and ΔcbrB mutants compared to wild type PAO1 in LB and BSM + succinate as determined by RT-qPCR.
| Gene | LBa | BSMa + succinate | ||||
| crc vs. wt | cbr vs. wt | crcZ vs. wt | crc vs. wt | cbr vs. wt | crcZ vs. wt | |
| estA | 2.6±0.4 | −26.3±0.3 | −4.9±0.3 | 2.5±0.4 | −4.1±0.2 | −7.2±0.1 |
| acsA | 4.3±0.2 | −10.1±0.7 | −7.5±0.1 | 7.5±0.2 | −1.1±0.4 | 1.5±1.1 |
| bkdR | 2.3±0.4 | −1.7±0.9 | 1.5±0.9 | 1.4±1.0 | 1.5±1.0 | 1.1±0.8 |
| bkdA1 | 4.0±0.2 | −25.4±0.2 | −12.3±0.1 | 9.3±0.1 | −3.0±0.3 | −2.3±0.2 |
| ilvE | 3.2±0.2 | 1.4±0.2 | 1.8±0.6 | 1.2±0.9 | 2.3±1.2 | −1.2±0.5 |
| aroP2 | 2.7±0.4 | −8.4±1.4 | −2.4±0.6 | −1.4±1.0 | 1.0±0.9 | 1.4±0.9 |
Values are represented as averages of 3 independent replicates for every strain.
Regulation of uptake and degradation of poor carbon sources - Previously, only amiE and phzM mRNAs have been characterized as targets of the CbrAB/Crc system in P. aeruginosa [1], [18]. In this section, we will provide evidence that estA and acsA are also targets. EstA esterase is an autotransporter that possesses lipolytic enzyme activity [19] and contributes to the hydrolysis of glycerol esters with short-chain fatty acids, which can be further used as carbon sources by P. aeruginosa [20]. EstA is located in the outer membrane [21]. In our RT-qPCR analysis, estA mRNA appeared to be down-regulated in PAO1ΔcrcZ and PAO1ΔcbrB and up-regulated in PAO1Δcrc grown under both growth conditions (Table 2). A translational estA’-‘lacZ fusion was assayed for β-galactosidase activity in strain PAO1 and in the cbrB, crcZ and crc mutants, after growth in LB (Figure 2A) and BSM supplemented with 40 mM succinate as the sole carbon source (Figure 2B). Under both conditions, estA’-‘lacZ expression was repressed in PAO1ΔcrcZ and PAO1ΔcbrB and derepressed in PAO1Δcrc. However, the estA’-‘lacZ expression profiles showed quantitative differences in LB (Figure 2A) vs. BSM-succinate (Figure 2B), in agreement with the transcript analysis (Tables 1 and 2). To confirm that estA is a target of the CbrAB/Crc system we mutated the CA motif, which is located 17 nucleotides upstream of the estA start codon (Table 1). In the absence of the wild-type CA motif, the CbrAB/Crc system lost its ability to regulate estA completely (Figure 2C).
Figure 2. EstA esterase is a target of the CbrAB/Crc cascade.
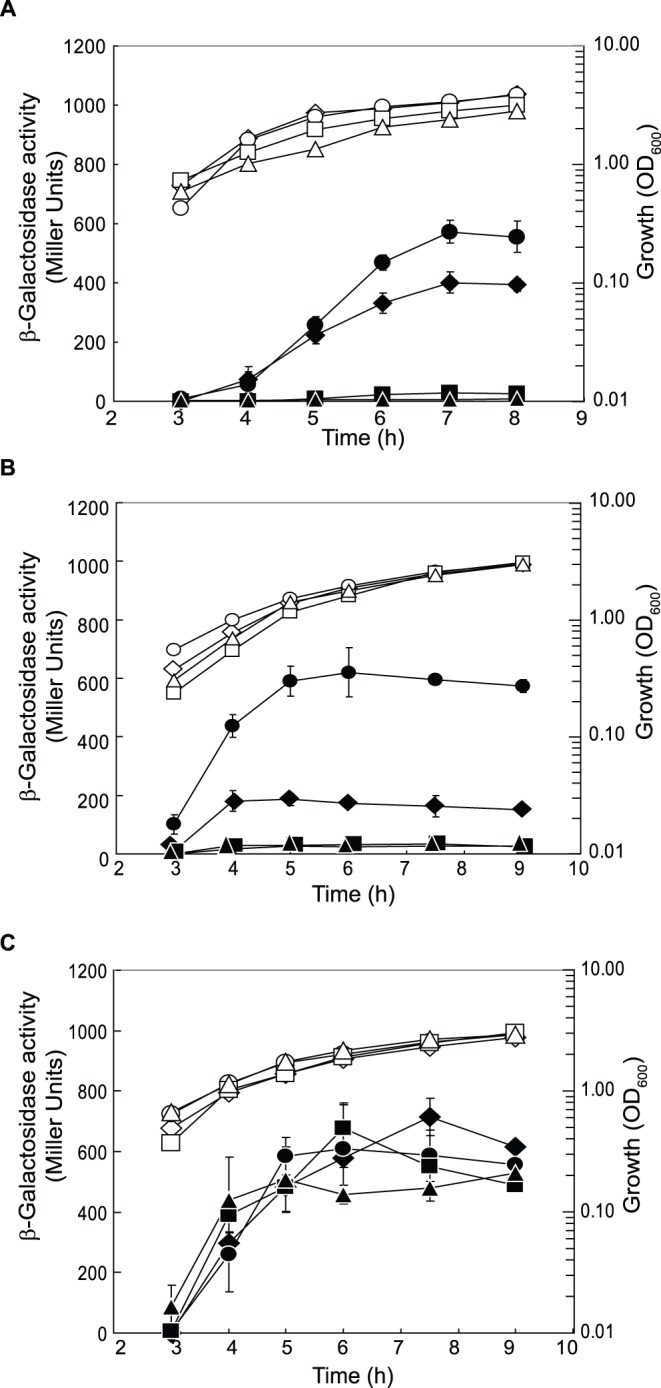
β-Galactosidase activities of an estA’-‘lacZ fusion were measured in PAO1 (black diamonds), PAO1ΔcbrB (black triangles), PAO1ΔcrcZ (black squares) and PAO1Δcrc (black circles) harbouring a plasmid with a translational estA’-‘lacZ fusion (pTLestA) (A) in LB medium and (B) in BSM medium supplemented with 40 mM succinate. (C) β-Galactosidase activities derived from a translational estA’-‘lacZ fusion with a mutated CA motif (pTLestA-ΔCA) were determined in PAO1 (black diamonds), PAO1ΔcbrB (black triangles), PAO1ΔcrcZ (black squares) and PAO1Δcrc (black circles). The strains were grown in BSM supplemented with 40 mM succinate. Cell growth was monitored by measuring the optical density at 600 nm (OD600) (white symbols).
P. aeruginosa can use acetate as a sole carbon and energy source, by converting acetate to acetyl-CoA, which is further metabolized via the tricarboxylic acid and glyoxylic acid cycles to generate energy and cell components. The initial reaction is catalyzed by acetyl-CoA synthetase, the product of the acsA gene. Acetyl-CoA synthetase activity is induced during growth on acetate or on ethanol [16], [22]. In our transcript analysis, acsA was differentially expressed in the wild type PAO1 and the crc, crcZ and cbrB mutants (Tables 1 and 2). The unexpected and unexplained finding here is that the cbrB and crcZ mutants on the one hand and the crc mutant on the other did not show opposite effects on ascA expression (Table 1). To demonstrate that the CbrAB/Crc system regulates acsA, we measured the β-galactosidase activity of a translational acsA’-‘lacZ fusion in these strains. As strain PAO1 lacking crcZ or cbrB showed a severe growth defect on acetate as the sole carbon source (Figure 3A), we grew the cells in BSM supplemented with 40 mM acetate and 5 mM succinate. As expected, ascA’-‘lacZ expression was completely repressed in PAO1ΔcrcZ and PAO1ΔcbrB, and increased in PAO1Δcrc, by comparison with PAO1 (Figure 3B). The acsA mRNA harbours an extended CA motif (AACAAAAACAA) located 25 nucleotides upstream of the acsA start codon. Mutation of the CA motif in the acsA leader of an acsA’-‘lacZ construct resulted in loss of catabolite repression mediated by Crc (Figure 3C).
Figure 3. Growth on acetate and acsA expression are under CbrAB/Crc control.
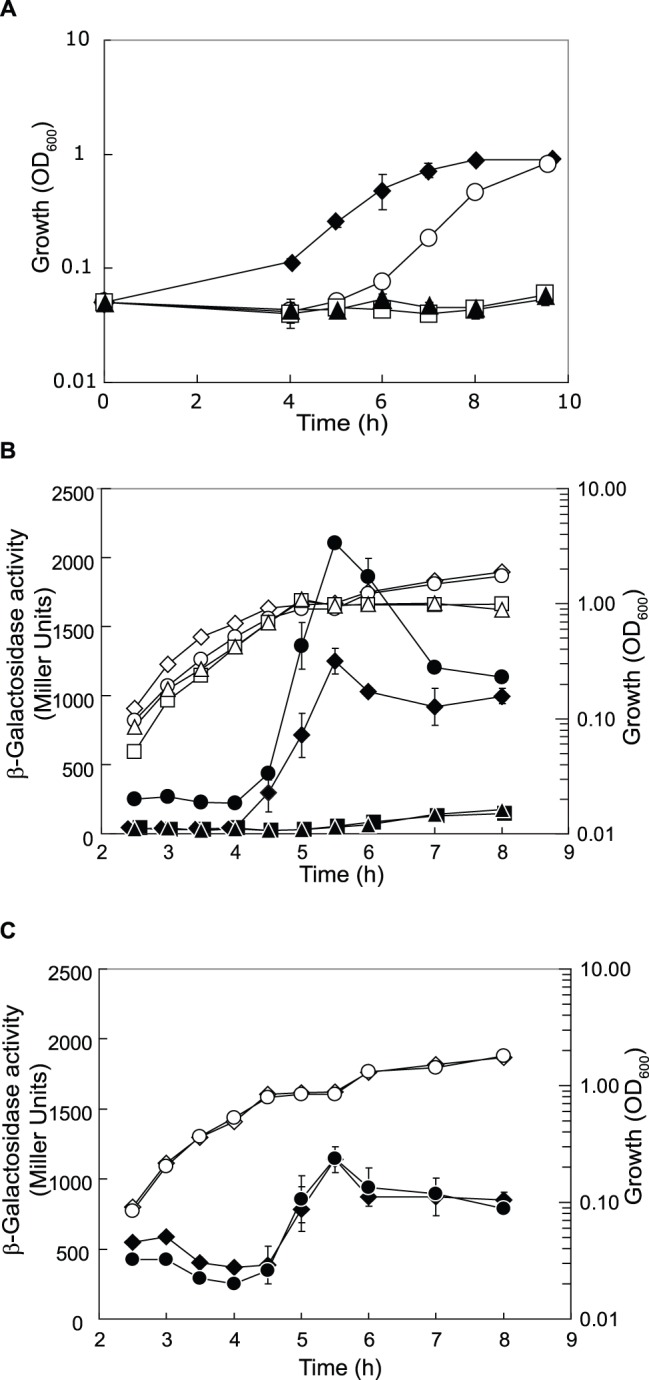
(A) Growth of PAO1 (black diamonds), PAO1ΔcbrB (black triangles), PAO1ΔcrcZ (white squares) and PAO1Δcrc (white circles) was measured in BSM supplemented with 40 mM acetate. (B) β-Galactosidase expression of a translational acsA’-‘lacZ fusion (pME10044) was monitored in PAO1 (black diamonds), PAO1ΔcbrB (black triangles), PAO1ΔcrcZ (black squares) and PAO1Δcrc (black circles). (C) Expression of a translational acsA’-‘lacZ fusion where the CA motif had been mutated (pME10045) was followed in PAO1 (black diamonds) and PAO1Δcrc (black circles). Cells were grown in BSM supplemented with 5 mM succinate and 40 mM acetate. The corresponding growth curves are shown in white symbols.
Targets of the CbrAB/Crc system in the uptake and metabolism of amino acids - The CbrAB two-component system is important for maintaining the C to N balance during amino acid utilization by P. aeruginosa [23]. In a seminal study, Crc was found to be a post-transcriptional regulator of the bkd operon, which is involved in the utilization of branched-chain amino acids in P. putida and P. aeruginosa [24]. Furthermore, a recent proteomic analysis [7] revealed that Crc is involved in the regulation of several transporters required for the uptake of amino acids in P. aeruginosa. Our transcriptome analysis revealed a number of CbrAB/Crc-regulated genes and operons (e.g., bkdA1A2B-lpdV, braBCDEDG, aroP2, aruBEFG, aotP, arcBC) that are involved in the utilization and transport of amino acids. In agreement with the original observations of Hester et al. [24], we found that the CbrAB/Crc system is involved in the regulation of branched-chain amino acid utilization in P. aeruginosa. In BSM supplemented with valine, isoleucine and leucine as the sole carbon sources, strains PAO1ΔcrcZ and PAO1ΔcbrB were not able to grow, whereas strains PAO1 and PAO1Δcrc did grow (Figure 4A). Branched-chain amino acid assimilation requires an initial deamination step performed by the branched-chain amino acid transaminase (encoded by ilvE), followed by decarboxylation by the branched-chain keto acid dehydrogenase (encoded by the bkdA1A2B-lpdV operon). The bkd operon is induced by branched-chain amino acids as well as branched-chain keto acids [25]. In P. putida, all five genes are repressed by Crc [26], whereas in P. aeruginosa only the bkd operon, but not the ilvE gene, seems to be under catabolite repression control by Crc (Tables 1 and 2). To verify the results from our transcriptome data, we analyzed the expression of a translational bkdA1’-‘lacZ fusion in LB supplemented with 23 mM leucine, 25.6 mM valine and 7.6 mM isoleucine. As shown in Figure 4B, bkdA1’-‘lacZ expression was slightly increased in PAO1Δcrc by comparison with that in PAO1, and strongly repressed in PAO1ΔcrcZ and PAO1ΔcbrB. In P. putida, a CA motif (AAAAACAA) is located 24 nucleotides upstream of the bkdA1 start codon. By contrast, in P. aeruginosa a putative CA motif (AAAAACAAAA) occurs 187 nucleotides upstream of the bkdA1 start codon and it is questionable whether this sequence would affect translation initiation of bkdA1. A more likely scenario pictures the bkdR gene, which lies upstream of the bkd operon, as a target of the CbrAB/Crc system. In P. putida, the transcriptional regulator BkdR positively controls expression of the bkd operon [27]. The BkdR protein content was elevated in a crc mutant while the bkdR mRNA levels were unchanged, whereas bkdA1 expression was affected at both the mRNA and the protein level [28].
Figure 4. Utilization of branched-chain amino acids is under CbrAB/Crc control.
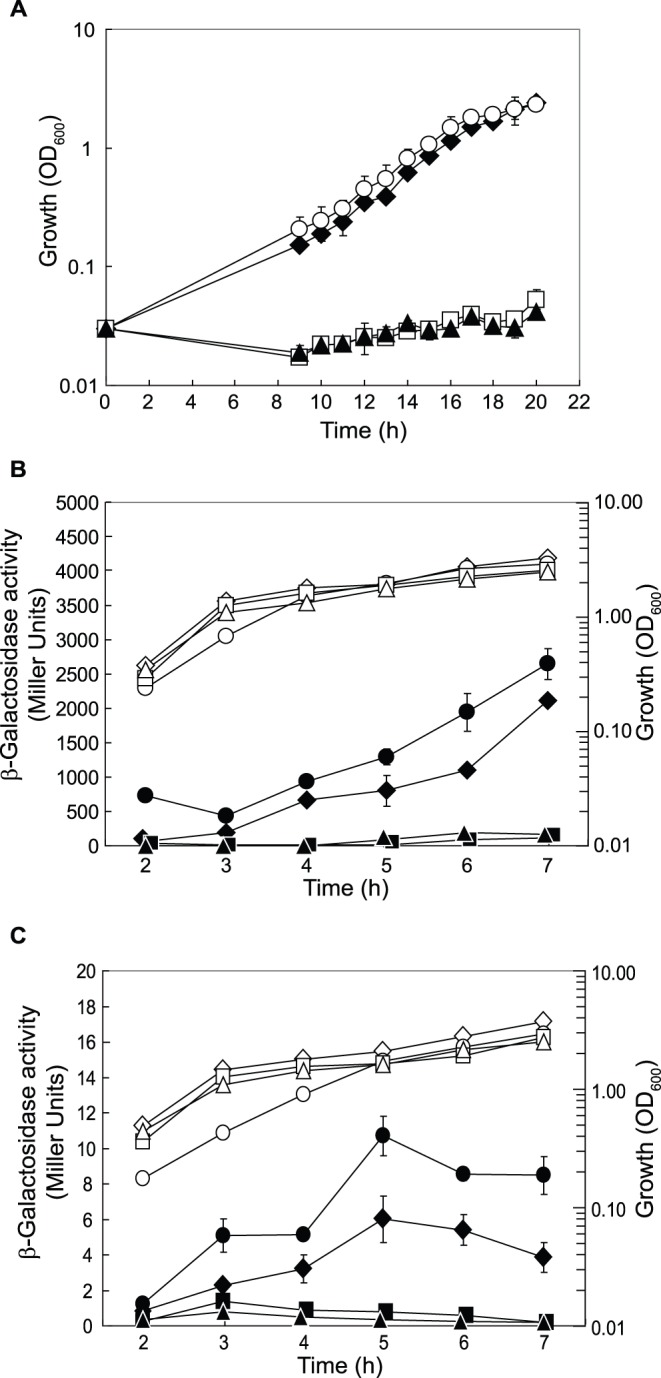
(A) Growth of PAO1 (black diamonds), PAO1ΔcbrB (black triangles), PAO1ΔcrcZ (white squares) and PAO1Δcrc (white circles) was monitored in BSM supplemented with 23 mM leucine, 25.6 mM valine and 7.6 mM isoleucine as the sole carbon sources. β-Galactosidase activities conferred by (B) a translational bkdA1’-‘lacZ fusion (pME10049) and (C) a translational bkdR’-‘lacZ fusion (pME10048) were measured in PAO1 (black diamonds), PAO1ΔcbrB (black triangles), PAO1ΔcrcZ (black squares) and PAO1Δcrc (black circles). Cells were grown in LB supplemented with 23 mM leucine, 25.6 mM valine and 7.6 mM isoleucine. The corresponding growth curves are shown in white symbols.
In P. aeruginosa, two putative CA motifs can be found in the untranslated leader region of bkdR (AACUACAAGAA at −36 to −26 and AACAAGAGAAACAA at −20 to −7). To see whether the CbrAB/Crc system regulates the expression of bkdR, we performed β-galactosidase assays of a translational bkdR’-‘lacZ fusion in LB supplemented with 23 mM leucine, 25.6 mM valine and 7.6 mM isoleucine. The activity of this fusion was 1.5- to 2.0-fold increased in PAO1Δcrc compared to PAO1 and was practically undetectable in PAO1ΔcrcZ and PAO1ΔcbrB (Figure 4C), confirming that bkdR is a target of the CbrAB/Crc system in P. aeruginosa.
Another good candidate for interaction with the CbrAB/Crc system was the aroP2 gene, which encodes a transporter for aromatic amino acids. A growth experiment was performed with PAO1 and its crc, crcZ, and cbrB deletion mutants in BSM supplemented with 4 mM tyrosine as the sole carbon source. The crcZ and cbrB mutants did not grow, whereas PAO1 and PAO1Δcrc reached an OD600 of 1 after 9 h; PAO1Δcrc even grew slightly faster than the wild type strain (Figure 5A). The expression of a translational aroP2’-‘lacZ fusion was very low in LB and in BSM amended with tyrosine (data not shown), as would be expected for a gene specifying an inner membrane protein. It has been shown previously that aroP2 expression is up-regulated in a lyophilized CF sputum medium, which is rich in amino acids [29]. Therefore, we used a synthetic CF sputum medium (SCFM; [30]) for measuring the β-galactosidase activities conferred by the aroP2’-‘lacZ fusion. Although expression was still very low under these conditions, a 2- to 4-fold higher expression of the aroP2’-‘lacZ fusion was measured in PAO1Δcrc, compared with PAO1; in the crcZ and cbrB mutants aroP2 was fully repressed (Figure 5B). The deletion of the CA motif in the fusion construct resulted in loss of regulation by Crc (Figure 5C) and confirmed that aroP2 is a target of the CbrAB/Crc system.
Figure 5. Tyrosine uptake is under CbrAB/Crc control.
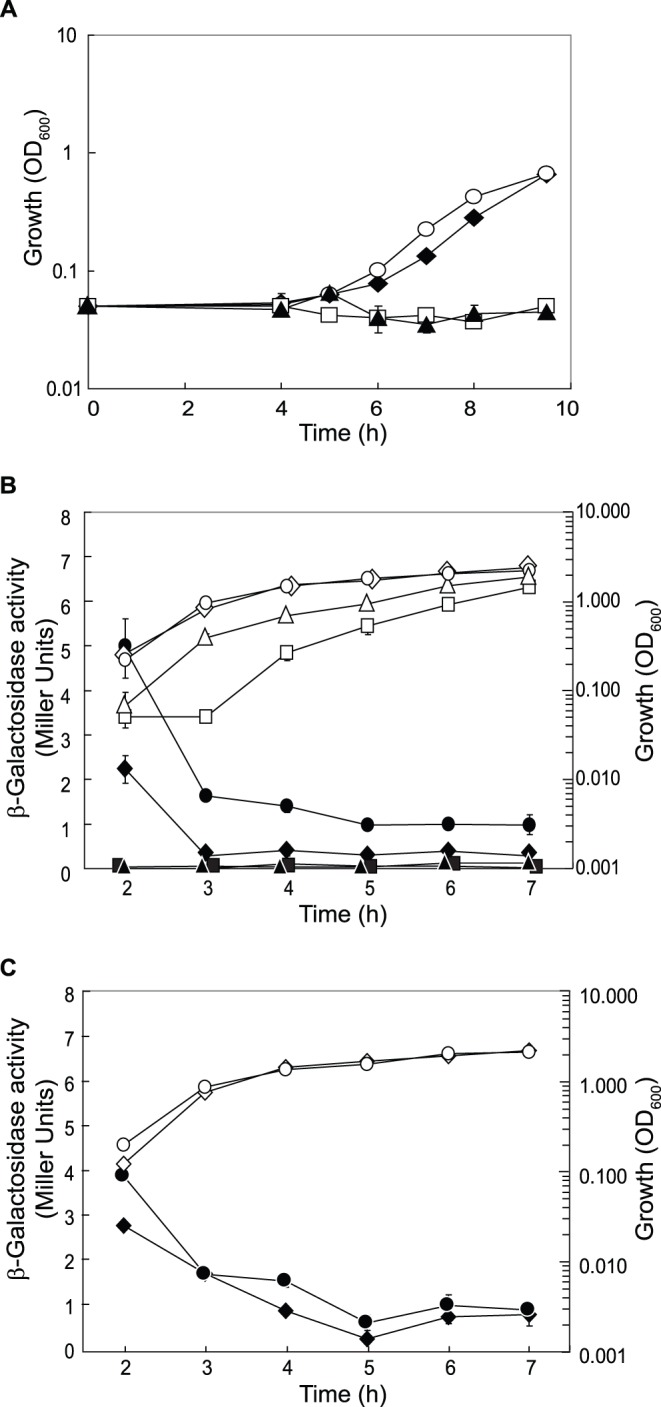
(A) Growth of PAO1 (black diamonds), PAO1ΔcbrB (black triangles), PAO1ΔcrcZ (white squares) and PAO1Δcrc (white circles) was followed in BSM supplemented with 4 mM tyrosine as the sole carbon source. (B) β-Galactosidase activities conferred by the translational aroP2’-‘lacZ fusion (pME10046) were determined in PAO1 (black diamonds), PAO1ΔcbrB (black triangles), PAO1ΔcrcZ (black squares) and PAO1Δcrc (black circles). (C) Expression of a translational aroP2’-‘lacZ fusion with a mutated CA motif (pME10047) was similarly followed in PAO1 (black diamonds) and PAO1Δcrc (black circles). Cells were grown in synthetic sputum medium (SCFM). The corresponding growth curves are shown in white symbols.
Consequences of catabolite repression for environmental adaptation of P. aeruginosa - As we have shown that the CbrAB/Crc system strongly affects the utilization and transport of various less preferred carbon and nitrogen sources, the question arises as to what benefits P. aeruginosa may obtain from this regulation in natural environments, e.g. in the CF lung where amino acids are important C sources [29], [30]. To investigate the impact of catabolite repression under such conditions, we cultivated PAO1, PAO1Δcrc, PAO1ΔcrcZ and PAO1ΔcbrB aerobically in the artificial sputum medium (SCFM). The crcZ and cbrB deletion mutants grew more slowly than the PAO1 wild type, whereas the crc deletion strain grew similarly to PAO1 (Figure 6). In this sputum medium, tyrosine and phenylalanine are important C sources and degradation of both amino acids is induced in P. aeruginosa [31]. Moreover, leucine (1.6 mM), isoleucine (1.1 mM) and valine (1.1 mM) are present at high concentrations in this medium [30]. As we have shown above, tyrosine uptake (Figure 4) and utilization of branched-chain amino acids (Figure 5) are tightly controlled by the CbrAB/Crc system. The growth patterns seen in SCFM (Figure 6) are fully consistent with this regulation. As during chronic infection P. aeruginosa forms biofilms [32], we also measured biofilm formation in PAO1, PAO1Δcrc, PAO1ΔcrcZ and PAO1ΔcbrB in SCFM using a static biofilm assay. The crcZ and cbrB mutants were impaired in biofilm formation whereas the biofilm formed by PAO1Δcrc was increased ∼5-fold, by comparison with the wild type (Figure S2). In BSM-succinate medium we observed the same pattern as that already seen in SCFM (Figure S2). However, when we used BSM medium supplemented with an intermediate (glucose) or non-preferred carbon source (mannitol), the repressing effect of Crc on biofilm formation was lost (Figure S2). In conclusion, global regulation by the CbrAB/Crc system may give the wild type P. aeruginosa a selective advantage over crcZ or cbrB mutants in the CF lung and may help the bacterium to persist in CF patients.
Figure 6. Growth of P. aeruginosa in sputum medium is under CbrAB/Crc control.
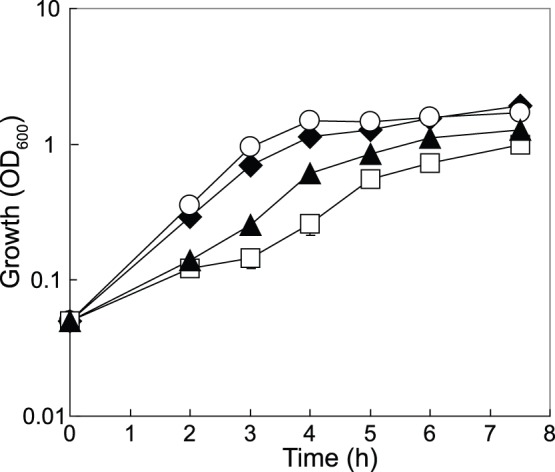
Growth curves of PAO1 (black diamonds), PAO1ΔcbrB (black triangles), PAO1ΔcrcZ (white squares) and PAO1Δcrc (white circles) were obtained in SCFM.
Discussion
In our present transcriptome analysis, the expression of ≥380 genes was affected by mutations in the CbrAB/Crc system in P. aeruginosa when we take into account data obtained in LB and in the defined medium BSM-succinate. Thus, in spite of a major output at the level of translation, the CbrAB/Crc signal transduction pathway clearly has a global impact on transcript abundance. In agreement with earlier phenotypic data [1], the present transcriptomic data indicate that most CbrB activity is linked to CrcZ activity (Figure 1) in that transcriptomic responses were similar, but not identical in cbrB and crcZ mutants (Tables S1 and S2). It is therefore possible that CbrB could act as a transcriptional regulator of genes other than the previously recognized crcZ, lipA and hutU genes [5]. However, such novel CbrB target genes remain to be identified. Whether CrcZ regulates mRNA expression independently of Crc is another question that cannot be answered by the experiments of this study.
The overlap between Crc- and CrcZ-regulated transcripts was unexpectedly low (Figure 1). There may be several reasons for this finding. In general, mRNAs are more stable when they are translated [33]. Nevertheless, in a crc mutant, derepressed translation may not always result in more stable, hence more abundant transcripts, whereas in a crcZ mutant, in which translational initiation of target mRNAs is repressed, the (negative) effects on transcript abundance may be more obvious. However, under both growth conditions used here, expression of crcZ is low and thus Crc-mediated repression of target mRNAs by Crc is strong [1]. Therefore, a crcZ mutation may result only in minor and possibly undetectable changes of the expression of some genes. As shown in Table 2, RT-qPCR (as a more sensitive technique than the microarray analysis) established that the estA, acsA, bkdA1 and aroP2 transcripts were regulated by Crc and CrcZ/CbrB in an opposite manner. However, there are also limitations. For example, bkdR did not reveal changes of transcript levels, whereas a bkdR’-‘lacZ reporter fusion was regulated (Table 2). Finally, many genes were not induced at the transcriptional level under the growth conditions used, making changes due to catabolite repression difficult to detect. Despite such limitations, we have been able to use transcriptome analysis as a tool to identify several novel targets of the CbrAB/Crc system.
The main targets of the CbrAB/Crc system seem to be metabolic transcripts, which is expected for a catabolite repression control system. Moreover, as noted before [6], [7], [34], the CbrAB/Crc system not only regulates genes for the utilization of less preferred C sources, but also dramatically influences motility and biofilm development, traits that are involved in the pathogenicity of P. aeruginosa and that depend on nutritional conditions. For example, a defect in CbrAB-mediated regulation of aromatic and branched-chain amino acid utilization affected growth and biofilm development of P. aeruginosa in sputum medium (Figures 6 and S2). In addition to aroP2 and bkdR (Figures 4 and 5), the braC gene and the braDEFG operon (encoding transport proteins with high affinity for the branched-chain amino acid leucine, isoleucine and valine and with low affinity for alanine and threonine; [35], [36]), were down-regulated in the crcZ mutant in both media and up-regulated in the crc mutant in BSM (Table 1). An incomplete CA motif (UACAACAA at +23 to +30) in the coding region of braD as well as an extended, but imperfect CA motif (ACAACAAUGACAACA at −13 to −6) upstream of the braC start codon suggests that the CbrAB/Crc system also controls these genes. However, further investigation is required to confirm this regulation.
In sputum medium a crc mutant had no growth advantage over the wild type (Figure 6) and there is currently no evidence that colonization of the CF lung by P. aeruginosa would select for crc mutants. However, both the crcZ and the cbrB mutant were partially deficient for growth in SCFM and may therefore be affected in virulence.
It has been known for some time that nutritional conditions influence biofilm development [37], [38] and that the CbrAB/Crc system is an important regulator for biofilm formation, although some published results appear to be strain-specific [6], [7], [34], [39]. For example, a PA14 Δcrc mutant produced less biofilm in minimal media containing glucose [6], [34] whereas a PAO1 Δcrc strain showed enhanced biofilm formation in LB compared with the wild type strain [7]. Our data indicate that the impact of the CbrAB/Crc system on biofilm development depends on media and carbon sources (Figure S2). In BSM medium, different carbon sources clearly affected the formation of static biofilms (Figure S2), which is consistent with different carbon sources having different regulatory effects on targets of the CbrAB/Crc system. By contrast, in LB medium the typical pattern of upregulation in the crc mutant and strong repression in the crcZ and cbrB deletion strains was not observed. In conclusion, the CbrAB/Crc system acts on a variety of different transcripts involved in biofilm development. Finding out more about the relationships between nutrient availability, catabolite repression control and regulation of virulence and biofilm development in P. aeruginosa may ultimately lead to a better understanding of the bacterium’s pathogenicity and intrinsic resistance to antimicrobial agents.
Methods
Bacterial strains and growth conditions - The strains and plasmids used in this study are listed in Table S3. Unless indicated otherwise, cells were grown in Luria Broth (LB) [12], [40] or in a minimal medium (BSM) [41] supplemented with 40 mM succinate [1]. For the investigation of special targets and growth behavior we used BSM amended with either 4 mM tyrosine, 5 mM succinate +40 mM acetate, 40 mM acetate or 23 mM leucine +25.6 mM valine +7.6 mM isoleucine as the sole carbon sources and LB supplemented with 23 mM leucine +25.6 mM valine +7.6 mM isoleucine. The synthetic sputum medium SCFM has been described by Palmer et al. [30]. When required, antibiotics were added to the media at the following concentrations: 100 µg ml−1 ampicillin for Escherichia coli and 125 µg ml−1 tetracycline for P. aeruginosa.
Transcriptome analysis - Overnight cultures of P. aeruginosa PAO1, the ΔcbrB mutant PAO6711, the Δcrc mutant PAO6673 and the ΔcrcZ mutant PAO6679 were diluted to an initial OD600 of 0.05 in 20 ml of LB or BSM amended with 40 mM succinate. The cultures were grown at 37°C with vigorous shaking until they reached an OD600 of 1.6. RNA purification, cDNA synthesis and cDNA hybridization were performed as described previously [42]. Processing of the P. aeruginosa GeneChip Array (Affymetrix) was performed at the University of Lausanne Center for Integrative Genomics. For each condition, cultures were grown in triplicate, and RNAs from these cultures were pooled before proceeding to cDNA synthesis. In addition, one biological replicate for each condition was performed on a separate day and run on separate microarray chips. Transcripts tabulated in Tables S1 and S2 meet the following criteria: (i) the P value for each transcript analyzed is ≤0.05 and (ii) the change in transcript level is ≥2.0-fold. In compliance to MIAME guidelines. The data has been deposited in the GEO database, GEO accession number: GSE33245.
Real-time quantitative PCR (RT-qPCR) – PAO1, PAO1ΔcbrB mutant PAO6711, PAO1Δcrc mutant PAO6673 and PAO1ΔcrcZ mutant PAO6679 were grown in LB medium or BSM supplemented with 40 mM succinate at 37°C with vigorous shaking until they reached an OD600 of 1.6. Cells were harvested using the RNA bacteria protect solution (QIAGEN). Total RNA was extracted with the RNA RNeasy kit (QIAGEN), treated with RQ1 DNase (Promega) to remove contaminating genomic DNA and subsequently re-purified using phenol-chlorophorm extraction. cDNA from each sample was obtained as previously described [16] for every sample 500 ng of total RNA was used. The resulting cDNAs were used as templates for qPCR and quantitated with a Bio-Rad iCycler machine using a Sybr Green Quantitect kit (QIAGEN). Fold changes were estimated by a comparative threshold cycle method with the anr gene PA1544 as a standard [43]. The primer pairs, termed gene name fw and gene name rev, used for qRT-PCR are shown in Table S4.
Construction of plasmids - To construct translational ‘lacZ fusions to estA, bkdA and bkdR, fragments of 592 bp, 354 bp and 368 bp, respectively, were amplified by PCR using the primer pairs Q67_estAfw/R67_estArev (estA), PA2246fw/PA2246rev (bkdA) and PA2247fw/PA2247rev (bkdR) (Table S4) and chromosomal DNA of strain PAO1 as template. The fragments containing the respective authentic promoter regions and the translation initiation sites were fused in-frame after the 6th codon of estA and the 4th codon of the bkdA and bkdR genes to the 8th codon of lacZ. These fragments were digested with EcoRI and BamHI and cloned into the corresponding sites of pME6015 generating pTLestA (estA’-‘lacZ), pME10049 (bkdA1’-‘lacZ) and pME10048 (bkdR’-‘lacZ). The translational acsA’-‘lacZ and aroP2’-‘lacZ fusions were constructed as described above by amplifying a 202-bp and 316-bp PCR fragment with primers AcsA1/AcsA2 and AroP21/AroP22 (Table S4), respectively. These fragments were digested with EcoRI and PstI and cloned into the corresponding sites of pME6015, resulting in plasmid pME10044 (acsA’-‘lacZ) and pME10046 (aroP2’-‘lacZ).
The CA motif mutations (TCAGTAGC instead of AAAAACAA) were introduced into pTLestA according to the QuikChange(R) site-directed mutagenesis protocol (http://www.stratagene.com/manuals/200518.pdf) with the mutagenesis primers L71_estAmut1a and M71_estAmut1b (Table S4). The parental DNA template was digested with DpnI, and the mutated plasmid was transformed into E. coli XL1-Blue, generating pTLestA-ΔCA. The same procedure was used to mutate the CA motif sequences AACAAAAACAA of acsA and AACAATAA of aroP2 with the mutagenesis primer pairs AcsAmutfw/AcsAmutrev and AroP2mutfw/AroP2mutrev (Table S4), respectively. The resulting plasmids were named pME10045 (acsA-ΔCA) and pME10047 (aroP2-ΔCA). All mutations were confirmed by sequencing.
β-Galactosidase assays - β-Galactosidase activities were quantified by the Miller method [40], using cells permeabilized with 5% toluene or 5% (v/v) chloroform and 0.01% SDS. Values shown were derived from three independent experiments and are means ± standard deviation.
Static biofilm assay - A static-culture biofilm assay in microtiter plates was performed as described previously [44]. Cells were grown in LB, SCFM or BMS medium supplemented with 40 mM succinate, glucose or mannitol, respectively, for 24 h and biofilms were stained with 0.1% crystal violet solution. The dye bound, which is proportional to the biofilm produced, was solubilized with 96% (v/v) ethanol and the absorption was photometrically measured at 540 nm (A540).
Supporting Information
β-Galactosidase activity measurements of amiE’-‘lacZ (pME9655) in PAO1 (black diamonds) and PAO1Δcrc (white circles) performed in LB.
(EPS)
Biofilm formation of PAO1 and Δcrc , ΔcrcZ , ΔcbrB mutant strains. Static biofilm development of PAO1 (black bar), PAO1Δcrc (white bar), PAO1ΔcrcZ (grey bars) and PAO1ΔcbrB (dashed bar) was measured after growth for 24 h in SCFM or BSM supplemented with 40 mM succinate (Succinate) or 40 mM glucose (Glucose) or 40 mM mannitol (Mannitol) in a polyethylene microtiter plate. The biofilm was stained with crystal violet and after stripping with 96% (v/v) ethanol the A540 was photometrically determined.
(EPS)
Transcripts which were at least two-fold differentially expressed in PAO1Δ crc compared to PAO1.
(DOC)
Transcripts which were differentially regulated in the cbrB and crcZ mutants compared to PAO1.
(DOC)
Strains and plasmids used in this study.
(DOC)
DNA oligonucleotides used in this study.
(DOC)
Acknowledgments
We thank Otto Hagenbüchle, Mélanie Dupasquier and Sylvain Pradervand for performing the microarray analyses and Karl Perron and Verena Ducret for help with the RT-qPCR experiments.
Funding Statement
Elisabeth Sonnleither was supported by the Hertha-Firnberg Research fellowship T448-B20 from the Austrian Science Fund. Karine Lapouge was supported by the Sandoz Family Foundation (Programme for academic promotion) and the Swiss National Foundation for Scientific Research (project 31003A-127587). Dieter Haas was supported by the Swiss National Foundation for Scientific Research (project 3100A0-100180). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sonnleitner E, Abdou L, Haas D (2009) Small RNA as global regulator of carbon catabolite repression in Pseudomonas aeruginosa . Proc Natl Acad Sci USA 106: 21866–21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moreno R, Marzi S, Romby P, Rojo F (2009) The Crc global regulator binds to an unpaired A-rich motif at the Pseudomonas putida alkS mRNA coding sequence and inhibits translation initiation. Nucleic Acids Res 37: 7678–7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rojo F (2010) Carbon catabolite repression in Pseudomonas: optimizing metabolic versatility and interactions with the environment. FEMS Microbiol Rev 34: 658–684. [DOI] [PubMed] [Google Scholar]
- 4. Sonnleitner E, Haas D (2011) Small RNAs as regulators of primary and secondary metabolism in Pseudomonas species. Appl Microbiol Biotechnol 91: 63–79. [DOI] [PubMed] [Google Scholar]
- 5. Abdou L, Chou H-T, Haas D, Lu C-D (2011) Promoter recognition and activation by the global response regulator CbrB in Pseudomonas aeruginosa . J Bacteriol 193: 2784–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yeung AT, Bains M, Hancock RE (2011) The sensor kinase CbrA is a global regulator that modulates metabolism, virulence, and antibiotic resistance in Pseudomonas aeruginosa . J Bacteriol 193: 918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Linares JF, Moreno R, Fajardo A, Martínez L, Escalante R, et al. (2010) The global regulator Crc modulates metabolism, susceptibility to antibiotics and virulence in Pseudomonas aeruginosa . Environ Microbiol 12: 3196–3212. [DOI] [PubMed] [Google Scholar]
- 8. Burrowes E, Baysse C, Adams C, O’Gara F (2006) Influence of the regulatory protein RsmA on cellular functions in Pseudomonas aeruginosa PAO1, as revealed by transcriptome analysis. Microbiology 152: 405–418. [DOI] [PubMed] [Google Scholar]
- 9. Brencic A, Lory S (2009) Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA. Mol Microbiol 72: 612–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brencic A, McFarland KA, McManus HR, Castang S, Mogno I, et al. (2009) The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol Microbiol 73: 434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hassan KA, Johnson A, Shaffer BT, Ren Q, Kidarsa TA, et al. (2010) Inactivation of the GacA response regulator in Pseudomonas fluorescens Pf-5 has far-reaching transcriptomic consequences. Environ Microbiol 12: 899–915. [DOI] [PubMed] [Google Scholar]
- 12. Sezonov G, Joseleau-Petit D, D’Ari R (2007) Escherichia coli physiology in Luria-Bertani broth. J Bacteriol 189: 8746–8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drew R, Haq M (2004) Lessons from the ami operon. In: Ramos J-L, editor. Pseudomonas: Virulence and gene regulation, volume 2. New York: Kluwer Academic/Plenum. 425–449.
- 14.Itoh Y, Nishijyo T, Nakada Y (2007) Histidine catabolism and catabolic regulation, In: Ramos J-L, Filloux A, editors. Pseudomonas - A model system in biology, volume 5. Dordrecht: Springer. 371–395.
- 15. Heeb S, Fletcher MP, Chhabra SR, Diggle SP, Williams P, et al. (2011) Quinolones: from antibiotics to autoinducers. FEMS Microbiol Rev 35: 247–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Görisch H (2003) The ethanol oxidation system and its regulation in Pseudomonas aeruginosa . Biochim Biophys Acta 1647: 98–102. [DOI] [PubMed] [Google Scholar]
- 17. Gliese N, Khodaverdi V, Schobert M, Görisch H (2004) AgmR controls transcription of a regulon with several operons essential for ethanol oxidation in Pseudomonas aeruginosa ATCC 17933. Microbiology 150: 1851–1857. [DOI] [PubMed] [Google Scholar]
- 18. Huang J, Sonnleitner E, Ren B, Xu Y, Haas D (2012) Catabolite repression control of pyocyanin biosynthesis at an intersection of primary and secondary metabolism in Pseudomonas aeruginosa. . Appl Environ Microbiol 78: 5016–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilhelm S, Rosenau F, Kolmar H, Jaeger KE (2011) Autotransporters with GDSL passenger domains: molecular physiology and biotechnological applications. Chembiochem 12: 1476–1485. [DOI] [PubMed] [Google Scholar]
- 20. Stuer W, Jaeger KE, Winkler UK (1986) Purification of extracellular lipase from Pseudomonas aeruginosa . J Bacteriol 168: 1070–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilhelm S, Tommassen J, Jaeger KE (1999) A novel lipolytic enzyme located in the outer membrane of Pseudomonas aeruginosa . J Bacteriol 181: 6977–6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kretzschmar U, Khodaverdi V, Adrian L (2010) Transcriptional regulation of the acetyl-CoA synthetase gene acsA in Pseudomonas aeruginosa . Arch Microbiol 192: 685–690. [DOI] [PubMed] [Google Scholar]
- 23. Nishijyo T, Haas D, Itoh Y (2001) The CbrA-CbrB two-component regulatory system controls the utilization of multiple carbon and nitrogen sources in Pseudomonas aeruginosa . Mol Microbiol 40: 917–931. [DOI] [PubMed] [Google Scholar]
- 24. Hester KL, Lehman J, Najar F, Song L, Roe BA, et al. (2000) Crc is involved in catabolite repression control of the bkd operons of Pseudomonas putida and Pseudomonas aeruginosa . J Bacteriol 182: 1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin RR, Marshall VD, Sokatch JR, Unger L (1973) Common enzymes of branched-chain amino acid catabolism in Pseudomonas putida . J Bacteriol 115: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moreno R, Martínez-Gomariz M, Yuste L, Gil C, Rojo F (2009) The Pseudomonas putida Crc global regulator controls the hierarchical assimilation of amino acids in a complete medium: evidence from proteomic and genomic analyses. Proteomics 9: 2910–2928. [DOI] [PubMed] [Google Scholar]
- 27. Madhusudhan KT, Lorenz D, Sokatch JR (1993) The bkdR gene of Pseudomonas putida is required for expression of the bkd operon and encodes a protein related to Lrp of Escherichia coli . J Bacteriol 175: 3934–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hester KL, Madhusudhan KT, Sokatch JR (2000) Catabolite repression control by crc in 2xYT medium is mediated by posttranscriptional regulation of bkdR expression in Pseudomonas putida . J Bacteriol 182: 1150–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palmer KL, Mashburn LM, Singh PK, Whiteley M (2005) Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol 187: 5267–5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmer KL, Aye LM, Whiteley M (2007) Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol 189: 8079–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Palmer GC, Palmer KC, Jorth PA, Whiteley M (2010) Characterization of the Pseudomonas aeruginosa transcriptional response to phenylalanine and tyrosine. J Bacteriol 192: 2722–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Kievit TR (2009) Quorum sensing in Pseudomonas aeruginosa biofilms. Environ Microbiol 11: 279–288. [DOI] [PubMed] [Google Scholar]
- 33. Kaberdin VR, Bläsi U (2006) Translation initiation and the fate of bacterial mRNAs. FEMS Microbiol Rev 30: 967–979. [DOI] [PubMed] [Google Scholar]
- 34. O’Toole GA, Gibbs KA, Hager PW, Phibbs PV Jr, Kolter R (2000) The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. . J Bacteriol 182: 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoshino T, Kageyama M (1980) Purification and properties of a binding protein for branched-chain amino acids in Pseudomonas aeruginosa . J Bacteriol 141: 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoshino T, Kose K (1990) Cloning, nucleotide sequences, and identification of products of the Pseudomonas aeruginosa PAO bra genes, which encode the high-affinity branched-chain amino acid transport system. J Bacteriol 172: 5531–5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49: 711–745. [DOI] [PubMed] [Google Scholar]
- 38. O’Toole GA, Kolter R (1998) Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol 28: 449–461. [DOI] [PubMed] [Google Scholar]
- 39. Amador CI, Canosa I, Govantes F, Santero E (2010) Lack of CbrB in Pseudomonas putida affects not only amino acids metabolism but also different stress responses and biofilm development. Environ Microbiol 12: 1748–1761. [DOI] [PubMed] [Google Scholar]
- 40.Miller JH (1972) Experiments in Molecular Genetics. Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
- 41. Durham DR, Phibbs PV Jr (1982) Fractionation and characterization of the phosphoenolpyruvate: fructose 1-phosphotransferase system from Pseudomonas aeruginosa . J Bacteriol 149: 534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sonnleitner E, Gonzalez N, Sorger-Domenigg T, Heeb S, Richter AS, et al. (2011) The small RNA PhrS stimulates synthesis of the Pseudomonas aeruginosa quinolone signal. Mol Microbiol 80: 868–885. [DOI] [PubMed] [Google Scholar]
- 43. Caille O, Rossier C, Perron K (2007) A copper-activated two-component system interacts with zinc and imipenem resistance in Pseudomonas aeruginosa. . J Bacteriol 189: 4561–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merritt JH, Kadouri DE, O’Toole GA (2005) Growing and analyzing static biofilms. Current Protocols in Microbiology 1B.1.1–1B.1.17. [DOI] [PMC free article] [PubMed]
- 45.Winsor GL, Lam DK, Fleming L, Lo R, Whiteside MD, et al.. (2011) Nucleic Acids Res. 39 (Database issue): D596–600. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
β-Galactosidase activity measurements of amiE’-‘lacZ (pME9655) in PAO1 (black diamonds) and PAO1Δcrc (white circles) performed in LB.
(EPS)
Biofilm formation of PAO1 and Δcrc , ΔcrcZ , ΔcbrB mutant strains. Static biofilm development of PAO1 (black bar), PAO1Δcrc (white bar), PAO1ΔcrcZ (grey bars) and PAO1ΔcbrB (dashed bar) was measured after growth for 24 h in SCFM or BSM supplemented with 40 mM succinate (Succinate) or 40 mM glucose (Glucose) or 40 mM mannitol (Mannitol) in a polyethylene microtiter plate. The biofilm was stained with crystal violet and after stripping with 96% (v/v) ethanol the A540 was photometrically determined.
(EPS)
Transcripts which were at least two-fold differentially expressed in PAO1Δ crc compared to PAO1.
(DOC)
Transcripts which were differentially regulated in the cbrB and crcZ mutants compared to PAO1.
(DOC)
Strains and plasmids used in this study.
(DOC)
DNA oligonucleotides used in this study.
(DOC)