Abstract
Chronic inflammation in the stomach can lead to gastric cancer. We previously reported that gastrin-deficient (Gast−/−) mice develop bacterial overgrowth, inflammatory infiltrate, increased Il-1β expression, antral hyperplasia and eventually antral tumors. Since Hedgehog (Hh) signaling is active in gastric cancers but its role in precursor lesions is poorly understood, we examined the role of inflammation and Hh signaling in antral hyperplasia. LacZ reporter mice for Sonic hedgehog (Shh), Gli1, and Gli2 expression bred onto the Gast−/− background revealed reduced Shh and Gli1 expression in the antra compared to wild type controls (WT). Gli2 expression in the Gast−/− corpus was unchanged. However in the hyperplastic Gast−/− antra, Gli2 expression increased in both the mesenchyme and epithelium, whereas expression in WT mice remained exclusively mesenchymal. These observations suggested that Gli2 is differentially regulated in the hyperplastic Gast−/− antrum versus the corpus and by a Shh ligand-independent mechanism. Moreover, the proinflammatory cytokines Il-1β and Il-11, which promote gastric epithelial proliferation, were increased in the Gast−/− stomach along with Infγ. To test if inflammation could account for elevated epithelial Gli2 expression in the Gast−/− antra, the human gastric cell line AGS was treated with IL-1β and was found to increase GLI2 but decrease GLI1 levels. IL-1β also repressed human GAST gene expression. Indeed, GLI2 but not GLI1 or GLI3 expression repressed gastrin luciferase reporter activity by ∼50 percent. Moreover, chromatin immunoprecipitation of GLI2 in AGS cells confirmed that GLI2 directly binds to the GAST promoter. Using a mouse model of constitutively active epithelial GLI2 expression, we found that activated GLI2 repressed Gast expression but induced Il-1β gene expression and proliferation in the gastric antrum, along with a reduction of the number of G-cells. In summary, epithelial Gli2 expression was sufficient to stimulate Il-1β expression, repress Gast gene expression and increase proliferation, leading to antral hyperplasia.
Introduction
The two histologically and physiologically distinct compartments of the mouse glandular gastric epithelium are: the proximal corpus/fundus (oxyntic) mucosa characterized by the presence of acid-producing parietal cells, and the distal endocrine mucosa (antrum) composed of enteroendocrine cells (G cells) that secrete the hormone gastrin (Gast) [1]. Gast stimulates the parietal cells in the corpus to secrete acid. In addition, the hormone is considered to be a growth factor for the gastrointestinal tract [2], [3], and on that basis has been implicated in gastrointestinal cancers [4], [5].
In the normal gastric corpus, Hedgehog (Hh) ligands such as Sonic hedgehog (Shh) are produced, but then decrease with chronic inflammation, loss of acid secretion (hypochlorhydria), which leads to gastric metaplasia, a precursor lesion for gastric cancer [6], [7], [8]. Nevertheless, Hh signaling remains active in gastric cancers [9], suggesting differences in the regulation of the Hh pathway in normal stomach compared to gastric carcinogenesis. We and others have analyzed the role of Hh signaling in the gastric corpus [6], but information on Hh signaling in the gastric antrum and its participation in antral tumor formation is scarce. In addition, Shh, the major Hh ligand expressed in the corpus, subsequently diminishes in the distal stomach (antrum) despite persistent expression of Hh gene targets, e.g., Gli1 and Gli2 [10], [11], [12], suggesting differential Hh signaling pathways operating in these two regions of the stomach.
Gastric cancer is among the more prevalent cancers worldwide, with a survival rate of 27% [13]. Interestingly, a shift in the most frequent site of gastric cancer from the distal stomach (antrum) to the more proximal corpus and cardia has been observed over the past 10 years, possibly reflecting differences in cancer etiology and risk factors for these two regions of the stomach [14]. Mouse models of gastric tumorigenesis frequently exhibit changes in the gastric corpus/fundus with little or no changes in the antrum. However to accurately compare the etiologic differences in cancer development between these two anatomic sites, further dissection of the mechanisms leading to hyperplasia and eventually tumorigenesis in the antrum is needed. Currently, different genetic models of antral cancer have been described and include loss of trefoil factor 1 (TFF1) [15], aberrant activation of the gp130 cytokine receptor [16] and loss of the hormone gastrin (Gast−/−) [17], yet none have examined a specific role for Hh signaling.
In a previous study, we reported that increased expression of Il-1β, and the Tgfβ- family members activin A (AcA) and follistatin (Fst) precede gastric transformation in the Gast−/− mice [18]. Tumors in this model occur when mice are older than 9 months and their development has been associated with bacterial overgrowth [19] and inflammation [20], [21]. By the time antral tumors are detected, mice may have also developed corpus atrophy due to hypochlorhydria [18], [20]. Therefore to better define the changes that are associated with the initiation of antral tumors, we analyzed Gast−/− mice between 9 and 13 months of age, which showed only antral hyperplasia without obvious histological changes in the corpus, for Hh signaling. We report here that Gli2 was induced in early antral lesions in a Shh-independent manner. Moreover, proinflammatory cytokines increased along with proliferative indicators while Gast gene expression decreased.
Methods
Ethics Statement
All animal procedures were approved by the University of Michigan Animal Care and Use Committee (DHHS Animal Welfare Assurance A3114-01).
Animals
Gastrin deficient (Gast−/−) mice [17], [18] were bred to mice carrying the bacterial β-galactosidase (lacZ) gene that was either inserted, together with an IRES element into the 3′ untranslated region of the Shh gene (ShhlacZ) [22], or disrupted one Gli1 (Gli1lacZ) or Gli2 (Gli2lacZ) [23] allele. Animals were conventionally housed in microisolator cages in nonbarrier mouse rooms.
Inducible GLI2 Transgene Expressing Mice
To activate GLI2 expression in vivo, we generated a doxycycline-inducible mouse model carrying a MYC-tagged, activated form of GLI2, designated GLI2?N. Generation of Shh-Cre;R26-LSL-rtTA;tetOGLI2ΔN triple allele transgenic mice has been previously described [24], [25]. This model utilizes mice carrying 3 alleles: (a) a tissue-specific Cre driver (Shh-Cre); (b) the Cre-inducible R26-LSL-rtTA strain [25]; and (c) a tetO-GLI2ΔN. Mice were bred according to standard protocols to generate triple-transgenic mice. To induce transgene expression, mice were fed chow containing 1 g doxycycline/kg chow (Bio-Serv, Frenchtown, NJ) and 200 µg/ml doxycycline (Sigma-Aldrich, St. Louis, MO) in their drinking water with 5% sucrose.
X-gal Staining
β-galactosidase activity (LacZ) was detected in whole stomach X-gal staining as previously described [6]. Briefly, stomachs were fixed in 4% buffered paraformaldehyde for 1 h at 4°C, washed in phosphate-buffered saline (PBS) with 0.01% sodium deoxycholate and 0.02% NP-40), and then stained overnight at 4°C with 1 mg/ml of the X-gal substrate in fresh 5 mM potassium ferrocyanide and 5 mM potassium ferricyanide. After staining, samples were washed and post-fixed in 4% buffered formaldehyde and processed for paraffin embedding.
Immunohistochemistry and Immunofluorescence
Stomachs were fixed in 4% buffered formaldehyde and paraffin-embedded. Longitudinal sections (5 µm) were deparaffinized and antigen retrieval was performed using by boiling the slides in 10 mM sodium citrate buffer, pH 6 for 40 min. Rabbit anti-gastrin (Dako, Carpinteria, CA), rabbit anti-Ki-67 (Thermo Scientific, Fremont, CA), rabbit anti-MYC (Cell Signaling, Danvers, MA). Donkey antibodies conjugated to Alexa-488 or Alexa-594 (Jackson ImmunoResearch Laboratories, West Grove, PA) were the secondary antibodies used to detect the primary antibody by immunofluorescence. Nuclei were counterstained with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI).
Cell Culture
The gastric cancer cell lines AGS and NCI-N87 were purchased from the American Type Culture Collection (ATCC, Manassas, VA) then grown to 80% confluence in RPMI-1640 media supplemented with 10% FBS and 1% antibiotics. Following serum starvation for 24 h, cells were treated with IL-1β (0.1 ng/ml, R&D Systems, Minneapolis, MN) in serum-free culture medium.
Quantitative RT-PCR
For mRNA extraction, samples were homogenized in Trizol reagent (Invitrogen, Carlsbad, CA) followed by phenol-chloroform RNA extraction, and purification with the RNeasy Mini Kit (Qiagen, Valencia, CA). First strand cDNA was synthesized using i-script (BioRad, Hercules, CA) according to the manufacturer’s protocol. Triplicates for each sample were amplified by qPCR in a BioRad iCycler using SYBR green. The primer sequences for PCR amplification are shown in Table S1.
Plasmids, Transfections and Luciferase Assay
All plasmid constructs used in the experiments have been described previously [26], [27], [28], [29], [30]. AGS cells were transiently transfected for 48 h with the indicated plasmids using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Cell lysates were harvested to determine luciferase activity using the Dual-Luciferase Reporter Assay kit (Promega, Madison, WI) and PerkinElmer (Waltham, MA) Wallac Victor3 luminometer. Luciferase activity was normalized to total protein content, which was determined by Bradford colorimetric assay (BCA) Protein Assay Kit (Thermo Scientific, Waltham, MA). The data were expressed as the mean ±SEM for three independent experiments performed in triplicate.
Chromatin-Immunoprecipitation (ChIP) Assay
The ChIP kit from Millipore (Millipore, Temecula, CA) was according to the manufacturer’s instructions. Briefly, after crosslinking with formaldehyde, AGS cells were collected, lysed then sonicated to shear DNA to an average fragment size of 200–1000 bp. For the “input,” 1% of the lysate was removed for PCR analysis and the remainder was used for immunoprecipitation overnight at 4°C with either rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-ZBP-89 [31] or anti-GLI2 antibodies (Abcam, Cambridge, MA). The crosslinking was reversed and DNA was recovered using QIAquick PCR Purification Kit (Qiagen, Valencia, CA). The following primers were used to amplify 216 bp of the proximal gastrin promoter (from 5′−218 and 3′−2 bp upstream of the transcriptional start site): forward 5′-GCTCCAGCCCCTCACCATGAAG-3′; reverse 5′-TTGATGCTCCAGGCCTGCCTTA-3′.
Western Blot
Cells were treated with IL-1β after serum starvation. Proteins were lysed using RIPA buffer (Sigma-Aldrich), quantified using the BCA Protein Assay Kit (Thermo Scientific) and then resolved in SDS-PAGE gels. After transfer to PVDF membranes, the membrane was blotted with primary antibodies overnight for anti-GLI2 (Abcam) and for 1 h with anti-GAPDH (Santa Cruz Biotechnology). Peroxidase-labeled seconday antibodies (Santa Cruz Biotechnology) were incubated with the membranes for an additional hour then bands were visualized using the Supersignal West Pico chemiluminescent substrate (Thermo Scientific).
Statistics
Gene expression was normalized to HPRT, and changes were calculated using the 2-ΔΔC(T) method [32]. Data are presented as the mean ± S.E.M., and were analyzed by one-way ANOVA, Mann-Whitney or Student’s-t tests using Prism software (GraphPad Software, Inc., La Jolla, CA, USA). P values ≤0.05 were considered significant.
Results
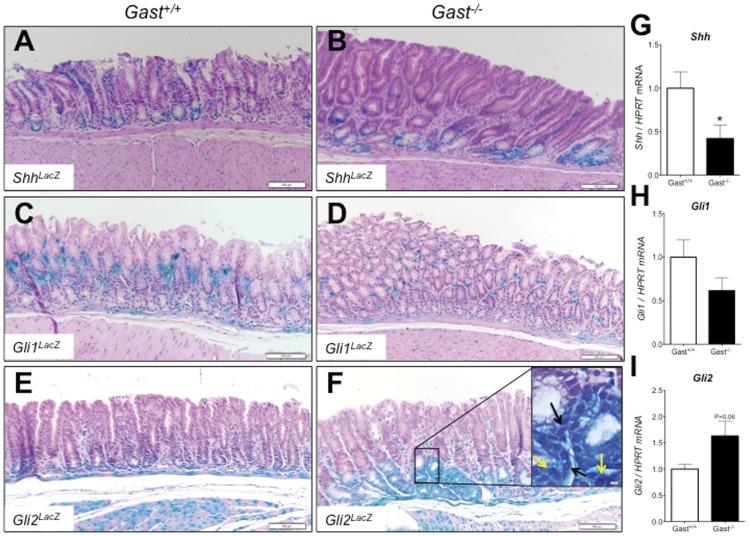
To better define the early mechanisms involved in gastric antral transformation, we studied the antra of 9–13 month-old Gast−/− mice that showed hyperplastic epithelium but not frank tumors (Fig. 1A–F). The contribution of Hh signaling during development of antral hyperplasia was assessed using three LacZ reporter mice bred onto the Gast−/− mouse genetic background. ShhLacZ reporter mice confirmed the restricted epithelial expression of Shh to corpus and antral glands. Collectively, we observed a reduction in antral Shh expression (P = 0.03) in the Gast−/− mice compared to their WT littermates (Fig. 1A, B and G). Analysis of reporter mice for Gli1 expression (Gli1LacZ) showed Gli1 expression restricted to stromal cells in both the normal (Gli1LacZ;Gast+/+) and hyperplastic antra (Gli1LacZ;Gast−/−). Moreover, Gli1 expression tended to decrease in the antral hyperplastic regions (P = 0.14), suggesting a reduction of Hh signaling in the Gast−/− antra (Fig. 1C, D and H). The Gli2LacZ;Gast−/− mice showed both mesenchymal and epithelial Gli2 expression, in contrast to exclusively mesenchymal expression in WT mouse antra (Fig. 1E and F). The expression of Gli2 in the Gast−/− stomach trended higher, although the expression levels did not achieve statistical significance (P = 0.06) (Fig. 1I). We observed nuclear (yellow arrows, Fig. 1F insert) and perinuclear (black arrows, Fig. 1F insert) β-gal staining in the epithelial cells exhibiting the highest Gli2-LacZ expression along with cytoplasmic accumulation. These results suggested that the increased Gli2 expression in the antral epithelium of the Gast−/− mouse was not the result of elevated Shh ligand expression and Hh canonical signaling.
Figure 1. Epithelial expression of Gli2 in the Gast−/− antrum.
The antral expression of Hedgehog pathway molecules was determined in 9–13 month-old littermate controls (Gast+/+) (panels A, C and E) and Gast−/− (panels B, D and F) mice by X-gal staining of LacZ reporter mice for Sonic hedgehog (Shh) (A and B), Gli1 (C and D) and Gli2 (E and F). A high power field of Gli2-LacZ staining is shown in F, where nuclear (yellow arrows) and perinuclear (black arrows) staining was observed along with cytoplasmic reporter accumulation. Whole stomachs from Gast+/+ and Gast−/− were analyzed for gene expression of Shh (G), Gli1 (H), and Gli2 (I). Bars in panels A to F are 100 µm. Data presented as mean±SEM. N = 8 per group. *P≤0.05.
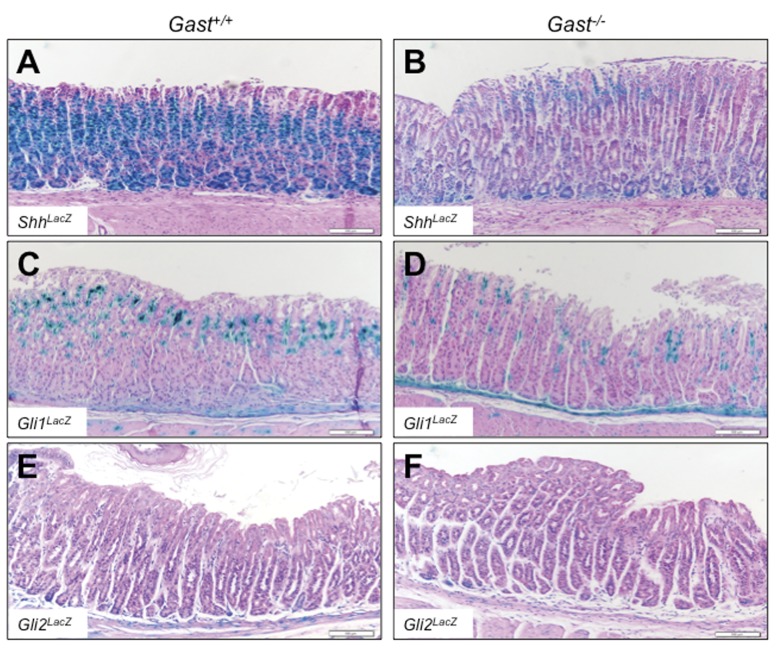
The adjacent corpi of the Gast−/− mice showed no hyperplastic or other significant histological changes (Fig. 2). However, ShhLacZ expression in the corpi of Gast−/− mice was lower than that of the Gast+/+ mice (Fig. 2A and B), accounting for the significant reduction in Shh mRNA expression (Fig. 1G) and consistent with the profound hypochlorhydria as previously reported [6]. Expression in the Gli1LacZ (Fig. 2C and D) and Gli2LacZ mice (Fig. 2E and F) trended slightly lower in the Gast−/− corpi (Fig. 2D and F) compared to Gast+/+ (Fig. 2C and E) mice. In contrast to expression in the antrum (Fig. 1F), we did not observe changes in the Gli2LacZ Gast−/− mouse corpi (Fig. 2F), where Gli2LacZ expression was restricted to the mesenchyme, suggesting differential regulation of Gli2 gene expression in the corpus compared to the hyperplastic antrum.
Figure 2. Gli2 expression is not increased in the Gast−/− corpus.
Representative X-gal staining of corpi of Gast+/+ and Gast−/− mice harboring the LacZ reporter for Sonic hedgehog (Shh) (A and B), Gli1 (C and D) and Gli2 (E and F). Bars are 100 µm.
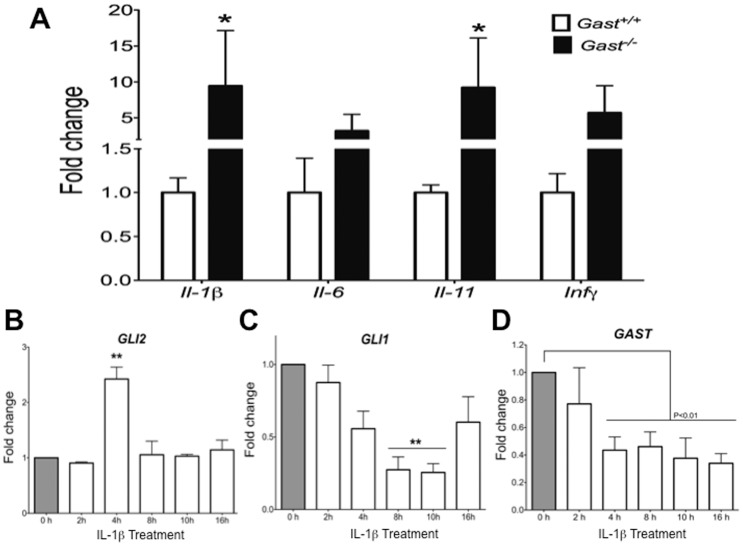
Since inflammatory cytokines, i.e. Il-1β [6], Il-6 [16] and Il-11 [21] have been associated with development of gastric tumors, we analyzed the hyperplastic antra of Gast−/− mice for the proinflammatory cytokines. Il-1β, Il-6, Il-11 and Infγ mRNA expression tended to increase in the Gast−/− antra, achieving statistical significance for Il-1β (P = 0.006) and Il-11 (P = 0.04) (Fig. 3A). To determine if the observed increase in antral Gli2 expression in the Gast−/− epithelium could be due to inflammation, the AGS human gastric cell line was treated with IL-1β. IL-1β induced a significant increase in GLI2 (P = 0.02) (Fig. 3B), while GLI1 mRNA expression decreased (P = 0.01) (Fig. 3C) further supporting the concept that GLI2 expression in gastric epithelial cells can be modulated in a Hh-independent manner. Treatment with IL-1β also induced GLI2 expression in the gastric cell line NCI-N87 (Fig. S1), which exhibits characteristics of epithelial cells in the deep antral glands [33]. These results demonstrated that GLI2 gene expression can be induced in gastric cells by proinflammatory cytokines.
Figure 3. IL-1β induced Gli2, but not Hh signaling in gastric epithelial cells.
A) Gene expression of inflammatory cytokines IL-1β, IL-6, IL-11 and INFγ were determined in the stomachs of Gast+/+ and Gast−/− mice by qRT-PCR. The gastric cell line AGS was treated with 0.1 ng/ml of IL-1β for different time points and their gene expression of GLI2 (B), GLI1 (C) and GAST (D) were measured. Data presented as mean ± SEM. N = 8 per group in panel A. B, C and D: N = 3 independent experiments. *P≤0.05, **P≤0.01.
It has been reported that gastrin promotes the development of gastric cancer [34], [35]. Specifically, Datta et al. reported that GAST mRNA expression can be repressed by IL-1β via Smad7 or NFκB activation [36], [37]. Therefore we tested whether IL-1β suppresses GAST gene expression. Treating AGS cells with IL-1β, which express but do not secrete gastrin [38], confirmed that IL-1β does indeed suppress GAST mRNA expression (P = 0.001) (Fig. 3D). In the Gast−/− hyperplastic antrum, the expanded epithelial expression of Gli2 occurred in the lower portion of the antral gland below the proliferative area, where gastrin-expressing cells are normally located. Since we showed that IL-1β stimulates GLI2 gene expression but reduces GAST expression, we tested the possibility that GLI2 might mediate IL-1β repression of GAST.
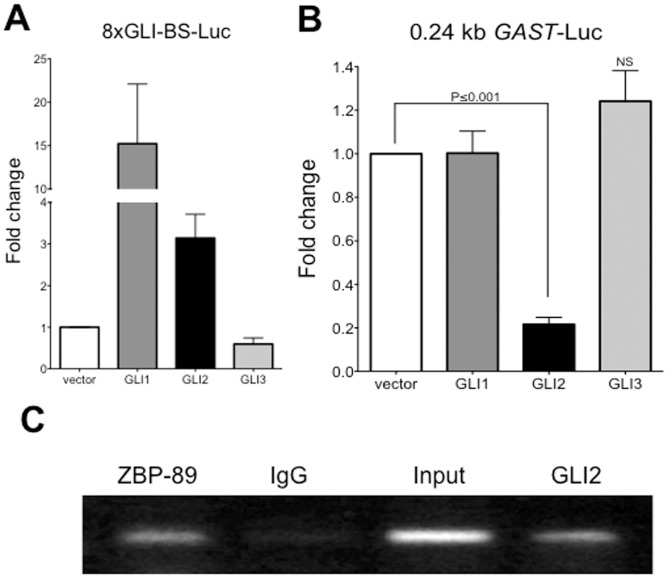
We co-transfected the expression vectors for GLI1, GLI2 and GLI3 with the 0.24 kb gastrin luciferase reporter (GAST-Luc) or the control GLI-responsive reporter plasmid 8xGli-BS-Luc (Fig. 4A) into AGS cells. Indeed, we observed a 50 percent decrease (P = 0.001) in GAST promoter activity when transfected with GLI2, but not GLI1 or GLI3, suggesting a GLI2-specific transcriptional regulatory effect on the GAST promoter (Fig. 4B). To determine if GLI2 directly binds the GAST promoter, we performed chromatin immunoprecipitation (ChIP) for GLI2 in AGS cells and found that GLI2 binds the proximal human gastrin promoter (Fig. 4C). In particular, GLI2 bound to the GAST promoter to a similar extent as ZBP-89 (Fig. 4C), another zinc finger protein that we have previously identified as a transcriptional repressor of the GAST promoter through a GC-rich element in the proximal promoter [31].
Figure 4. GLI2 repressed GAST expression.
A) AGS cells were co-transfected with GLI1, GLI2 and GLI3 expression vectors and the Shh pathway readout construct (8xGLI-BS-Luc) and tested for promoter activation by luciferase assay. B) Luciferase assay of AGS cells co-transfected with the 0.24 kb GAST-Luc construct and GLI1, GLI2, and GLI3 expression vectors. C) Chromatin immunoprecipitation of AGS cells DNA-protein complexes using anti-ZBP89, rabbit (IgG), and anti-GLI2 antibodies followed by amplification of 216 bp of the proximal gastrin promoter. Data presented as mean±SEM. N = 3 independent experiments with triplicates.
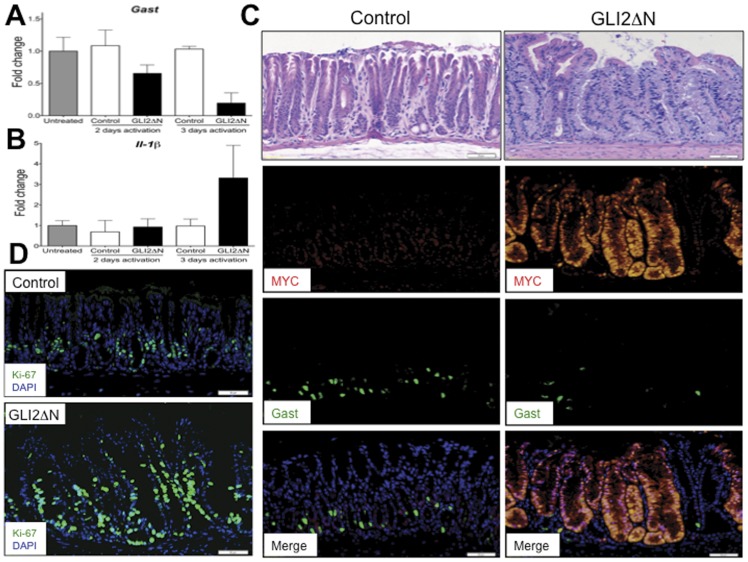
Next we considered the possibility that Gli2 might repress Gast gene expression in vivo, and thus induce a phenotype similar to that observed in the Gast−/− mice. To test this hypothesis, we examined the level of Gast gene expression in the Shh-Cre;R26-LSL-rtTA;tetO-GLI2ΔN (GLI2ΔN) mice, which conditionally express constitutively-active MYC-tagged GLI2 (GLI2ΔN) in the epithelium in the presence of doxycycline. Indeed, Gast gene expression (P = 0.056) (Fig. 5A) and the number of gastrin-expressing cells (Fig. 5C, green) decreased in the induced GLI2ΔN mice after only 3 days of doxycycline treatment, while Il-1β gene expression tended to increase (P = 0.38) (Fig. 5B). We also observed increased proliferation (Fig. 5D) and distorted gland morphology over the same time period (Fig. 5C top panel). However, changes in Il-6 or Il-11 mRNA expression (data not shown) or a significant inflammatory infiltrate was not observed (Fig. 5C, top panel). Therefore, we concluded that epithelial GLI2 activation and Il-1β can induce loss of Gast gene expression while increasing proliferation, leading to dysplastic changes in the gastric antrum.
Figure 5. Epithelial activation of Gli2 induced Il-1β and reduced Gast expression and G-cell number.
A) Gast gene expression is reduced after Gli2 activation in Shh-Cre;R26-LSL-rtTA;tetO-GLI2ΔN (GLI2ΔN) mice after 3 days of treatment with doxycycline. B) Il-1β was induced in the antra of GLI2ΔN mice antra. C) Representative images of hematoxylin-eosin staining (top panels), MYC staining to detect epitope-tagged GLI2?N (red), Gast staining (green) and merged images (lower panel) of the antrum of control and GLI2ΔN mice after 3 days of doxycycline. D) Representative images of proliferation marker Ki-67 staining in control and GLI2ΔN mice after 3 days of doxycycline. Data presented as mean±SEM. N = 2 mice per group per time. Bars are 100 µm in panel C) and 50 µm in panel D).
Discussion
Hh signaling is important for maintenance of the gastric mucosa [10], [11]. However it remains unclear whether deregulation of the Hh signal leads to preneoplastic changes and eventually gastric cancer. Therefore, the goal of our study was to determine if Hh signaling contributed to early preneoplastic changes in the antrum where the etiology of gastric cancers has not been well-established. Normal Shh expression is highest in the corpus and decreases in the antrum [9]. In addition, expression during Helicobacter infection also reduces ligand expression especially in the corpus [6]. It is important to note that we did not observe histological changes in the corpus when Hh signaling was examined on a Gast−/− background in which the stomach was hypochlorhydric [6], [39]. Consistent with this finding there was a decrease in Gli1 expression, in the absence of an obvious inflammatory infiltrate. However in contrast to the corpus, Gli2 reporter expression on the Gast−/− genetic background was increased in the epithelial cells of the deep antral glands where hyperplastic changes were also observed. Due to the dissociation between Shh and Gli1 compared to Gli2 expression, we concluded that the epithelial expression of Gli2 was likely Shh-independent.
The epithelial-specific expression of constitutively activated GLI2 (GLI2ΔN) in vivo proved to be sufficient to induce the loss of Gast gene expression and to induce Il-1β expression and antral hyperplasia. Our in vitro data demonstrated that IL-1β induces GLI2 expression in epithelial gastric cells, while in vivo GLI2ΔN activation resulted in a significant induction of Il-1β expression, similar to what has been reported in the skin of mice expressing Gli2 [40]. However, whether there is reciprocity between Gli2 and Il-1β expression requires further investigation.
Antral tumorigenesis in the Gast−/− mice has been associated with bacterial overgrowth [19] and inflammation [20], [21]. Our previous report on antral tumors in the Gast−/− mice showed that increased expression of Il-1β, of the Tgfβ -family member activin A (AcA) and follistatin (Fst), the bmp/activin antagonist, preceded transformation in the Gast−/− mice antrum [18], suggesting that there are multiple signal transduction pathways that contribute to the development of gastric cancer. There are different reports supporting the possible interactions. First, IL-1β is known to induce the expression of AcA in different cell types [41], [42]. Second, there is evidence of a strong link between TGFβ signaling and GLI2, such that TGFβ-activation of the Smad signaling cascade inducing GLI2 expression [43], [44]. This induction has been shown to be important for cancer development in organs other than the stomach [45], [46], [47]. Furthermore, Gli2 has been shown to induce the expression of Fst [48], which probably serves as a negative feedback in response to the increase in activins or BMPs.
In the present study, we focused on the early changes observed in hyperplastic antra of Gast−/− mice between 9 and 13 months of age that had no evidence of histological changes in the corpus. The difficulty of analyzing early lesions is the limited ability to achieve statistical differences for some markers that have been shown to be important in gastric cancer development. This is especially important for the observed increase in Il-6 expression that did not reach statistical significance in our study, but has previously been reported to be important for tumor formation in the stomach [21]. Interestingly, we consistently observed significant increases in Il-11 mRNA, suggesting that this cytokine is of importance in the development of antral tumors, and might participate in the regulation of parietal cell function and acid secretion in the corpus [49].
Our novel finding of Gli2 being a regulator of Gast expression potentially complements the previous work by Datta et al. showing that both TGFβ and IL-1β negatively regulate GAST expression [36]. However, our previous report suggests that the factors involved in antral changes are the Tgfβ-related molecules AcA and its inhibitor Fst [18]. Although the mechanisms triggering AcA expression and initiating Gli2 and Il-1β induction remain to be defined, bacterial overgrowth in the hypochlorhydric Gast−/− stomach is a potential culprit. When Jones et al. challenged mice with LPS, they observed a rapid increase in circulating AcA mediated by TLR4 activation, that was soon followed by increased levels of circulating Fst, Il-6 and Il-1β [50]. It is thus conceivable that gastric bacteria stimulate AcA and Il-1β expression, which in turn induces Gli2 in gastric epithelial cells, leading to Fst expression as a negative feedback and a reduction of Gast expression, further altering gastric homeostasis.
Overall, our study provides evidence of inflammation-driven Hh-independent induction of Gli2 in the gastric epithelium and indicates that Gli2 is a direct negative regulator of Gast. As a result, inflammatory mediators, such as Il-1β, Il-11 and AcA, along with epithelial Gli2, appear to be important epithelial drivers of the histologic changes during antral transformation.
Supporting Information
IL-1β induces GLI2 expression in NCI-N87 cells. The gastric cell line NCI-N87 was treated with different doses of IL-1β for 24 hr. Protein was resolved by SDS-PAGE, transferred to PVDF membrante and then blotted for GLI2 and GAPDH as the loading control.
(TIF)
Primer sequences.
(DOCX)
Funding Statement
This study was funded by the National Institutes of Health grant P01-DK62041. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Walsh JH, Grossman MI (1975) Gastrin (first of two parts). N Engl J Med 292: 1324–1334. [DOI] [PubMed] [Google Scholar]
- 2. Majumdar AP, Johnson LR (1982) Gastric mucosal cell proliferation during development in rats and effects of pentagastrin. Am J Physiol 242: G135–139. [DOI] [PubMed] [Google Scholar]
- 3. Dembinski AB, Johnson LR (1979) Growth of pancreas and gastrointestinal mucosa in antrectomized and gastrin-treated rats. Endocrinology 105: 769–773. [DOI] [PubMed] [Google Scholar]
- 4. Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, et al. (2000) Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 118: 36–47. [DOI] [PubMed] [Google Scholar]
- 5. Singh P, Dai B, Wu H, Owlia A (2000) Role of autocrine and endocrine gastrin-like peptides in colonic carcinogenesis. Curr Opin Gastroenterol 16: 68–77. [DOI] [PubMed] [Google Scholar]
- 6.Waghray M, Zavros Y, Saqui-Salces M, El-Zaatari M, Alamelumangapuram CB, et al.. (2010) Interleukin-1beta promotes gastric atrophy through suppression of Sonic Hedgehog. Gastroenterology 138: 562–572, 572 e561–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Merchant JL (2005) Inflammation, atrophy, gastric cancer: connecting the molecular dots. Gastroenterology 129: 1079–1082. [DOI] [PubMed] [Google Scholar]
- 8. Correa P (1988) A human model of gastric carcinogenesis. Cancer Res 48: 3554–3560. [PubMed] [Google Scholar]
- 9. Saqui-Salces M, Merchant JL (2010) Hedgehog signaling and gastrointestinal cancer. Biochim Biophys Acta 1803: 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramalho-Santos M, Melton DA, McMahon AP (2000) Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 127: 2763–2772. [DOI] [PubMed] [Google Scholar]
- 11. Kim SK, Hebrok M, Li E, Oh SP, Schrewe H, et al. (2000) Activin receptor patterning of foregut organogenesis. Genes Dev 14: 1866–1871. [PMC free article] [PubMed] [Google Scholar]
- 12. Kolterud A, Grosse AS, Zacharias WJ, Walton KD, Kretovich KE, et al. (2009) Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology 137: 618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howlader NNA, Krapcho M, Neyman N, Aminou R, Altekruse SF, et al.. (eds) (2012) SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations), National Cancer Institute, Bethesda, MD. Available: http://seer.cancer.gov/csr/1975_2009_pops09/. Based on November 2011 SEER data submission. Accessed 2012 Apr.
- 14. Camargo MC, Anderson WF, King JB, Correa P, Thomas CC, et al. (2011) Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut 60: 1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lefebvre O, Chenard MP, Masson R, Linares J, Dierich A, et al. (1996) Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science 274: 259–262. [DOI] [PubMed] [Google Scholar]
- 16. Judd LM, Alderman BM, Howlett M, Shulkes A, Dow C, et al. (2004) Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology 126: 196–207. [DOI] [PubMed] [Google Scholar]
- 17. Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, et al. (1998) Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol 274: G561–568. [DOI] [PubMed] [Google Scholar]
- 18. Kang W, Saqui-Salces M, Zavros Y, Merchant JL (2008) Induction of follistatin precedes gastric transformation in gastrin deficient mice. Biochem Biophys Res Commun 376: 573–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zavros Y, Rieder G, Ferguson A, Samuelson LC, Merchant JL (2002) Genetic or chemical hypochlorhydria is associated with inflammation that modulates parietal and G-cell populations in mice. Gastroenterology 122: 119–133. [DOI] [PubMed] [Google Scholar]
- 20. Zavros Y, Eaton KA, Kang W, Rathinavelu S, Katukuri V, et al. (2005) Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene 24: 2354–2366. [DOI] [PubMed] [Google Scholar]
- 21. Howlett M, Giraud AS, Lescesen H, Jackson CB, Kalantzis A, et al. (2009) The interleukin-6 family cytokine interleukin-11 regulates homeostatic epithelial cell turnover and promotes gastric tumor development. Gastroenterology 136: 967–977. [DOI] [PubMed] [Google Scholar]
- 22. Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP (2004) Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol 270: 393–410. [DOI] [PubMed] [Google Scholar]
- 23. Bai CB, Auerbach W, Lee JS, Stephen D, Joyner AL (2002) Gli2, but not Gli1, is required for initial Shh signaling and ectopic activation of the Shh pathway. Development 129: 4753–4761. [DOI] [PubMed] [Google Scholar]
- 24. Grachtchouk M, Pero J, Yang SH, Ermilov AN, Michael LE, et al. (2011) Basal cell carcinomas in mice arise from hair follicle stem cells and multiple epithelial progenitor populations. J Clin Invest 121: 1768–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, et al. (2005) Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res 33: e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shiotani A, Merchant JL (1995) cAMP regulates gastrin gene expression. Am J Physiol 269: G458–464. [DOI] [PubMed] [Google Scholar]
- 27. Sasaki H, Hui C, Nakafuku M, Kondoh H (1997) A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development 124: 1313–1322. [DOI] [PubMed] [Google Scholar]
- 28. Ruppert JM, Vogelstein B, Arheden K, Kinzler KW (1990) GLI3 encodes a 190-kilodalton protein with multiple regions of GLI similarity. Mol Cell Biol 10: 5408–5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kinzler KW, Vogelstein B (1990) The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Mol Cell Biol 10: 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roessler E, Ermilov AN, Grange DK, Wang A, Grachtchouk M, et al. (2005) A previously unidentified amino-terminal domain regulates transcriptional activity of wild-type and disease-associated human GLI2. Hum Mol Genet 14: 2181–2188. [DOI] [PubMed] [Google Scholar]
- 31. Merchant JL, Iyer GR, Taylor BR, Kitchen JR, Mortensen ER, et al. (1996) ZBP-89, a Kruppel-like zinc finger protein, inhibits epidermal growth factor induction of the gastrin promoter. Mol Cell Biol 16: 6644–6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 33. Kang W, Rathinavelu S, Samuelson LC, Merchant JL (2005) Interferon gamma induction of gastric mucous neck cell hypertrophy. Lab Invest 85: 702–715. [DOI] [PubMed] [Google Scholar]
- 34. Takaishi S, Tu S, Dubeykovskaya ZA, Whary MT, Muthupalani S, et al. (2009) Gastrin is an essential cofactor for helicobacter-associated gastric corpus carcinogenesis in C57BL/6 mice. Am J Pathol 175: 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tomita H, Takaishi S, Menheniott TR, Yang X, Shibata W, et al. (2011) Inhibition of gastric carcinogenesis by the hormone gastrin is mediated by suppression of TFF1 epigenetic silencing. Gastroenterology 140: 879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Datta De D, Bhattacharjya S, Maitra M, Datta A, Choudhury A, et al. (2011) IL1B induced Smad 7 negatively regulates gastrin expression. PLoS One 6: e14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chakravorty M, Datta De D, Choudhury A, Roychoudhury S (2009) IL1B promoter polymorphism regulates the expression of gastric acid stimulating hormone gastrin. Int J Biochem Cell Biol 41: 1502–1510. [DOI] [PubMed] [Google Scholar]
- 38. Ford MG, Valle JD, Soroka CJ, Merchant JL (1997) EGF receptor activation stimulates endogenous gastrin gene expression in canine G cells and human gastric cell cultures. J Clin Invest 99: 2762–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zavros Y, Waghray M, Tessier A, Bai L, Todisco A, et al. (2007) Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem 282: 33265–33274. [DOI] [PubMed] [Google Scholar]
- 40. Depianto D, Kerns ML, Dlugosz AA, Coulombe PA (2010) Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat Genet 42: 910–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Florio P, Rossi M, Vigano P, Luisi S, Torricelli M, et al. (2007) Interleukin 1beta and progesterone stimulate activin a expression and secretion from cultured human endometrial stromal cells. Reprod Sci 14: 29–36. [DOI] [PubMed] [Google Scholar]
- 42. Yoshino O, Izumi G, Shi J, Osuga Y, Hirota Y, et al. (2011) Activin-A is induced by interleukin-1beta and tumor necrosis factor-alpha and enhances the mRNA expression of interleukin-6 and protease-activated receptor-2 and proliferation of stromal cells from endometrioma. Fertil Steril 96: 118–121. [DOI] [PubMed] [Google Scholar]
- 43. Javelaud D, Alexaki VI, Dennler S, Mohammad KS, Guise TA, et al. (2011) TGF-beta/SMAD/GLI2 signaling axis in cancer progression and metastasis. Cancer Res 71: 5606–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, et al. (2007) Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res 67: 6981–6986. [DOI] [PubMed] [Google Scholar]
- 45. Dennler S, Andre J, Verrecchia F, Mauviel A (2009) Cloning of the human GLI2 Promoter: transcriptional activation by transforming growth factor-beta via SMAD3/beta-catenin cooperation. J Biol Chem 284: 31523–31531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnson RW, Nguyen MP, Padalecki SS, Grubbs BG, Merkel AR, et al. (2011) TGF-beta promotion of Gli2-induced expression of parathyroid hormone-related protein, an important osteolytic factor in bone metastasis, is independent of canonical Hedgehog signaling. Cancer Res 71: 822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Javelaud D, Alexaki VI, Pierrat MJ, Hoek KS, Dennler S, et al. (2011) GLI2 and M-MITF transcription factors control exclusive gene expression programs and inversely regulate invasion in human melanoma cells. Pigment Cell Melanoma Res 24: 932–943. [DOI] [PubMed] [Google Scholar]
- 48. Eichberger T, Kaser A, Pixner C, Schmid C, Klingler S, et al. (2008) GLI2-specific transcriptional activation of the bone morphogenetic protein/activin antagonist follistatin in human epidermal cells. J Biol Chem 283: 12426–12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howlett M, Chalinor HV, Buzzelli JN, Nguyen N, van Driel IR, et al.. (2011) IL-11 is a parietal cell cytokine that induces atrophic gastritis. Gut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jones KL, Mansell A, Patella S, Scott BJ, Hedger MP, et al. (2007) Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc Natl Acad Sci U S A 104: 16239–16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IL-1β induces GLI2 expression in NCI-N87 cells. The gastric cell line NCI-N87 was treated with different doses of IL-1β for 24 hr. Protein was resolved by SDS-PAGE, transferred to PVDF membrante and then blotted for GLI2 and GAPDH as the loading control.
(TIF)
Primer sequences.
(DOCX)