Abstract
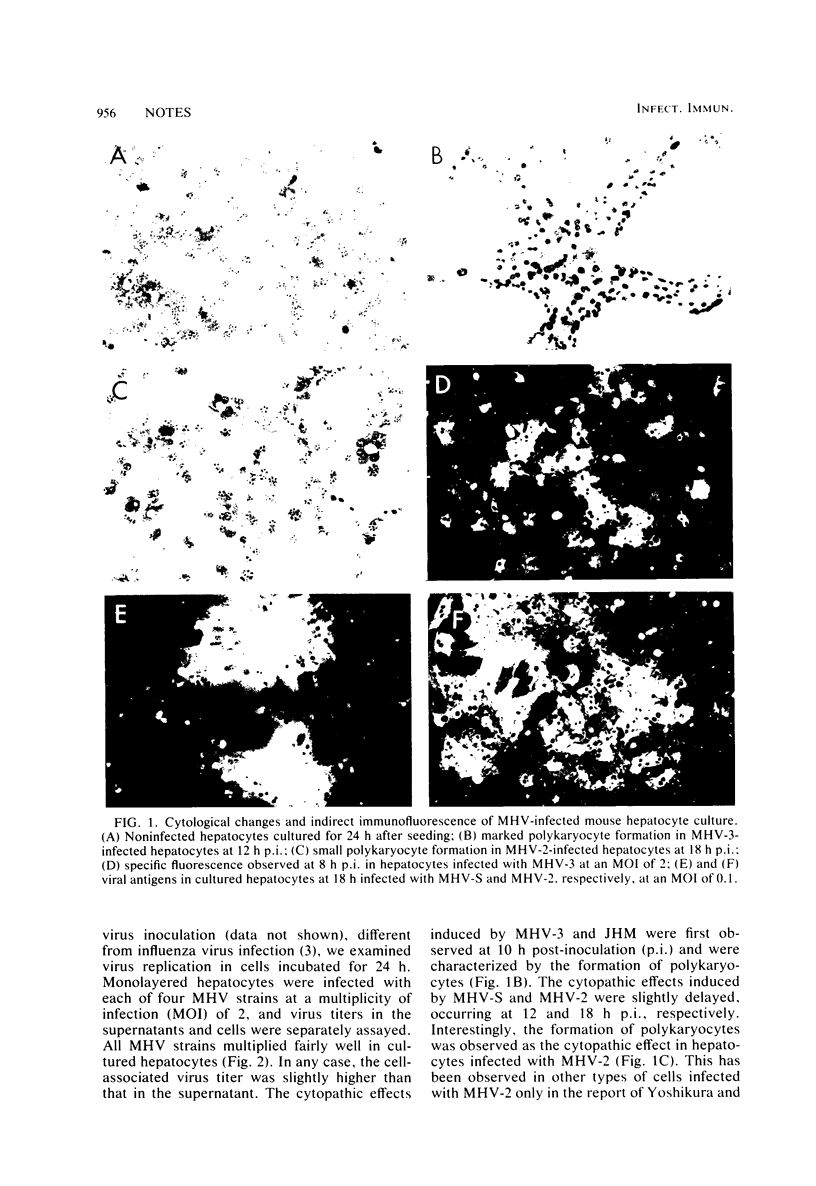
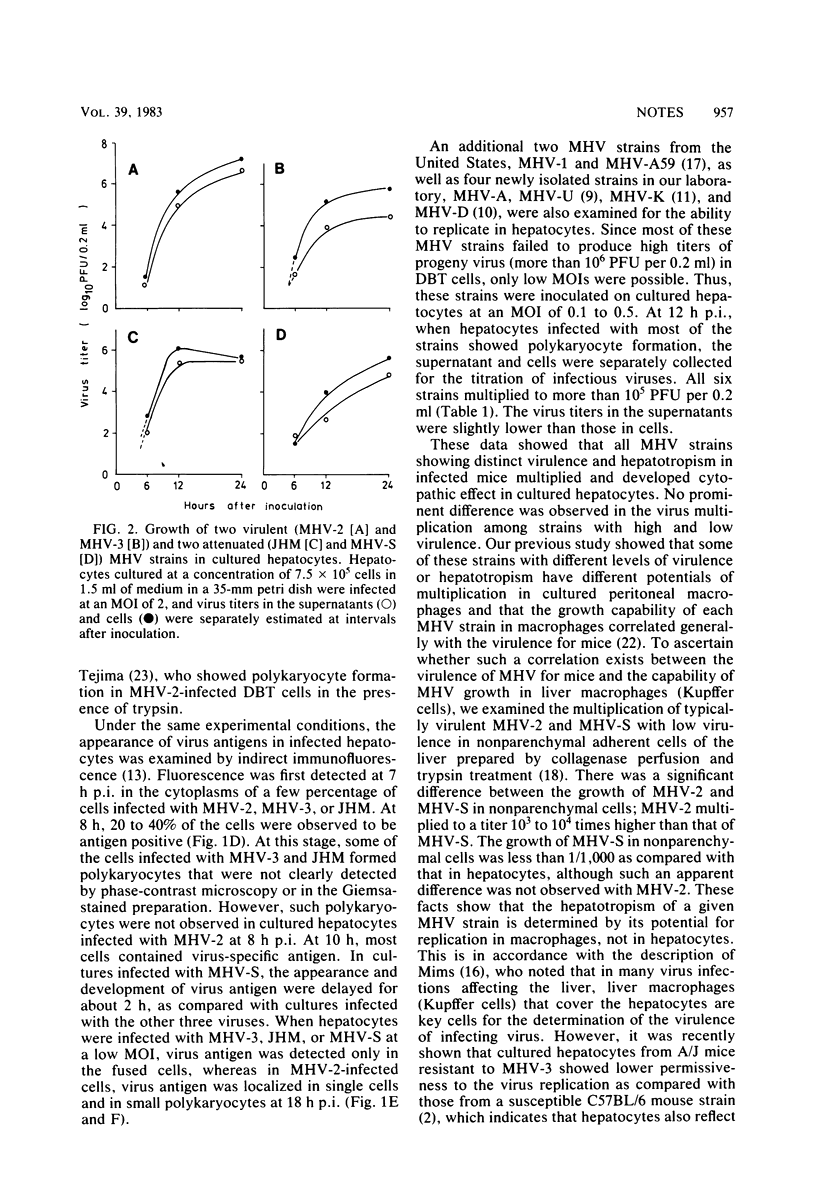
Ten strains of mouse hepatitis virus with different levels of virulence and hepatotropism were examined for the ability to replicate in cultured mouse hepatocytes. All of these viruses multiplied well in hepatocytes, attaining a maximum of 105 to 107 PFU per 0.2 ml, with cytopathic effects characterized by the formation of polykaryocytes. However, in nonparenchymal adherent cells of the liver (liver macrophages), highly virulent mouse hepatitis virus type 2 multiplied to a titer 1,000 times higher than that of mouse hepatitis virus S with a low virulence. These results suggest that the virulence of mouse hepatitis virus in infected mice is determined by its potential for replication not in hepatocytes, but in macrophages, including Kupffer cells in the liver.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheiter H., Baechi T., Haller O. Adult mouse hepatocytes in primary monolayer culture express genetic resistance to mouse hepatitis virus type 3. J Immunol. 1982 Sep;129(3):1275–1281. [PubMed] [Google Scholar]
- Arnheiter H., Haller O., Lindenmann J. Host gene influence on interferon action in adult mouse hepatocytes: specificity for influenza virus. Virology. 1980 May;103(1):11–20. doi: 10.1016/0042-6822(80)90122-1. [DOI] [PubMed] [Google Scholar]
- Arnheiter H. Primary monolayer culture of adult mouse hepatocytes -- a model for the study of hepatotropic viruses. Arch Virol. 1980;63(1):11–22. doi: 10.1007/BF01320757. [DOI] [PubMed] [Google Scholar]
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN G. T., KOPROWSKI H. Study of the mechanism of innate resistance to virus infection. J Cell Comp Physiol. 1962 Jun;59:333–373. doi: 10.1002/jcp.1030590313. [DOI] [PubMed] [Google Scholar]
- Haller O., Arnheiter H., Lindenmann J. Natural, genetically determined resistance toward influenza virus in hemopoietic mouse chimeras. Role of mononuclear phagocytes. J Exp Med. 1979 Jul 1;150(1):117–126. doi: 10.1084/jem.150.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano N., Fujiwara K., Hino S., Matumoto M. Replication and plaque formation of mouse hepatitis virus (MHV-2) in mouse cell line DBT culture. Arch Gesamte Virusforsch. 1974;44(3):298–302. doi: 10.1007/BF01240618. [DOI] [PubMed] [Google Scholar]
- Hirano N., Murakami T., Taguchi F., Fujiwara K., Matumoto M. Comparison of mouse hepatitis virus strains for pathogenicity in weanling mice infected by various routes. Arch Virol. 1981;70(1):69–73. doi: 10.1007/BF01320795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano N., Tamura T., Taguchi F., Ueda K., Fujiwara K. Isolation of low-virulent mouse hepatitis virus from nude mice with wasting syndrome and hepatitis. Jpn J Exp Med. 1975 Oct;45(5):429–432. [PubMed] [Google Scholar]
- Ishida T., Taguchi F., Lee Y. S., Yamada A., Tamura T., Fujiwara K. Isolation of mouse hepatitis virus from infant mice with fatal diarrhea. Lab Anim Sci. 1978 Jun;28(3):269–276. [PubMed] [Google Scholar]
- Ishida T., Tamura T., Ueda K., Fujiwara K. Hepatosplenic myelosis in naturally occurring mouse hepatitis virus infection in the nude mouse. Nihon Juigaku Zasshi. 1978 Dec;40(6):739–743. doi: 10.1292/jvms1939.40.739. [DOI] [PubMed] [Google Scholar]
- KANTOCH M., WARWICK A., BANG F. B. The cellular nature of genetic susceptibility to a virus. J Exp Med. 1963 May 1;117:781–798. doi: 10.1084/jem.117.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmann J., Deuel E., Fanconi S., Haller O. Inborn resistance of mice to myxoviruses: macrophages express phenotype in vitro. J Exp Med. 1978 Feb 1;147(2):531–540. doi: 10.1084/jem.147.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Taguchi F., Aiuchi M., Fujiwara K. Age-dependent response of mice to a mouse hepatitis virus, MHV-S. Jpn J Exp Med. 1977 Apr;47(2):109–115. [PubMed] [Google Scholar]
- Taguchi F., Yamada A., Fujiwara K. Resistance to highly virulent mouse hepatitis virus acquired by mice after low-virulence infection: enhanced antiviral activity of macrophages. Infect Immun. 1980 Jul;29(1):42–49. doi: 10.1128/iai.29.1.42-49.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F., Yamaguchi R., Makino S., Fujiwara K. Correlation between growth potential of mouse hepatitis viruses in macrophages and their virulence for mice. Infect Immun. 1981 Dec;34(3):1059–1061. doi: 10.1128/iai.34.3.1059-1061.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H., Tejima S. Role of protease in mouse hepatitis virus-induced cell fusion. Studies with a cold-sensitive mutant isolated from a persistent infection. Virology. 1981 Sep;113(2):503–511. doi: 10.1016/0042-6822(81)90178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]