Abstract
Wheat (Triticum aestivum L.) was grown under CO2 partial pressures of 36 and 70 Pa with two N-application regimes. Responses of photosynthesis to varying CO2 partial pressure were fitted to estimate the maximal carboxylation rate and the nonphotorespiratory respiration rate in flag and preceding leaves. The maximal carboxylation rate was proportional to ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) content, and the light-saturated photosynthetic rate at 70 Pa CO2 was proportional to the thylakoid ATP-synthase content. Potential photosynthetic rates at 70 Pa CO2 were calculated and compared with the observed values to estimate excess investment in Rubisco. The excess was greater in leaves grown with high N application than in those grown with low N application and declined as the leaves senesced. The fraction of Rubisco that was estimated to be in excess was strongly dependent on leaf N content, increasing from approximately 5% in leaves with 1 g N m−2 to approximately 40% in leaves with 2 g N m−2. Growth at elevated CO2 usually decreased the excess somewhat but only as a consequence of a general reduction in leaf N, since relationships between the amount of components and N content were unaffected by CO2. We conclude that there is scope for improving the N-use efficiency of C3 crop species under elevated CO2 conditions.
The effects of the increasing atmospheric CO2 concentration on photosynthesis has stimulated an enormous volume of research, which has been extensively reviewed (Lawlor and Mitchell, 1991; Bowes, 1993; Drake et al., 1997). One aspect of particular interest for the possibility of crop improvement is the way in which a change in CO2 partial pressure alters the balance of limitation between processes that determine the instantaneous A in C3 plants. At high light, photosynthesis can be limited by the carboxylation capacity or by the light-saturated capacity for regeneration of RuBP, and the optimal use of resources invested in photosynthetic components (i.e. that which gives the maximal rate of photosynthesis) for a given environment occurs when these capacities are balanced (Farquhar and Sharkey, 1994). When CO2 partial pressure increases, the carboxylation capacity increases more than the light-saturated capacity for RuBP regeneration; therefore, the theoretical optimal balance of investment in photosynthetic components is altered (Sage, 1994; Webber et al., 1994; Medlyn, 1996).
Optimization of these components has considerable implications for N-use efficiency of leaf photosynthesis (Makino et al., 1997), since photosynthetic components involved in determining the carboxylation capacity (Rubisco) and light-saturated RuBP-regeneration capacity (e.g. thylakoid ATP-synthase and the Cyt b/f complex) account for a substantial fraction of leaf N (Evans and Seemann, 1989). For a doubling of CO2 partial pressure from the current ambient conditions (36 Pa), a 30% to 40% increase in the ratio of light-saturated RuBP-regeneration capacity to carboxylation capacity has been predicted for optimal N-use efficiency (Webber et al., 1994; Medlyn, 1996). Since N is likely to be more limiting to growth at elevated CO2, this increase in ratio would normally be expected to be achieved by a specific decrease in the amount of Rubisco.
Experimental evidence from gas-exchange studies suggests that such optimization occurs only infrequently in C3 plants grown at elevated CO2 (Makino, 1994; Sage, 1994; Medlyn, 1996). However, many studies have found evidence of acclimatory decreases in A and in amount of components on a leaf-area basis in response to growth at elevated CO2 (for review, see Webber et al., 1994; Drake et al., 1997). Similarly, at subambient CO2, Rubisco content per unit leaf area may increase (Rowland-Bamford et al., 1991), but there is little evidence for an increase in carboxylation capacity relative to other capacities (Sage and Reid, 1992). It therefore seems that many of the observations reflect a general reduction in all photosynthetic components due to a source-sink imbalance at higher CO2 rather than to an optimization between photosynthetic components. This is consistent with the hypothesis that the mechanism for the effect of elevated CO2 on amounts of photosynthetic components is a response to carbohydrate buildup in the leaves (van Oosten and Besford, 1996). Transcription for both a subunit of thylakoid ATP-synthase and the Rubisco small subunit was decreased by cold-girdling, which decreased carbohydrate export from the leaf (Krapp and Stitt, 1995).
In one study (Nie et al., 1995) there was apparent support for an optimization response in wheat crops grown in a free-air CO2-enrichment facility. While there was no effect of elevated CO2 on the amount of Rubisco when the leaf emerged, there was a subsequent effect, because Rubisco was decreased relative to other photosynthetic components. However, important recent work (Nakano et al., 1997) has shown that in young rice leaves the effect of elevated CO2 partial pressure appears to operate entirely via a reduction in leaf N. Since leaves with less N contain a smaller fraction of Rubisco, there is a de facto readjustment in favor of RuBP regeneration compared with carboxylation capacity, which is dependent on N-supply conditions. This result could explain the variability in experimental findings on the occurrence of the theoretical optimization (Sage, 1994), since any rebalancing will depend on whether a reduction in leaf N occurs at elevated CO2 under the experimental conditions.
The issue is of particular importance for C3 crop species, because if they do not optimize their investment in photosynthetic components in response to growth CO2 partial pressure, there may be opportunities to increase the N-use efficiency of photosynthesis by genetic manipulation for the higher CO2 environments of the future. Specifically, if there is no optimization under agronomic conditions, there will be excess investment in Rubisco relative to RuBP-regeneration capacity at high CO2 concentrations. Makino et al. (1997) recently demonstrated that N-use efficiency of leaf photosynthesis in rice, measured during short-term exposure to elevated CO2 concentrations, was greater in lines that had been transformed to specifically reduce the amount of Rubisco compared with the wild type. The extent to which this would be advantageous in plants grown at elevated CO2 is dependent on how much Rubisco remains in excess when given the opportunity to acclimate. The aim of this study was to estimate the amount of Rubisco that was in excess of the requirement for leaf photosynthesis in wheat plants grown at elevated CO2 partial pressures and various N-supply treatments under conditions representative of the field. To this end, a large number of gas-exchange measurements were combined with estimations of Rubisco and thylakoid ATP-synthase contents, the latter being chosen as the largest single component of RuBP regeneration (Evans and Seemann, 1989).
MATERIALS AND METHODS
Plant Culture
Data are presented from three experiments on spring wheat (Triticum aestivum L. cv Minaret) grown in controlled environments. Experiments 1 and 2 were designed to estimate the amount of excess Rubisco at elevated CO2 under different N-supply regimes, and experiment 3 was designed to relate the estimated excess Rubisco to its activation state. For all experiments, nine pregerminated seeds were sown in each 10-L plastic bag containing sintered agrillite rooting medium and Perlite. Groups of six bags were placed together in boxes on wheels, arranged to form four replicate arrays of three by four boxes, each assigned to a separate growth chamber (described by Lawlor et al., 1993). In experiments 1 and 2, plants were grown at two N supplies and at CO2 partial pressures of 36 Pa (ambient) or 70 Pa (elevated). In experiment 3, all plants were grown with a total N supply of 10 g m−2 and a CO2 partial pressure of 36 Pa (ambient) or 100 Pa (elevated); the more extreme elevated CO2 was chosen to ensure significant effects on activation.
Nutrient solution (described by Delgado et al., 1994) was applied twice weekly from approximately 10 to 70 d after sowing; the nitrate concentrations were 12.5 and 4.6 mm for high- and low-N regimes, respectively, for experiments 1 and 2, and 6.25 mm for experiment 3. Nitrate concentrations were varied by substituting chloride. Following eight nutrient applications, the concentrations of solutions were quadrupled in all experiments for the remaining applications. The total N application was equivalent to 8 and 18 g m−2 in experiment 1, 8 and 21 g m−2 in experiment 2 for the low- and high-N treatments, and 9.8 g m−2 in experiment 3. Although the total N applied was similar in experiments 1 and 2, the nature of the application regimes were different, since the last application was 30 d before anthesis in experiment 1 and 8 d before anthesis in experiment 2. The exact nature of the N-application regimes was not critical for the hypotheses being tested, but this change between experiments was made to induce a wider range of leaf N contents in experiment 2. Plants were watered daily with demineralized water and grown with day/night temperature regimes of 20°C/7°C from the start of tillering until maturity (before this time the temperatures were 12°C/6°C), a 16-h photoperiod, and constant artificial PPFD of 580 μmol quanta m−2 s−1 at plant height. To minimize positional effects, boxes were moved within and between chambers every 10 to 11 d. To mimic a continuous crop with an even canopy structure, reflective screens were placed from the soil surface to the top of the canopy around the arrays. Gaps caused by destructive sampling were filled with bags containing plants of the same treatment.
Gas-Exchange Measurement and Analyses
The response of A to varying pi in the leaf was determined in a six-chamber, open-circuit gas-exchange system as previously described (Lawlor et al., 1989), with a leaf temperature of 20°C, a leaf-to-air vapor-pressure deficit of 1.5 kPa, and a PPFD of 1400 to 1500 μmol quanta m−2 s−1. Saturating PPFD was used because the study concerned the effect of growth CO2 on the balance of carboxylation and RuBP-regeneration capacities, which is dictated by the amount of the components. Responses were determined in six replicate leaves for each treatment and occasion. In experiment 1, responses were determined in flag−1 (leaf preceding flag) and flag leaves on three and four occasions, respectively; in experiment 2 responses were determined in flag−2 (leaf preceding flag−1), flag−1, and flag leaves on three, three, and six occasions, respectively; and in experiment 3 responses were determined in flag leaves on seven occasions.
The first measurement of each leaf was when the ligule had just become visible. Light-saturated photosynthesis was estimated at a pi of approximately 2, 4, 7, 10, 14, 25, 50, 60, and 68 Pa for each leaf. In addition, the sensitivity of leaf photosynthesis to changing O2 partial pressure from ambient (20 kPa) to 2 kPa was determined at a pa of 70 Pa. Light-saturated photosynthesis was assumed to be determined by the RuBP-saturated kinetics of Rubisco for pi <35 Pa (Makino et al., 1988), and points in this region were fitted to Equation 1 below. In about 10% of cases this procedure gave a poor fit (r2 < 0.90), in which case the fit was restricted to points with a pi < 25 Pa, which invariably increased r2 to >0.90. The responses were fitted assuming a finite gw (Loreto et al., 1992) so that Ag is given by:
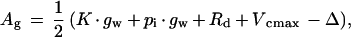 |
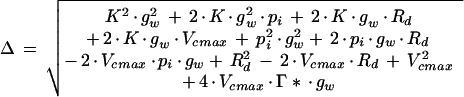 |
1 |
where K is the effective Michaelis-Menten constant for CO2 at 20 kPa O2, Γ* is the photorespiratory compensation point, Vcmax is the maximal carboxylation rate, and Rd is the rate of nonphotorespiratory respiration. A similar equation was derived by von Caemmerer and Evans (1991). The values of K and Γ* at 20°C were assumed to be constant for all leaves and were taken from the literature: K of 47.0 Pa is the value for wheat at 25°C taken from Makino et al. (1988); temperature dependence is from A.J. Keys, (unpublished data) and Machler et al. (1980); and Γ* of 3.4 Pa is from Brooks and Farquhar (1985). The value of gw cannot practically be estimated by fitting A − pi responses (von Caemmerer et al., 1994); therefore, three different approaches were tried for this parameter for all response curves. In the first, gw was assumed to have a constant value for all leaves; in the second, it varied between leaves such that the CO2 partial pressure at the site of carboxylation was 0.55 of atmospheric ambient (i.e. 20 Pa when pa = 36 Pa; Farquhar and Sharkey, 1994); in the third, an initial value of 5.0 μmol m−2 s−1 Pa−1 at the time of leaf emergence, which declined as the leaf senesced to a value of 2.0 μmol m−2 s−1 Pa−1, was used based on the dependence on leaf age found for wheat flag leaves (Loreto et al., 1994). Since the last approach gave the best r2 values (>0.95 for 96% and >0.99 for 74% of curves), all of the data presented from the 508 response curves in the three experiments were obtained using this approach. (However, because gw was not measured, the sensitivity of our conclusions to the assumed value of gw is addressed in Discussion.) For each A − pi response curve, nonlinear regression (Genstat5, Rothamsted Experimental Station, Hertfordshire, UK) was used to estimate the values of only two parameters in Equation 1, Vcmax and Rd.
For any observed value of A at a given pi, the minimum value of Vcmax required to achieve this (Vcmax,req) from Equation 1 is:
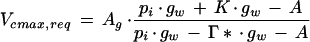 |
2 |
The amount of apparent excess investment in Rubisco (rxs) compared with that needed to maintain the A is given by:
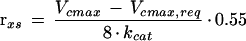 |
3 |
where kcat is the maximal rate of carboxylation per active site on fully activated Rubisco, 8 is the number of active sites per molecule, and 0.55 is the molecular mass (g μmol−1) for Rubisco. Equations 2 and 3 were used to estimate the theoretical excess in Rubisco content compared with that required to maintain the observed assimilation rate with the pa set at the elevated CO2 growth value. Thus, Equation 2 was used with the observed pi and A values corresponding to pa = 70 Pa for experiments 1 and 2 and pa = 100 Pa for experiment 3. In only 4% of the A − pi responses were these estimated values of rxs less than 0, and these were within the error of the fitting procedure, suggesting that the assumed values of K, Γ*, and gw were reasonable.
The kcat for Rubisco was estimated by the coefficient relating Vcmax to the Rubisco content, estimated by linear regression forced through the origin, and converted to moles of CO2 per mole active site per second. The kcat for thylakoid ATP-synthase can be estimated from the relationship between Ag,70 and ATP-synthase content. The rate of ATP synthesis (VATP) required to maintain a A at pa = 70 Pa was reported previously (eq. 16.27, Farquhar and von Caemmerer, 1982):
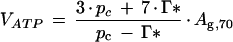 |
4 |
where pc is the CO2 partial pressure at the site of carboxylation, given by pi − A/gw. Since estimates of pc did not vary much, VATP was well approximated by 3.68(Ag,70). Linear regression forced through the origin of Ag,70 on the amount of thylakoid ATP-synthase gives a coefficient that is therefore approximately proportional to kcat. This is multiplied by the 3.68 factor and by 0.555 g μmol−1 to convert to moles of ATP per mole of ATP-synthase per second.
Biochemical Assays
The activity and amount of Rubisco and the amounts of thylakoid ATP-synthase (specifically the CF1 α- and β-subunits), soluble protein, chlorophyll, and total leaf N were determined for the same leaf samples that had been used for gas-exchange measurement. Unless otherwise stated, all operations were done between 0°C and 4°C. The leaves were cut into strips and divided for analysis of total N, Rubisco, and ATP-synthase. Requirement for material meant that only two of these were possible on a given sample. Total N, chlorophyll, and soluble protein contents were determined by the Dumas combustion method and the Arnon and Bradford methods, as previously described (Lawlor et al., 1989).
The amount of Rubisco was determined from the binding of [14C]CABP using a modified method of Yokota and Canvin (1985). One-half of the blade from each leaf sample (approximately 50 mm2) was homogenized in 2 mL of a 50 mm Bicine buffer, pH 7.5, containing 20 mm MgCl2, 1 mm EDTA, and 50 mm β-mercaptoethanol. Part of this homogenate was removed for the determination of chlorophyll and soluble protein content. The remainder was clarified at 10,000g for 3 min, and 200 μL of the supernatant was incubated for 15 min in 100 μL of a 4× activating buffer containing 400 mm Bicine, pH 8.0, 80 mm MgCl2, 40 mm NaHCO3, and 200 mm β-mercaptoethanol. To this 40 μL of 1 m Na2SO4, 35 μL of water, and 25 μL of 2.3 mm [14C]CABP (37 GBq mol−1) were added. Protein was precipitated by adding 288 μL of 60% (w/v) PEG, which was left to stand on ice for 30 min, followed by clarification at 10,000g for 10 min. The pellet was washed twice in 20% (w/v) PEG and then resuspended in 1 mL of 1% (v/v) Triton X-100 and left overnight at room temperature prior to the addition of scintillant and counting (model 2500 TR liquid-scintillation analyzer, Packard Instruments, Meriden, CT).
Initial, total, and maximal activities of Rubisco were determined using the method of Parry et al. (1997). Rubisco from leaves (40–50 mm−2) was rapidly extracted in 1 mL of CO2-free buffer containing 50 mm Bicine, pH 8.0, 20 mm MgCl2, 1 mm EDTA, and 50 mm β-mercaptoethanol. Extracts were clarified at 10,000g for 2 min and the supernatant was immediately assayed for Rubisco activity. Initial activity was determined at 25°C by adding 20 μL of extract to 980 μL of a CO2-free assay buffer containing 100 mm Bicine, pH 8.2, and 20 mm MgCl2 to which [14C]NaHCO3 (4.6 kBq μmol−1) and RuBP had been added to concentrations of 10 and 0.4 mm, respectively, immediately prior to adding the extract. Total activity was determined at 25°C by incubating 20 μL of extract for 3 min in 980 μL of the same assay buffer without RuBP and with free CO2 to allow for the carbamylation of all available active sites. The assay was started by adding RuBP to 0.4 mm as above.
For determination of maximal activity, 100 μL of extract was incubated for 30 min in an equal volume of 400 mm Na2SO4 at 4°C to remove any tight-binding inhibitors that may have been present. This was followed by centrifugation at 400g for 2 min at 4°C using Sephadex G25 medium (Pharmacia) equilibrated with extraction buffer in a small polystyrene column (Pierce). Maximal activity was determined using the protocol for total activity, beginning with the 3-min incubation in the absence of RuBP. All assays were stopped after 1 min by adding 100 μL of 10 m formic acid to liberate any unfixed 14CO2 and thereafter evaporated to dryness. Determination of 14C was by liquid-scintillation spectrometry.
The content of CF1 α- and β-subunits was quantified in a selection of the remaining leaf halves by extraction in a 50 mm Bicine buffer, pH 7.6, which contained 20 mm EDTA, 1 mm MgCl2, and 50 mm β-mercaptoethanol. Again, part of the homogenate was removed for the determination of chlorophyll and soluble protein, and each sample was clarified at 10,000g for 3 min. Samples were solubilized at a SDS to protein ratio of 4:1 in 250 mm Tris buffer, pH 7.6, containing 250 mm DTT, 10% (w/v) SDS, 0.2% (w/v) bromphenol blue, and 10% (w/v) glycerol. Denatured CF1 was electrophoresed on a 12% discontinuous gradient of SDS-polyacrylamide minigel (Bio-Rad), and separated proteins were electroblotted for 90 min at 1.8 mA cm−2 onto a PVDF membrane (Millipore) in a cell (TransBlot, Bio-Rad) containing 25 mm Tris, pH 8.3, 192 mm Gly, and 20% methanol. The membrane was blocked and developed with a chemiluminescent detection system (Aurora, ICN) using primary polyclonal antibody against the α- and β-subunits of CF1 and the manufacturer's protocol.
Bands visualized onto radiographic film were scanned and quantified using a software package (SigmaGel, Jandel Scientific, Sausalito, CA). CF1 standard run alongside samples was prepared to approximately 90% purity from 10-d-old wheat seedlings using the method of Moase and Green (1981). The standard was further purified on a Sephacryl S-300 column (Pharmacia) that had been preequilibrated with several washes of buffer containing 40 mm Tricine-NaOH, pH 8.0, 1 mm ATP, and 1 mm EDTA. Crude CF1 was applied to the column and eluted using a 0.1 to 0.5 m NaCl gradient. Total CF1 obtained was assessed at 98% purity, of which 63% represented the α- and β-subunits. Assuming that all CF1 α- and β-subunits are present in the thylakoid ATP-synthase complex, the amount of this complex was estimated by multiplying CF1 α and β content by 1.61 g ATP-synthase per g CF1 α and β (Moase and Green, 1981).
RESULTS
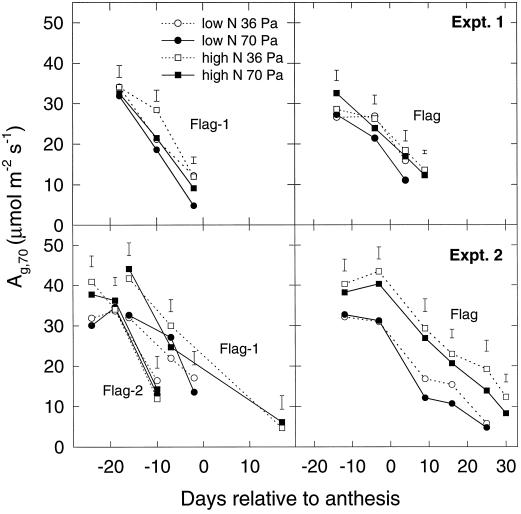
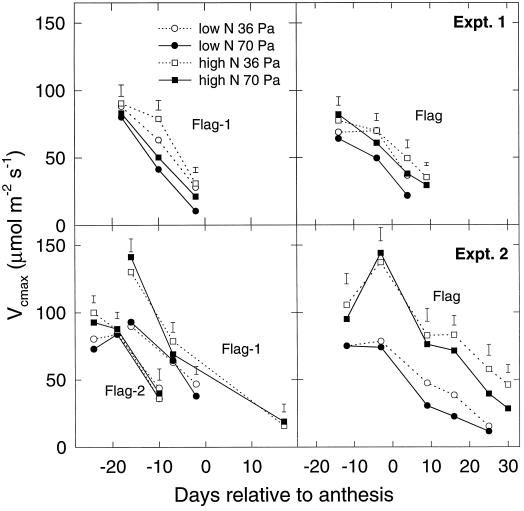
The As measured at 70 Pa pa and high light corrected for nonphotorespiratory respiration (Ag,70) are shown in Figure 1 for various leaves in experiments 1 and 2. In each case, the first point for each leaf corresponds approximately to full leaf expansion (ligule just visible), and there was no significant effect of growth-CO2 conditions on Ag at this time. When there were significant effects (e.g. at 15 d after anthesis in experiment 2), growth in elevated CO2 decreased the A. The effect of the N treatment was more pronounced in flag leaves in experiment 2 than for the other leaves in experiments 1 and 2. The pattern of effects was very similar for the carboxylation capacity, Vcmax, as estimated from A − pi responses (Fig. 2). Again, elevated CO2 treatment did not affect leaves at full expansion or shortly afterward but did induce a more rapid subsequent decline.
Figure 1.
The estimated rate (see text) of Ag,70 versus time as days relative to anthesis in wheat leaves in two experiments. Data for flag leaves are presented in separate graphs from preceding leaves for clarity. Plants were grown in different N-application and CO2 environments, as denoted in the key. For all plants, photosynthesis was measured at a leaf temperature of 20°C, a PPFD of 1500 μmol quanta m−2 s−1, and a leaf-to-air vapor-pressure deficit of 1.5 kPa. Error bars represent se of differences between mean values shown from the CO2 × N analysis of variance for each occasion (n = 6).
Figure 2.
The estimated rate (see text) of Vcmax versus time as days relative to anthesis for wheat leaves in two experiments. Treatments were as described in Figure 1. Error bars are defined as in Figure 1.
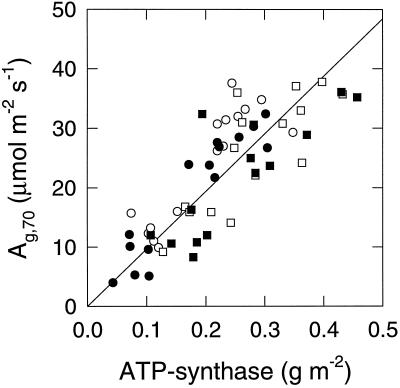
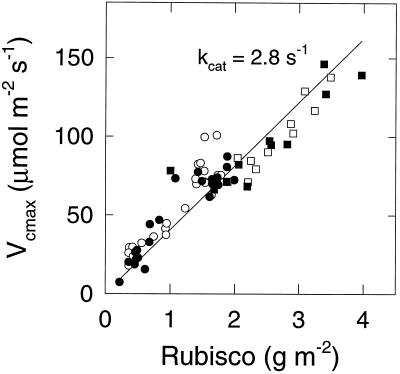
The relationship between Ag measured at 70 Pa CO2 partial pressure and the ATP-synthase content of the same leaf samples is shown in Figure 3. The line shown is a linear regression through the origin (r2 = 0.65), and from the slope, kcat was estimated using Equation 4 at 200 mol ATP mol−1 ATP-synthase s−1 (se = 6). The relationship between Vcmax estimated from A − pi responses and Rubisco content of the same leaf samples was close to the theoretical proportionality (Fig. 4). The linear regression through the origin (r2 = 0.83) gave an overall kcat estimate of 2.79 s−1 (se = 0.06).
Figure 3.
Rate of Ag,70 versus ATP-synthase content for flag 1 and flag leaves in experiment 1. Photosynthesis was measured at a leaf temperature of 20°C, a PPFD of 1500 μmol PPFD m−2 s−1, and a leaf-to-air vapor-pressure deficit of 1.5 kPa. Plants were grown with a total application of eight (circles) and 18 (squares) g N m−2 under 36 (open symbols) and 70 (closed symbols) Pa CO2. The line is a linear regression forced through the origin: y = 158x (r2 = 0.65).
Figure 4.
Rate of estimated (see text) Vcmax versus Rubisco content for the first four occasions of flag leaves from experiment 2. Conditions and treatments were as described in Figure 3. The line is a linear regression forced through the origin: y = 40.6x (r2 = 0.83).
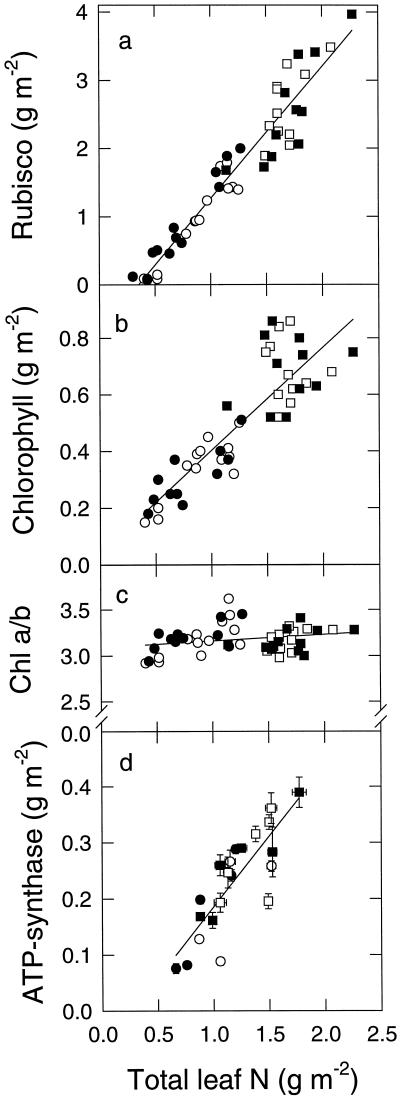
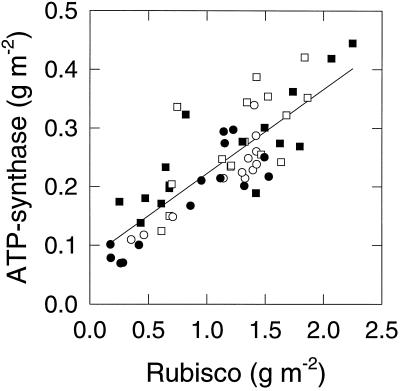
Chlorophyll, Rubisco, and ATP-synthase content were related to leaf N content (Fig. 5). Chlorophyll was approximately proportional to the leaf N content (Fig. 5b), whereas Rubisco had a positive x intercept (Fig. 5a), suggesting that the fraction of leaf N represented by Rubisco increases as leaf N content increases. ATP-synthase against leaf N (Fig. 5d) had a smaller x intercept than for Rubisco. Figure 6 shows the relationship between ATP-synthase and Rubisco content determined in the same flag−1 and flag leaves of various ages in the different treatments. It is clear that Rubisco and ATP-synthase are not simply proportional and that the mass ratio of Rubisco to ATP-synthase increases from about 3.8 at low Rubisco content to 5.75 at high Rubisco content. There were no significant effects of N or CO2 treatment on this relationship.
Figure 5.
Amounts of Rubisco and chlorophyll, chlorophyll a/b ratio, and ATP-synthase content versus total leaf N content. For ATP-synthase (d), points are the means of three N values versus the means of three ATP-synthase values in leaves of the same occasion and treatment, with se shown for both axes of each. Plants were grown at either low (circles) or high (squares) N applications and at 36 (open symbols) or 70 Pa CO2 (closed symbols). For Rubisco (a), y = 1.944x − 0.684 (r2 = 0.92). For chlorophyll (b), y = 0.364x (r2 = 0.75). For ATP-synthase (d), y = 0.15x − 0.04 (r2 = 0.69).
Figure 6.
The relationship between ATP-synthase and Rubisco contents measured in the same leaf (flag−1 or flag) of experiment 1. The line is a linear regression: y = 0.14x + 0.08 (r2 = 0.68). Symbols are as described in Figure 5.
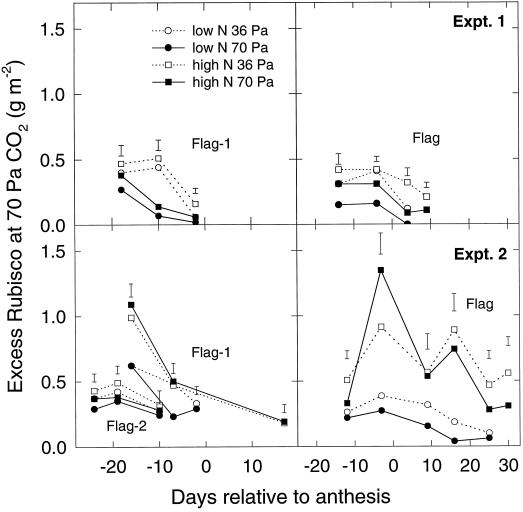
Given the relationship between Vcmax and Rubisco content (Fig. 4), Equation 3 was used to estimate the apparent excess Rubisco content compared with that required to maintain the observed rate of photosynthesis at 70 Pa atmospheric CO2 (Fig. 7). For all leaves and for both experiments, the excess was consistently less at low N. Where elevated CO2 treatment had a significant effect (flag−1 leaves in experiment 1 and flag leaves in all experiments), it decreased the excess. In some cases (e.g. experiment 2: low N, 70 Pa, flag leaves 15 d after anthesis), the estimated excess was not significantly greater than 0. It is also possible to use Equation 3 to estimate excess Rubisco at ambient CO2. This was only significantly different from 0 for the last three points for high-N flag leaves in experiment 2, decreasing from approximately 0.4 to approximately 0.2 g m−2 (data not shown).
Figure 7.
The estimated excess (see text) of Rubisco at 70 Pa CO2 atmospheric partial pressure versus time as days relative to anthesis for various wheat leaves from two experiments. Treatments were as described in Figure 1. Error bars are defined as in Figure 1.
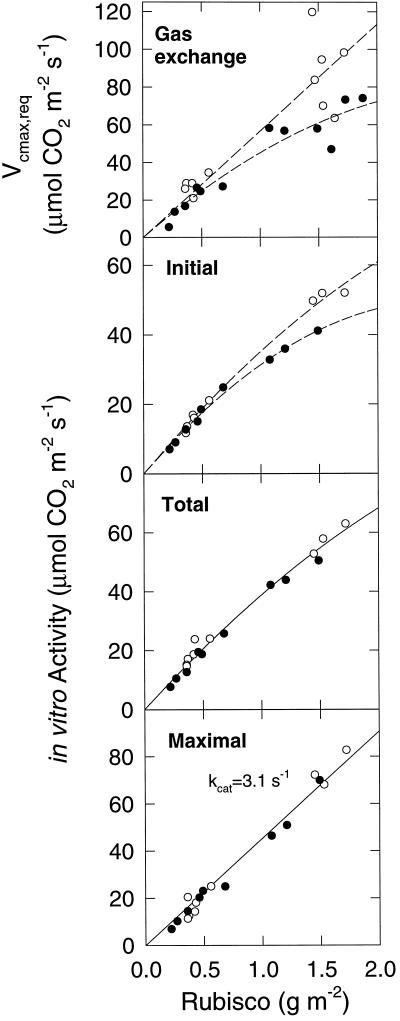
In experiment 3 leaves used for gas exchange were freeze-clamped at high light and at their growth pa and the activity of isolated Rubisco was determined in vitro, which was related to the Rubisco content. In Figure 8 the in vitro activities are compared with the Vcmax required to achieve the observed A measured at the growth CO2 partial pressure, estimated from the gas-exchange parameters using Equation 2. Total activity refers to that determined after complete activation of the enzyme and maximal activity after treatment to remove any inhibitors bound to the active site (Parry et al., 1997). The initial and total activities show a distinct curvilinear relationship, with the amount determined by [14C]CABP binding, whereas maximal activity is proportional, giving an estimated kcat of 3.1 s−1 at 25°C. Although the relationships between total and maximal activity and Rubisco content are independent of growth-CO2 treatment, the lines for initial activity for the two treatments diverge at high Rubisco content. A similar pattern is seen for the estimates of required Vcmax, which diverge at higher Rubisco contents.
Figure 8.
Estimates of in vivo and in vitro Rubisco activity versus Rubisco content in flag leaves of different ages measured between 22 d before anthesis and 18 d after anthesis in experiment 3. Plants were grown at 36 (open symbols) and 100 (closed symbols) Pa CO2 partial pressure. Top, Vcmax required to maintain observed A at a PPFD of 1500 μmol quanta m−2 s−1 and the growth CO2 partial pressure estimated from the A − pi response using Equation 2. Bottom, Initial, total, and maximal in vitro activities determined at 25°C of Rubisco isolated from leaves freeze-clamped under a PPFD of 1500 μmol quanta m−2 s−1 and the growth atmospheric CO2 partial pressure. Lines are regressions forced through the origin. Separate regressions were used for the two treatments where this improved the r2 value (dashed lines); otherwise a single regression was used (solid line). Second-order linear regressions were used where this improved the r2 value (gas-exchange, elevated-CO2 treatment, and initial and total, both treatments); otherwise, first-order regression was used.
DISCUSSION
A − pi Responses
The effect of elevated-CO2 growth treatment on the A measured at pa = 70 Pa was small, and where there was an effect, it was almost invariably a decrease (Fig. 1). Carboxylation capacity, as estimated by the Vcmax parameter, was also decreased (Fig. 2). Many studies of the effects of elevated CO2 have observed decreases in photosynthetic capacity (Sage, 1994; Webber et al., 1994; Sicher and Bunce, 1997), except when N supply was not limited (Habash et al., 1995). We found no consistent differences in the effect of CO2 on these parameters between N treatments. The application of N ended 30 d before anthesis in experiment 1 compared with 8 d before anthesis in experiment 2, and this resulted in the N treatment exerting more of an effect on leaf area in experiment 1 and more on leaf N content in experiment 2. Consequently, effects of N treatment on leaf-photosynthetic parameters are greater in experiment 2. The effect of CO2 treatment also differed somewhat between experiments; in experiment 2 there was no effect on Ag,70 or Vcmax in leaves prior to the flag leaf or in flag leaves up to 10 d after anthesis (similar to Delgado et al., 1994), whereas effects appeared earlier in experiment 1. There was no significant effect of CO2 in any leaf at full emergence (the first point measured).
Amounts of Photosynthetic Components
Ag at 70 Pa CO2 and ATP-synthase content were well correlated (Fig. 3) and resulted in a kcat estimate for ATP-synthase (200 s−1) comparable to that obtained in vitro (160 s−1; Fromme and Graber, 1989). A strong relationship was expected because effectively all products of photosynthetic electron transport are used in RuBP regeneration for photosynthesis and photorespiration (Habash et al., 1995), and the A is usually limited by RuBP regeneration at the pi used here (45–55 Pa) (Makino et al., 1988). Although under these conditions the rate can also be limited by triose-phosphate-utilization capacity (Farquhar and Sharkey, 1994), in our experiments the stimulation of Ag,70 on decreasing O2 partial pressure to 2 kPa was mostly close to the theoretical expectation for RuBP-regeneration-limited photosynthesis (mean stimulation 18%; data not shown).
The relationship between the estimated in vivo Vcmax and the Rubisco content used to derive the estimate of Rubisco kcat of 2.8 s−1 at 20°C (Fig. 4) was strong (r2 = 0.83). The value is in line with in vivo estimates for tobacco of 3.5 s−1 (25°C; von Caemmerer et al., 1994) and for wheat of 3.3 s−1 (23°C; Evans and Austin, 1986) and an in vitro estimate for wheat of 3.0 s−1 (25°C; Makino et al., 1988), given the temperature dependence that would be expected to increase the value by 40% going from 20°C to 25°C (Machler et al., 1980; Makino et al., 1988). The fraction of leaf N invested in Rubisco increases with leaf N content from 21% to 26% in the range 1 to 2 g N m−2 (Fig. 5d) in a manner similar to previous findings for wheat flag leaves of varying age (Makino et al., 1988; Lawlor et al., 1989).
The relationship between ATP-synthase and Rubisco content determined in experiment 1 (Fig. 6) was such that the ratio of Rubisco to ATP-synthase increased with increasing Rubisco content independently of treatment. Since the amounts of these components are expected to reflect carboxylation and RuBP-regeneration capacities (supported by the data in Figs. 3 and 4), this suggests that carboxylation capacity increases relative to RuBP-regeneration capacity in leaves with high N content and that this is not affected by elevated CO2. This is in close agreement with the results of Nakano et al. (1997) using rice. Also, a field experiment on wheat revealed that elevated CO2 did not affect the ratio of Rubisco to other components, including CF1, at flag leaf emergence but did induce a decrease in this ratio during grain fill (Nie et al., 1995), which could be interpreted as a more rapid decline in leaf N in the elevated-CO2-grown leaves.
Excess Investment in Rubisco
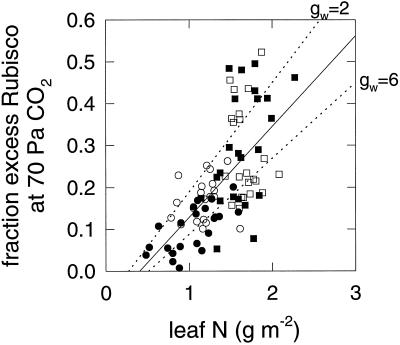
The amount of Rubisco in excess of the requirement to maintain photosynthesis at high light and pa = 70 Pa estimated from the gas-exchange data (Fig. 7) showed a clear pattern in all leaves: the excess was greater in high-N compared with low-N leaves and declined as leaves senesced. The effect of elevated CO2 treatment was more variable but generally decreased the excess. It is therefore clear that some rebalancing of capacities does occur in the elevated-CO2 treatment, since the excess is lower, but there is still some excess until well into senescence in the low-N treatments and throughout in the high-N treatments. From the results presented above and from the conclusions of Nakano et al. (1997), such rebalancing can be explained purely in terms of elevated-CO2 treatment inducing a reduction in leaf N. This is associated with a greater than proportional reduction in Rubisco (Fig. 5a) compared with all of the other photosynthetic components, particularly ATP-synthase (Fig. 6); therefore, there is a de facto rebalancing away from carboxylation to RuBP-regeneration capacities. This hypothesis is supported by the relationship between the data in Figure 7, expressed as the fraction of Rubisco in excess at 70 Pa CO2 versus leaf N (Fig. 9). The fraction increases from approximately 5% in leaves with 1 g N m−2 to approximately 40% in leaves with 2 g N m−2 regardless of CO2 treatment. Thus, any decrease in excess Rubisco due to elevated growth-CO2 partial pressure appears to be an indirect consequence of decreased leaf N.
Figure 9.
The fraction of Rubisco estimated to be in excess of that required to maintain A at 70 Pa atmospheric CO2 partial pressure versus total leaf N content, for all leaves in experiments 1 and 2. Plants were grown at low (circles) and high (squares) N applications and at 36 (open symbols) or 70 (closed symbols) Pa CO2. The solid line is linear regression of all points (r2 = 0.41; n = 87). Dotted lines are equivalent regressions for same data when recalculated with gw = 2 and 6 μmol m−2 s−1 Pa−1 (r2 = 0.56 and 0.45, respectively).
The estimates of excess Rubisco refer to the conditions used for the determination of steady-state photosynthesis. The conditions of high light intensity and moderate temperature used are those under which Rubisco would be expected to be the least in excess; therefore, the excess would presumably be greater under variable field conditions at elevated CO2. (However, this conclusion would not hold for conditions that decrease stomatal conductance, such as water stress.)
Sensitivity to gw
The above analysis was based on assuming a variable gw, as described in Methods. To investigate the sensitivity of the conclusions based on this assumption, the analysis was repeated with constant gw values of 2.0 and 6.0 μmol m−2 s−1 Pa−1, spanning the range estimated in measurements (von Caemmerer and Evans, 1991; Loreto et al., 1994) or infinity. For the regression between Vcmax and Rubisco content, kcat estimates of 3.71, 2.53, 2.17 s−1 with r2 = 0.75, 0.67, 0.56, respectively, were derived, compared with 2.79 s−1 with r2 = 0.83 for the data shown in Figure 4. The assumption of a finite gw, therefore, improved the fit between Rubisco content and photosynthetic parameters, as was found previously for wheat (Evans and Austin, 1986; Makino et al., 1988). Whereas the effect on kcat was quite marked, the value of gw had much less effect on the main aim of estimating the fraction of Rubisco that is in excess at elevated CO2. The dotted lines in Figure 9 show the corresponding linear regressions with gw = 2 and 6 μmol m−2 s−1 Pa−1. These values would alter the estimate of 40% excess Rubisco at 2 g N m−2 to 50% and 30%, respectively. Even making the unlikely assumption of an infinite gw gave the same highly significant trend and a corresponding fraction excess of 20% (data not shown).
Rubisco Activation
It is usually assumed that Rubisco in excess of that required to maintain carboxylation will be deactivated, because RuBP concentrations are stable (Sage et al., 1990) and a reduction in the Rubisco activation state at elevated CO2 has been found in some (Sage et al., 1990; Nakano et al., 1997) but not all (Sicher et al., 1995) studies. The estimated value of the Vcmax required to maintain observed A (Vcmax,req from Eq. 2) should therefore be directly comparable to the initial activity of Rubisco. Activity parameters determined for Rubisco isolated from leaf sections freeze-clamped under high light and growth-CO2 partial pressure were compared with Vcmax,req values estimated for the same leaf sections under these conditions (Fig. 8).
The pattern of Vcmax,req was similar to initial activity, with elevated CO2 points lying increasingly below ambient CO2 points as Rubisco content increased, although the absolute initial in vitro activities were much lower. The apparent decrease in activation state (initial activity/maximal activity) with increasing Rubisco content in ambient CO2 leaves to 60% to 70% was unexpected, since it has been assumed that all Rubisco is utilized at high light and pa = 36 Pa, for which there is good evidence (Makino et al., 1988; Sage et al., 1990) and which resulted in a close relationship between estimated Vcmax and Rubisco content (Fig. 4). Part of the decrease in activation state is associated with an inhibitor, as demonstrated by the difference between total and maximal activity (Parry et al., 1997), but initial activity was also somewhat lower than total. Although the conditions used were similar to those used in many other studies, initial activity is known to be affected by pretreatment of the extraction buffer and by the time taken to determine activity (Sage et al., 1993), which may have lowered absolute initial activities in our material, but the difference between treatments probably reflects the in vivo state.
The in vitro maximal activity of Rubisco was closely correlated (r2 = 0.98) with its amount, and the estimated kcat of 3.1 s−1 was the same as that estimated previously for wheat at 25°C (Makino et al., 1988). However, even the maximal activity was somewhat less (20% lower at 1.5 g m−2 Rubisco) than the Vcmax,req for ambient leaves (Fig. 8); estimates of Rubisco activity are often higher in vivo than in vitro (von Caemmerer et al., 1994).
CONCLUSIONS
We have found evidence that there is excess investment in Rubisco over that required for maintaining A at elevated CO2 and that this excess is greater at high N. Long-term growth at elevated CO2 does reduce this excess somewhat but apparently only as an indirect consequence of elevated CO2 causing a decreased leaf N content, which agrees with the findings of Nakano et al. (1997). There is therefore no evidence of a direct mechanism optimizing the balance between carboxylation and RuBP-regeneration capacities in response to long-term growth at elevated CO2. Makino et al. (1997) observed an increase in A per unit leaf N in rice plants genetically manipulated to reduce Rubisco expression, which was measured under conditions of short-term exposure to elevated CO2. In agreement with the relationship shown in Figure 9, they found the greatest advantage in leaves with high N content. The work presented here suggests that most of this advantage would be preserved if the plants were grown under conditions of elevated CO2, since there is still excess investment in Rubisco under these conditions. There is therefore potential for improving the adaptation of crop plants to growth at elevated CO2.
ACKNOWLEDGMENTS
The kind gift of antisera against CF1 from Dr. J.C. Gray (University of Cambridge, UK) is gratefully acknowledged. We thank Dr. P.J. Andralojc for assistance and advice with the ATP-synthase work and S.P. Driscoll and V.J. Mitchell for technical assistance.
Abbreviations:
- A
photosynthetic rate
- Ag
gross photosynthetic rate
- CABP
2-carboxyarabinitol-1,5-bisphosphate
- CF1
thylakoid coupling factor 1
- gw
conductance for diffusion of CO2 from the intercellular space to the carboxylation site
- pa
external CO2 partial pressure
- pi
internal CO2 partial pressure
- RuBP
ribulose-1,5-bisphosphate
Footnotes
J.C.T. was supported by a grant from the European Union as part of the European Stress Physiology and Climate Experiment. Wheat project (contract no. EV5V-C793-0301). IACR-Rothamsted receives grant-aided support from the Biotechnology and Biological Sciences Research Council of the United Kingdom.
LITERATURE CITED
- Bowes G. Facing the inevitable—plants and increasing atmospheric CO2. Annu Rev Plant Physiol Mol Biol. 1993;44:309–332. [Google Scholar]
- Brooks A, Farquhar GD. Effect of temperature on the CO2/O2 specificity of ribulose-1,5- bisphosphate carboxylase oxygenase and the rate of respiration in the light—estimates from gas-exchange measurements on spinach. Planta. 1985;165:397–406. doi: 10.1007/BF00392238. [DOI] [PubMed] [Google Scholar]
- Delgado E, Mitchell RAC, Parry MAJ, Driscoll SP, Mitchell VJ, Lawlor DW. Interacting effects of CO2 concentration, temperature and nitrogen supply on the photosynthesis and composition of winter-wheat leaves. Plant Cell Environ. 1994;17:1205–1213. [Google Scholar]
- Drake BG, Gonzalez-Meler MA, Long SP. More efficient plants: a consequence of rising atmospheric CO2? Annu Rev Plant Physiol Mol Biol. 1997;48:609–639. doi: 10.1146/annurev.arplant.48.1.609. [DOI] [PubMed] [Google Scholar]
- Evans JR, Austin RB. The specific activity of ribulose-1,5-bisphosphate carboxylase in relation to genotype in wheat. Planta. 1986;167:344–350. doi: 10.1007/BF00391337. [DOI] [PubMed] [Google Scholar]
- Evans JR, Seemann JR (1989) The allocation of protein nitrogen in the phototsynthetic apparatus: costs, consequences and control. In W Briggs, ed, Photosynthesis. Alan R Liss, New York, pp 183–205
- Farquhar GD, von Caemmerer S. Modelling of photosynthetic response to environmental conditions. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Physiological Plant Ecology, Vol 12. Berlin: Springer-Verlag; 1982. pp. 549–587. [Google Scholar]
- Farquhar GD, Sharkey TD (1994) Photosynthesis and carbon assimilation. In KJ Boote, JM Bennett, TR Sinclair, GM Paulson, eds, Physiology and Determination of Crop Yield. American Society of Agronomy, Madison, WI, pp 187–210
- Fromme P, Graber P. Heterogeneity of ATP-hydrolyzing sites on reconstituted CF0CF1. FEBS Lett. 1989;259:33–36. [Google Scholar]
- Habash DZ, Paul MJ, Parry MAJ, Keys AJ, Lawlor DW. Increased capacity for photosynthesis in wheat grown at elevated CO2—the relationship between electron-transport and carbon metabolism. Planta. 1995;197:482–489. [Google Scholar]
- Krapp A, Stitt M. An evaluation of direct and indirect mechanisms for the sink-regulation of photosynthesis in spinach—changes in gas-exchange, carbohydrates, metabolites, enzyme-activities and steady-state transcript levels after cold-girdling source leaves. Planta. 1995;195:313–323. [Google Scholar]
- Lawlor DW, Konturri M, Young AT. Photosynthesis by flag leaves of wheat in relation to protein, ribulose bisphospate carboxylase activity and nitrogen supply. J Exp Bot. 1989;40:43–52. [Google Scholar]
- Lawlor DW, Mitchell RAC. The effects of increasing CO2 on crop photosynthesis and productivity—a review of field studies. Plant Cell Environ. 1991;14:807–818. [Google Scholar]
- Lawlor DW, Mitchell RAC, Franklin J, Mitchell VJ, Driscoll SP, Delgado E. Facility for studying the effects of elevated carbon-dioxide concentration and increased temperature on crops. Plant Cell Environ. 1993;16:603–608. [Google Scholar]
- Loreto F, Dimarco G, Tricoli D, Sharkey TD. Measurements of mesophyll conductance, photosynthetic electron-transport and alternative electron sinks of field-grown wheat leaves. Photosynth Res. 1994;41:397–403. doi: 10.1007/BF02183042. [DOI] [PubMed] [Google Scholar]
- Loreto F, Harley PC, Dimarco G, Sharkey TD. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiol. 1992;98:1437–1443. doi: 10.1104/pp.98.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machler F, Keys AJ, Cornelius MJ. Activation of ribulose bisphosphate carboxylase purified from wheat leaves. J Exp Bot. 1980;31:7–14. [Google Scholar]
- Makino A. Biochemistry of C3-photosynthesis in high CO2. J Plant Res. 1994;107:79–84. [Google Scholar]
- Makino A, Mae T, Ohira K. Differences between wheat and rice in the enzymic properties of ribulose-1,5-bisphosphate carboxylase oxygenase and the relationship to photosynthetic gas-exchange. Planta. 1988;174:30–38. doi: 10.1007/BF00394870. [DOI] [PubMed] [Google Scholar]
- Makino A, Shimada T, Takumi S, Kaneko K, Matsuoka M, Shimamoto K, Nakano H, Miyao-Tokutomi M, Mae T, Yamamoto N. Does decrease in ribulose-1,5-bisphosphate carboxylase by antisense RbcS lead to a higher N-use efficiency of photosynthesis under conditions of saturating CO2 and light in rice plants? Plant Physiol. 1997;114:483–491. doi: 10.1104/pp.114.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlyn BE. The optimal allocation of nitrogen within the C3 photosynthetic system at elevated CO2. Aust J Plant Physiol. 1996;23:593–603. [Google Scholar]
- Moase EH, Green BR. Isolation and properties of chloroplast coupling factor from wheat. Eur J Biochem. 1981;119:145–150. doi: 10.1111/j.1432-1033.1981.tb05587.x. [DOI] [PubMed] [Google Scholar]
- Nakano H, Makino A, Mae T. The effect of elevated partial pressures of CO2 on the relationship between photosynthetic capacity and N content in rice leaves. Plant Physiol. 1997;115:191–198. doi: 10.1104/pp.115.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie GY, Long SP, Garcia RL, Kimball BA, Lamorte RL, Pinter PJ, Wall GW, Webber AN. Effects of free-air CO2 enrichment on the development of the photosynthetic apparatus in wheat, as indicated by changes in leaf proteins. Plant Cell Environ. 1995;18:855–864. [Google Scholar]
- Parry MAJ, Andralojc PJ, Parmar S, Keys AJ, Habash D, Paul MJ, Alred R, Quick WP, Servaites JC. Regulation of Rubisco by inhibitors in the light. Plant Cell Environ. 1997;20:528–534. [Google Scholar]
- Rowland-Bamford AJ, Baker JT, Allen LH, Bowes G. Acclimation of rice to changing atmospheric carbon-dioxide concentration. Plant Cell Environ. 1991;14:577–583. [Google Scholar]
- Sage RF. Acclimation of photosynthesis to increasing atmospheric CO2—the gas-exchange perspective. Photosynth Res. 1994;39:351–368. doi: 10.1007/BF00014591. [DOI] [PubMed] [Google Scholar]
- Sage RF, Reid CD. Photosynthetic acclimation to sub-ambient CO2 (20 Pa) in the C3 annual Phaseolus vulgaris L. Photosynthetica. 1992;27:605–617. [Google Scholar]
- Sage RF, Reid CD, Moore BD, Seemann JR. Long-term kinetics of the light-dependent regulation of ribulose-1,5-bisphosphate carboxylase oxygenase activity in plants with and without 2-carboxyarabinitol 1-phosphate. Planta. 1993;191:222–230. [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR. Regulation of ribulose-1,5-bisphosphate carboxylase activity in response to light intensity and CO2 in the C3 annuals Chenopodium album and Phaseolus vulgaris. Plant Physiol. 1990;94:1735–1742. doi: 10.1104/pp.94.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher RC, Bunce JA. Relationship of photosynthetic acclimation to changes of Rubisco activity in field-grown winter wheat and barley during growth in elevated carbon dioxide. Photosynth Res. 1997;52:27–38. [Google Scholar]
- Sicher RC, Kremer DF, Bunce JA. Photosynthetic acclimation and photosynthate partitioning in soybean leaves in response to carbon-dioxide enrichment. Photosynth Res. 1995;46:409–417. doi: 10.1007/BF00032295. [DOI] [PubMed] [Google Scholar]
- van Oosten JJ, Besford RT. Acclimation of photosynthesis to elevated CO2 through feedback-regulation of gene-expression—climate of opinion. Photosynth Res. 1996;48:353–365. doi: 10.1007/BF00029468. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR. Determination of the average partial-pressure of CO2 in chloroplasts from leaves of several C3 plants. Aust J Plant Physiol. 1991;18:287–305. [Google Scholar]
- von Caemmerer S, Evans JR, Hudson GS, Andrews TJ. The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in-vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta. 1994;195:88–97. [Google Scholar]
- Webber AN, Nie GY, Long SP. Acclimation of photosynthetic proteins to rising atmospheric CO2. Photosynth Res. 1994;39:413–425. doi: 10.1007/BF00014595. [DOI] [PubMed] [Google Scholar]
- Yokota A, Canvin DT. Ribulose bisphosphate carboxylase oxygenase content determined with [C-14]carboxypentitol bisphosphate in plants and algae. Plant Physiol. 1985;77:735–739. doi: 10.1104/pp.77.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]