Abstract
Recent studies have shown that a tumor-supportive microenvironment is characterized by high levels of pro-inflammatory and pro-angiogenic eicosanoids derived from omega-6 (n−6) arachidonic acid (AA). Although the metabolic pathways (COX, LOX, and P450) that generate these n−6 AA eicosanoids have been targeted, the role of endogenous AA production in tumorigenesis remains unexplored. Delta-6 desaturase (D6D) is the rate-limiting enzyme responsible for the synthesis of n−6 AA and increased D6D activity can lead to enhanced n−6 AA production. Here, we show that D6D activity is upregulated during melanoma and lung tumor growth and that suppressing D6D activity, either by RNAi knockdown or a specific D6D inhibitor, dramatically reduces tumor growth. Accordingly, the content of AA and AA-derived tumor-promoting metabolites is significantly decreased. Angiogenesis and inflammatory status are also reduced. These results identify D6D as a key factor for tumor growth and as a potential target for cancer therapy and prevention.
Introduction
The identification of factors that modulate tumorigenesis is crucial for cancer prevention and treatment. Most studies on cancer drug discovery have tried to target cell proliferation using cell- based screening systems to identify anti-cancer compounds; however, the translation of such discoveries into cancer therapy has had limited success [1], [2]. Current research shows that targeting cancer metabolism or factors that modulate the tumor microenvironment may be promising venues for cancer therapy [3], [4].
Arachidonic acid (AA), an omega-6 (n−6) polyunsaturated fatty acid, is converted through three major pathways– the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 epoxygenase pathways–into bioactive lipid mediator eicosanoids, including prostaglandins (PGs), leukotrienes (LTs), and epoxyeicosatrienoids (EETs), respectively [5], [6] (Figure S1). These metabolites have crucial roles in chronic inflammation and cancer [5]–[7]. Increased AA metabolism and eicosanoid formation is a common feature of various types of cancer cells [8], [9]. AA-derived pro-inflammatory eicosanoids, particularly PGE2 and LTB4 which are produced by tumor cells and their surrounding stromal cells, are key mediators in their crosstalk and can accelerate tumor growth and metastasis through several mechanisms [5], including: 1) directly activating their receptors on tumor cells to induce cell proliferation, survival, migration, and invasion through multiple signaling pathways in both autocrine and paracrine manners, 2) directly inducing cancer cells to secrete growth factors, pro-inflammatory mediators and angiogenic factors that turn a normal microenvironment into one that supports tumor growth and spread, and 3) directly binding receptors on stromal cells to promote a tumor-supportive microenvironment by inducing angiogenesis and evading attack by the immune system [5]. Certain hydroxyeicosatetraenoic acids (HETEs) and EETs derived from AA through the LOX and cytochrome P450 epoxygenase pathways have also been shown to promote angiogenesis, tumor growth and metastasis [10], [11]. Evidently, AA metabolites play a significant role in tumorigenesis, mainly through the promotion of inflammation and angiogenesis.
Given the importance of AA metabolites in cancer biology, many studies have developed anti-cancer drugs targeting the major pathways (COX, LOX, or P450) of AA metabolism [5]. Although some of the drugs targeting eicosanoid signaling, such as aspirin and celecoxib, have shown efficacy in suppressing cancer, they have also been associated with unacceptable toxicity [5], [12]; thus, other strategies must be identified for targeting AA metabolism. Regarding tumorigenesis, the role of endogenous AA production, which occurs upstream of these metabolic pathways (Figure S1), remains unexplored.
AA, which is highly abundant in the modern Western diet and in body tissues [13], [14], is synthesized from linoleic acid (LA) through a series of elongation and desaturation enzyme systems. Among these enzymes, delta-6 desaturase (D6D) is the first and rate-limiting step in the production of AA (Figure S1). Thus far, the role of D6D in cancer development has not been established. Here, we show that D6D activity is up-regulated during the growth of melanoma and lung tumors and that suppressing D6D activity dramatically reduces tumor growth. Our results suggest that D6D is a key factor for tumor growth, and therefore, it is a potential target for cancer therapy and prevention.
Methods
Ethics Statement
All animal procedures were carried out in accordance with the guidelines set by the Massachusetts General Hospital Animal Committee and with IACUC approval (Protocol # 2010N000038). All efforts possible were made to limit animal suffering.
D6D RNAi Lentivirus Vector and Stable Cell Lines
The Bam HI site of the transfer plasmid pHRST-IRES-GFP was inactivated by blunting the sticky ends with Klenow I and religating with T4 DNA ligase. Then, the RNAi lentivirus vector pLu6-RNAi was obtained by inserting two copies of the hU6 promoter into the Eco RI site of the plasmid. The D6D RNAi lentivirus vector pLu6-RNAi-D6D was obtained by ligating short hairpin mouse D6D oligonucleotides with the large fragment of pLu6-RNAi after Bam HI and Xho I digestion and agarose gel electrophoresis (Figure S2). 293T cells (ATCC: CRL-11268, Manassas, VA, authenticated prior to accession by DNA fingerprinting) were transfected with pLu6-RNAi or pLu6-RNAi-D6D transfer plasmid and pHDM-G, pHDM-tat1b, pHDM-Hgpm2, and pRC/CMV-rev1B helper plasmids using SuperFect transfection reagent. Twenty-four and 48 hours after transfection, the lentivirus-containing supernatant was harvested and added to B16-F0 (ATCC: CRL-6322, Manassas, VA) and LLC cells (ATCC: CRL-1642, Manassas, VA) in a media supplemented with 8 µg/ml polybrene. The GFP-positive cells of vector control and D6D-RNAi were sorted on FACSAriaII (BD Biosciences (Billerica, MA) 7 days after virus infection.
Mouse Tumor Models
Female C57BL6 mice were used for this study. Animals were fed a diet high in n−6 and very low in n−3 fatty acids (Table S1) (Mod AIN-76A with safflower oil; Land O’Lakes Purina Feed, LLC, Richmond, IN) until the desired age (10–12 weeks) for experiments was reached. Each mouse was injected subcutaneously into both sides of the lateral abdomen with 2×106 B16-F0 or LLC cells suspended in 50 µl of PBS. To obtain tumor tissues of different sizes, the mice were sacrificed and the tumor tissues were harvested at 2- to 4-day intervals. To investigate the antitumor effects of SC-26196, wild type B16-F0 or LLC cancer cell-bearing mice were intragastrically injected with SC-26196 (100 mg/kg body weight/day; the SC-26196 was kindly provided by the Chemical Synthesis and Drug Supply Program (CSDSP) of the National Institute of Mental Health (NIMH), Bethesda, MD, USA) that was suspended in 0.5% methyl cellulose. Tumor volume, based on caliper measurements, was calculated every day (B16-F0) or every 2 days (LLC) according to the following formula: tumor volume = the shortest diameter2× the largest diameter×0.5. After 14 days (B16-F0) or 25 days (LLC) of inoculation, the mice were sacrificed, and the tumor tissues were harvested and stored at −70°C.
Analysis of Fatty Acids and Eicosanoids
The fatty acid composition of cultured cells and tissues was analyzed by gas chromatography as previously described [15]. For the measurement of eicosanoids, a quantity of 50 mg of tumor tissue was homogenized in 2 ml water on ice for 45 s. Methanol and water were added to make the total 3 ml 15% methanol. The homogenate was incubated for 1 h on ice after adding 30 ng D4-PGE2 and 300 ng D4-LA (internal standards) and vortexing. The pH was adjusted to 3.0 with 0.2 M HCl. The mixture was loaded on a SPE column preconditioned with 2 ml methanol and 2 ml water. The SPE column was washed with 1 ml water, and the eicosanoids were eluted with 3 ml ethyl acetate. The ethyl acetate solution was dried under nitrogen. The residue was dissolved in 200 µl methanol, and 50 µl were subjected to LC/MS analysis. The Agilent 1200 Series Liquid Chromatography/Mass Selective Detector (LC/MSD) system was used for separation and detection. A gradient chromatographic separation was performed on a ZORBAX Eclipse XDB-C18 column (Agilent, 5 µm, 75×4.6 mm) at 25°C. The detection was made in the negative mode. D4-PGE2 was used as an internal standard for quantification of all eicosanoids. The concentrations of eicosanoids in the samples were calculated by comparing their ratios of the peak areas of the compounds to the internal standards (Figure S3).
Angiogenesis Assay
Microvessel density in tumors was determined by immunohistological staining using an anti-CD31 antibody and expressed as a percentage of CD31 stained area per section, as described previously [16]. An in vivo angiogenesis assay was performed as described by Ito et al. [17] with the following modifications. Geltrex™ Basement Membrane Matrix 0.4 mL premixed with bFGF (5 µg/ml) with and without 100 µM SC-26196 was injected subcutaneously into C57BL6 mice (3 mice/group). Mice were sacrificed 7 days after injection and dissected to expose the implants for recording.
Statistical Analysis
Comparisons were made between the 2 treatments using Student’s t tests. Differences in tumor growth rate between the control and treatment (D6D-RNAi or D6D inhibitor) groups or between B16 melanoma and LLC lung cancer groups was evaluated by two-way repeated measures ANOVA analyses followed by Bonferroni tests. Linear regression analysis was used for determining the significance of associations between log-transformed tumor size and D6D activity or expression. Differences were considered significant at the level of P<0.05. Statistical analysis was performed using GraphPad Prism 5 (La Jolla, CA).
Results
D6D Activity is Higher in Tumor Tissue than in Adjacent Non-tumor Tissue
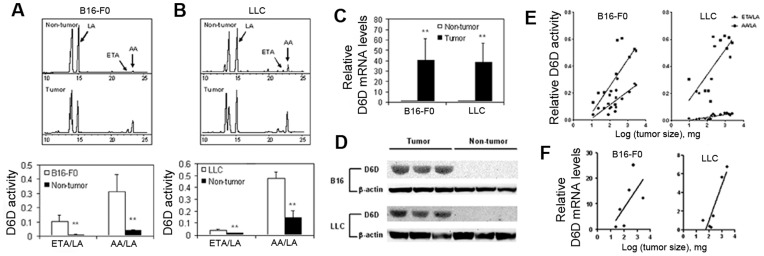
To determine whether there is a difference in D6D activity between tumor and non-tumor tissues, we analyzed the activity of biomarkers of the D6D enzyme and expression levels in tumor and non-tumor tissues from B16 melanoma and Lewis lung cancer (LLC) tumors implanted in C57B6 mice. Since D6D catalyzes the first rate-limiting step of the enzymatic conversion of LA to AA, with the formation of eicosatrienoic acid (ETA) as an intermediate product, the ratios of ETA to LA and AA to LA reflect the activity of the biomarkers of D6D [18]. The results show that the tumor tissue content of AA was 4 times greater for B16 melanoma ( Figure 1A ) and 2 times greater for LLC tumors ( Figure 1B ), compared to adjacent non-tumor tissues. Accordingly, the ETA/LA and AA/LA ratios were significantly higher in both tumor tissues than in their adjacent non-tumor tissues ( Figures 1A, 1B ). To determine whether the higher D6D activity observed was due to higher expression of D6D, we measured D6D mRNA and protein levels. As seen in Figure 1 , the D6D mRNA expression levels were 40 times higher in tumor tissues than in adjacent non-tumor tissues ( Figure 1C ) whereas D6D protein was abundantly expressed in tumor tissues but almost undetectable in adjacent normal tissues ( Figure 1D ). These data indicate that D6D is up-regulated in tumor tissue.
Figure 1. D6D activity is higher in tumor tissue than in adjacent non-tumor tissue.
(a) The activity of biomarkers of D6D in B16 melanoma. Upper: Gas chromatography showing the differences in LA, ETA and AA content between B16 melanoma and adjacent non-tumor tissue. Lower: The ratios of ETA/LA and AA/LA indicate D6D enzyme activity. **P<0.01, n = 4. (b) Activity of biomarkers of D6D in LLC tumors. Upper: Gas chromatography showing the differences in LA, ETA and AA content between LLC tumor and adjacent non-tumor tissue. Lower: The ratios of ETA/LA and AA/LA indicate D6D enzyme activity. **P<0.01, n = 4. (c) D6D mRNA levels in tumor and adjacent non-tumor tissue. **P<0.01, n = 3. (d) Western blot showing D6D protein levels in tumor and adjacent non-tumor tissue. (e) Correlation of the activity of biomarkers of D6D (based on ETA/LA and AA/LA ratios) with tumor size. (f) Correlation of D6D mRNA levels with tumor size.
We also examined whether there was a correlation between tumor size and D6D activity. The activity of the biomarkers of D6D was analyzed in B16 and LLC tumors of different sizes. As shown in Figure 1E , the ETA/LA and AA/LA ratios were positively correlated with the sizes of both the B16 and LLC tumors (ETA/LA in B16 melanoma: P = 0.0015, r 2 = 0.58; AA/LA in B16 melanoma: P = 0.0025, r 2 = 0.55; ETA/LA in LLC tumors: P = 0.0001, r 2 = 0.70; AA/LA in LLC tumors: P = 0.0017, r 2 = 0.54). D6D mRNA expression levels were also correlated with tumor size in the B16 melanoma (P = 0.0017, r 2 = 0.93) and LLC tumors (P = 0.0139, r 2 = 0.81) ( Figure 1F ). These results suggest a positive correlation between the activity of the biomarkers of D6D and tumor growth.
Reducing D6D Activity Suppresses Tumor Growth
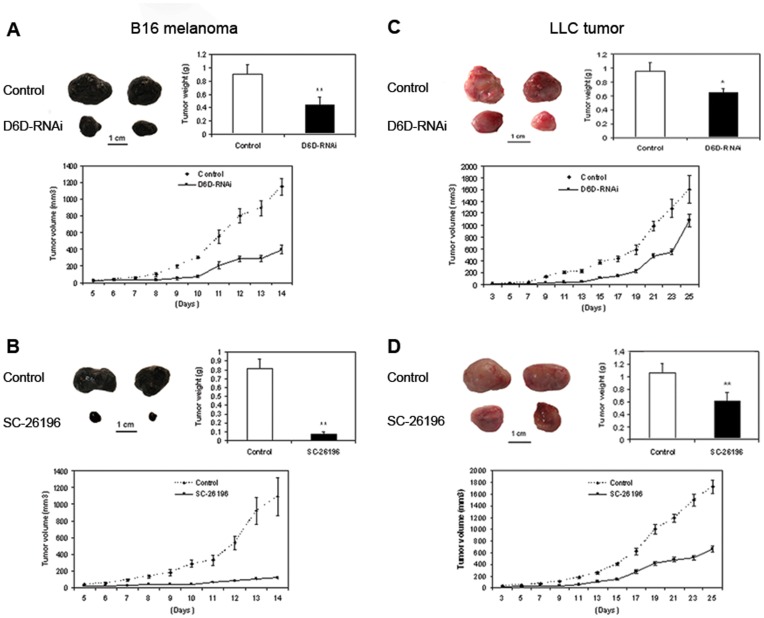
To examine the role of D6D in tumorigenesis, we reduced D6D activity either by knocking down D6D expression with RNAi or by inhibiting D6D enzyme activity with SC-26196, a highly selective inhibitor of D6D [19]. We then measured the growth rate of the B16 melanoma and LLC tumors in mice. Our data indicate that treatment with RNAi or SC-26196 can effectively reduce D6D activity (Figures S4, S5 and S6).
D6D-RNAi or the D6D selective inhibitor SC-26196 remarkably suppressed the growth of the B16 melanoma and LLC tumors ( Figure 2 ). For the B16 melanoma model, all control groups of mice developed palpable B16 melanoma tumors by day 7 whereas the treatment groups (D6D-RNAi or SC-26196) did not develop palpable tumors until days 10 ( Figure 2A ) and 12 ( Figure 2B ), respectively, over an observation period of 14 days. The tumor growth rate of B16 melanoma treated with D6D-RNAi or SC-26196 was much slower than that of the control group ( Figures 2A, 2B ). The percent inhibition of B16 melanoma growth (based on tumor weight after harvest) by D6D-RNAi and SC-26196 was 53±13% (P<0.01) and 91±6% (P<0.01), respectively. The mice treated with SC-26196 showed no signs of significant toxicity, which may include reduced mobility, reduced body weight, piloerection, hunched-back posture, anorexia, diarrhea, or somnolence.
Figure 2. Reducing D6D expression or activity suppresses tumor growth.
(a) Effect of D6D-RNAi knockdown on B16 melanoma growth. **P<0.001; n = 8 for both groups. (b) Effect of the D6D selective inhibitor SC-26196 on B16 melanoma growth. **P<0.0001; control, n = 7; treated, n = 8. (c) Effect of D6D-RNAi knockdown on LLC tumor growth. *P<0.05; n = 8 for both groups. (d) Effect of the D6D selective inhibitor SC-26196 on LLC tumor growth. **P<0.01; control, n = 7; treated, n = 10. (a–d) Upper left: Representative tumor sizes. Upper right: Average tumor weight at the end of the experiment. Lower: Tumor growth rate during the 14-day experimental period.
Similarly, for the LLC tumor model, all control groups of mice developed palpable tumors by day 7 whereas the treatment groups (SC-26196 or D6D-RNAi) developed palpable tumors by days 13 ( Figure 2C ) and 15 ( Figure 2D ), respectively, over an observation period of 25 days. The growth rates of LLC tumors treated with D6D-RNAi or SC-26196 were both significantly slower when compared to that of the control groups ( Figures 2C, 2D ). The percent inhibition of LLC tumor growth (based on tumor weight after harvest) by D6D-RNAi and SC-26196 were 32±6% (P<0.05) and 42±5% (P<0.01), respectively. These data indicate that reduction of D6D activity is highly effective in suppressing tumor growth, especially for B16 melanoma.
Decreased D6D Reduces the Levels of AA and AA-derived Eicosanoids in Tumor Tissue
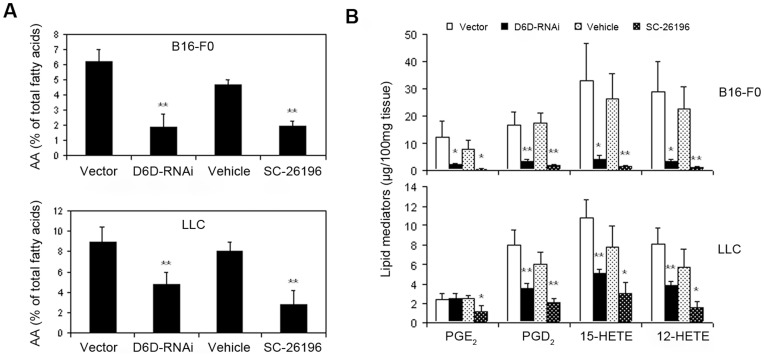
To test whether decreased D6D results in reduced production of AA and its metabolites in tumor tissue, we measured the levels of AA and AA-derived eicosanoids in tumor tissues by gas chromatography (GC) and liquid chromatography-mass spectrometry (LC-MS). As shown in Figure 3A and Table 1 , the tumor content of AA decreased by 47–69% in D6D-RNAi- or SC-26196-treated B16 melanoma and LLC tumors as compared to the control groups. The levels of AA-derived eicosanoids (including PGD2, PGE2, 12-HETE, and 15-HETE) decreased by 80–95% in D6D-RNAi- or SC-26196-treated B16 melanoma compared to the control groups ( Figure 3B ). A significant reduction of AA-derived eicosanoids was also observed in D6D-RNAi- and SC-26196-treated LLC tumors; however, the degree of inhibition was lower compared with that observed in B16 melanoma. These results are consistent with the inhibitory effects of reduced D6D on tumor growth in the treated B16 and LLC tumors.
Figure 3. Decreased D6D reduces the levels of AA and AA-derived eicosanoids in tumor tissue.
Knocking down D6D (with RNAi) or inhibiting D6D (with selective inhibitor SC-26196) decreased (a) AA and (b) AA metabolite levels in mouse models of B16 melanoma and LLC. *P<0.05; **P<0.01; n = 3–5.
Table 1. Fatty acid composition of B16 and LLC tumor tissues (% of total fatty acids).
| C18∶1 (OA) | C18∶2n6(LA) | C18∶3n3 (ALA) | C20∶3n6 (ETA) | C20∶4n6(AA) | C20∶5n3 (EPA) | C22∶4n6 (DTA) | C22∶5n6 (DPAn6) | C22∶5n6 (DPAn3) | C22∶6n6 (DHA) | ||
| B16 | Vehicle | 24.58±1.55 | 16.41±4.31 | 0.12±0.04 | 1.54±0.16 | 4.66±0.32 | UD | 0.64±0.06 | 0.03±0.04 | 0.12±0.01 | 0.37±0.04 |
| SC-26196 | 27.3±2.24 | 18.53±2.81 | 0.13±0.02 | 1.01±0.28a | 1.94±0.31b | UD | 0.27±0.03b | UD | UD | 0.04±0.07b | |
| Vector | 24.05±1.63 | 12.63±1.48 | 0.14±0.02 | 1.71±0.24 | 6.18±0.67 | 0.18±0.04 | 0.66±0.09 | UD | 0.17±0.08 | 0.31±0.14 | |
| RNAi | 30.28±3.21a | 19.75±1.42b | 0.11±0.05b | 0.88±0.29b | 2.01±0.64b | 0.12±0.09 | 0.06±0.1b | UD | UD | 0.07±0.09b | |
| LLC | Vehicle | 11.82±1.18 | 15.25±1.23 | 0.9±0.35 | 0.67±0.09 | 7.98±0.78 | 0.09±0.04 | 2.94±0.54 | 0.79±0.47 | 0.55±0.09 | 2.11±0.31 |
| SC-26196 | 19.18±1.42b | 25.54±3.98a | 1.02±0.76 | 0.43±0.29 | 2.76±1.17b | 0.03±0.01a | 0.79±0.32b | 0.16±0.08 | 0.28±0.13 | 0.56±0.25b | |
| Vector | 13.95±1.95 | 16.59±2.22 | 1.2±0.46 | 0.68±0.13 | 8.95±1.47 | UD | 4.51±0.58 | UD | 0.25±0.36 | 1.35±0.39 | |
| RNAi | 21.28±3.81b | 21.39±3.18b | 2.29±0.77a | 0.42±0.14b | 4.73±1.17b | UD | 2.46±0.44b | UD | UD | 0.55±0.18b |
The data are expressed as means ± SD. UD: undetectable.
P<0.05,
P<0.01, compared to vehicle or vector control. Student’s t-test.
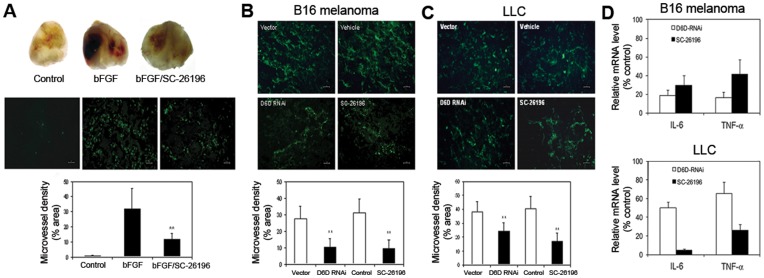
Decreased D6D Suppresses Tumor Angiogenesis and Inflammation
Because D6D inhibition does not seem to significantly affect cell proliferation in in vitro models (Figure S7), we suspect that the anti-tumor effects of D6D inhibition may be due to its influence on characteristics of the tumor microenvironment, such as angiogenesis and inflammation. Therefore, we examined whether decreased D6D affects the status of tumor angiogenesis and inflammation. The effects of D6D inhibition on angiogenesis status were measured by both a Matrigel plug assay and immunohistological staining (IHS). As shown in Figure 4A , SC-26196 at 100 µM significantly inhibited basic fibroblast growth factor (bFGF)-induced angiogenesis in a Matrigel plug assay by 64% (P<0.01). Furthermore, the angiogenesis evaluated by IHS was significantly decreased in D6D-RNAi- or SC-26196-treated B16 melanoma ( Figure 4B ) and LLC tumors ( Figure 4C ) compared to their respective control groups (P<0.01). The inhibitory effects on angiogenesis in treated B16 melanoma were stronger than those in treated LLC tumors. The inflammation status in B16 melanoma and LLC tumors with and without D6D inhibition was determined by measuring gene expression of the inflammatory cytokines IL-6 and TNF-alpha. As shown in Figure 4D , knocking down or inhibiting D6D reduced mRNA expression of IL-6 and TNF-alpha expression in B16 and LLC tumors, suggesting a reduced inflammatory state in the treated tumors. The results support an anti-angiogenic and anti-inflammatory effect of D6D inhibition.
Figure 4. Decreased D6D suppresses tumor angiogenesis and inflammation.
(a) SC-26196, a D6D selective inhibitor, at 100 µM significantly inhibited basic fibroblast growth factor (bFGF)-induced angiogenesis in a Matrigel plug assay (**P<0.01; n = 3). (b, c) Immunohistological staining of microvessel density in tumors. Knocking down D6D (with RNAi) or inhibiting D6D (with selective inhibitor SC-26196) reduced angiogenesis in (b) B16 melanoma and (c) LLC tumors (**P<0.01; n = 3). (d) The mRNA expression of the inflammatory factors interleukin-6 (IL-6) and TNF-α is down-regulated in B16 melanoma and LLC lung tumors treated with D6D-RNAi or SC-26196, shown here by percent of control. **P<0.01; n = 4.
Discussion
Tumor initiation and progression require a tumor-supporting microenvironment, such as inflammation and angiogenesis [4], [5]. Metabolites of AA have long been known to be critical pro-angiogenic and pro-inflammatory mediators [5]. Therefore, inhibitors of cyclooxygenase such as COX-2 have been a subject of intense research, and COX-2 has been well recognized as an anti-cancer therapy target [5], [8], [9]. However, there is little information on whether the endogenous synthesis of AA affects eicosanoid production and tumorigenesis. In this study, we show that D6D, the rate-limiting enzyme for AA synthesis, is up-regulated in tumor tissues and that suppression of its expression or activity results in a remarkable reduction of tumor growth associated with a decrease in AA-derived eicosanoids, angiogenesis, and inflammation in tumor tissues. These results demonstrate that D6D is a potentially critical factor for tumorigenesis and that targeting this enzyme is effective in suppressing tumor growth.
D6D catalyzes the first and rate-limiting step of the conversion of essential fatty acids, which is converting n−6 linoleic acid (LA, 18∶2n−6) and n−3 α-linolenic acid (ALA, 18:n−3) to the longer chain polyunsaturated fatty acids (LC-PUFA) arachidonic acid (AA, 20∶4n−6) and eicosapentaenoic acid (EPA, 20∶5n−3), respectively. These two classes of PUFA compete with each other for the same metabolizing enzymes but their metabolites are functionally distinct and often have opposing physiological effects [6], [7], [20]. For example, the n−6 PUFA-derived metabolites promote inflammation and angiogenesis, whereas those derived from n−3 PUFA have anti-inflammatory and anti-angiogenic properties [7], [21], [22]. Although D6D prefers ALA as a substrate [23], LA (the n−6 substrate of D6D) is much more abundant in the modern Western diet and body tissues than ALA (the n−3 substrate of D6D), with an n−6/n−3 fatty acid ratio of >10∶1 [13], [14]. Therefore, high levels of endogenous AA are synthesized and contribute to eicosanoid production. It has been suggested that endogenous and exogenous AA are metabolized differently, with more endogenous AA being metabolized into pro-inflammatory eicosanoids [24]. Thus, increased D6D predominantly favors the LA-AA pathway and greatly increases the amount of endogenous AA available for the production of pro-cancer eicosanoids.
Our findings that the expression and activity of the biomarkers of D6D and the levels of AA are remarkably higher in tumor than adjacent normal tissues, as well as our observation that tumor size is positively correlated with higher activity of the biomarkers of D6D activity, suggest that D6D plays a key role in tumorigenesis. Our consistent results in the increased expression and functional activity of D6D in two different cancer types (melanoma and lung cancer) point to the possibility that up-regulation of D6D is a common characteristic of malignant tumors. This possibility is supported by previous studies in humans using microarray analysis that have shown a significant difference in D6D mRNA between tumor and normal tissues (>2-fold higher in tumors than in normal tissue) from patients with breast cancer or brain tumors [25], [26]. Nevertheless, further study is warranted to examine the D6D status of human tumor samples of various cancer types. We noticed that there was a considerable amount of AA in adjacent normal tissues in the context of almost undetectable D6D expression. We assumed that this AA was synthesized in other tissues or organs (e.g., the liver) and transferred through the circulation system. The mechanisms by which D6D is up-regulated during tumor development remain to be explored. We speculate that D6D is up-regulated by increased activity of SREBP-1c or PPARα in a tumor microenvironment characterized by hypoxia and aberrant energy metabolism. Previous studies have shown that D6D expression may be regulated by SREBP-1c [27] and that SREBP-1c can be stimulated under hypoxic conditions via hypoxia inducible factor-1alpha (HIF-1α) [28]. In addition, SREBP-1c is the key factor for up-regulating fatty acid synthesis in many cancers [29]. Given the fact that hypoxia and increased ROS are common conditions in the tumor microenvironment, it is possible that D6D expression in tumors results from the following pathway: Hypoxia/ROS → HIF-1α → SREBP-1c → D6D. Thus, the discovery of increased D6D activity in tumors opens up a new field of investigation in cancer biology. Our findings suggest that increased expression and activity of D6D is potentially a new cancer biomarker.
Our results showing that inhibition of D6D activity, by either RNAi-knockdown or a selective inhibitor, is highly effective in suppressing tumor growth indicate that D6D is a target for cancer therapy. Given that D6D is the rate-limiting enzyme for AA synthesis and that LA is the major substrate for generating AA in our bodies, it is conceivable that the activity of D6D is critical for the production of AA and AA-derived pro-cancer eicosanoids and thereby affects tumorigenesis. From knowledge of these biochemical pathways, it is logical to speculate that D6D inhibition was effective in this study due to its inhibition of AA and its metabolites. D6D inhibition, which has a target upstream of AA metabolism, is more efficient in reducing pro-cancer eicosanoid production compared to the inhibition of individual pathways such as COX, LOX, or P450 (by which pro-cancer AA metabolites form), thus making D6D inhibition potentially more effective in suppressing cancer development. Thus far, therapeutic agents that target AA production/D6D activity do not exist, so the development of such drugs may provide effective therapeutic options, as demonstrated in the present study by the high efficacy of SC-26196, a selective D6D inhibitor. This idea also supported by a previous study showing that SC-26196 impeded intestinal tumorigenesis by inhibiting the synthesis of AA [30].
During treatment with SC-26196, we did not observe any signs of toxicity in the mice such as reduced mobility, piloerection, hunched-back posture, etc. In addition, D6D-RNAi and SC-26196 at concentrations of up to 128 µM did not show significant growth suppression in both B16 and LLC cells in vitro (Figure S7), suggesting that blocking D6D is not directly toxic to cancer cells. However, cell proliferation is not necessarily related to tumor growth and is probably not an accurate measure of the effects of D6D inhibition on cancer. Rather, the anti-tumor effect of D6D inhibition is likely due to its impact on the tumor microenvironment, such as angiogenesis and inflammation, which are the key factors that facilitate tumor growth and survival [4], [5]. It is well documented that AA-derived eicosanoids play a central role in inflammation [5]–[7], [31]. Inflammatory cells (e.g., macrophages, neutrophils, natural killer cells, etc.) and factors (e.g., PGE2, matrix metallopeptidases, interleukins, chemokines, etc.) are well-known to induce angiogenesis [32]–[34]. A growing body of evidence has demonstrated that AA-derived eicosanoids stimulate angiogenesis by up-regulating angiogenic factors. PGE2 stimulates angiogenesis by activating the fibroblast growth factor receptor (FGFR), epidermal growth factor receptor (EGFR) and β3 integrin through E prostanoid 2 (EP2)- and EP4-mediated pathways [5], [35], [36]. Lipoxygenase metabolites, including 12-HETE and 15-HETE, have also been reported to promote angiogenesis by inducing FGF [37], interleukin-8 (IL-8, a macrophage-derived mediator of angiogenesis) [38], and vascular endothelial growth factor (VEGF) [39]. Our results revealed that the pro-angiogenic, AA-derived eicosanoids PGE2, 12-HETE, and 15-HETE, as well as the key inflammatory factors interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α), were dramatically reduced in B16 melanoma and LLC tumors treated with D6D-RNAi or SC-26196 ( Figures 3B , 4D ). This suggests that blocking D6D can inhibit inflammation and angiogenesis. Indeed, a remarkable reduction in tumor angiogenesis, as evaluated by immunohistological staining and an in vivo angiogenesis model, was observed in the tumors treated with D6D-RNAi or SC-26196 ( Figures 4A–C ). Altogether, our results demonstrate that inhibition of D6D can diminish the production of AA and AA-derived eicosanoids, leading to the reduction of inflammation and angiogenesis and consequently, the suppression of tumor growth.
A potential problem is that D6D inhibition may cause a deficiency of long chain n−6 and n−3 fatty acids (i.e., AA and EPA) in normal tissue. However, because the content of AA in the modern Western diet and body tissues is very high (and even excessive for most people), D6D inhibition would not deplete all AA but simply reduce its endogenous production. In fact, our animals treated with the D6D inhibitor showed no significant side effects. Regarding the long chain n−3 fatty acids (i.e., EPA, DHA), these are mainly derived from specific foods (fish and fish oils), and dietary supplementation of the n−3 fatty acids can readily prevent a deficiency. Given the capability of n−3 fatty acids to compete with n−6 fatty acids for metabolism, thereby reducing AA-derived eicosanoids and their anti-cancer properties, the supplementation of n−3 fatty acids together with the use of D6D inhibitors might not only make D6D inhibition safer, but also enhance its anti-cancer efficacy. As the exogenous AA from animal products may still interfere with the outcome of D6D-inhibition therapy to some extent, the consumption of AA-containing products must be restricted during the treatment. Still, we acknowledge that AA and its metabolites are naturally occurring and essential factors in maintaining normal functioning of the immune system, heart, brain, testes, etc. At present, it is unknown whether there are detrimental effects of significantly reduced AA content due to D6D inhibition, and how long-term use of a D6D inhibitor might impact health. These are important issues that will need to be carefully addressed in future studies.
Our discovery of D6D’s role in tumorigenesis also points to the importance of tissue n−6 and n−3 fatty acid status in cancer prevention. Because of the competition between n−6 and n−3 fatty acids for D6D and other metabolizing enzymes, the relative amounts, or the ratio, of these two classes of fatty acids may be a key factor for tumorigenesis. The severe imbalance between n−6 and n−3 fatty acids (n−6/n−3>10) in the modern Western diet and human body tissue has been thought to contribute to today’s increased risk of diseases, including cancer [14], [18]. Evidence from recent studies using the transgenic fat-1 mouse model, which can endogenously convert n−6 to n−3 fatty acids and has a balanced n−6/n−3 ratio in its body tissues [40], strongly supports this notion [41]–[48]. These studies have demonstrated that decreasing the tissue ratio of n−6/n−3 can significantly reduce the formation and growth of various cancers, which is associated with reduced levels of cancer-related eicosanoids and genes [41]–[48], suggesting that the tumor-promoting effect of increased D6D activity can be diminished by decreasing tissue n−6/n−3 fatty acid ratio. While further study is warranted to explore this exciting field, the results of the present study increase our understanding of the importance of D6D as well as that of the tissue ratio of n−6/n−3 in cancer prevention and also point to a potentially safe and effective approach to cancer prevention through balancing dietary intake of n−6/n−3.
In summary, the results presented here demonstrate an important role for the D6D enzyme in tumorigenesis and reveal a link between lipid metabolism and cancer biology. D6D may serve as a new cancer biomarker and a potential target for the development of novel anti-cancer drugs. The results also highlight the potential utility of n−3 fatty acid supplementation and balancing the tissue ratio of n−6/n−3 fatty acids for cancer prevention and treatment.
Supporting Information
Metabolism of polyunsaturated fatty acids. The long-chain polyunsaturated fatty acids n−6 arachidonic acid (AA) and n−3 eicosapentaenoic acid (EPA) are derived from LA and ALA through a series of desaturation and chain-elongation enzyme systems and are metabolized through the three major pathways cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 epoxygenase. Please note that delta-6 desaturase (D6D) is the rate-limiting enzyme for the synthesis of both n−6 AA and n−3 EPA. Furthermore, the two classes of fatty acids (n−6 and n−3) compete for the same enzymes for both synthesis and metabolism.
(TIF)
The schematic structure of the pLu6-RNAi-D6D plasmid.
(TIF)
Identification and quantification of AA-derived eicosanoids were determined by LC- MS. A gradient chromatographic separation was performed on a ZORBAX Eclipse XDB-C18 column (Agilent, 5 µm, 75×4.6 mm) at 25°C. The detection was made in the negative mode. D4-PGE2 was used as an internal standard for quantification of all eicosanoids. The concentrations of eicosanoids in the samples were calculated by comparing their ratios of peak areas of compounds to the internal standards.
(TIF)
D6D mRNA expression in B16 and LLC cells treated with D6D-RNAi in vitro .
(TIF)
D6D activity in B16 and LLC cells treated with D6D-RNAi in vitro .
(TIF)
Gas chromatography showing the differences in LA, ETA and AA content in treated (with D6D-RNAi or SC-26196) or non-treated tumors.
(TIF)
Viability of B16 and LLC cells treated with D6D-RNAi and SC-26196 in vitro . P >0.05; n = 4.
(TIF)
Dietary fatty acid composition for C57BL6 mice.
(TIF)
Acknowledgments
The D6D selective inhibitor SC-26196 was kindly provided by Chemical Synthesis and Drug Supply Program (CSDSP) of National Institute of Mental Health (NIMH). The human U6 snRNA promoter was a kind gift from Dr. Haitao Zhang (Guangdong Medical College). The recombinant lentiviral system including pHDM-G, pHDM-tat1b, pHDM-Hgpm2, pHRST-IRES-GFP, and pRC/CMV-rev1B plasmids were kindly provided by Dr. Jeng-Shin Lee at Harvard Gene Therapy Initiative. We are also grateful to Sarah Brigandi, Erin Gleason, Marina Kang and Marilla Pender-Cudlip for their assistance in the preparation of the manuscript.
Funding Statement
This work was supported by National Institutes of Health grant CA113605 to JXK. No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Editorial (2011) Cancer drugs: remedy required. Nature 17: 231–232. [DOI] [PubMed] [Google Scholar]
- 2. Polyak K, Garber J (2011) Targeting the missing links for cancer therapy. Nat Med 17: 283–284. [DOI] [PubMed] [Google Scholar]
- 3. Prensner JR, Chinnaiyan AM (2011) Metabolism unhinged: IDH mutations in cancer. Nat Med 17: 291–293. [DOI] [PubMed] [Google Scholar]
- 4. Bissell MJ, Hines WC (2011) Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 17: 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, DuBois RN (2010) Eicosanoids and cancer. Nat Rev Cancer 10: 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weylandt KH, Kang JX (2005) Rethinking lipid mediators in physiology and pathophysiology. Lancet 366: 618–620. [DOI] [PubMed] [Google Scholar]
- 7. Kang JX, Weylandt KH (2008) Modulation of inflammatory cytokines by omega-3 Fatty acids. Subcell Biochem 49: 133–143. [DOI] [PubMed] [Google Scholar]
- 8. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, et al. (1994) Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarinomas. Gastroenterology 107: 1183–1188. [DOI] [PubMed] [Google Scholar]
- 9. Mazhar D, Ang R, Waxman J (2006) COX inhibitors and breast cancer. Br J Cancer 94: 346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang B, Cao H, Rao GN (2005) 15(S)-Hydroxyeicosatetraenoic acid induces angiogenesisvia activation of PI3K-Akt-mTOR-S6K1 signaling. Cancer Res 65: 7283–7291. [DOI] [PubMed] [Google Scholar]
- 11. Jiang JG, Ning YG, Chen C, Ma D, Liu ZJ, et al. (2007) Cytochrome p450 epoxygenase promotes human cancer metastasis. Cancer Res 67: 6665–6674. [DOI] [PubMed] [Google Scholar]
- 12. Gravitz L (2011) Chemoprevention: First line of defence. Nature 471: S5–S7. [DOI] [PubMed] [Google Scholar]
- 13. Leaf A, Weber PC (1987) A new era for science in nutrition. Am J Clin Nutr 45: 1048–1053. [DOI] [PubMed] [Google Scholar]
- 14. Simopoulos AP (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med 233: 674–688. [DOI] [PubMed] [Google Scholar]
- 15. Kang JX, Wang J (2005) A simplified method for analysis of polyunsaturated fatty acids. BMC Biochem 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hlatky L, Hahnfeldt P, Folkman J (2002) Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst 94: 883–893. [DOI] [PubMed] [Google Scholar]
- 17. Ito Y, Iwamoto Y, Tanaka K, Okuyama K, Sugioka Y (1996) A quantitative assay using basement membrane extracts to study tumor angiogenesis in vivo. Int J Cancer 67: 148–152. [DOI] [PubMed] [Google Scholar]
- 18. Cho HP, Nakamura MT, Clarke SD (1999) Cloning, expression, and nutritional regulation of the mammalian Delta-6 desaturase. J Biol Chem 274: 471–477. [DOI] [PubMed] [Google Scholar]
- 19. Obukowicz MG, Welsch DJ, Salsgiver WJ, Martin-Berger CL, Chinn KS, et al. (1998) Novel, selective delta6 or delta5 fatty acid desaturase inhibitors as antiinflammatory agents in mice. J Pharmacol Exp Ther 287: 157–166. [PubMed] [Google Scholar]
- 20. Simopoulos AP (1999) Essential fatty acids in health and chronic disease. Am J Clin Nutr 70: 560S–569S. [DOI] [PubMed] [Google Scholar]
- 21. Kang JX (2011) The Omega-6/Omega-3 Fatty Acid Ratio in Chronic Diseases: Animal Models and Molecular Aspects. World Rev Nutr Diet 102: 22–29. [DOI] [PubMed] [Google Scholar]
- 22. Spencer L, Mann C, Metcalfe M, Webb M, Pollard C, et al. (2009) The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur J Cancer 45: 2077–2086. [DOI] [PubMed] [Google Scholar]
- 23. Burdge GC, Calder PC (2005) Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod Nutr Dev 45: 581–597. [DOI] [PubMed] [Google Scholar]
- 24. Sala A, Zarini S, Folco G, Murphy RC, Henson PM (1999) Differential Metabolism of Exogenous and Endogenous Arachidonic Acid in Human Neutrophils. J Biol Chem 274: 28264–28269. [DOI] [PubMed] [Google Scholar]
- 25. Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, et al. (2006) Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 9: 287–300. [DOI] [PubMed] [Google Scholar]
- 26. Zhao H, Langerød A, Ji Y, Nowels KW, Nesland JM, et al. (2004) Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell 15: 2523–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakamura MT, Nara TY (2003) Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot Essent Fatty Acids 68: 145–150. [DOI] [PubMed] [Google Scholar]
- 28. Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, et al. (2008) Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res 68: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 29. Swinnen JV, Heemers H, Deboel L, Foufelle F, Heyns W, et al. (2000) Stimulation of tumor-associated fatty acid synthase expression by growth factor activation of the sterol regulatory element-binding protein pathway. Oncogene 19: 5173–5181. [DOI] [PubMed] [Google Scholar]
- 30. Hansen-Petrik MB, McEntee MF, Johnson BT, Obukowicz MG, Masferrer J, et al. (2002) Selective inhibition of Delta-6 desaturase impedes intestinal tumorigenesis. Cancer Lett 175: 157–163. [DOI] [PubMed] [Google Scholar]
- 31. Funk CD (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294: 1871–1875. [DOI] [PubMed] [Google Scholar]
- 32. Noonan DM, De Lerma Barbaro A, Vannini N, Mortara L, Albini A (2008) Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev 27: 31–40. [DOI] [PubMed] [Google Scholar]
- 33. Ruegg C (2006) Leukocytes, inflammation, and angiogenesis in cancer: fatal attractions. J Leukoc Biol 80: 682–684. [DOI] [PubMed] [Google Scholar]
- 34. Costa C, Incio J, Soares R (2007) Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis 10: 149–166. [DOI] [PubMed] [Google Scholar]
- 35. Jain S, Chakraborty G, Raja R, Kale S, Kundu GC (2008) Prostaglandin E2 regulates tumor angiogenesis in prostate cancer. Cancer Res 68: 7750–7759. [DOI] [PubMed] [Google Scholar]
- 36. Finetti F, Solito R, Morbidelli L, Giachetti A, Ziche M, et al. (2008) Prostaglandin E2 regulates angiogenesis via activation of fibroblast growth factor receptor-1. J Biol Chem 283: 2139–2146. [DOI] [PubMed] [Google Scholar]
- 37. Kundumani-Sridharan V, Niu J, Wang D, Van Quyen D, Zhang Q, et al. (2010) 15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis requires Src-mediated Egr-1-dependent rapid induction of FGF-2 expression. Blood 115: 2105–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheranov SY, Wang D, Kundumani-Sridharan V, Karpurapu M, Zhang Q, et al. (2009) The 15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis requires Janus kinase 2-signal transducer and activator of transcription-5B-dependent expression of interleukin-8. Blood 113: 6023–6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nie D, Krishnamoorthy S, Jin R, Tang K, Chen Y, et al. (2006) Mechanisms regulating tumor angiogenesis by 12-lipoxygenase in prostate cancer cells. J Biol Chem 281: 18601–18609. [DOI] [PubMed] [Google Scholar]
- 40. Kang JX, Wang J, Wu L, Kang ZB (2004) Transgenic mice: fat-1 mice convert n−6 to n−3 fatty acids. Nature 427: 504. [DOI] [PubMed] [Google Scholar]
- 41. Xia S, Lu Y, Wang J, He C, Hong S, et al. (2006) Melanoma growth is reduced in fat-1 transgenic mice: impact of omega-6/omega-3 essential fatty acids. Proc Natl Acad Sci USA 103: 12499–12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nowak J, Weylandt KH, Habbel P, Wang J, Dignass A, et al. (2007) Colitis-associated colon tumorigenesis is suppressed in transgenic mice rich in endogenous n−3 fatty acids. Carcinogenesis 28: 1991–1995. [DOI] [PubMed] [Google Scholar]
- 43. Jia Q, Lupton JR, Smith R, Weeks BR, Callaway E, et al. (2008) Reduced colitis-associated colon cancer in fat-1 (n−3 fatty acid desaturase) transgenic mice. Cancer Res 68: 3985–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berquin IM, Min Y, Wu R, Wu J, Perry D, et al. (2007) Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest 117: 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weylandt KH, Krause LF, Gomolka B, Chiu CY, Bilal S, et al. (2011) Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF- α. Carcinogenesis 32: 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lim K, Han C, Dai Y, Shen M, Wu T (2009) Omega-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking beta-catenin and cyclooxygenase-2. Mol Cancer Ther 8: 3046–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lu Y, Nie D, Witt WT, Chen Q, Shen M, et al. (2008) Expression of the fat-1 gene diminishes prostate cancer growth in vivo through enhancing apoptosis and inhibiting GSK-3 beta phosphorylation. Mol Cancer Ther 7: 3203–3211. [DOI] [PubMed] [Google Scholar]
- 48. Griffitts J, Saunders D, Tesiram YA, Reid GE, Salih A, et al. (2010) The non-mammalian fat-1 gene prevents neoplasia when introduced to a mouse hepatocarcinogenesis model. Biochim Biophys Acta 1801: 1133–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Metabolism of polyunsaturated fatty acids. The long-chain polyunsaturated fatty acids n−6 arachidonic acid (AA) and n−3 eicosapentaenoic acid (EPA) are derived from LA and ALA through a series of desaturation and chain-elongation enzyme systems and are metabolized through the three major pathways cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 epoxygenase. Please note that delta-6 desaturase (D6D) is the rate-limiting enzyme for the synthesis of both n−6 AA and n−3 EPA. Furthermore, the two classes of fatty acids (n−6 and n−3) compete for the same enzymes for both synthesis and metabolism.
(TIF)
The schematic structure of the pLu6-RNAi-D6D plasmid.
(TIF)
Identification and quantification of AA-derived eicosanoids were determined by LC- MS. A gradient chromatographic separation was performed on a ZORBAX Eclipse XDB-C18 column (Agilent, 5 µm, 75×4.6 mm) at 25°C. The detection was made in the negative mode. D4-PGE2 was used as an internal standard for quantification of all eicosanoids. The concentrations of eicosanoids in the samples were calculated by comparing their ratios of peak areas of compounds to the internal standards.
(TIF)
D6D mRNA expression in B16 and LLC cells treated with D6D-RNAi in vitro .
(TIF)
D6D activity in B16 and LLC cells treated with D6D-RNAi in vitro .
(TIF)
Gas chromatography showing the differences in LA, ETA and AA content in treated (with D6D-RNAi or SC-26196) or non-treated tumors.
(TIF)
Viability of B16 and LLC cells treated with D6D-RNAi and SC-26196 in vitro . P >0.05; n = 4.
(TIF)
Dietary fatty acid composition for C57BL6 mice.
(TIF)